A Review on Biomedical Applications of Plant Extract-Mediated Metallic Ag, Au, and ZnO Nanoparticles and Future Prospects for Their Combination with Graphitic Carbon Nitride
Abstract
1. Introduction
2. Different Synthesis Methods Used for Nanoparticles Investigation
Merits of Biological Methods for Sustainable Approach
3. Methodology of Plant-Based Synthesis of Nanoparticles and Their Mechanisms
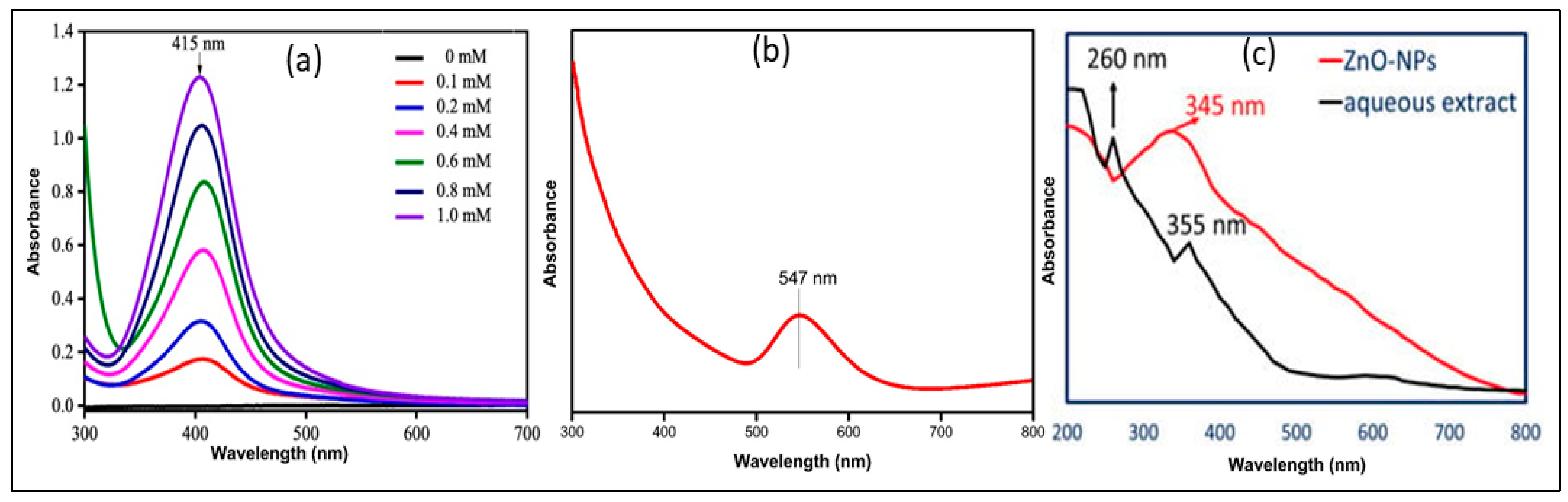
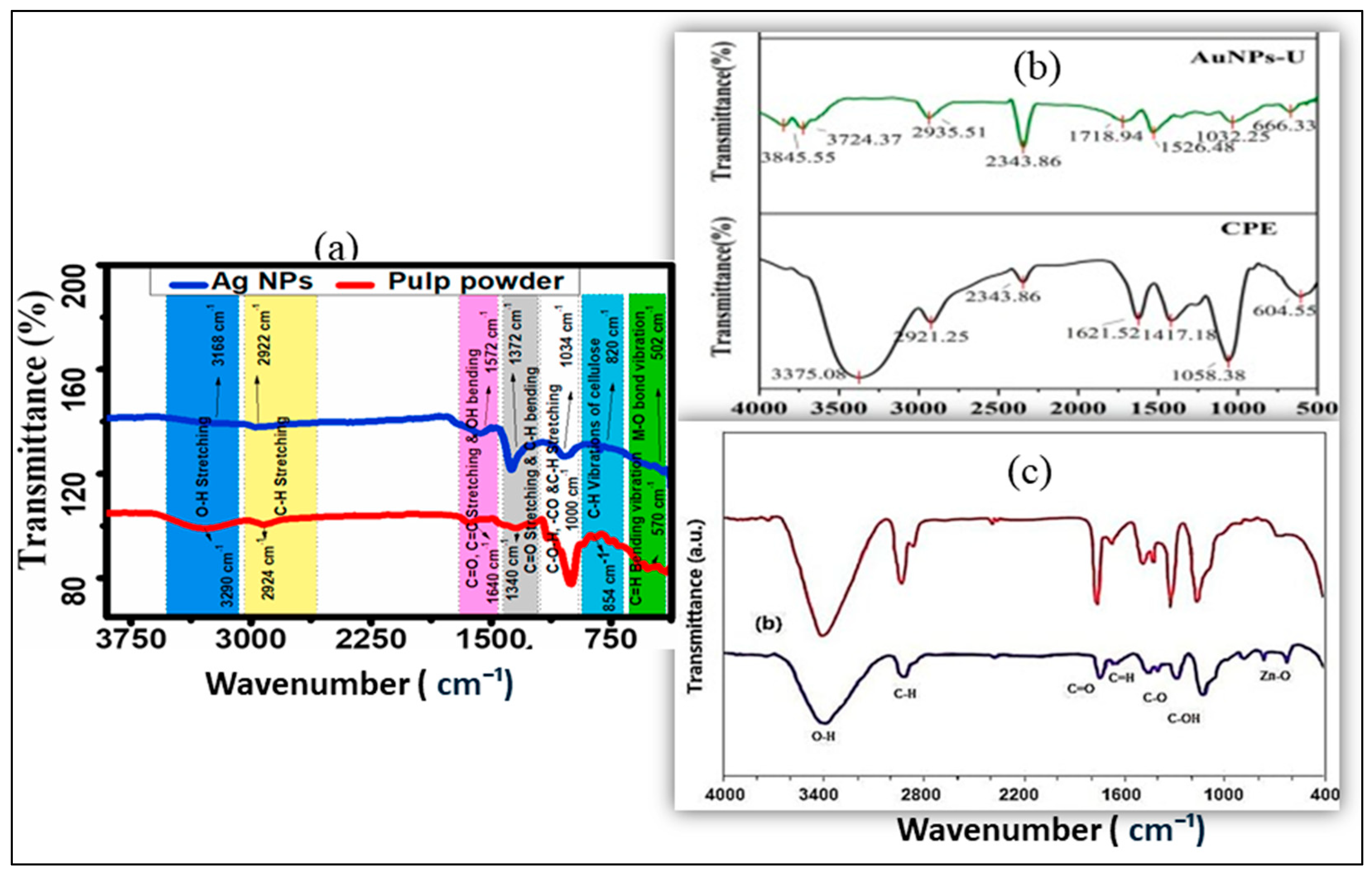
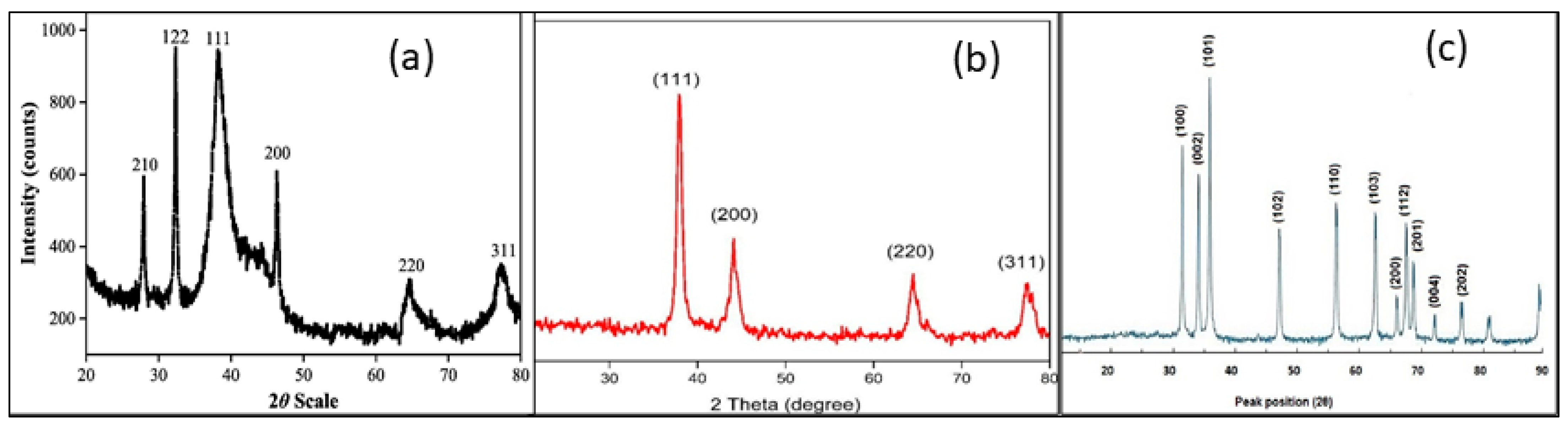
3.1. Characterization Methods to Control the Stability and the Properties
3.2. Morphology and Size Distribution
4. Literature Study
4.1. Pioneering Studies on Ag-NPs Derived from Plant Extracts
4.2. Studies on Au-NPs Synthesized from Plant Extract
4.3. Exploratory Studies on ZnO-NPs Produced from Plant Extracts
4.4. Biomedical Applications
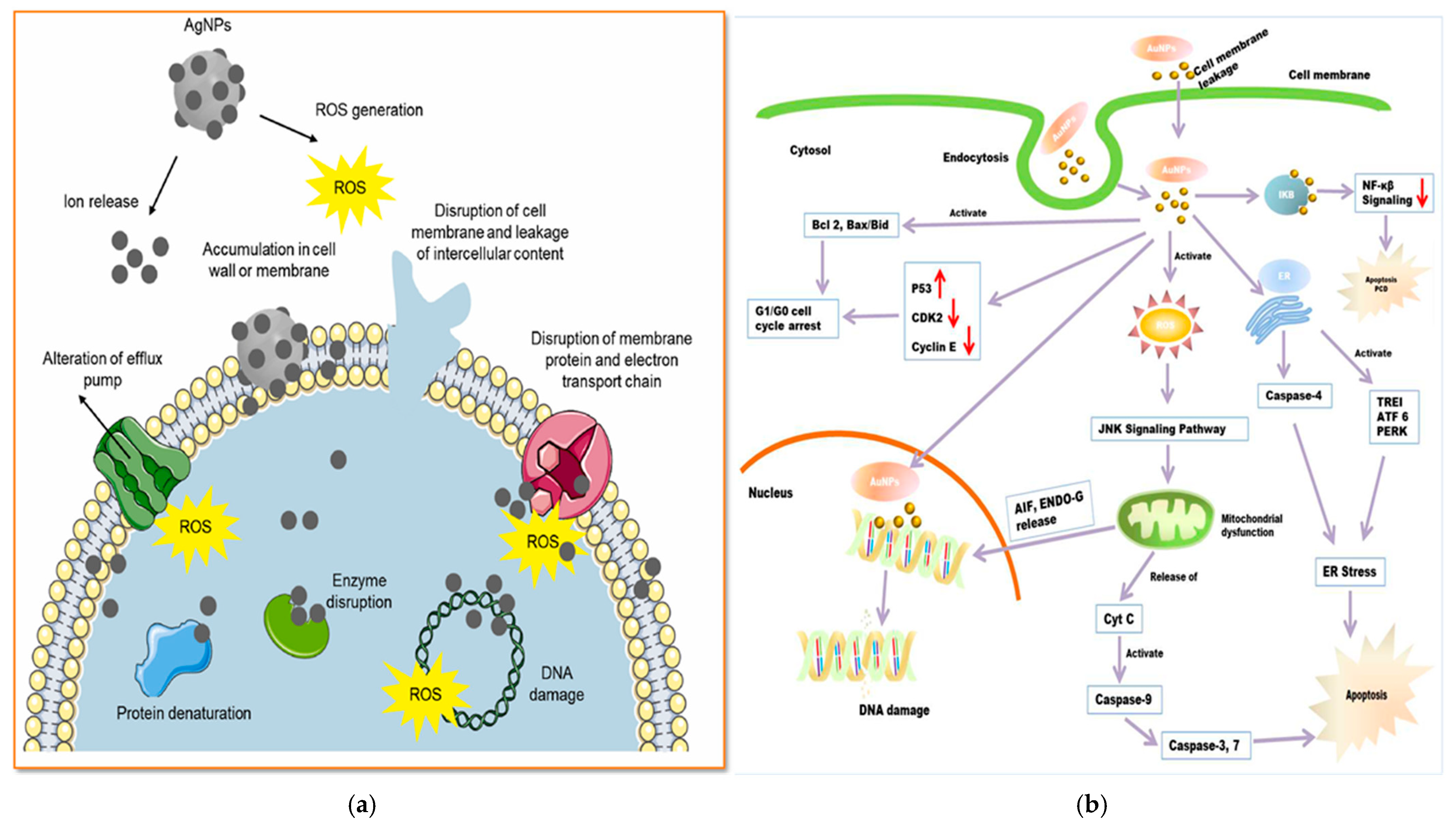
4.4.1. Antimicrobial and Antioxidant Activities
4.4.2. Anticancer Activities
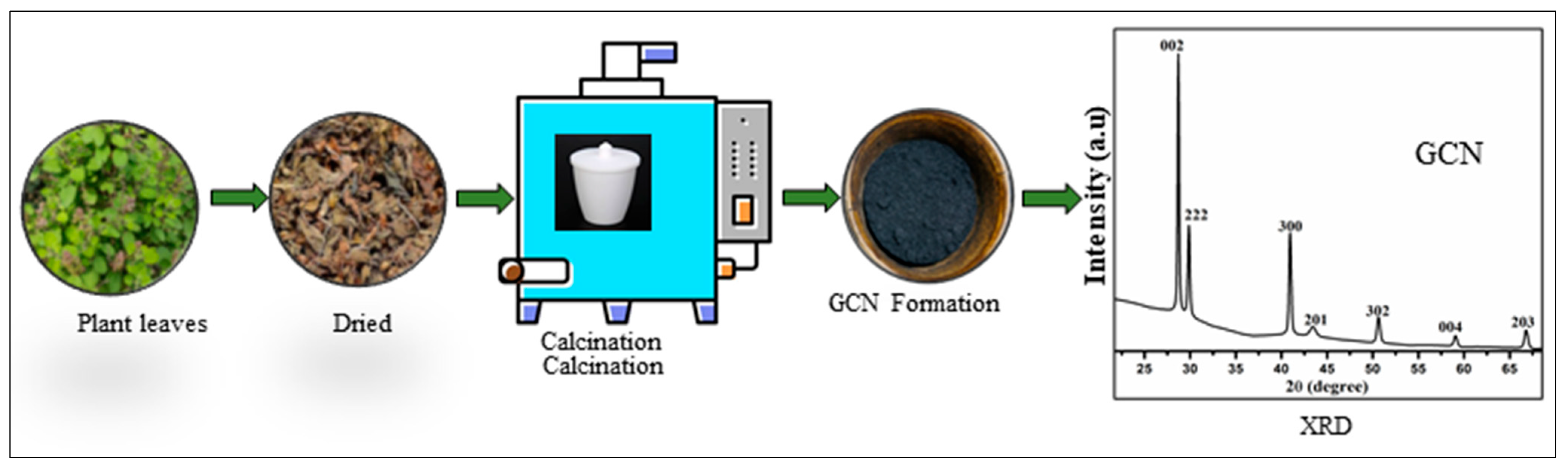
5. Future Outlook of Hybrid Nanocomposites with Graphitic Carbon Nitride (GCN)
Our Current Research Approach and Innovations
6. Limitations of Green-Synthesized Nanoparticles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Bacha, M.; Shah, M.R.; Shah, W.; Kubra, K.; Khan, A.; Ahmad, M.; Latif, A.; Ali, M. Green synthesis of silver and gold nanoparticles using Crataegus oxyacantha extract and their urease inhibitory activities. Biotechnol. Appl. Biochem. 2021, 68, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fozia Jabeen, M.; Haq, Z.U.; Ahmad, I.; Wahab, A.; Islam, Z.U.; Ullah, R.; Bari, A.; Abdel-Daim, M.M.; El-Demerdash, F.M.; et al. Green fabrication of silver nanoparticles using Euphorbia serpens Kunth aqueous extract, their characterization, and investigation of its in vitro antioxidative, antimicrobial, insecticidal, and cytotoxic activities. BioMed Res. Int. 2022, 2022, 5562849. [Google Scholar] [CrossRef]
- Alhomaidi, E.; Jasim, S.A.; Amin, H.I.M.; Lima Nobre, M.A.; Khatami, M.; Jalil, A.T.; Hussain Dilfy, S. Biosynthesis of silver nanoparticles using Lawsonia inermis and their biomedical application. IET Nanobiotechnol. 2022, 16, 284–294. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Griškonis, E.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Boruah, J.S.; Devi, C.; Hazarika, U.; Reddy, P.V.B.; Chowdhury, D.; Barthakur, M.; Kalita, P. Green synthesis of gold nanoparticles using an antiepileptic plant extract: In vitro biological and photo-catalytic activities. RSC Adv. 2021, 11, 28029–28041. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green biosynthesis of flaxseed gold nanoparticles (Au-NPs) as a potent anti-cancer agent against breast cancer cells. J. Saudi Chem. Soc. 2021, 25, 101243. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Q.; Wang, Z.; Ju, F. Oak gum mediated sustainable synthesis of gold nanoparticles (Au NPs): Evaluation of its antioxidant and anti-colon cancer effects. J. Exp. Nanosci. 2022, 17, 377–388. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Ramesh, A.M.; Purushotham, D.; Kodandaram, A.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Gowtham, H.G.; Rahdar, A.; Kaviyarasu, K.; Murali, M. Visible light driven photocatalytic and competent antioxidant properties of phyto-fabricated zinc oxide nanoparticles (ZnO-NPs) from Borreria hispida. J. Mol. Struct. 2023, 1293, 136152. [Google Scholar] [CrossRef]
- Awan, S.S.; Khan, R.T.; Mehmood, A.; Hafeez, M.; Abass, S.R.; Nazir, M.; Raffi, M. Ailanthus altissima leaf extract mediated green production of zinc oxide (ZnO) nanoparticles for antibacterial and antioxidant activity. Saudi J. Biol. Sci. 2023, 30, 103487. [Google Scholar] [CrossRef]
- Chunchegowda, U.A.; Shivaram, A.B.; Mahadevamurthy, M.; Ramachndrappa, L.T.; Lalitha, S.G.; Krishnappa, H.K.N.; Anandan, S.; Sudarshana, B.S.; Chanappa, E.G.; Ramachandrappa, N.S. Biosynthesis of zinc oxide nanoparticles using leaf extract of Passiflora subpeltata: Characterization and antibacterial activity against Escherichia coli isolated from poultry faeces. J. Clust. Sci. 2021, 32, 1663–1672. [Google Scholar] [CrossRef]
- Dappula, S.S.; Kandrakonda, Y.R.; Shaik, J.B.; Mothukuru, S.L.; Lebaka, V.R.; Mannarapu, M.; Amooru, G.D. Biosynthesis of zinc oxide nanoparticles using aqueous extract of Andrographis alata: Characterization, optimization and assessment of their antibacterial, antioxidant, antidiabetic and anti-Alzheimer’s properties. J. Mol. Struct. 2023, 1273, 134264. [Google Scholar] [CrossRef]
- Gamedze, N.P.; Mthiyane, D.M.N.; Mavengahama, S.; Singh, M.; Onwudiwe, D.C. Biosynthesis of ZnO nanoparticles using the aqueous extract of Mucuna pruriens (utilis): Structural characterization, and the anticancer and antioxidant activities. Chem. Afr. 2024, 7, 219–228. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium favum leaf extract. Appl. Nanosci. 2023, 13, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Dulta, K.; Ağçeli, G.K.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K. A novel approach of synthesis zinc oxide nanoparticles by Bergenia ciliata rhizome extract: Antibacterial and anticancer potential. J. Inorg. Organomet. Polym. Mater. 2021, 31, 180–190. [Google Scholar] [CrossRef]
- Palithya, S.; Gaddam, S.A.; Kotakadi, V.S.; Penchalaneni, J.; Golla, N.; Naidu Krishna, S.B.; Naidu, C.V. Green synthesis of silver nanoparticles using flower extracts of Aerva lanata and their biomedical applications. Part. Sci. Technol. 2022, 40, 84–96. [Google Scholar] [CrossRef]
- Darwesh, M.Y.; Ibrahim, S.S.; Mohammed, M.A. Plant extract mediated green synthesis of zinc oxide nanoparticles and their biomedical applications. Results Chem. 2024, 7, 101368. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ.-Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Du, M.; Wang, L. Eco-friendly synthesis of size-controlled silver nanoparticles by using Areca catechu nut aqueous extract and investigation of their potent antioxidant and antibacterial activities. Arab. J. Chem. 2022, 15, 103763. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumar, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity, and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Surendra, B.S.; Mallikarjunaswamy, C.; Pramila, S.; Rekha, N.D. Bio-mediated synthesis of ZnO nanoparticles using Lantana Camara flower extract: Its characterizations, photocatalytic, electrochemical and anti-inflammatory applications. J. Clust. Sci. 2021, 32, 647–663. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Varghese, B.A.; Nair, R.V.R.; Jude, S.; Varma, K.; Amalraj, A.; Kuttappan, S. Green synthesis of gold nanoparticles using Kaempferia parviflora rhizome extract and their characterization and application as an antimicrobial, antioxidant, and catalytic degradation agent. J. Taiwan Inst. Chem. Eng. 2021, 126, 166–172. [Google Scholar] [CrossRef]
