Preparation and Evaluation of an Oral Administration System of Albendazole-Metal-Organic Framework Based on Dual Response to pH and Enzymes
Abstract
1. Introduction
2. Results and Discussion
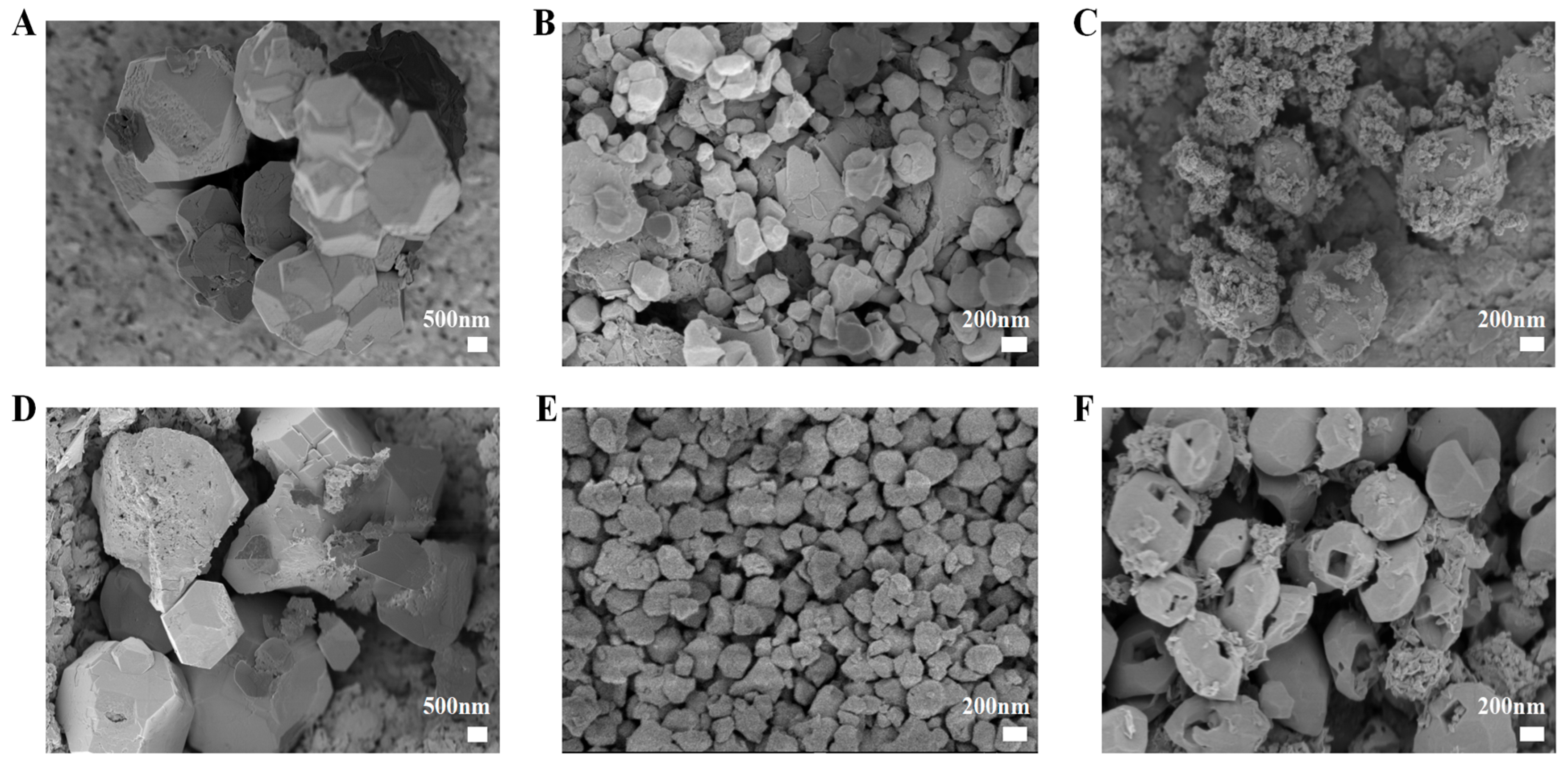
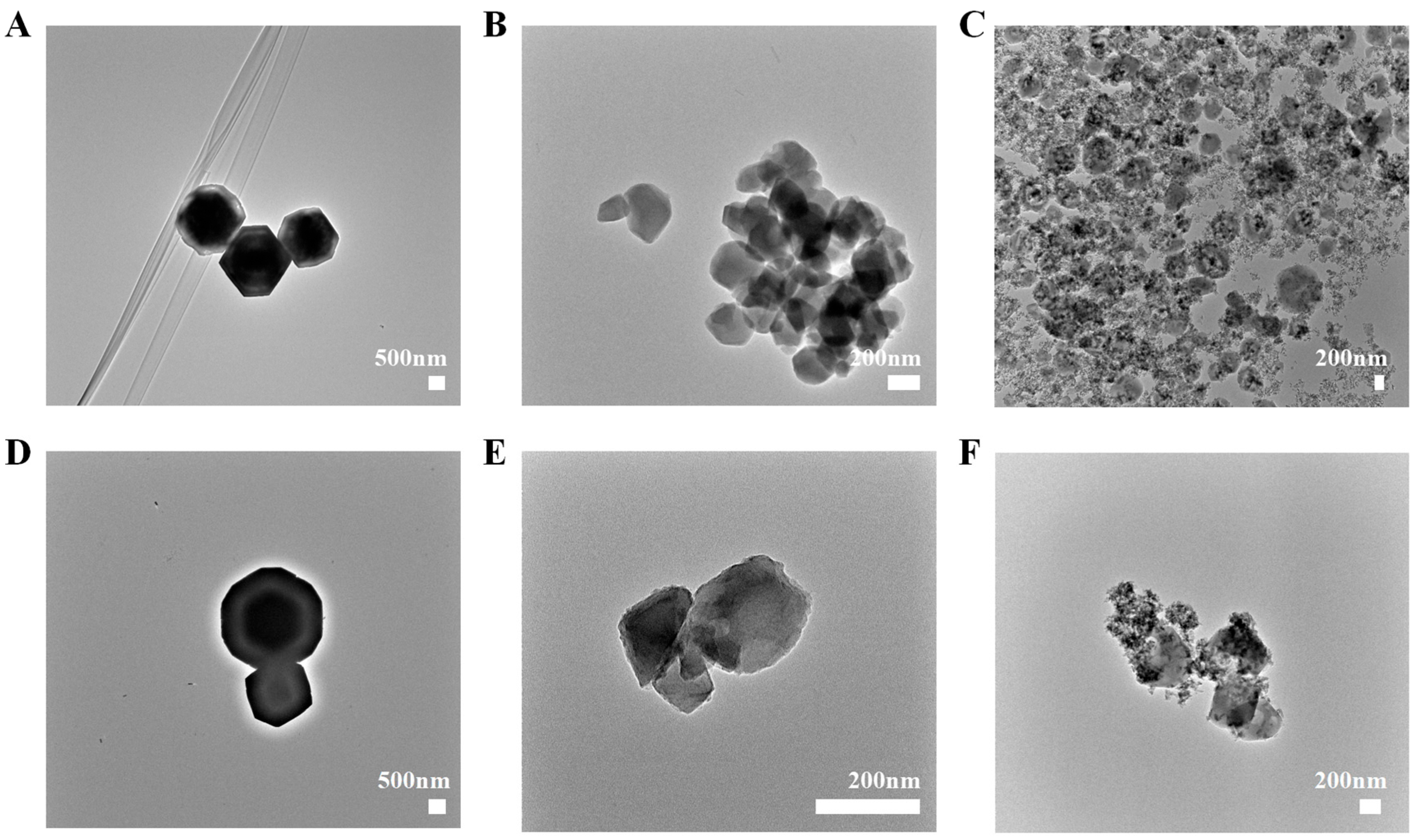
2.1. Particle Size, Zeta Potential, and Morphology
