DFT and Molecular Docking Study of HA-Conjugated SWCNTs for CD44-Targeted Delivery of Platinum-Based Chemotherapeutics
Abstract
1. Introduction
- Evaluate the stability and encapsulation efficiency of drug-loaded DDS1 and DDS2 via DFT calculations,
- Analyze the binding affinity and interaction profiles of DDSs with the CD44 receptor through molecular docking simulations,
- Assess the influence of HA conjugation on the targeting specificity and biocompatibility of SWCNT-based DDSs.
2. Results
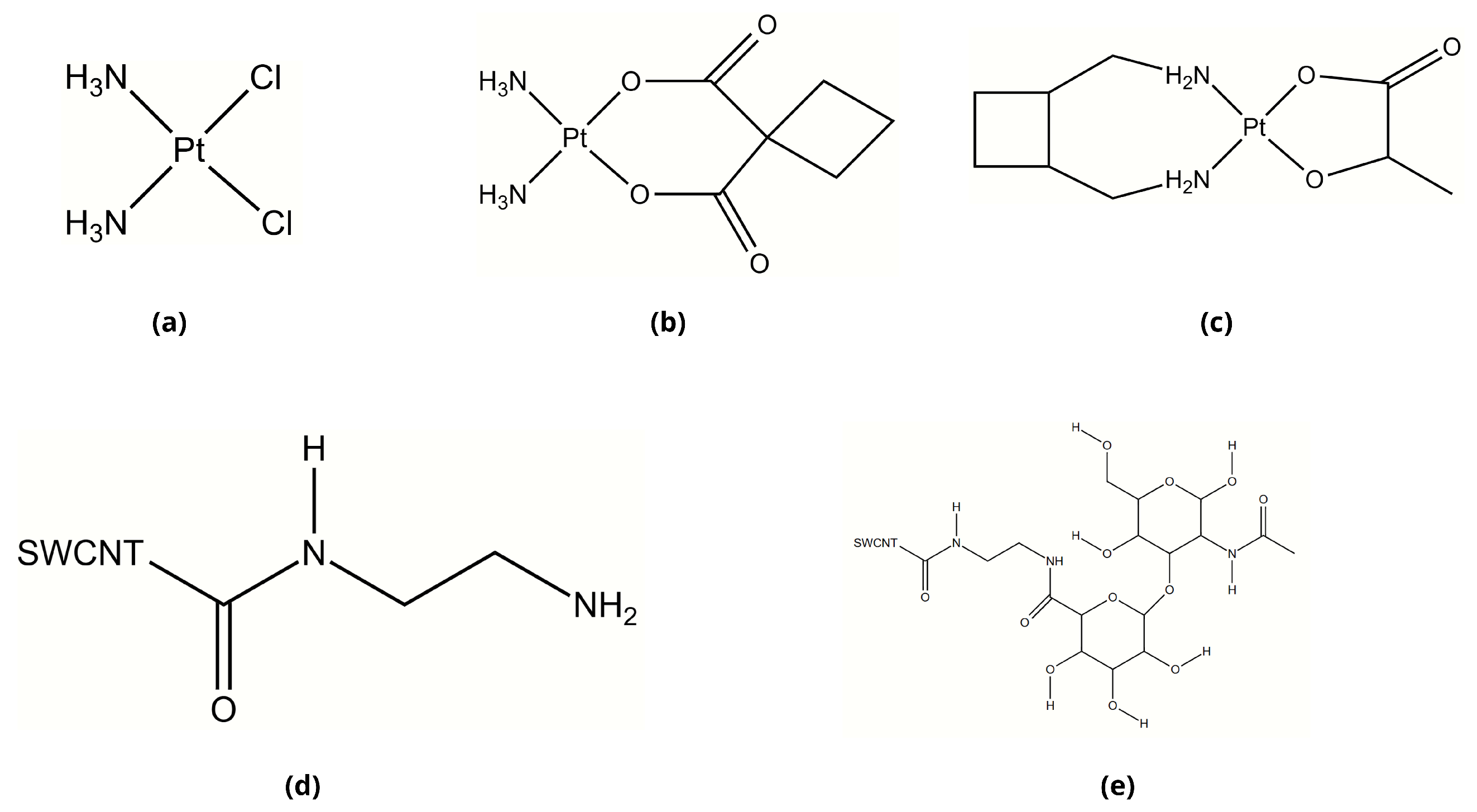
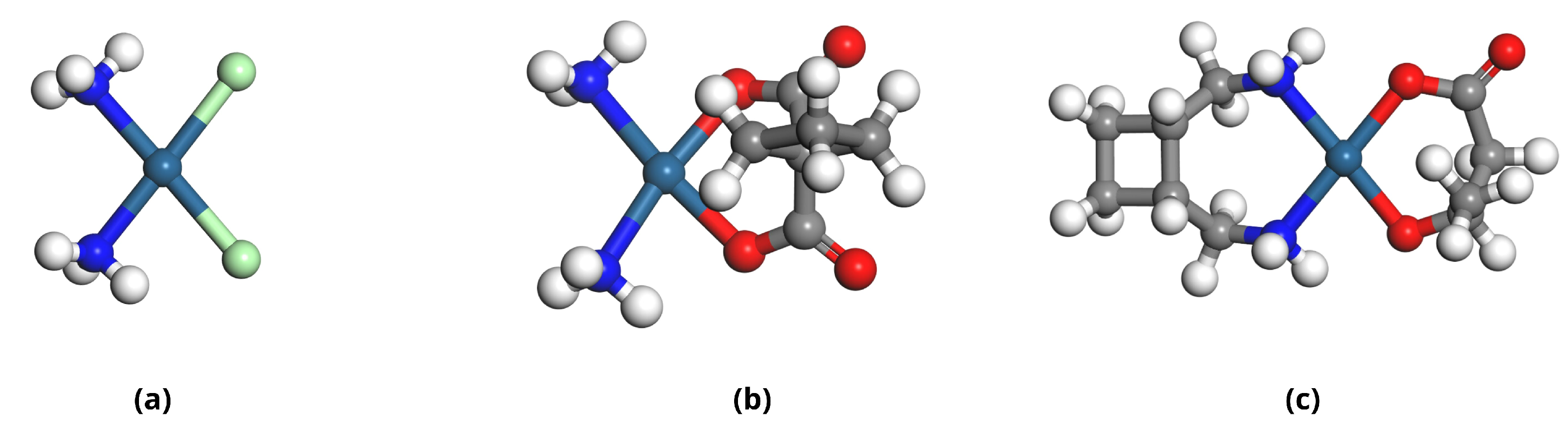
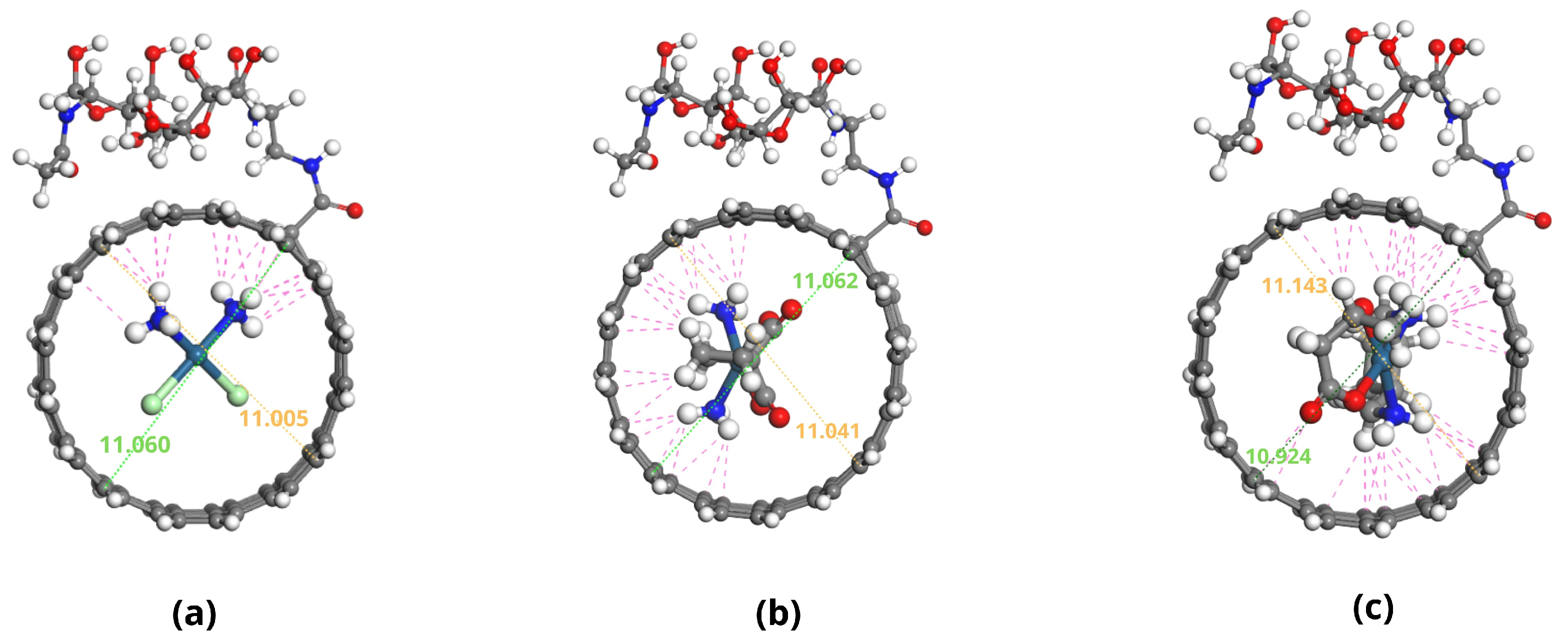
2.1. Molecular Structures of Investigated Compounds