- Devanesan, S.; Al Salhi, M.S. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int. J. Nanomed. 2021, 16, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Jalab, J.; Abdelwahed, W.; Kitaz, A.; Al-Kayali, R. Green synthesis of silver nanoparticles using aqueous extract of Acacia cyanophylla and its antibacterial activity. Heliyon 2021, 7, e08033. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green fabrication of zinc oxide nanoparticles using Phlomis leaf extract: Characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef]
- Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Hoşgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green synthesis of silver nanoparticles from Allium cepa L. peel extract, their antioxidant, antipathogenic, and anticholinesterase activity. Molecules 2023, 28, 2310. [Google Scholar] [CrossRef]
- Efati, Z.; Shahangian, S.S.; Darroudi, M.; Amiri, H.; Hashemy, S.I.; Aghamaali, M.R. Green chemistry synthesized zinc oxide nanoparticles in Lepidium sativum L. seed extract and evaluation of their anticancer activity in human colorectal cancer cells. Ceram. Int. 2023, 49, 32568–32576. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Ma, H.; Chen, X. Ultrasound-assisted green synthesis of gold nanoparticles using citrus peel extract and their enhanced anti-inflammatory activity. Ultrason. Sonochem. 2022, 83, 105940. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Giri, S.; Nandi, G.; Sahu, R.; Shaw, T.K.; Paul, P. Green synthesis of silver nanoparticles using Eupatorium adenophorum leaf extract: Characterizations, antioxidant, antibacterial and photocatalytic activities. Chem. Pap. 2023, 77, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Hasan, M.; Zafar, A.; Imran, M.; Iqbal, K.J.; Tariq, T.; Iqbal, J.; Shaheen, A.; Hussain, R.; Anjum, S.I.; Shu, X. Crest to trough cellular drifting of green-synthesized zinc oxide and silver nanoparticles. ACS Omega 2022, 7, 34770–34778. [Google Scholar] [CrossRef]
- Mishra, S.; Das, A.; Mohanty, S. Green synthesis of silver nanoparticles using Phyllanthus niruri and their biomedical applications. ACS Omega 2023, 8, 1528–1537. [Google Scholar] [CrossRef]
- Nazir, R.; Malik, M. Green synthesis of silver nanoparticles using Camellia sinensis leaf extract and their antibacterial properties. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100641. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Virijević, K.; Arsenijević, D.; Avdović, E.; Živanović, M.; Filipović, N.; Ćirić, A.; Petrović, I. Silver nanoparticles from Ocimum basilicum L. tea: A green route with potent anticancer efficacy. Colloid Interface Sci. Commun. 2024, 59, 100771. [Google Scholar] [CrossRef]
- Vinayagam, R.; Santhoshkumar, M.; Lee, K.E.; David, E.; Kang, S.G. Bioengineered gold nanoparticles using Cynodond actylon extract and its cytotoxicity and antibacterial activities. Bioprocess Biosyst. Eng. 2021, 44, 1253–1262. [Google Scholar] [CrossRef]
- Younas, M.; Rasool, M.H.; Khurshid, M.; Khan, A.; Nawaz, M.Z.; Ahmad, I.; Lakhan, M.N. Moringa oleifera leaf extract mediated green synthesis of silver nanoparticles and their antibacterial effect against selected gram-negative strains. Biochem. Syst. Ecol. 2023, 107, 104605. [Google Scholar] [CrossRef]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization, and biological applications. J. Clust. Sci. 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green synthesis and characterization of zinc oxide nanoparticles using Camellia sinensis tea leaf extract and their antioxidant, anti-bactericidal and anticancer efficacy. Res. Chem. Intermed. 2022, 48, 4769–4783. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.A.; Marchal, J.A.; Morales-Luckie, R.A.; Boulaiz, H. Silver nanoparticles from Annona muricata peel and leaf extracts as a potential potent, biocompatible, and low-cost antitumor tool. Nanomaterials 2021, 11, 1273. [Google Scholar] [CrossRef] [PubMed]
- Donga, S.; Chanda, S. Caesalpinia crista seeds mediated green synthesis of zinc oxide nanoparticles for antibacterial, antioxidant, and anticancer activities. BioNanoScience 2022, 12, 451–462. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-based synthesis of zinc oxide nanoparticles (ZnO NPs) using aqueous leaf extract of Aquilegia pubiflora: Their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxid. Med. Cell. Longev. 2021, 2021, 4786227. [Google Scholar] [CrossRef]
- Jobie, F.N.; Ranjbar, M.; Moghaddam, A.H.; Kiani, M. Green synthesis of zinc oxide nanoparticles using Amygdalus scoparia Spach stem bark extract and their applications as an alternative antimicrobial, anticancer, and anti-diabetic agent. Adv. Powder Technol. 2021, 32, 2043–2052. [Google Scholar] [CrossRef]
- Nour, M.; Hamdy, O.; Faid, A.H.; Eltayeb, E.A.; Zaky, A.A. Utilization of gold nanoparticles for the detection of squamous cell carcinoma of the tongue based on laser-induced fluorescence and diffuse reflectance characteristics: An in vitro study. Laser Med. Sci. 2022, 37, 3551–3560. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Diez-Pascual, A.M.; Bilal, M. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Biogenic synthesis of gold nanoparticles mediated by Spondias dulcis (Anacardiaceae) peel extract and its cytotoxic activity in human breast cancer cell. Toxicol. Rep. 2022, 9, 1092–1098. [Google Scholar] [CrossRef]
- D’Souza, J.N.; Nagaraja, G.K.; Prabhu, A.; Navada, K.M.; Kouser, S.; Manasa, D.J. Sauropus androgynus (L.) leaf phytochemical activated biocompatible zinc oxide nanoparticles: An antineoplastic agent against human triple negative breast cancer and a potent nanocatalyst for dye degradation. Appl. Surf. Sci. 2021, 552, 149429. [Google Scholar] [CrossRef]
- Alhumaydhi, F.A.; Khan, I.; Rauf, A.; Qureshi, M.N.; Aljohani, A.S.; Khan, S.A.; Khalil, A.A.; El-Esawi, M.A.; Muhammad, N. Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles. Green Process. Synth. 2021, 10, 230–245. [Google Scholar] [CrossRef]