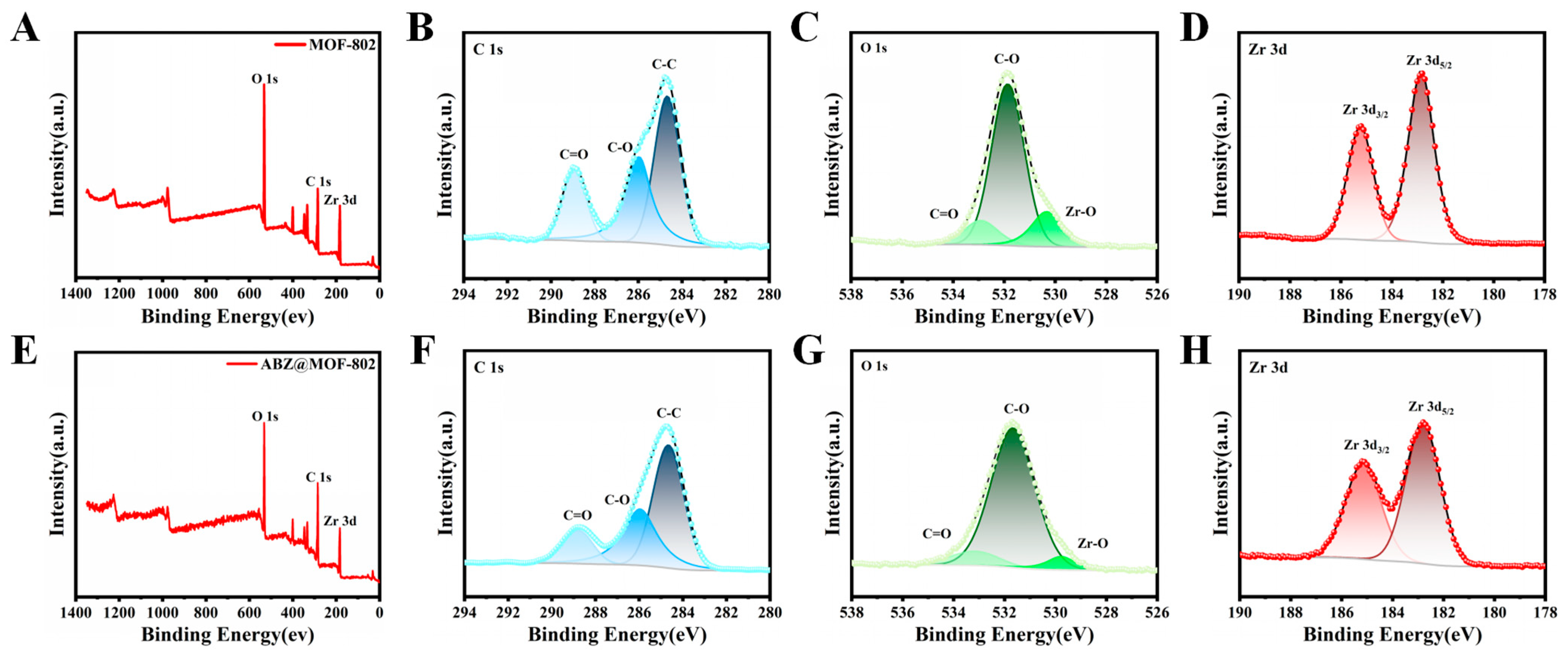
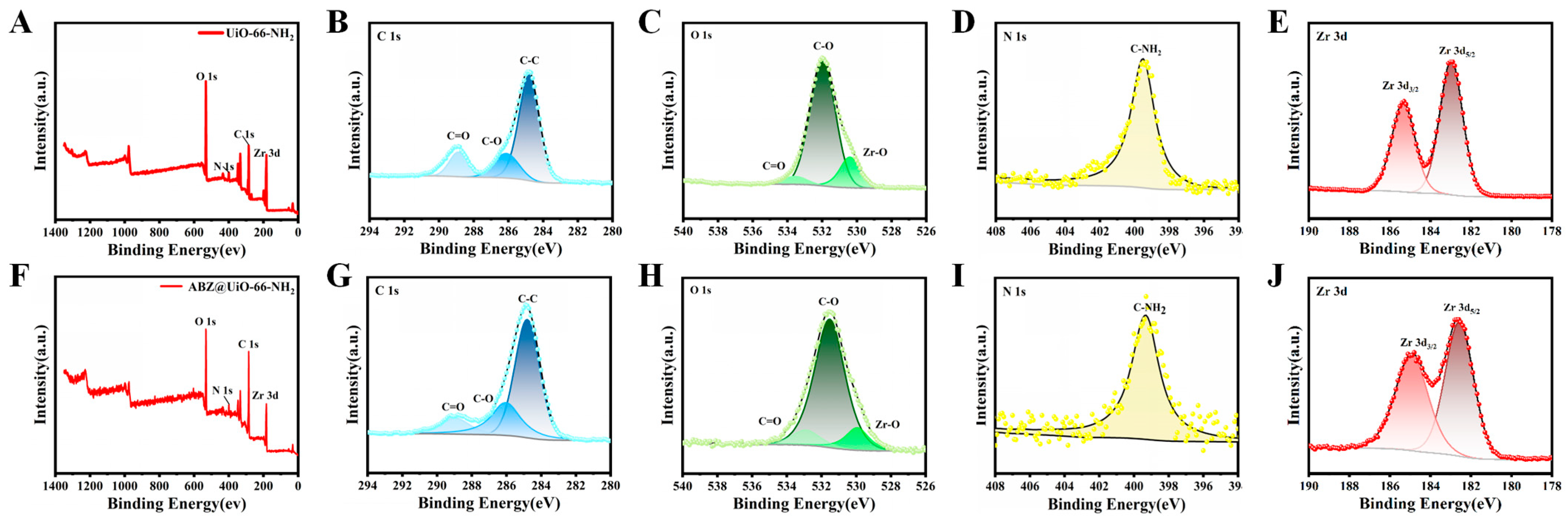
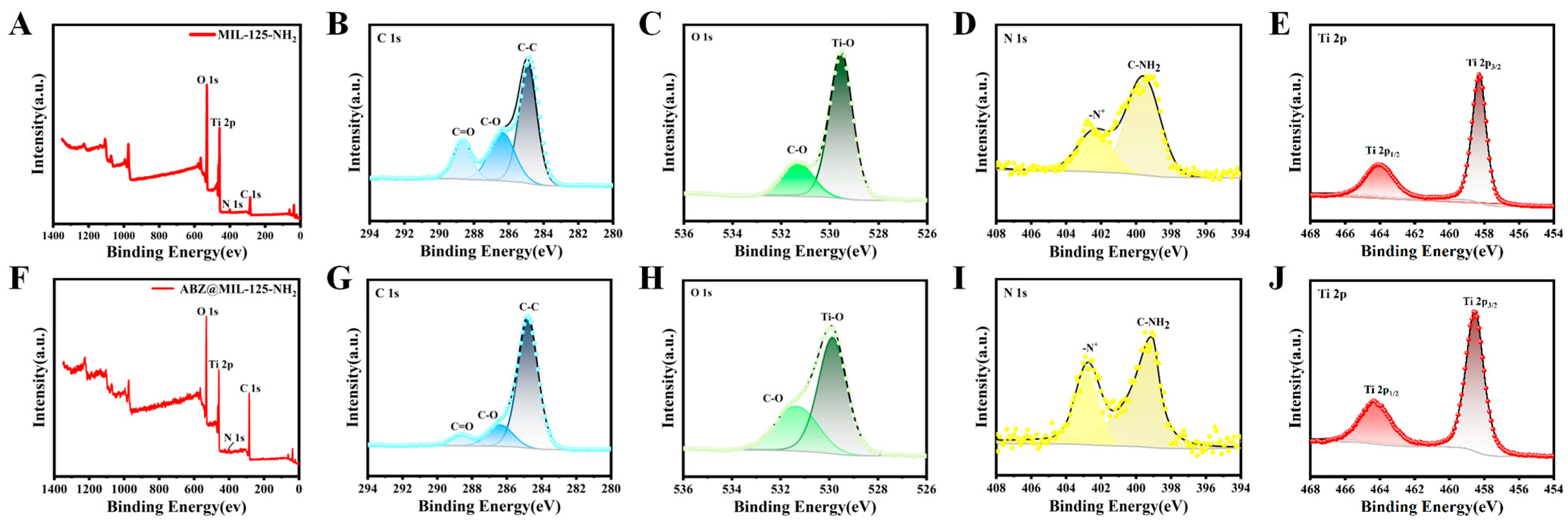
2.2. Characterization of ABZ@MOFs
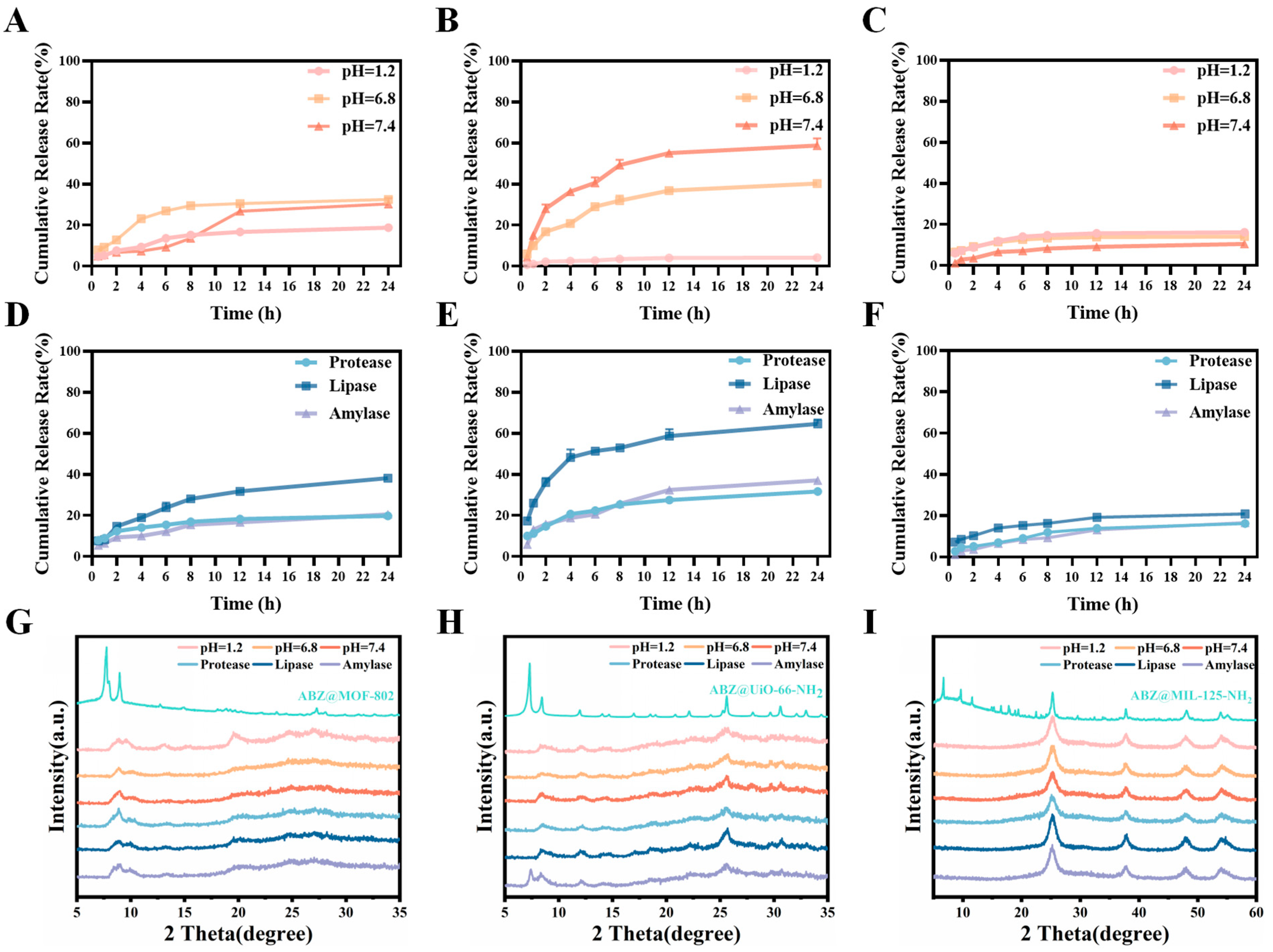
2.3. Drug Loading and Responsive Release of ABZ@MOFs
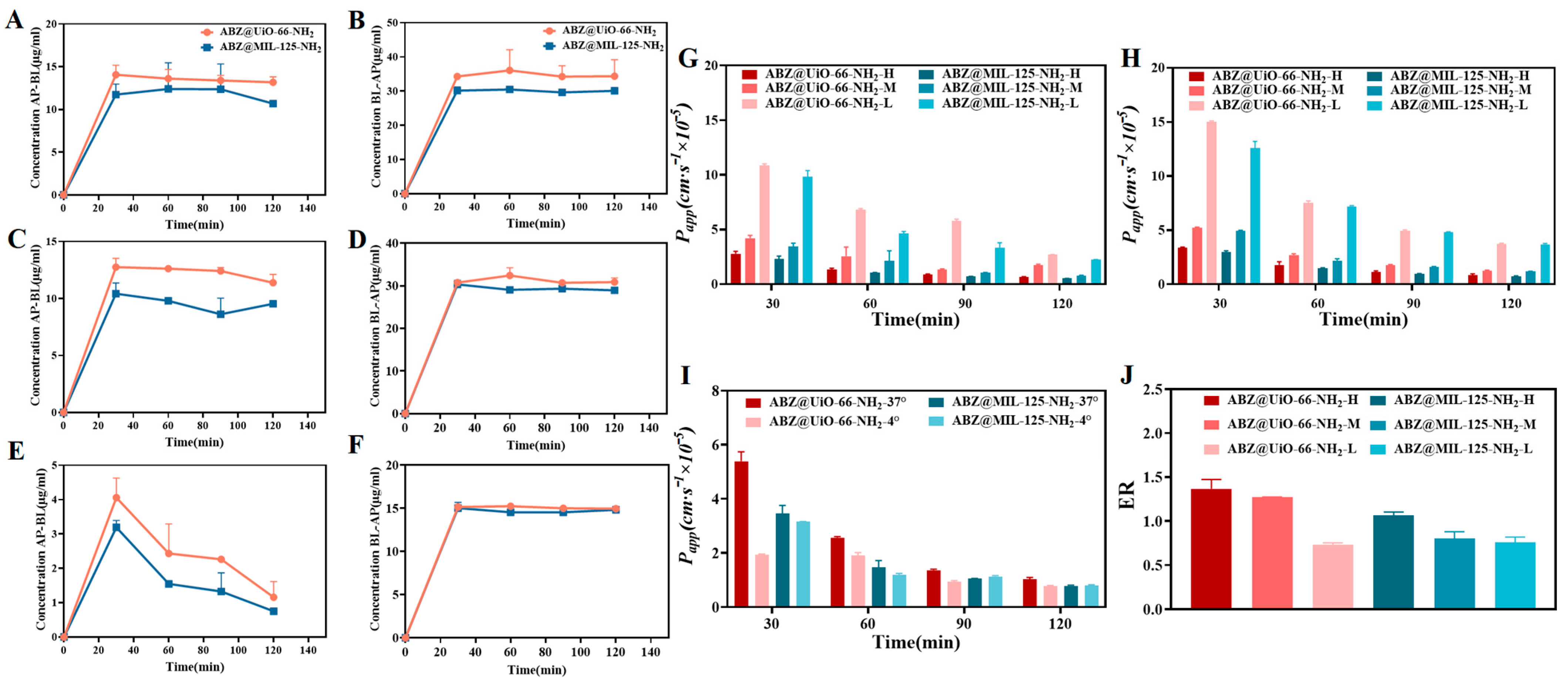
2.4. Study of Transmembrane Transport In Vitro
2.5. In Vivo Pharmacokinetic Study
3. Materials and Methods
3.1. Materials
3.2. Instrument
3.3. Cell Lines and Experimental Animals
3.4. Synthesis of MOF-802 and ABZ@MOF-802
3.5. Synthesis of UiO-66-NH2 and ABZ@UiO-66-NH2
3.6. Synthesis of MIL-125-NH2 and ABZ@MIL-125-NH2
3.7. Particle Size and Zeta Potential
3.8. Morphological Characterization
3.9. Powder X-Ray Diffraction (PXRD)
3.10. Fourier Transform Infrared Spectroscopy (FT-IR)
3.11. N2 Adsorption-Desorption Isotherms
3.12. X-Ray Photoelectron Spectroscopy (XPS)
3.13. Thermal Stability Analysis
3.14. Drug Loading Content (DL) and Encapsulation Efficiency (EE)
3.15. In Vitro Release Rate
3.16. Transmembrane Transport Study
3.17. Bioavailability of ABZ@MOFs
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mcmanus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Diseases Burden Epidemiology Reference Group 2007–2015 [EB/OL] (2015-12-01). Available online: https://www.who.int/publications/i/item/9789241565165 (accessed on 5 April 2023).