2.2. Molecular Descriptor Analysis
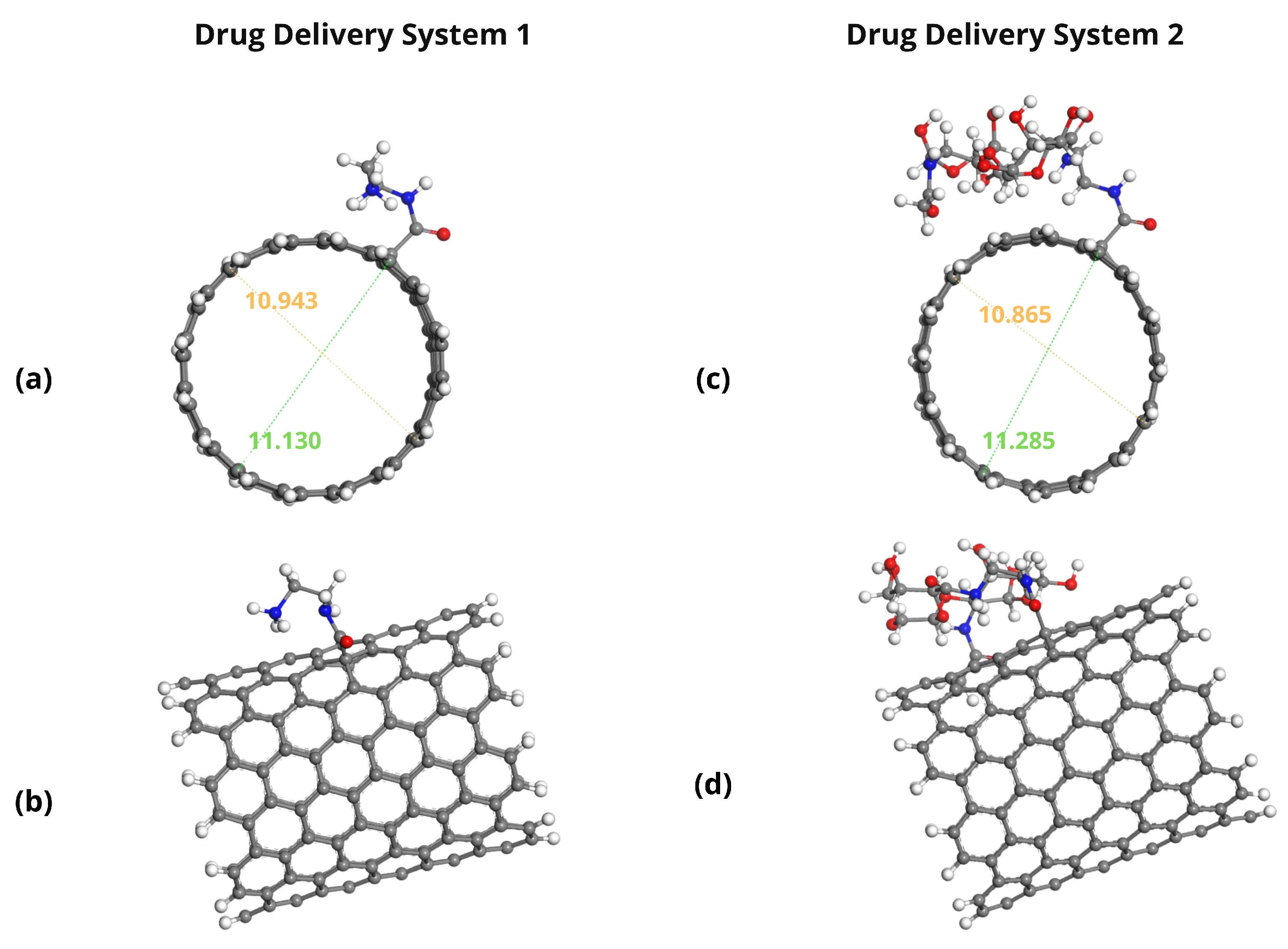
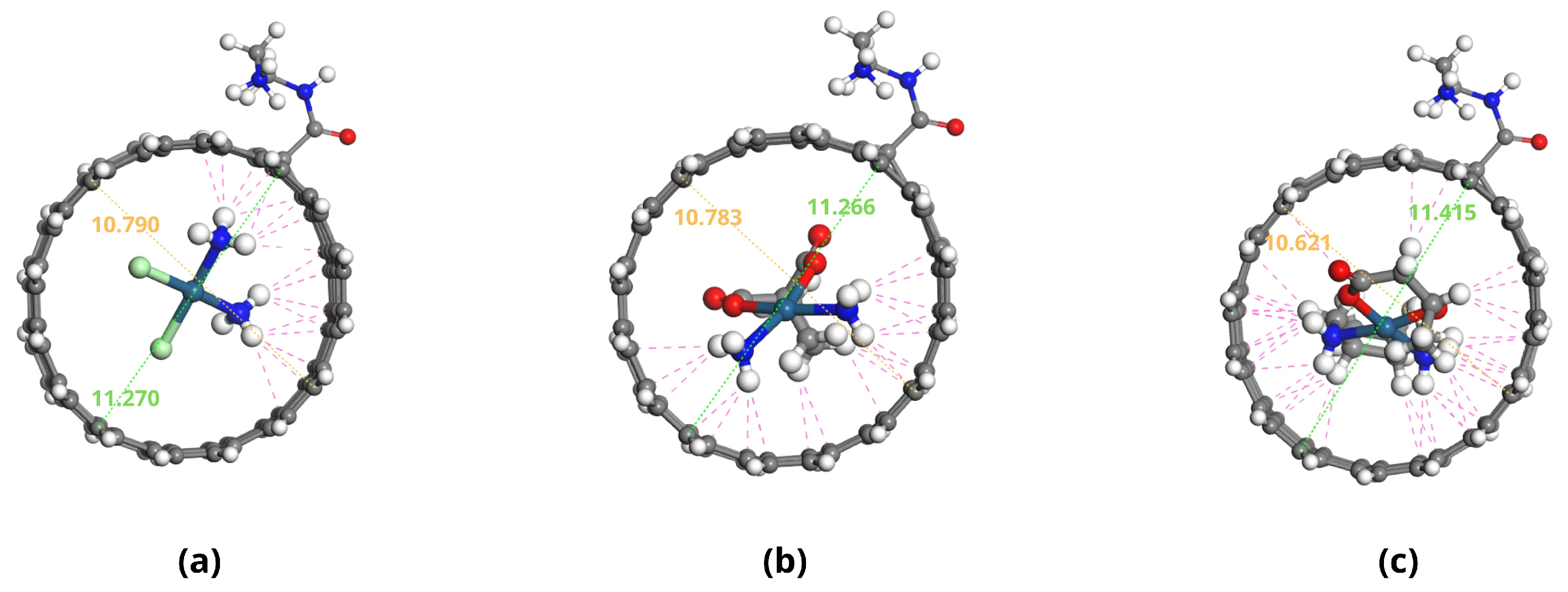
2.3. Adsorption Energy Calculations
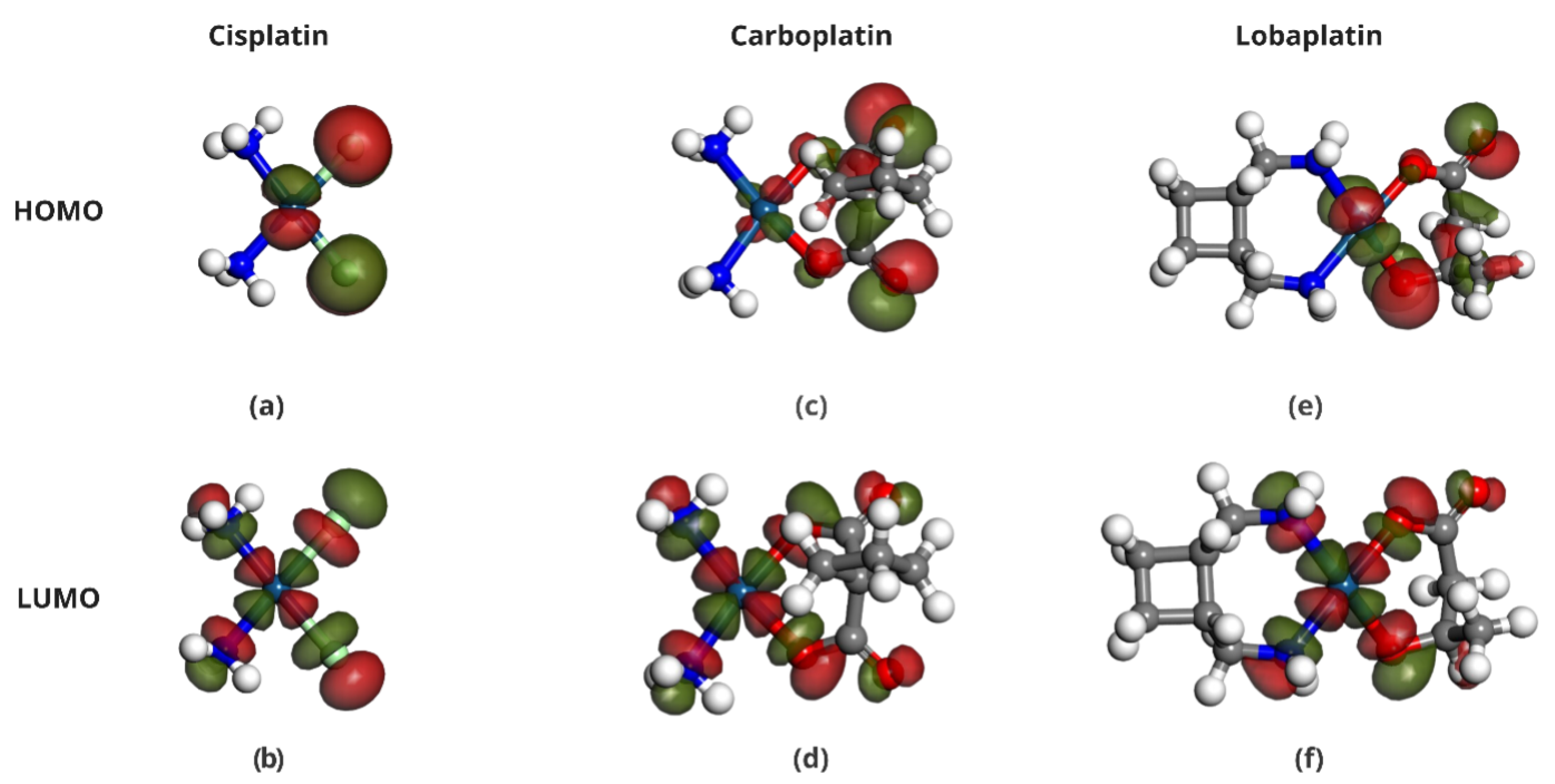
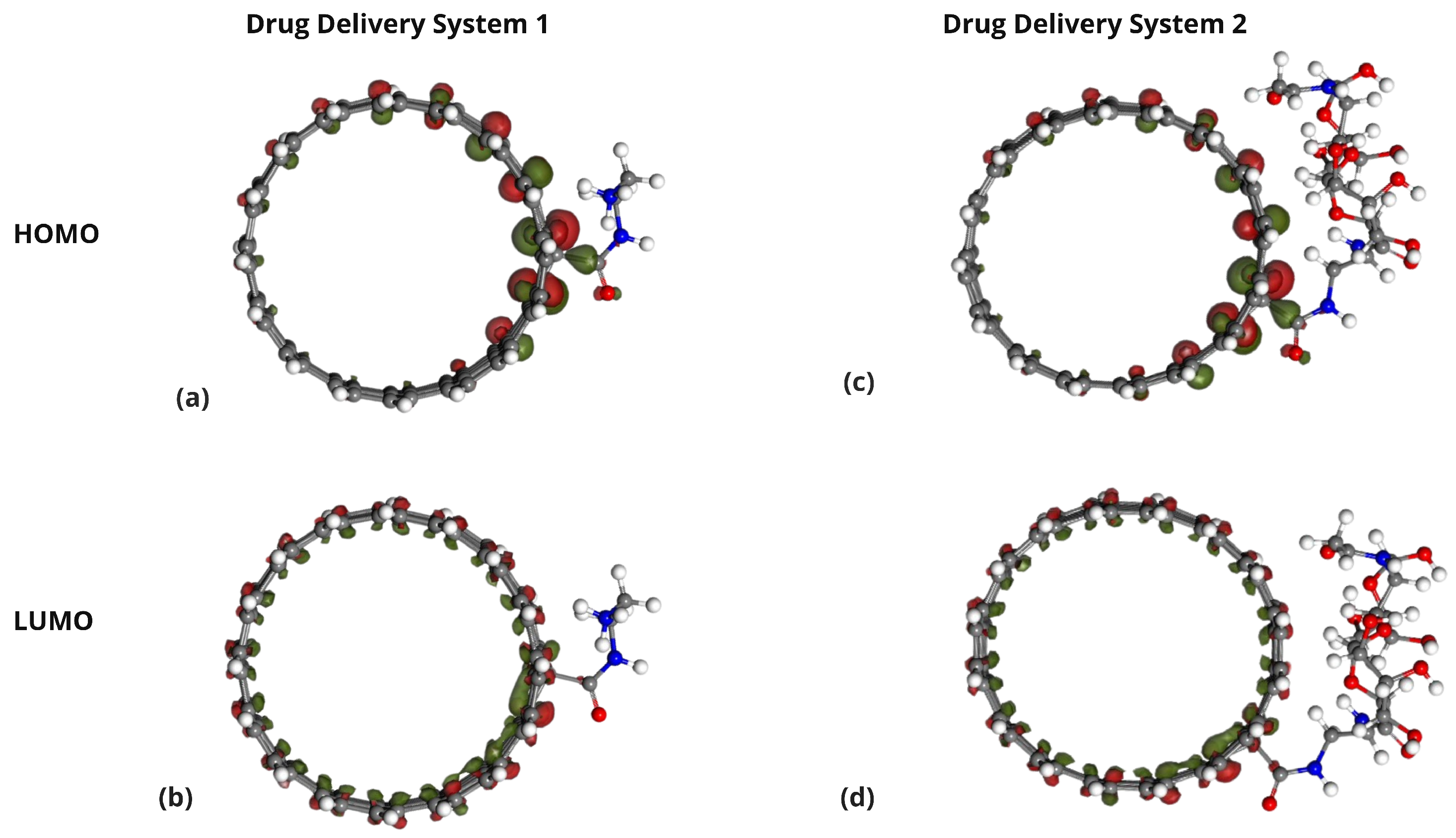
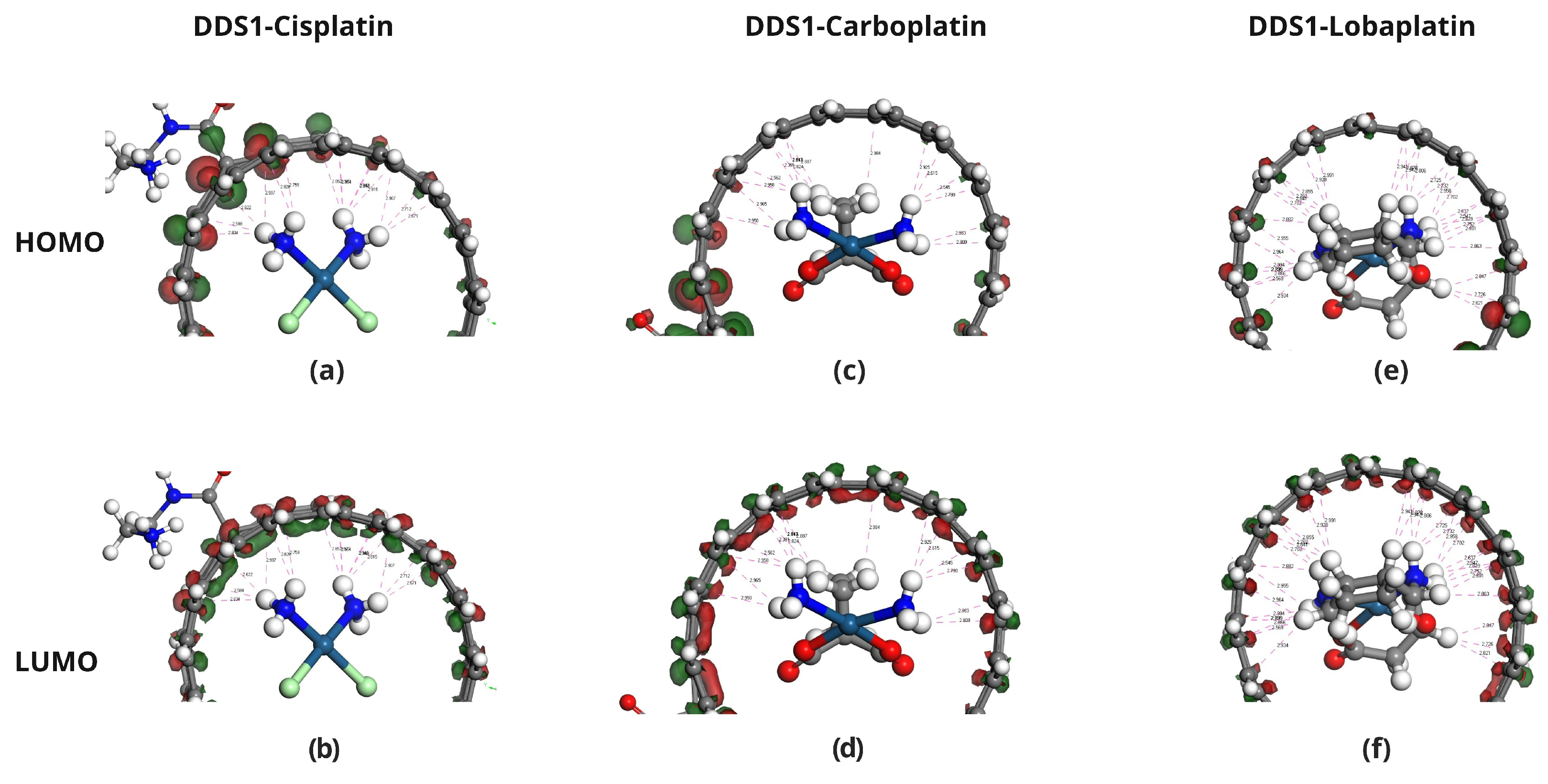
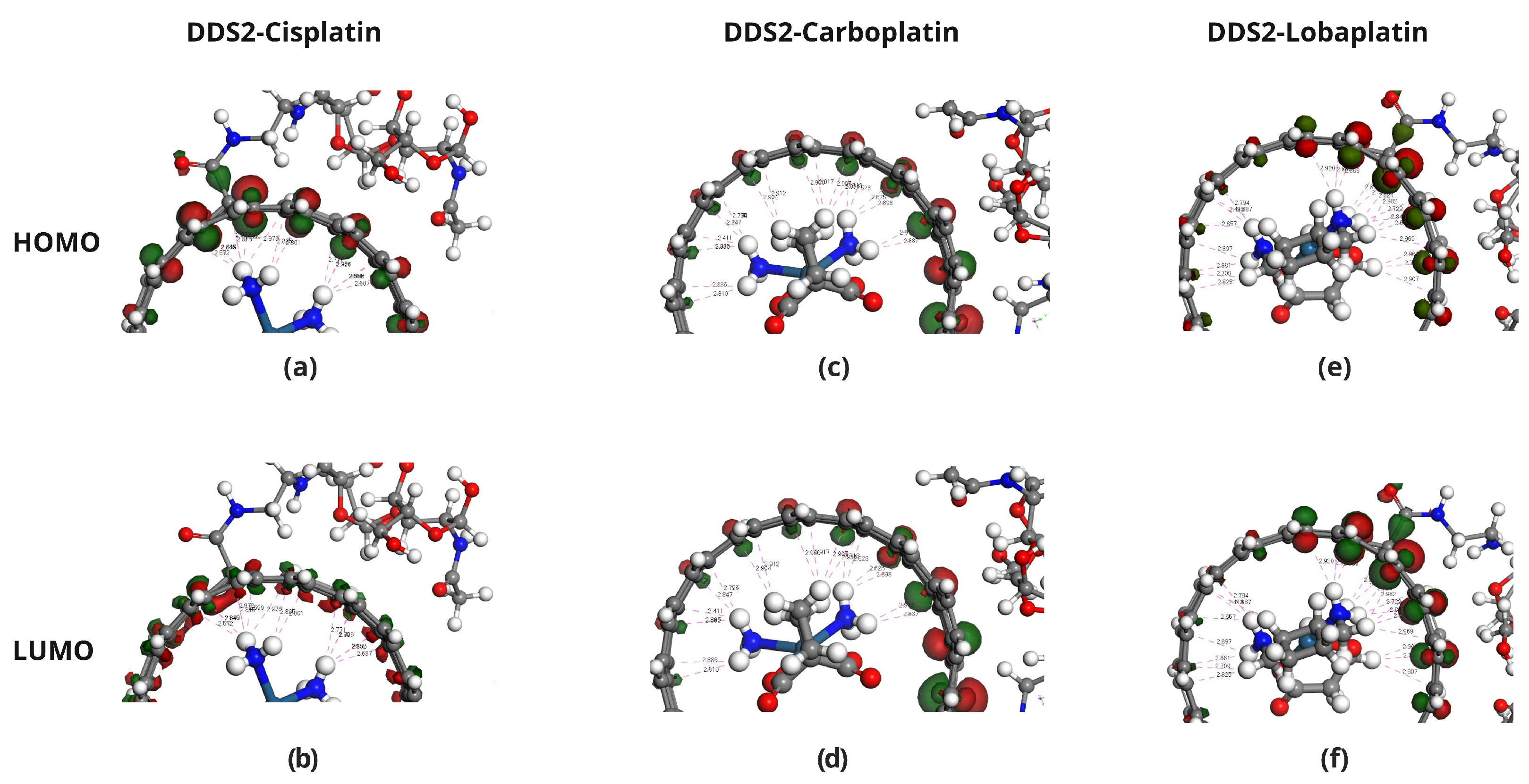
2.4. Frontier Molecular Orbital Analysis
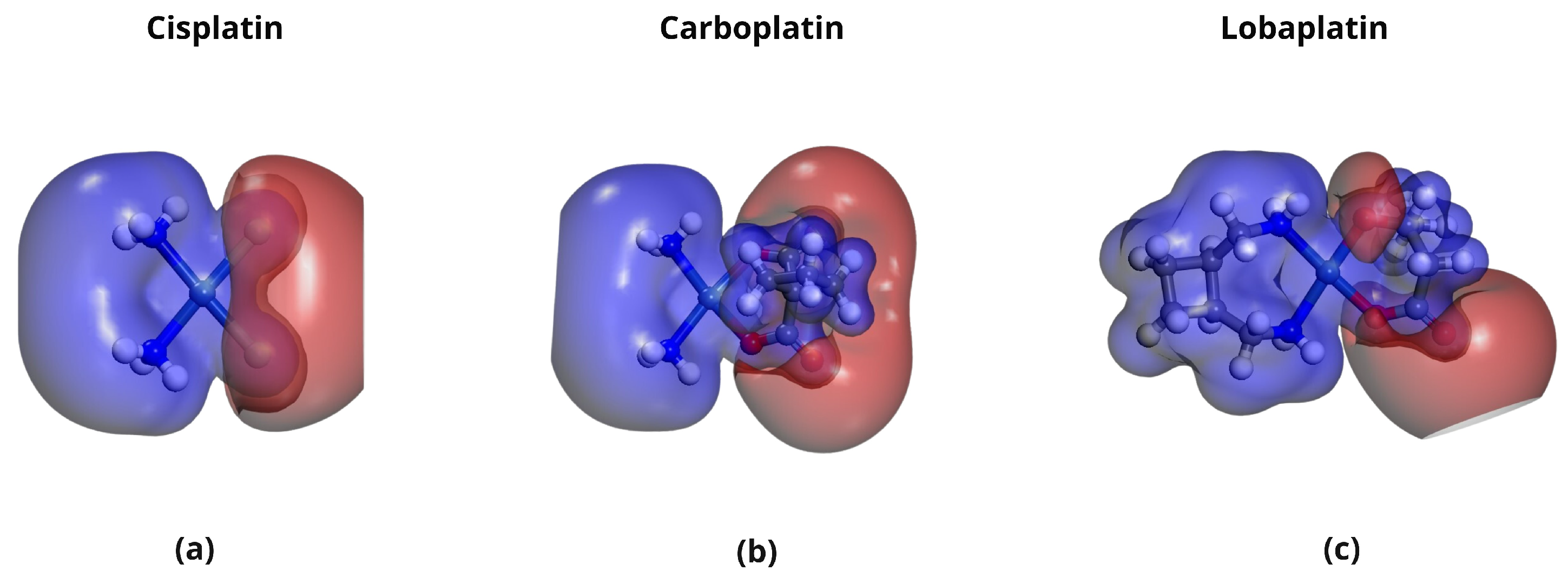
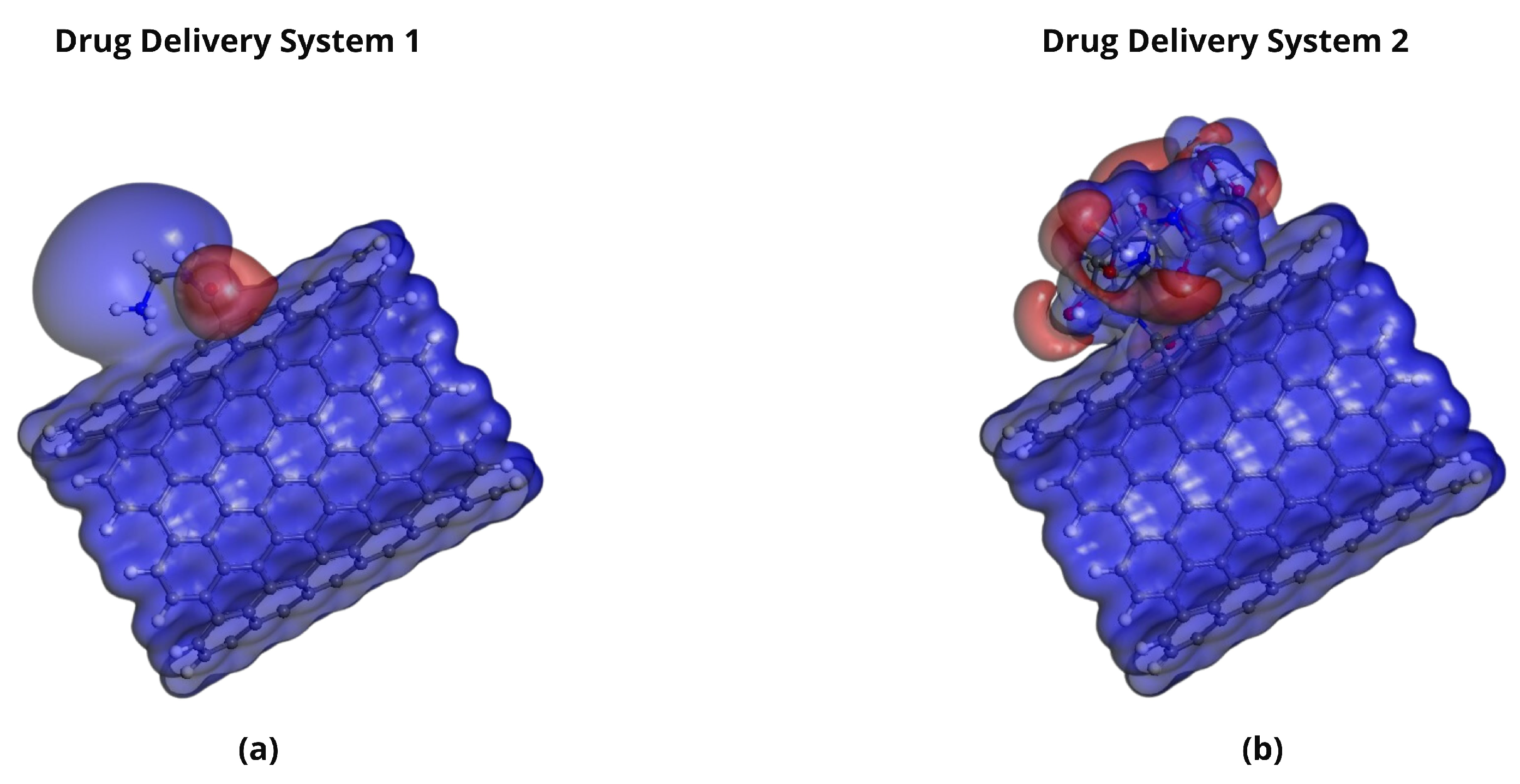
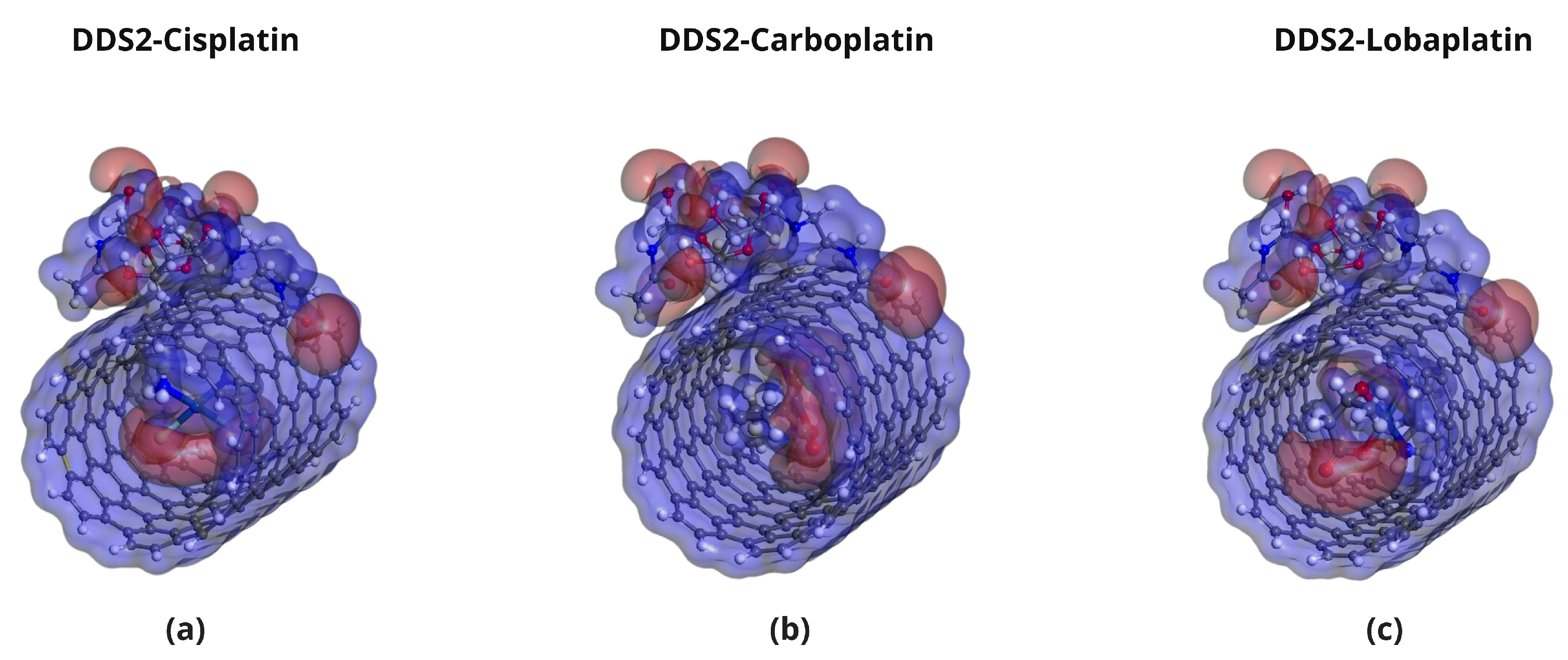
2.5. Molecular Electrostatic Potential (MEP) Analysis
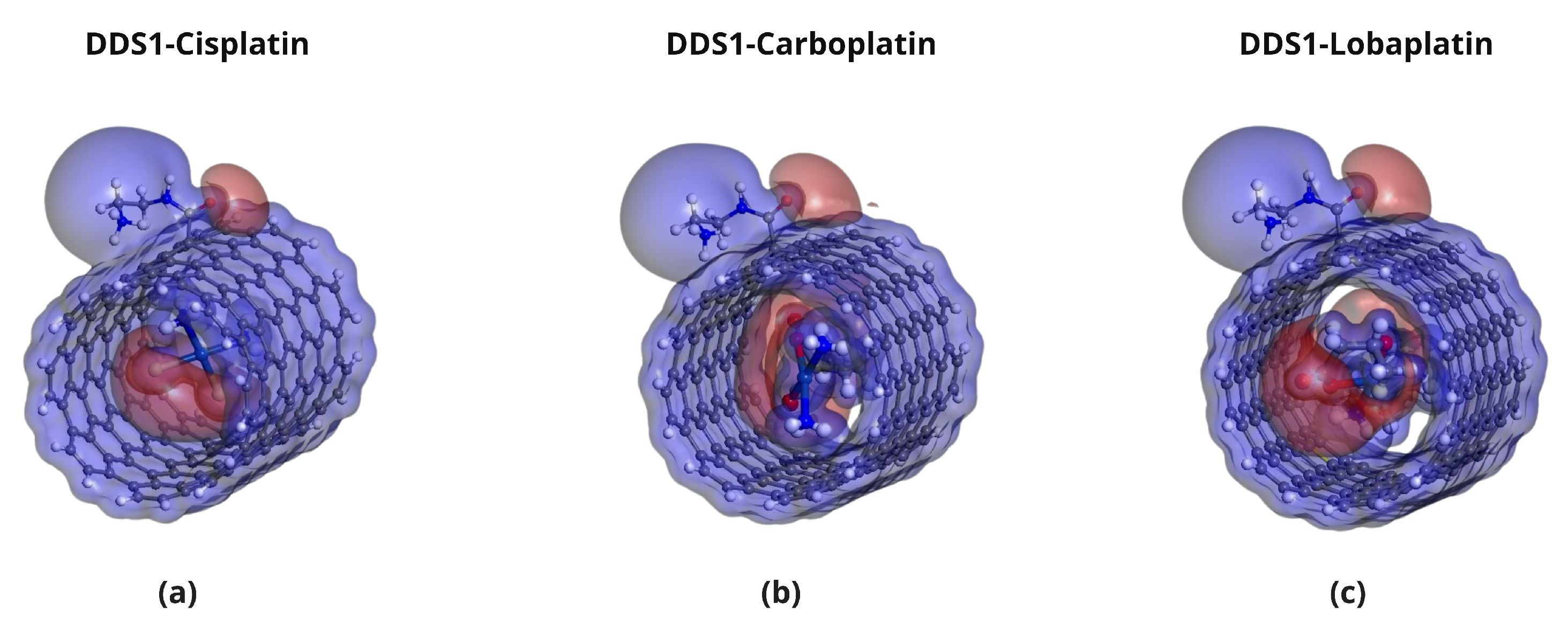
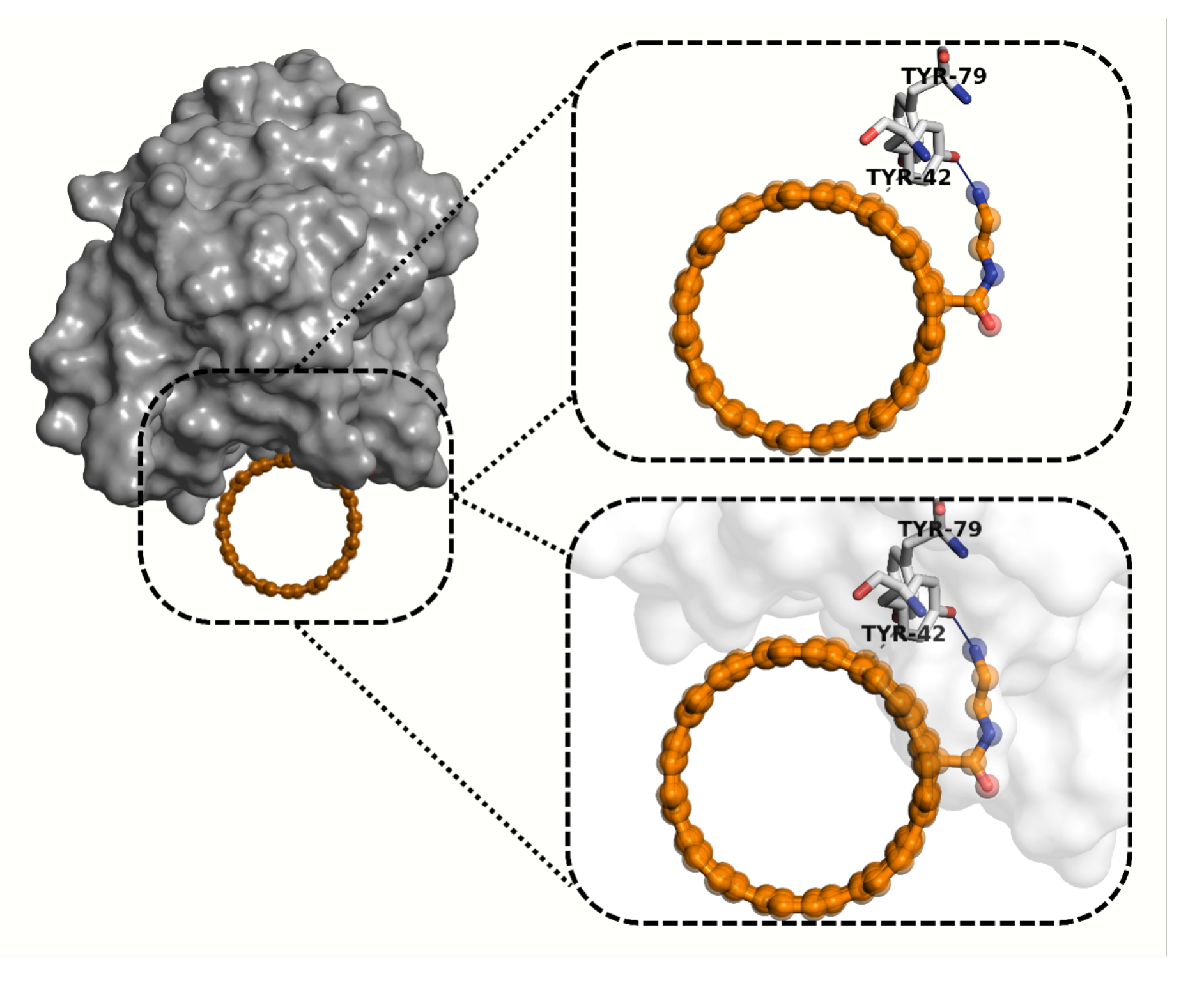
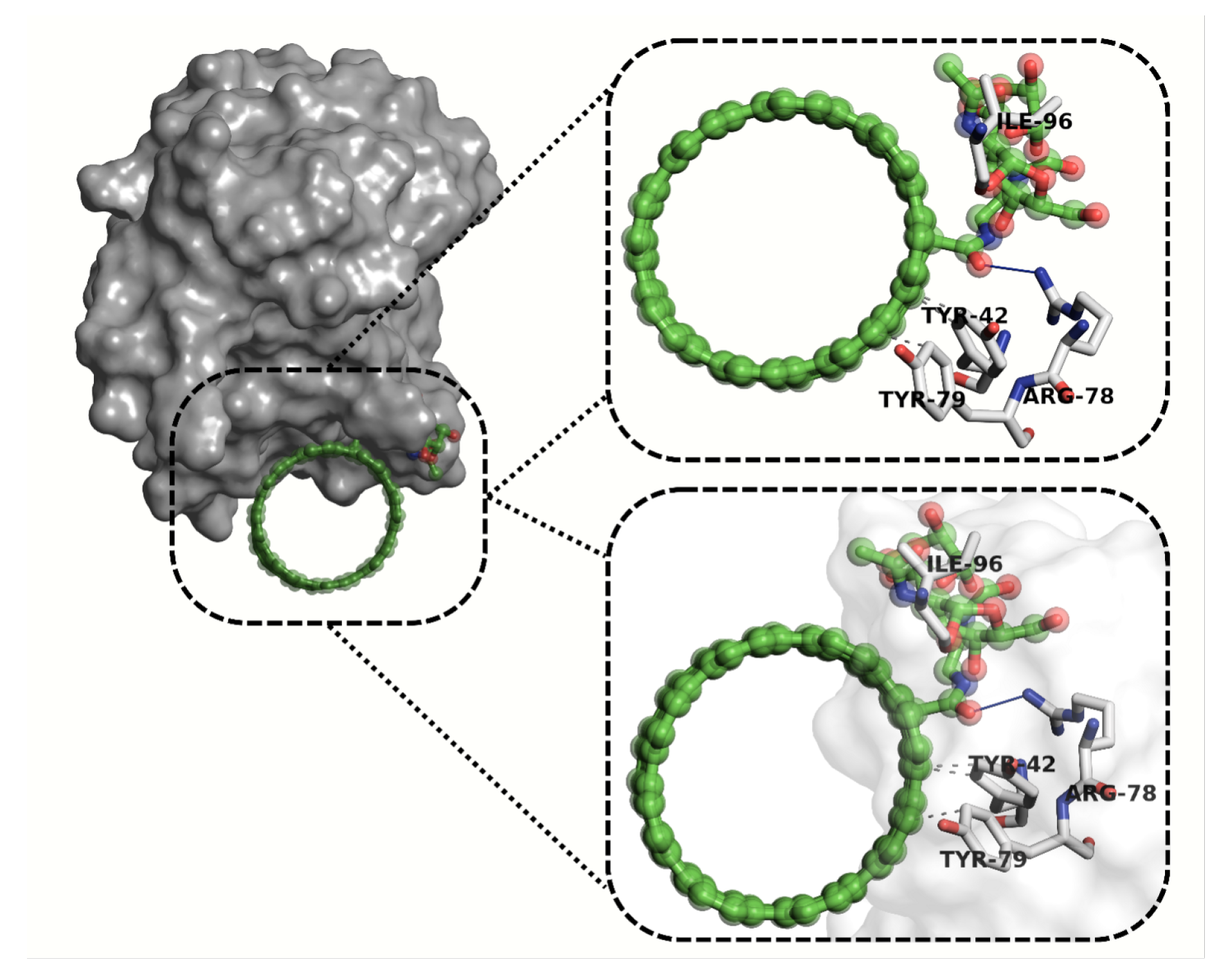
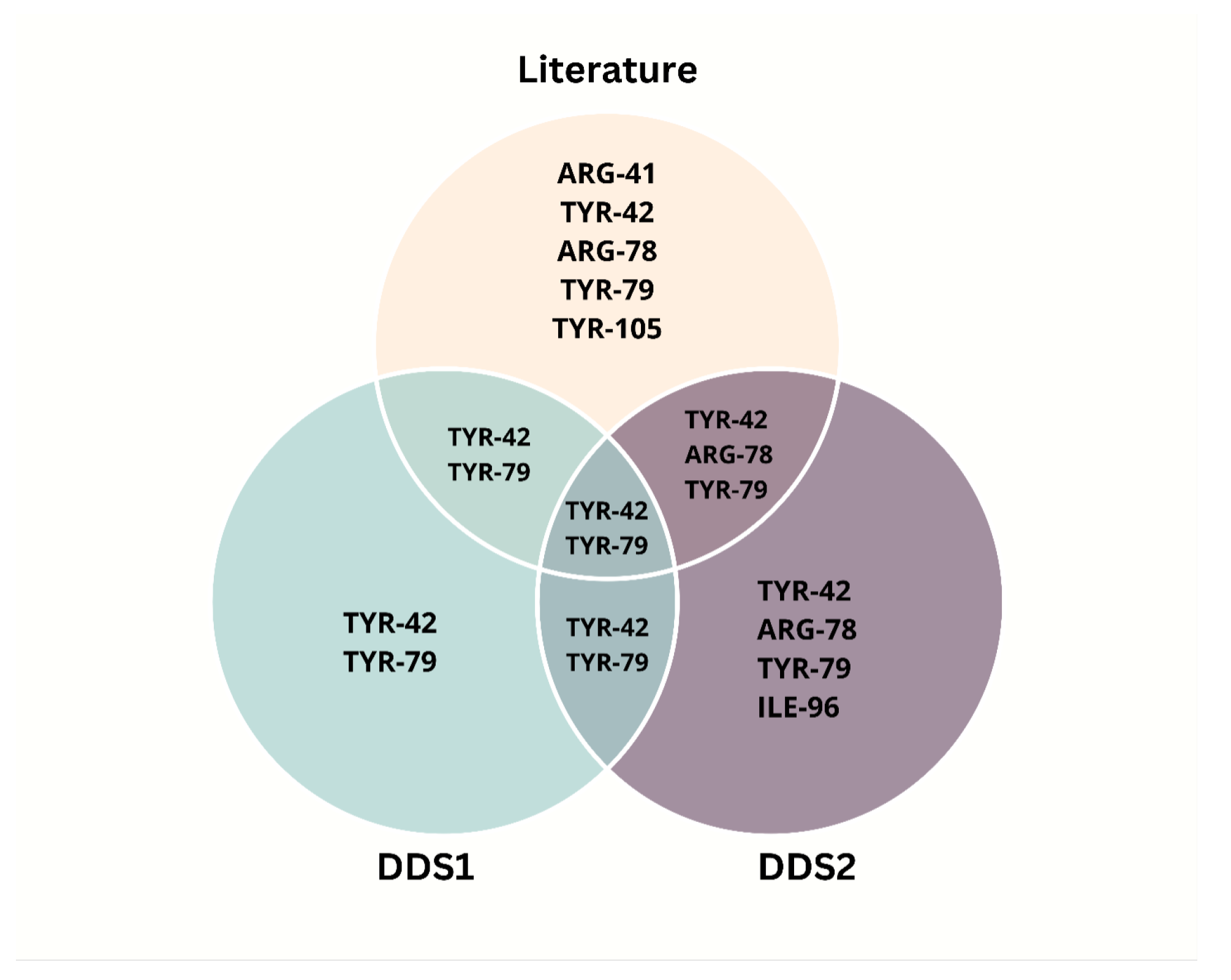