- Neamah, S.A.; Albukhaty, S.; Falih, I.Q.; Dewir, Y.H.; Mahood, H.B. Biosynthesis of Zinc Oxide Nanoparticles Using Capparis spinosa L. Fruit Extract: Characterization, Biocompatibility, and Antioxidant Activity. Appl. Sci. 2023, 13, 6604. [Google Scholar] [CrossRef]
- Muchinthala, P.K.; Losetty, V. Role of phytochemical mediated zinc oxide nanoparticles on biomedical and industrial wastewater treatment: A green approach by experimental and molecular docking exploration. J. Environ. Chem. Eng. 2025, 13, 115333. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Mohamed, A.M.H.A.; Sharaky, M.; Mohamed, N.A.; Diab, Y.M. Green approach for the synthesis of ZnO nanoparticles using Cymbopogon citratus aqueous leaf extract: Characterization and evaluation of their biological activities. Chem. Biol. Technol. Agric. 2023, 10, 63. [Google Scholar] [CrossRef]
- Ohiduzzaman, M.; Khan, M.N.I.; Khan, K.A.; Paul, B. Biosynthesis of silver nanoparticles by banana pulp extract: Characterizations, antibacterial activity, and bioelectricity generation. Heliyon 2024, 10, e25520. [Google Scholar] [CrossRef]
- Ahmad, N.; Ali, S.; Abbas, M.; Fazal, H.; Saqib, S.; Ali, A.; Ullah, Z.; Zaman, S.; Sawati, L.; Zada, A.; et al. Antimicrobial efficacy of Mentha piperata-derived biogenic zinc oxide nanoparticles against UTI-resistant pathogens. Sci. Rep. 2023, 13, 14972. [Google Scholar] [CrossRef]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Sharma, V.; Al-Tawaha, A.R.M.S.; Sivanesan, I. Nanotechnology for healthcare: Plant-derived nanoparticles in disease treatment and regenerative medicine. Pharmaceuticals 2024, 17, 1711. [Google Scholar] [CrossRef]
- Kaushal, P.; Maity, D.; Awasthi, R. Nano-green: Harnessing the potential of plant extracts for sustainable antimicrobial metallic nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 94, 105488. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the green synthesis of nanoparticles: Uncovering influences of plant extracts as reducing agents for enhanced synthesis efficiency and its biomedical applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Afonso, I.S.; Cardoso, B.; Nobrega, G.; Minas, G.; Ribeiro, J.E.; Lima, R.A. Green synthesis of nanoparticles from olive oil waste for environmental and health applications: A review. J. Environ. Chem. Eng. 2024, 12, 114022. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; El-Monaem, E.M.A.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Coogan, Á.; Botov, D.; Gromova, Y.; Ushakova, E.V.; Gun’ko, Y.K. Expanding the horizons of machine learning in nanomaterials to chiral nanostructures. Adv. Mater. 2024, 36, 2308912. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sharma, R.; Gautam, P.; Panchal, P.; Chaudhary, S.; Sharma, A.; Almáši, M.; Nehra, S.P. Eco-friendly phytofabrication of Ficus Benjamina, L. based ZnO-doped g-C3N4 nanocomposites for remarkable photocatalysis and antibacterial applications. Chemosphere 2023, 339, 139707. [Google Scholar] [CrossRef]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharma, A. ZnO-modified g-C3N4: A potential photocatalyst for environmental application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Parameswari, R.P.; Sandhiya, D.; Al-Ghanim, K.A.; Nicoletti, M.; Govindarajan, M. Green Synthesis, Characterization and Bioactivity of Mangifera indica Seed-Wrapped Zinc Oxide Nanoparticles. Molecules 2023, 28, 2818. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Sharma, R.; Singh, S.; Singh, P.; Panchal, P.; Sharma, A.; Devi, P.; Nehra, S.P. Mg/Li Co-doped g-C3N4: An excellent photocatalyst for waste water remediation and hydrogen production applications towards sustainable development. Int. J. Hydrogen Energy 2023, 48, 37746–37761. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Rao, V.S.; Panchal, P.; Gautam, S.; Sharma, A.; Nehra, S.P. Mg/Li@ GCN as highly active visible light responding 2D photocatalyst for waste water remediation application. Environ. Sci. Pollut. Res. 2023, 30, 98540–98547. [Google Scholar] [CrossRef]
- Ullah, M.; Ali, M.E.; Hamid, S.B.A. Surfactant-assisted ball milling: A novel route to novel materials with controlled nanostructure—A review. Rev. Adv. Mater. Sci. 2014, 37, 1–14. [Google Scholar]
- Yeganeh-Faal, A.; Bordbar, M.; Negahdar, N.; Nasrollahzadeh, M. Green synthesis of the Ag/ZnO nanocomposite using Valeriana officinalis L. root extract: Application as a reusable catalyst for the reduction of organic dyes in a very short time. IET Nanobiotechnol. 2017, 11, 669–676. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef]
- Enan, E.T.; Ashour, A.A.; Basha, S.; Felemban, N.H.; Gad El-Rab, S.M.F. Antimicrobial activity of biosynthesized silver nanoparticles, amoxicillin, and glass-ionomer cement against Streptococcus mutans and Staphylococcus aureus. Nanotechnology 2021, 32, 215101. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J. A review of silver nanoparticles: Synthesis methods, properties, and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Ashkarran, A.A. A novel method for synthesis of colloidal silver nanoparticles by arc discharge in liquid. Curr. Appl. Phys. 2010, 10, 1442–1447. [Google Scholar] [CrossRef]
- Uttayarat, P.; Eamsiri, J.; Tangthong, T.; Suwanmala, P. Radiolytic synthesis of colloidal silver nanoparticles for antibacterial wound dressings. Adv. Mater. Sci. Eng. 2015, 2015, 376082. [Google Scholar] [CrossRef]
- Keskar, M.; Sabatini, C.; Cheng, C.; Swihart, M.T. Synthesis and characterization of silver nanoparticle-loaded amorphous calcium phosphate microspheres for dental applications. Nanoscale Adv. 2019, 1, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Dos Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Milea, C.A.; Bogatu, C.; Duta, A. The influence of parameters in silica sol-gel process. Eng. Sci. 2011, 4, 59–66. [Google Scholar]
- Piszczek, P.; Radtke, A. Silver nanoparticles fabricated using chemical vapor deposition and atomic layer deposition techniques: Properties, applications, and perspectives: Review. In Noble Precious Metals: Properties, Nanoscale Effects, and Applications; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Shahzadi, S.; Fatima, S.; Qurat ul Ain Shafiq, Z.; Janjua, M.R.S.A. A review on green synthesis of silver nanoparticles (SNPs) using plant extracts: A multifaceted approach in photocatalysis, environmental remediation, and biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, H.; Khan, I.; Alghamdi, S.; Almehmadi, M.; Ali, M.; Ullah, A.; Hussain, H.; Khan, N.; Ali, F. Green synthesis of gold nanoparticles using Delphinium chitralense tuber extracts, their characterization, and enzyme inhibitory potential. Braz. J. Biol. 2022, 82, e257622. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Saleem, M.; Pasha, U.; Sadaf, S. Synthesis of Aloe vera-conjugated silver nanoparticles for use against multidrug-resistant microorganisms. Electron. J. Biotechnol. 2022, 55, 55–64. [Google Scholar] [CrossRef]