- Nunnari, G.; Pinzone, M.R.; Gruttadauria, S.; Celesia, B.M.; Madeddu, G.; Malaguarnera, G.; Pavone, P.; Cappellani, A.; Cacopardo, B. Hepatic echinococcosis: Clinical and therapeutic aspects. World J. Gastroenterol. 2012, 18, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Gil-Grande, L.A.; Rodriguez-Caabeiro, F.; Prieto, J.G.; Sánchez-Ruano, J.J.; Brasa, C.; Aguilar, L.; Garcίa-Hoz, F.; Casado, N.; Bárcena, R.; Alvarez, A.I.; et al. Randomised controlled trial of efficacy of albendazole in intra-abdominal hydatid disease. Lancet 1993, 342, 1269–1272. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Wang, W.; Dai, Y.; Sun, H.; Li, J.; Wang, S.; Li, F. Status and prospect of novel treatment options toward alveolar and cystic echinococcosis. Acta Trop. 2022, 226, 106252. [Google Scholar] [CrossRef]
- Dong, C.L.; Zheng, S.D.; Liu, Y.Y.; Cui, W.Q.; Hao, M.Q.; God’spower, B.O.; Chen, X.Y.; Li, Y.H. Albendazole solid dispersions prepared using PEG6000 and Poloxamer188: Formulation, characterization and in vivo evaluation. Pharm. Dev. Technol. 2020, 25, 1043–1052. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Pang, M.; Zhang, Y.; Hu, C.; Fan, H. Metabolic mechanism and pharmacological study of albendazole in secondary hepatic alveolar echinococcosis (HAE) model rats. Antimicrob. Agents Chemother. 2024, 68, e0144923. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, X.; Liu, Y. Improving the Solubility, Stability, and Bioavailability of Albendazole through Synthetic Salts. Molecules 2024, 29, 3571. [Google Scholar] [CrossRef]
- Maqbool, F.; Moyle, P.M.; Tan, M.S.A.; Thurecht, K.J.; Falconer, J.R. Preparation of albendazole-loaded liposomes by supercritical carbon dioxide processing. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1186–S1192. [Google Scholar] [CrossRef]
- Li, H.; Song, T.; Shao, Y.; Aili, T.; Ahan, A.; Wen, H. Comparative Evaluation of Liposomal Albendazole and Tablet-Albendazole Against Hepatic Cystic Echinococcosis: A Non-Randomized Clinical Trial. Medicine 2016, 95, e2237. [Google Scholar] [CrossRef]
- Sawatdee, S.; Atipairin, A.; Sae, Y.A.; Srichana, T.; Changsan, N.; Suwandecha, T. Formulation Development of Albendazole-Loaded Self-Microemulsifying Chewable Tablets to Enhance Dissolution and Bioavailability. Pharmaceutics 2019, 11, 134. [Google Scholar] [CrossRef]
- Abulaihaiti, M.; Wu, X.W.; Qiao, L.; Lv, H.L.; Zhang, H.W.; Aduwayi, N.; Wang, Y.J.; Wang, X.C.; Peng, X.Y. Efficacy of Albendazole-Chitosan Microsphere-based Treatment for Alveolar Echinococcosis in Mice. PLoS Negl. Trop. Dis. 2015, 9, e0003950. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B. 2021, 11, 2505–2536. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Shah, S.; Patel, J. Solid dispersion technology as a formulation strategy for the fabrication of modified release dosage forms: A comprehensive review. Daru 2022, 30, 165–189. [Google Scholar] [CrossRef]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical liposomal delivery-specific considerations of innovation and challenges. Biomater. Sci. 2022, 11, 62–75. [Google Scholar] [CrossRef]