2.6. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Quantum Mechanical Studies and Model Validation
4.2. Molecular Docking Studies
4.2.1. Protein Preparation
- His-45 and His-102: assigned as -protonated (HIE),
- His-142: assigned as -protonated (HID).
4.2.2. Docking Protocol
4.2.3. Protocol Validation and Refinement
4.2.4. Docking Score Calibration
4.2.5. Scoring Function Overview
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, X.; Wu, F.; Li, Y.; Cai, X. Hyaluronic acid-functionalized halloysite nanotubes for targeted drug delivery to CD44-overexpressing cancer cells. Mater. Today Commun. 2021, 28, 102682. [Google Scholar] [CrossRef]
- Fu, C.P.; Cai, X.Y.; Chen, S.L.; Yu, H.W.; Fang, Y.; Feng, X.C.; Zhang, L.-M.; Li, C.-Y. Hyaluronic acid-based nanocarriers for anticancer drug delivery. Polymers 2023, 15, 2317. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Chiesa, E.; Greco, A.; Riva, F.; Dorati, R.; Conti, B.; Modena, T.; Genta, I. CD44-targeted carriers: The role of molecular weight of hyaluronic acid in the uptake of hyaluronic acid-based nanoparticles. Pharmaceuticals 2022, 15, 103. [Google Scholar] [CrossRef]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-coated nanoparticles for active tumor targeting: Influence of polysaccharide molecular weight on cell uptake. Colloids Surf. B. Biointerfaces 2022, 210, 112240. [Google Scholar] [CrossRef] [PubMed]