- Erenler, R.; Dag, B. Biosynthesis of silver nanoparticles using Origanum majorana L. and evaluation of their antioxidant activity. Inorg. Nano-Met. Chem. 2022, 52, 485–492. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, J.R.; Mavumengwana, V.; Oluwafemi, O.S. Facile green, room-temperature synthesis of gold nanoparticles using Combretum erythrophyllum leaf extract: Antibacterial and cell viability studies against normal and cancerous cells. Antibiotics 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved photocatalytic and antioxidant activity of olive fruit extract-mediated ZnO nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef]
- Singh, D.; Lodhi, R.; Nayak, S. A review on green synthesis and physiological properties of silver nanoparticles. Int. J. Res. Sci. Innov. 2025, 12, 1470–1479. [Google Scholar] [CrossRef]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–446. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Shaban, A.S.; Owda, M.E.; Basuoni, M.M.; Mousa, M.A.; Radwan, A.A.; Saleh, A.K. Punica granatum peel extract mediated green synthesis of zinc oxide nanoparticles: Structure and evaluation of their biological applications. Biomass Convers. Biorefin. 2024, 14, 12265–12281. [Google Scholar] [CrossRef]
- Ehsan, M.; Waheed, A.; Ullah, A.; Kazmi, A.; Ali, A.; Raja, N.I.; Mashwani, Z.U.R.; Sultana, T.; Mustafa, N.; Ikram, M.; et al. Plant-based bimetallic silver-zinc oxide nanoparticles: A comprehensive perspective of synthesis, biomedical applications, and future trends. BioMed Res. Int. 2022, 2022, 1215183. [Google Scholar] [CrossRef]
- Singh, S.P.; Mishra, A.; Shyanti, R.K.; Singh, R.P.; Acharya, A. Silver nanoparticles synthesized using Carica papaya leaf extract (AgNPs-PLE) causes cell cycle arrest and apoptosis in human prostate (DU145) cancer cells. Biol. Trace Elem. Res. 2021, 199, 1316–1331. [Google Scholar] [CrossRef]
- Lal, S.; Verma, R.; Chauhan, A.; Dhatwalia, J.; Guleria, I.; Ghotekar, S.; Thakur, S.; Mansi, K.; Kumar, R.; Kumari, A.; et al. Antioxidant, antimicrobial, and photocatalytic activity of green synthesized ZnO-NPs from Myrica esculenta fruits extract. Inorg. Chem. Commun. 2022, 141, 109518. [Google Scholar] [CrossRef]
- Mthana, M.S.; Mthiyane, D.M.N.; Onwudiwe, D.C.; Singh, M. Biosynthesis of ZnO nanoparticles using Capsicum chinense fruit extract and their in vitro cytotoxicity and antioxidant assay. Appl. Sci. 2022, 12, 4451. [Google Scholar] [CrossRef]
- Nawabjohn, M.S.; Sivaprakasam, P.; Anandasadagopan, S.K.; Begum, A.A.; Pandurangan, A.K. Green synthesis and characterisation of silver nanoparticles using Cassia tora seed extract and investigation of antibacterial potential. Appl. Biochem. Biotechnol. 2022, 194, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Küünal, S.; Visnapuu, M.; Volubujeva, O.; Soares Rosario, M.; Rauwel, P.; Rauwel, E. Optimisation of plant mediated synthesis of silver nanoparticles by common weed Plantago major and their antimicrobial properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 613, 012003. [Google Scholar] [CrossRef]
- Edith, E.I.; Uzor, P.F. Green synthesis of silver nanoparticles using Euphorbia hirta leaf extract and the determination of their antimicrobial activity. Adv. Biosci. Bioeng. 2021, 9, 124–129. [Google Scholar] [CrossRef]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Ali, A.A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Shorafa, E.; Omidifar, N.; Gholami, A. Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on human cell lines. J. Nanomater. 2021, 2021, 6676555. [Google Scholar] [CrossRef]
- Safi Ur Rehman Qamar, S.; Tanwir, S.; Khan, W.A.; Altaf, J.; Ahmad, J.N. Biosynthesis of silver nanoparticles using Ocimum tenuiflorum extract and its efficacy assessment against Helicoverpa armigera. Int. J. Pest Manag. 2024, 70, 375–383. [Google Scholar] [CrossRef]
- Hassan Afandy, H.; Sabir, D.K.; Aziz, S.B. Antibacterial Activity of the Green Synthesized Plasmonic Silver Nanoparticles with Crystalline Structure against Gram-Positive and Gram-Negative Bacteria. Nanomaterials 2023, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Thampy, A.; Anandan, S.; Aiyaz, M.; Shilpa, N.; Singh, S.B.; Gowtham, H.G.; Ramesh, A.M.; Rahdar, A.; Kyzas, G.Z. Competent antioxidant and antiglycation properties of zinc oxide nanoparticles (ZnO NPs) phyto-fabricated from aqueous leaf extract of Boerhaavia erecta L. Environ. Sci. Pollut. Res. 2023, 30, 56731–56742. [Google Scholar] [CrossRef]
- Güngörmüş, M. Synthesis and characterization of silver nanoparticles using Myrtus communis (myrtle) and grape seed extract. Gazi J. Eng. Sci. 2021, 7, 320–329. [Google Scholar] [CrossRef]
- Meera, S.D.; Naidoo, Y.; Dewir, Y.H.; Lin, J.; Rihan, H.Z. Green synthesis of silver nanoparticles from Heteropyxisnatalensis leaf extract and their potential antibacterial efficacy. ScienceAsia 2022, 48, 196–201. [Google Scholar] [CrossRef]
- Periasamy, S.; Jegadeesan, U.; Sundaramoorthi, K.; Rajeswari, T.; Tokala, V.N.B.; Bhattacharya, S.; Muthusamy, S.; Sankoh, M.; Nellore, M.K. Comparative analysis of synthesis and characterization of silver nanoparticles extracted using leaf, flower, and bark of Hibiscus rosasinensis and examine its antimicrobicidal activity. J. Nanomater. 2022, 2022, 8123854. [Google Scholar] [CrossRef]
- Singh, R.; Sagar, N.A.; Kumar, N. Bio-inspired green fabrication of silver nanoparticles (AgNPs) using aqueous leaf extract of Ipomoea carnea Jacq. to tackle multiple drug resistance MTCC bacterial strains. Eur. J. Med. Chem. Rep. 2022, 6, 100066. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, J.; Long, X.; Pan, C.; Liu, G.; Peng, J. Green Synthesis of Silver Nanoparticles Using Jasminum nudiflorum Flower Extract and Their Antifungal and Antioxidant Activity. Nanomaterials 2023, 13, 2558. [Google Scholar] [CrossRef]