- Chatterjee, B.; Almurisi, S.H.; Dukhan, A.A.M.; Mandal, U.K.; Sengupta, P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016, 23, 3639–3652. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Y. Metal-Organic Framework(MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.; Chou, L.; Liu, D.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Jiang, Y.; Zhou, F.; Wu, Y.; Jiang, H.; Wang, R.; Xu, Q.; Hua, C. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar] [CrossRef]
- Han, J.; Xiao, B.; Le, P.K.; Mangwandi, C. Enhancement of the Solubility of BS Class II Drugs with MOF and MOF/GO Composite Materials: Case Studies of Felodipine, Ketoprofen and Ibuprofen. Materials 2023, 16, 1554. [Google Scholar] [CrossRef]
- Lv, N.; Guo, T.; Liu, B.; Wang, C.; Singh, V.; Xu, X.; Li, X.; Chen, D.; Gref, R.; Zhang, J. Improvement in thermal stability of sucralose by γ-cyclodextrin metal-organic frameworks. Pharm. Res. 2017, 34, 269–278. [Google Scholar] [CrossRef]
- Lin, W.; Hu, Q.; Jiang, K.; Yang, Y.; Yang, Y.; Cui, Y.; Qian, G. A porphyrin-based metal-organic framework as a pH-responsive drug carrier. J. Solid State Chem. 2016, 237, 307–312. [Google Scholar] [CrossRef]
- Jiang, K.; Ni, W.; Cao, X.; Zhang, L.; Lin, S. A nanosized anionic MOF with rich thiadiazole groups for controlled oral drug delivery. Mater. Today Bio 2022, 13, 100180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, S.; Zhao, S.; Li, X.; Liu, B.; Liu, Y. Chitosan modified metal-organic frameworks as a promising carrier for oral drug delivery. RSC Adv. 2020, 10, 45130–45138. [Google Scholar] [CrossRef]
- Teplensky, M.H.; Fantham, M.; Li, P.; Wang, T.C.; Mehta, J.P.; Young, L.J.; Moghadam, P.Z.; Hupp, J.T.; Farha, O.K.; Kaminski, C.F.; et al. Temperature treatment of highly porous zirconium-containing metal-organic frameworks extends drug delivery release. J. Am. Chem. Soc. 2017, 139, 7522–7532. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, H.; Zhang, X.; Sun, X.; Liu, M.; Yang, D.; Liu, X.; Wei, J. Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-based micropore metal-organic frameworks as sustained-release anti⁃cancer drug carriers. Molecules 2018, 23, 2490. [Google Scholar] [CrossRef]
- Alsaedi, M.K.; Alothman, G.K.; Alnajrani, M.N.; Alsager, O.A.; Alshmimri, S.A.; Alharbi, M.A.; Alawad, M.O.; Alhadlaq, S.; Alharbi, S. Antibiotic Adsorption by Metal-Organic Framework (UiO-66): A Comprehensive Kinetic, Thermodynamic, and Mechanistic Study. Antibiotics 2020, 9, 722. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Homaeigohar, S. A hierarchical Ca/TiO/NH-MIL-125 nanocomposite photocatalyst for solar visible light induced photodegradation of organic dye pollutants in water. RSC Adv. 2020, 10, 29808–29820. [Google Scholar] [CrossRef]