- Labulo, A.H.; David, O.A.; Terna, A.D. Green synthesis and characterization of silver nanoparticles using Morinda lucida leaf extract and evaluation of its antioxidant and antimicrobial activity. Chem. Pap. 2022, 76, 7313–7325. [Google Scholar] [CrossRef]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Manojkumar, U.; Kaliannan, D.; Srinivasan, V.; Balasubramanian, B.; Kamyab, H.; Mussa, Z.H.; Palaniyappan, J.; Mesbah, M.; Chelliapan, S.; Palaninaicker, S. Green synthesis of zinc oxide nanoparticles using Brassica oleracea var. botrytis leaf extract: Photocatalytic, antimicrobial and larvicidal activity. Chemosphere 2023, 323, 138263. [Google Scholar] [CrossRef]
- Khatun, M.; Khatun, Z.; Karim, M.R.; Habib, M.R.; Rahman, M.H.; Aziz, M.A. Green synthesis of silver nanoparticles using extracts of Mikania cordata leaves and evaluation of their antioxidant, antimicrobial, and cytotoxic properties. Food Chem. Adv. 2023, 3, 100386. [Google Scholar] [CrossRef]
- Fatimah, I.; Hidayat, H.; Purwiandono, G.; Khoirunisa, K.; Zahra, H.A.; Audita, R.; Sagadevan, S. Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste. J. Funct. Biomater. 2022, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Tailor, G.; Yadav, B.L.; Chaudhary, J.; Joshi, M.; Suvalka, C. Green synthesis of silver nanoparticles using Ocimum canum and their antibacterial activity. Biochem. Biophys. Rep. 2020, 24, 100848. [Google Scholar] [CrossRef]
- Manikandan, D.B.; Sridhar, A.; Sekar, R.K.; Perumalsamy, B.; Veeran, S.; Arumugam, M.; Karuppaiah, P.; Ramasamy, T. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer, and photocatalytic reduction. J. Environ. Chem. Eng. 2021, 9, 104845. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F. Phytofabrication of Silver Nanoparticles (AgNPs) with Pharmaceutical Capabilities Using Otostegia persica (Burm.) Boiss. Leaf Extract. Nanomaterials 2021, 11, 1045. [Google Scholar] [CrossRef]
- Piramila, H.B.; Kavitha, R.; Oviya, B. Green synthesis of silver nanoparticles using Pisonia alba L. and its antioxidant activity. Res. J. Agric. Sci. 2024, 15, 1033–1036. [Google Scholar]
- Saxena, R.; Kotnala, S.; Bhatt, S.C.; Uniyal, M.; Rawat, B.S.; Negi, P.; Riyal, M.K. A review on green synthesis of nanoparticles toward sustainable environment. Sustain. Chem. Clim. Action 2025, 6, 100071. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Green synthesis of zinc oxide nanoparticles: Eco-friendly advancements for biomedical marvels. Resour. Chem. Mater. 2024, 3, 294–316. [Google Scholar] [CrossRef]
- Alyami, M.H.; Fakhry, A.M.; El Halfawy, N.M.; Toto, S.M.; Sedky, N.K.; Yassin, H.A.; Fahmy, S.A.; Mokhtar, F.A. Retama monosperma chemical profile, green synthesis of silver nanoparticles, and antimicrobial potential: A study supported by network pharmacology and molecular docking. RSC Adv. 2023, 13, 26213. [Google Scholar] [CrossRef]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for antioxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef]
- Lalsangpuii, F.; Rokhum, S.L.; Nghakliana, F.; Fakawmi, L.; Ruatpuia, J.V.L.; Laltlanmawii, E.; Lalfakzuala, R.; Siama, Z. Green synthesis of silver nanoparticles using Spilanthes acmella leaf extract and its antioxidant-mediated ameliorative activity against doxorubicin-induced toxicity in Dalton’s lymphoma ascites (DLA)-bearing mice. ACS Omega 2022, 7, 44346–44359. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, S.; Thangavel, N.; Sivalingam, A.M.; Lakshmanan, G.; Selvakumari, J.; Pandian, A. Green synthesis and characterization of silver nanoparticles with different solvent extracts of Sesbania grandiflora (L.) Poiret and assessment of their antibacterial and antioxidant potentials. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Karan, T.; Gonulalan, Z.; Erenler, R.; Kolemen, U.; Eminagaoglu, O. Green synthesis of silver nanoparticles using Sambucus ebulus leaves extract: Characterization, quantitative analysis of bioactive molecules, antioxidant and antibacterial activities. J. Mol. Struct. 2024, 1296 Pt 1, 136836. [Google Scholar] [CrossRef]
- Pérez-Marroquín, X.A.; Aguirre-Cruz, G.; Campos-Lozada, G.; Callejas-Quijada, G.; León-López, A.; Campos-Montiel, R.G.; García-Hernández, L.; Méndez-Albores, A.; Vázquez-Durán, A.; Aguirre-Álvarez, G. Green Synthesis of Silver Nanoparticles for Preparation of Gelatin Films with Antimicrobial Activity. Polymers 2022, 14, 3453. [Google Scholar] [CrossRef]
- Tharani, M.; Rajeshkumar, S.; Al-Ghanim, K.A.; Nicoletti, M.; Sachivkina, N.; Govindarajan, M. Terminalia chebula-Assisted Silver Nanoparticles: Biological Potential, Synthesis, Characterization, and Ecotoxicity. Biomedicines 2023, 11, 1472. [Google Scholar] [CrossRef]
- Bawazeer, S.; Rauf, A.; Shah, S.U.A.; Shawky, A.M.; Al-Awthan, Y.S.; Bahattab, O.S.; Uddin, G.; Sabir, J.; El-Esawi, M.A. Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies. Green Process. Synth. 2021, 10, 85–94. [Google Scholar] [CrossRef]
- Hamad, I.; Aleidi, S.M.; Alshaer, W.; Twal, S.; Al Olabi, M.; Bustanji, Y. Advancements and global perspectives in the green synthesis of silver nanoparticles: A two-decade analysis. Pharmacia 2025, 72, 1–13. [Google Scholar] [CrossRef]
- Fouda, A.; Saied, E.; Eid, A.M.; Kouadri, F.; Alemam, A.M.; Hamza, M.F.; Alharbi, M.; Elkelish, A.; Hassan, S.E.-D. Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. J. Funct. Biomater. 2023, 14, 205. [Google Scholar] [CrossRef]
- Annapoorani, A.; Koodalingam, A.; Beulaja, M.; Saiprasad, G.; Chitra, P.; Stephen, A.; Palanisamy, S.; Prabhu, N.M.P.; You, S.G.; Janarthanan, S.; et al. Eco-friendly synthesis of zinc oxide nanoparticles using Rivina humilis leaf extract and their biomedical applications. Process Biochem. 2022, 112, 192–202. [Google Scholar] [CrossRef]
- Murali, M.; Manjula, S.; Shilpa, N.; Ravishankar, D.K.; Shivakumara, C.S.; Thampy, A.; Ayeshamariam, A.; Pandey, S.; Anandan, S.; Amruthesh, K.N.; et al. Facile synthesis of ZnO-NPs from yellow creeping daisy (Sphagneticola trilobata L.) attenuates cell proliferation by inducing cellular level apoptosis against colon cancer. J. King Saud Univ.-Sci. 2022, 34, 102084. [Google Scholar] [CrossRef]
- Dey, A.; Somaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using leaf extract of Thryallis glauca (Cav.) Kuntze and their role as antioxidant and antibacterial. Microsc. Res. Tech. 2022, 85, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Rosario, M.S.; Praakle, K.; Rauwel, E.; Rauwel, P. Investigation of the optical and antibacterial properties of biosynthesized ZnO nanoparticles using Thuja tincture. J. Phys. Conf. Ser. 2022, 2315, 012016. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S.P. Biogenic mediated Ag/ZnO nanocomposites for photocatalytic and antibacterial activities towards disinfection of water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Gautam, S.; Meena, P.; Nehra, S.P.; Maken, S.; Sharma, A. Photocatalytic and antibacterial activities of green synthesized Ag doped MgO nanocomposites towards environmental sustainability. Chemosphere 2022, 297, 134182. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Hooda, D.; Yadav, R.; Meena, P.; Nehra, S.P. Phytoextract mediated ZnO/MgO nanocomposites for photocatalytic and antibacterial activities. J. Photochem. Photobiol. A Chem. 2019, 385, 112049. [Google Scholar] [CrossRef]
- Panchal, P.; Meena, P.; Nehra, S.P. A rapid green synthesis of Ag/AgCl-NC photocatalyst for environmental applications. Environ. Sci. Pollut. Res. Int. 2021, 28, 3972–3982. [Google Scholar] [CrossRef]
- Panchal, P.; Sharma, R.; Sudarshan Reddy, A.; Nehra, K.; Sharma, A.; Nehra, S.P. Eco-friendly synthesis of Ag-doped ZnO/MgO as a potential photocatalyst for antimicrobial and dye degradation applications. Coord. Chem. Rev. 2023, 493, 215283. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Sharma, A.; Panchal, P.; Singh, A.; Chaudhary, S.; Nehra, S.P. Structural properties of Mg − x wt% Co (x = 0, 5, 10 & 20) nanocomposites for hydrogen storage applications. Mater. Today Proc. 2021, 42, 1713–1717. [Google Scholar] [CrossRef]
- Garg, A.; Almáši, M.; Bednarčík, J.; Sharma, R.; Rao, V.S.; Panchal, P.; Jain, A.; Sharma, A. Gd(III) metal-organic framework as an effective humidity sensor and its hydrogen adsorption properties. Chemosphere 2022, 305, 135467. [Google Scholar] [CrossRef]
- Rauwel, E.; Arya, G.; Praakle, K.; Rauwel, P. Use of Aloe Vera Gel as Media to Assess Antimicrobial Activity and Development of Antimicrobial Nanocomposites. Int. J. Mol. Sci. 2024, 25, 5599. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Qi, K.; Khan, I.; Wang, A.; Liu, J.; Humayun, M.; Khan, A.; Bahadur, A.; Alanazi, A.F.; Bououdina, M. Eco-friendly graphitic carbon nitride nanomaterials for the development of innovative biomaterials: Preparation, properties, opportunities, current trends, and future outlook. J. Saudi Chem. Soc. 2023, 27, 101753. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Graphitic carbon nitride/zinc oxide-based Z-scheme and S-scheme heterojunction photocatalysts for the photodegradation of organic pollutants. Int. J. Mol. Sci. 2023, 24, 15021. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.P.; Sivashanmugam, P.; Mubashera, S.M.; Sagadevan, S.; Kulandaivelu, R. The development of ZnO nanoparticle-embedded graphitic-carbon nitride towards triple-negative breast cancer therapy. RSC Adv. 2023, 13, 24333–24342. [Google Scholar] [CrossRef] [PubMed]
- Tsekov, R.; Georgiev, P.; Simeonova, S.; Balashev, K. Quantifying the Blue Shift in the Light Absorption of Small Gold Nanoparticles. arXiv 2017, arXiv:1702.04513. [Google Scholar]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Lan, P.T.; Hao, N.H.; Hieu, N.T.; Ha, N.T.T.; Brown, C.T.; Cam, L.M. Graphitic carbon nitride supported silver nanoparticles (Ag-NPs/g-C3N4): Synthesis and photocatalytic behaviour in the degradation of 2,4-dichlorophenoxyacetic acid. RSC Adv. 2024, 14, 19014–19028. [Google Scholar] [CrossRef]
- Wang, X.; Tan, F.; Wang, W.; Qiao, X.; Qiu, X.; Chen, J. Anchoring of silver nanoparticles on graphitic carbon nitride sheets for the synergistic catalytic reduction of 4-nitrophenol. Chemosphere 2017, 172, 147–154. [Google Scholar] [CrossRef]
- Nunna, G.P.; Rosaiah, P.; Sangaraju, S.; Ramalingam, G.; Jwuiyad, A.; Adem, S.; Ko, T.J. Mesostructured graphitic carbon nitride composites with silver nanoparticle decoration as the best visible-light-driven photocatalysts for dye degradation and H₂ production. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132615. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Ahn, S.H.; Lee, C.S. Gold nanoparticle modified graphitic carbon nitride/multi-walled carbon nanotube (g-C3N4/CNTs/Au) hybrid photocatalysts for effective water splitting and degradation. RSC Adv. 2015, 5, 24281–24292. [Google Scholar] [CrossRef]
- Arushi Sharma, A.; Arora, A.; Mehta, N.; Kataria, R.; Mehta, S.K. Au nanoparticles decorated graphitic carbon nitride nanosheets as a sensitive and selective fluorescence probe for Fe³⁺ and dichromate ions in aqueous medium. Chemosphere 2024, 363, 142834. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Setiawan, K.I.; Avanti, C.; Kartini, K.; Rupa, E.J.; Yang, D.-C. Scale-up of green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S. Afr. J. Chem. Eng. 2021, 38, 1–8. [Google Scholar] [CrossRef]
- Panchal, P.; Rauwel, P.; Sharma, S.; Nehra, S.P.; Estephan, E.; Praakle, K.; Rauwel, E. Ocimum tenuiflorum leaf-mediated graphitic carbon nitride and ZnO/GCN nanohybrid: A sustainable approach for environmental applications. Environ. Sci. Pollut. Res. 2025, 32, 9945–9965. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Sastry, A.B.S.; Karthik Aamanchi, R.B.; Sree Rama Linga Prasad, C.; Murty, B.S. Large-scale green synthesis of Cu nanoparticles. Environ. Chem. Lett. 2013, 11, 183–187. [Google Scholar] [CrossRef]



| Methods | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Physical Methods | |||
| Ball milling | Low cost | Agglomeration of nanoparticles with low production | [68,69,70,71,72] |
| Evaporation | Large production of nanoparticles | Costly; Time-consuming; high operating temperature | [70] |
| Arc discharge | Found high purity of nanoparticles; large production | Large size distribution of nanoparticles | [70,71,72,73] |
| Laser ablation | Found small size of nanoparticles; narrow size distribution | Very expensive, large amount of energy used; Less production of nanoparticles | [70] |
| Spray pyrolysis | Found high purity of nanoparticles | Require very high operating temperature | [71,72] |
| Chemical methods | |||
| Sol-gel process | High-purity nanoparticles synthesized | Limited industrial applications; costly precursors; time-consuming process; difficult to handle | [77] |
| Chemical vapor deposition | Single deposition step is required for nanoparticles synthesis | High process cost; difficult to handle; Low yield | [78] |
| Reverse micelle | Simple process and easy to handle | Nanoparticle’s production is less with large size; toxic and hazardous chemicals are used as a stabilizing agent | [79] |
| Plant Species | Absorption (nm) | Refs. |