- Tripathi, S.; Sreenivasulu, B.; Suresh, A.; Rao, C.V.S.B.; Sivaraman, N. Assorted functionality-appended UiO-66-NH2 for highly efficient uranium(VI) sorption at acidic/neutral/basic pH. RSC Adv. 2020, 10, 14650–14661. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fan, W.; Wang, L.; Qi, J.; Lu, Y.; Wu, W. Effect of surface charges on oral absorption of intact solid lipid nanoparticles. Mol. Pharm. 2019, 16, 5013–5024. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N. Nanotechnology for delivery of natural therapeutic substances: A review. Environ. Chem. Lett. 2021, 19, 1097–1106. [Google Scholar] [CrossRef]

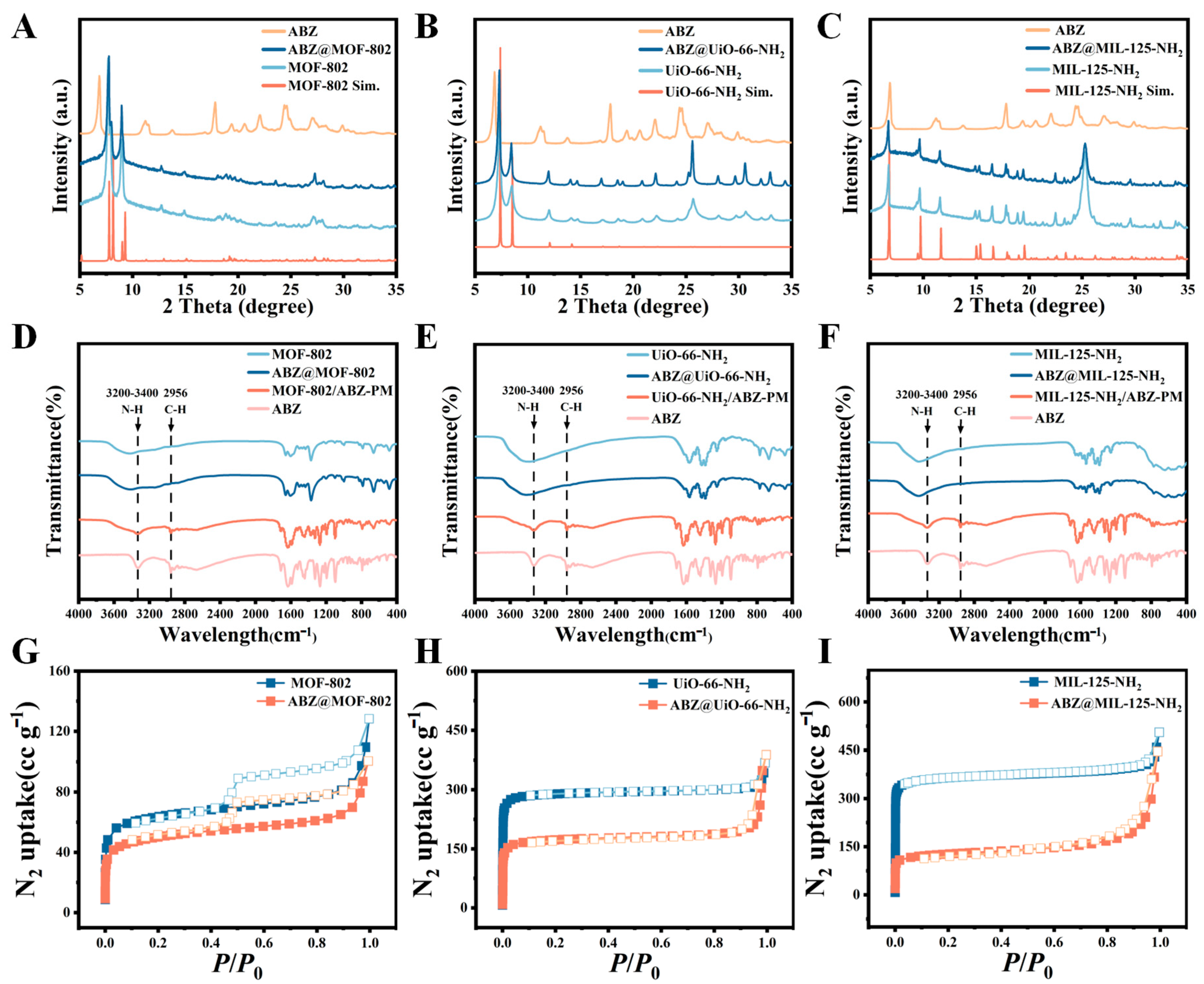


| BET Surface Area (m2·g−1) | Pore Volume (mL·g−1) | |
|---|---|---|
| MOF-802 | 238.71 | 0.18 |
| ABZ@MOF-802 | 184.58 | 0.15 |
| UiO-66-NH2 | 1179.78 | 0.55 |
| ABZ@UiO-66-NH2 | 684.77 | 0.57 |
| MIL-125-NH2 | 1103.10 | 0.74 |
| ABZ@MIL-125-NH2 | 484.15 | 0.68 |
| ABZ | ABZ@UiO-66-NH2 | ABZ@MIL-125-NH2 | |
|---|---|---|---|
| Cmax (μg·L−1) | 34.11 ± 5.19 | 81.21 ± 17.97 * | 77.20 ± 15.26 * |
| Tmax (h) | 2.00 ± 0.38 | 1.83 ± 0.40 | 2.00 ± 0.14 |
| t1/2 (h) | 2.00 ± 0.38 | 29.70 ± 17.44 ** | 4.89 ± 1.95 ** |
| CL (L·h−1·kg−1) | 0.09 ± 0.01 | 16.61 ± 12.16 ** | 65.25 ± 18.20 **** |
| AUC0–t (μg·L−1·h) | 216.00 ± 27.55 | 702.6 ± 54.83 * | 389.82 ± 111.67 * |
| AUC0–∞ (μg·L−1·h) | 219.91 ± 25.27 | 2269.66 ± 707.32 * | 409.82 ± 115.74 * |
| MRT0–t (h) | 6.16 ± 0.36 | 8.46 ± 2.78 | 5.90 ± 1.47 |
| MRT0–∞ (h) | 6.55 ± 0.37 | 37.89 ± 22.20 ** | 7.27 ± 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Guo, Z.; Zhang, Y.; Guo, Y.; Wang, T.; Liu, D.; Hu, C. Preparation and Evaluation of an Oral Administration System of Albendazole-Metal-Organic Framework Based on Dual Response to pH and Enzymes. Pharmaceuticals 2025, 18, 819. https://doi.org/10.3390/ph18060819
Liu W, Guo Z, Zhang Y, Guo Y, Wang T, Liu D, Hu C. Preparation and Evaluation of an Oral Administration System of Albendazole-Metal-Organic Framework Based on Dual Response to pH and Enzymes. Pharmaceuticals. 2025; 18(6):819. https://doi.org/10.3390/ph18060819
Chicago/Turabian StyleLiu, Weiqi, Zhimei Guo, Yong Zhang, Yufei Guo, Ting Wang, Dahuan Liu, and Chunhui Hu. 2025. "Preparation and Evaluation of an Oral Administration System of Albendazole-Metal-Organic Framework Based on Dual Response to pH and Enzymes" Pharmaceuticals 18, no. 6: 819. https://doi.org/10.3390/ph18060819
APA StyleLiu, W., Guo, Z., Zhang, Y., Guo, Y., Wang, T., Liu, D., & Hu, C. (2025). Preparation and Evaluation of an Oral Administration System of Albendazole-Metal-Organic Framework Based on Dual Response to pH and Enzymes. Pharmaceuticals, 18(6), 819. https://doi.org/10.3390/ph18060819