|---|---|---|
| Ag Nanoparticles | ||
| Euphorbia serpens Kunth | 420 | [2] |
| Areca catechu nut | 415 | [21] |
| Carica papaya | 419 | [92] |
| Ferula asafoetida | 410–420 | [101] |
| Ipomoea carnea Jacq. | 390–410 | [108] |
| Au Nanoparticles | ||
| Crataegus oxyacantha | 520–530 | [1] |
| Moringa oleifera | 535 | [6] |
| Flaxseed | 540 | [7] |
| Curcuma pseudomontana | 542 | [9] |
| Spondias dulcis | 536 | [49] |
| ZnO Nanoparticles | ||
| Phlomis Leaf | 360 | [29] |
| Cymbopogon citratus | 370 | [54] |
| Mentha piperata | 385 | [56] |
| Rivina humilis | 370 | [131] |
| Plant Species | Part of Plant | Size/Shape | Application | Ref. |
|---|---|---|---|---|
| Euphorbia serpens | Leaves | ~30–80 nm (Spherical) | Antimicrobial and Antibiofilm Activities | [2] |
| Lawsonia inermis | Leaves | ~40 nm (Spherical) | Antimicrobial Activity | [3] |
| Eucalyptus globulus and Salvia officinalis | Leaves | ~17.5 ± 5.89 nm and 34.3 ± 7.76 nm (Spherical) | Antioxidant and Antimicrobial Activities | [4] |
| Aerva lanata | Flower | ~7 ± 3 nm (Spherical) | Antibacterial and Antioxidant Activities | [17] |
| Areca catechu nut | Fruit | ~15–20 nm (Regular spherical) | Antioxidant and Antibacterial Activities | [21] |
| Abelmoschus esculentus | Flower | ~5.52 to 31.96 nm (Spherical) | Antimicrobial Activity | [27] |
| Acacia cyanophylla | Leaves, Flowers and stems | ~88.11 nm (Spherical) | Antibacterial Activity | [28] |
| Allium cepa L. | Bulb | ~19.47 ± 1.12 nm (spherical) | Antioxidant, Antipathogenic, Anticholinesterase | [30] |
| Eupatorium adenophorum | Leaves | ~117.75 nm (Spherical) | Antioxidant and Antibacterial Activities | [33] |
| Withania coagulans | seeds | ~25 nm (spherical) | Antioxidant Activity | [35] |
| Camellia sinensis | Leaves | ~8–26 nm (Spherical) | Antibacterial Activity | [37] |
| Ocimum basilicum L. | Leaves and stem | ~35 nm (Oval) | Anticancer Activity | [38] |
| Moringa oleifera | Leaves | ~25.235 ± 0.694 nm (spherical) | Antibacterial Activity | [40] |
| Annona muricata | Leaves | ~19.63 ± 3.7 nm and 16.56 ± 4.1 nm | Antitumor Activity | [43] |
| Banana | Pulp | ~42.97 nm (Spherical) | Antibacterial and Bioelectricity generation Activities | [55] |
| Cupressus macrocarpa | Leaves | ~13.5–25.8 nm (Spherical) | Antibacterial Activity | [71] |
| Aloe vera | Leaves | ~30–80 nm (spherical) | Antibacterial Activity | [83] |
| Origanum majorana L. | Leaves | ~72.01 nm (Spherical) | Antioxidant Activity | [84] |
| Carica papaya | Leaves | 10–25 nm (Round) | Anticancer Activity | [92] |
| Cassia tora | Seed | ~50–60 nm (spherical) | Antibacterial Activity | [95] |
| Eugenia roxburghii | Leaves | ~19–39 nm (Spherical) | Antimicrobial Activity | [96] |
| Euphorbia hirta | Leaves | ~90–120 nm (spherical) | Antimicrobial Activity | [99] |
| Flaxseed | Seed | ~46.98 ± 12.45 nm (Spherical) | Antibacterial and Antioxidant Activity | [100] |
| Ferula asafoetida | Leaves | ~10 ± 2.77 nm (Circular) | Antibacterial and Cytotoxicity Activities | [101] |
| Ocimum tenuiflorum | Leaves | ~10–65 nm (Round) | Helicoverpa Armigera Activity | [102] |
| Green Tea | ……. | ~50 nm (spherical) | Antimicrobial Activity | [103] |
| Grape seed | Seed | ~10 to 30 nm (spherical) | Antimicrobial Activity | [105] |
| Heteropyxis natalensis | Leaves | ~5–60 nm (spherical) | Antibacterial Activity | [106] |
| Hibiscus rosasinensis | Leaves, Flower, and Bark | ~200 nm to 1 μm (Spherical) | Antimicrobial Activity | [107] |
| Ipomoea carnea Jacq. | Leaves | ~11.21–46.90 nm (Spherical) | Antimicrobial Activity | [108] |
| Jasminum nudiflorum | Flower | ~13 nm (Spherical) | Antioxidant and Antifungal Activities | [109] |
| Morinda lucida | Leaves | ~11 nm (spherical) | Antioxidant and Antimicrobial Activities | [110] |
| Mussaenda frondosa | Leaves | ~30–60 nm (spherical) | Antioxidant Activity | [111] |
| Mikania cordata | Leaves | ~46–50 nm (spherical) | Antioxidant, Antimicrobial, Cytotoxic Activities | [113] |
| Curcuma longa | Leaves | ~5–25 nm (Spherical) | Antibacterial Activity | [114] |
| Ocimum canum | Leaves | ~15.76 nm (Rod and Spherical) | Antibacterial Activity | [115] |
| Ocimum americanum | Leaves | ~48.25 nm (spherical) | Antibacterial, Antioxidant, and Anticancer Activities | [116] |
| Otostegia persica | Leaves | ~36.5 ± 2.0 nm (Spherical) | Antibacterial, Antifungal, and Anti-inflammatory Activities | [117] |
| Pisonia alba L. | Leaves | Not mentioned (Spherical) | Antioxidant Activity | [118] |
| Retama monosperma | Root | ~9.87–21.16 nm (Spherical) | Antimicrobial Activity | [121] |
| Rosa canina | Fruit | ~13–31 nm (Spherical) | Antioxidant, Antimicrobial, and DNA cleavage | [122] |
| Spilanthes acmella | Flower | ~10–35 nm (Spherical and oval) | Antioxidant Activity | [123] |
| Sesbania grandiflora | Leaves | ~10–25 nm (Granular-like) | Antibacterial and Antioxidant Activities | [124] |
| Sambucus ebulus | Leaves | ~18.6 nm (Spherical) | Antioxidant and Antibacterial Activities | [125] |
| Thuja orientalis | Leaves | ~85.77 nm (spherical) | Antimicrobial Activity | [126] |
| Terminalia chebula | Fruit | ~10–30 nm (Spherical) | Antioxidant, Protein Leakage Analysis Antibacterial, Zebrafish Embryonic Toxicology | [127] |
| Tropaeolum majus | Leaves | ~25 nm (Crystalline) | Antimicrobial Activity | [128] |
| Aloe vera | Stems | Cubical, Spherical, and Triangles | Antibacterial Activity | [137] |
| Plant Species | Part of Plant | Size/Shape | Applications | Ref. |
|---|---|---|---|---|
| Crataegus oxyacantha | Twig | ~85 nm (Spherical) | Urease inhibitory Activities | [1] |
| Moringa oleifera | Leaves | ~14–30 nm | Antibacterial, Antioxidant, and Cytotoxicity Activities | [6] |
| Flaxseed | Seed | ~3.4–6.9 nm (Spherical and Triangular) 10–30 Spherical | Anticancer Activity | [7] |
| Oak gum | Fruit | ~10–15 nm (Spherical) | Antioxidant and Anti-colon cancer Activities | [8] |
| Curcuma pseudomontana | Flower | ~20 nm (Spherical) | Antimicrobial, antioxidant, anti-inflammatory activities | [9] |
| Glaucium flavum | Leaves | ~32 nm (Hexagonal, Triangular, and Spherical) | Anticancer Activity | [15] |
| Jatropha integerrima Jacq. | Flower | ~38.8 nm (Spherical) | Antibacterial activity | [20] |
| Licorice | Root | ~ 2.647 nm to 16.25 nm (Circular) | Antimicrobial and Anticancer Activities | [25] |
| Kaempferia parviflo | Rhizomes | ~ 44 ± 3 nm (Spherical) | Antimicrobial and Antioxidant Activities | [26] |
| Citrus peel | Fruit | ~13.65–16.80 nm (Spherical) | Anti-inflammatory activity | [32] |
| Cynodondactylon | Grass | ~21.33 nm (Spherical and Irregular) | Cytotoxicity and Antibacterial activities | [39] |
| Spondias dulcis | Fruit | ~36.75 ± 11.36 nm (Spherical) | Cytotoxic activity in human breast cancer cells | [49] |
| Saffron | Flower | ~25 nm (Spherical and Oval) 15–50 Spherical | Antioxidant and Cytotoxicity Activities | [51] |
| Delphinium chitralense | Tuber | ~100–300 nm (Cubic) | Enzyme inhibitory Activity | [82] |
| Combretum erythrophyllum | Leaves | ~13.20 nm (Spherical) | Antibacterial, Cell viability Activities | [85] |
| Plant Species | Part of Plant | Size/Shape | Applications | Ref. |
|---|---|---|---|---|
| Borreria hispida | Leaves | ~21.87 nm (Hexagonal) | Antioxidant Activity | [10] |
| Ailanthus altissima | Leaves | ~13.27 nm (Spherical) | Antibacterial and Antioxidant Activities | [11] |
| Passiflora subpeltata | Leaves | ~40–50 nm (Irregular) | Antibacterial Activity | [12] |
| Andrographis alata | Whole plant | ~45 ± 4.23 nm (Spherical, Oval, and Hexagonal) | Antibacterial, Antioxidant, Antidiabetic, and Anti-Alzheimer Activities | [13] |
| Mucuna pruriens | Peel | ~21.60 to 47.16 nm (Spherical) | Anticancer, antioxidant activity | [14] |
| Bergenia ciliata | Rhizome | (Flower-like bundles) | Antibacterial, anticancer potential | [16] |
| Berberis aristata | Leaves | ~5–25 nm (Needle) | Antibacterial activities and Antioxidant | [23] |
| Lantana camara | Flower | ~25 nm (Spherical) | anti-inflammatory | [24] |
| Phlomis leaf | Leaves | ~79 nm (Hexagonal) | Cytotoxicity and Antibacterial Activities | [29] |
| Lepidium sativum | Seeds | ~36.96–44.50 (Spherical) | Anticancer activity | [31] |
| Plantain peel | Peel | ~20 nm (Spherical) | Antibacterial Activity | [34] |
| Camellia sinensis | Leaves | ~6 to 112 nm (Spherical) | Antioxidant, Antibacterial, and Anticancer Activity | [42] |
| Caesalpinia crista | Seed | ~34.67 nm (Irregular) | Antibacterial, Antioxidant, and Anticancer Activities | [44] |
| Aquilegia pubiflora | Leaves | ~34.23 nm (Spherical or Elliptical) | Antiproliferative Activity | [45] |
| Amygdalus scoparia | Bark | ~15–40 nm (Spherical) | Antimicrobial, Anticancer, and Antidiabetic | [46] |
| Sauropus androgynus | Leaves | ~12 to 23 nm (Spherical) | Antineoplastic Activity | [50] |
| Capparis spinosa | Fruit | ~37.49 nm (Spherical) | Antioxidant Activity | [52] |
| Ipomoea Sagittifolia Burm.f | Leaves | ~51.2 ± 8.5 nm (Hexagonal) | Antibacterial, Antioxidant, and Anticancer Activities | [53] |
| Cymbopogon citratus | Aerial Part | ~21 nm (Spherical) | Antimicrobial and Anticancer Activity | [54] |
| Mentha piperata | Leaves | 15 to 27 nm (Globular and Oblong) | UTI-resistant pathogens | [56] |
| Mangifera indica | Seed | ~40–70 nm (Cylindrical) | Antibacterial and Antioxidant Activities | [65] |
| Olive fruit | Fruit | ~56.8 ± 0.6 (Spheroidal) | Antioxidant Activity | [86] |
| Punica granatum | Peel | ~20–40 nm (Spherical and Hexagonal) | Antimicrobial Activity | [90] |
| Myrica esculenta | Fruit | ~115 ± 3.21 nm (Pellets-like) | Antioxidant, antimicrobial, photocatalytic | [93] |
| Capsicum chinense | Fruit | ~12.7 nm (Spherical) | Cytotoxicity and Antioxidant Activities | [94] |
| Myristica fragrans | Fruit | ~43.3 to 83.1 nm (Semi spherical) | Antioxidant, antimicrobial, photocatalytic | [97] |
| Boerhaavia erecta | Leaves | ~20.55 nm (Spherical) | Antioxidant and Antiglycation Activities | [104] |
| Brassica oleracea | Leaves | ~52 nm (Flower-like) | Antimicrobial and Larvicidal Activities | [112] |
| Punica granatum | Peel | ~10.45 nm (Spherical) | Antimicrobial Activity | [130] |
| Rivina humilis | Leaves | ~14.4 nm (Irregular and Circular) | Anticancer, Cytotoxicity, and Antiproliferative Activities | [131] |
| Sphagneticola trilobata | Leaves | ~29.83 (Spherical) | Colon cancer and Antioxidant Activities | [132] |
| Thryallis glauca | Leaves | ~50 nm (Hexagonal wurtzite) | Antioxidant and Antibacterial | [133] |
| Thuja officinalis | Leaves | 4–5 nm | Antimicrobial | [134] |
| Ocimum tenuiflorum | Leaves | ~30 to 40 nm Flakes | Photocatalytic-Antimicrobial Activity | [136] |
| Ricinus Communis L. | Fruit | Hexagonal irregular | Antibacterial Activity | [138] |
| Application | Traditional Methods | Nanoparticle-Based Method | Advantages of Nanoparticles | Refs. |
|---|---|---|---|---|
| Cancer Treatment | Chemotherapy; radiotherapy | Targeted drug delivery; photothermal therapy | Minimize damage to healthy cells | [16,49,92,132] |
| Antibacterial | Broad-spectrum antibiotics | Ag-, ZnO-, Cu-NPs etc. | Effective against resistant strains | [2,27,138,154] |
| Biosensors | Enzyme and antibody-based detection | Nanoparticles-based sensors | Faster detection | [5,13,55,60] |
| Wound healing | Traditional dressing; antiseptic solution | Nanoparticles-embedded dressing such as Ag-, ZnO-NPs | Antibacterial, faster healing, reduced infection rate | [24,53,138] |
| Drug delivery | Oral intravenous drug | Targeted delivery | Site specific action, reduce side effects | [57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchal, P.; Rauwel, P.; Nehra, S.P.; Singh, P.; Karla, M.; Hermosa, G.; Rauwel, E. A Review on Biomedical Applications of Plant Extract-Mediated Metallic Ag, Au, and ZnO Nanoparticles and Future Prospects for Their Combination with Graphitic Carbon Nitride. Pharmaceuticals 2025, 18, 820. https://doi.org/10.3390/ph18060820
Panchal P, Rauwel P, Nehra SP, Singh P, Karla M, Hermosa G, Rauwel E. A Review on Biomedical Applications of Plant Extract-Mediated Metallic Ag, Au, and ZnO Nanoparticles and Future Prospects for Their Combination with Graphitic Carbon Nitride. Pharmaceuticals. 2025; 18(6):820. https://doi.org/10.3390/ph18060820
Chicago/Turabian StylePanchal, Priyanka, Protima Rauwel, Satya Pal Nehra, Priyanka Singh, Mamta Karla, Glemarie Hermosa, and Erwan Rauwel. 2025. "A Review on Biomedical Applications of Plant Extract-Mediated Metallic Ag, Au, and ZnO Nanoparticles and Future Prospects for Their Combination with Graphitic Carbon Nitride" Pharmaceuticals 18, no. 6: 820. https://doi.org/10.3390/ph18060820
APA StylePanchal, P., Rauwel, P., Nehra, S. P., Singh, P., Karla, M., Hermosa, G., & Rauwel, E. (2025). A Review on Biomedical Applications of Plant Extract-Mediated Metallic Ag, Au, and ZnO Nanoparticles and Future Prospects for Their Combination with Graphitic Carbon Nitride. Pharmaceuticals, 18(6), 820. https://doi.org/10.3390/ph18060820