- Chadar, R.; Afzal, O.; Alqahtani, S.M.; Kesharwani, P. Carbon nanotubes as an emerging nanocarrier for the delivery of doxorubicin for improved chemotherapy. Colloids Surf. B Biointerfaces 2021, 208, 112044. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Hou, L.; Yuan, Y.; Ren, J.; Zhang, Y.; Wang, Y.; Shan, X.; Liu, Q.; Zhang, Z. In vitro and in vivo comparative study of the phototherapy anticancer activity of hyaluronic acid-modified single-walled carbon nanotubes, graphene oxide, and fullerene. J. Nanoparticle Res. 2017, 19, 286. [Google Scholar] [CrossRef]
- Cavalcanti, A.D.D.; Santana, M.H.A. Structural and surface properties control the recovery and purity of bio-hyaluronic acid upon precipitation with isopropyl alcohol. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 112–118. [Google Scholar] [CrossRef]
- Hou, X.; Zhong, D.; Chen, H.; Gu, Z.; Gong, Q.; Ma, X.; Zhang, H.; Zhu, H.; Luo, K. Recent advances in hyaluronic acid-based nanomedicines: Preparation and application in cancer therapy. Carbohydr. Polym. 2022, 292, 119662. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan 1. Faseb J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Y.; Zhang, H.; Qin, Y.; Li, D.; Gan, L.; Wu, F. Using hyaluronic acid-functionalized pH stimuli-responsive mesoporous silica nanoparticles for targeted delivery to CD44-overexpressing cancer cells. Int. J. Nanomed. 2016, 2016, 6485–6497. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Nan, Y.; Jia, L.; Sun, J.; Zhang, L.; Wang, Y. Hyaluronidase and pH dual-responsive nanoparticles for targeted breast cancer stem cells. Front. Oncol. 2021, 11, 760423. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronidases in cancer biology. Hyaluronan Cancer Biol. 2008, 18, 207–220. [Google Scholar]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and palladium complexes as promising sources for antitumor treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W. Density functional and density matrix method scaling linearly with the number of atoms. Phys. Rev. Lett. 1996, 76, 3168. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface modification of lipid-based nanocarriers: A potential approach to enhance targeted drug delivery. ACS Omega 2022, 8, 74–86. [Google Scholar] [CrossRef]
- Mohammadi, A.; Bagheri, F.; Abutalebi, Y.; Aghaei, A.; Danafar, H. Platinum nanoparticles-embedded single-walled carbon nanotubes as a new carrier for curcumin delivery and investigating its anticancer effect on cell line 4T1. Heliyon 2024, 10, e33703. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Makki, M.M.A.; Hashem, A.R.H.; Dhiyab, A.A.K. Carbon Nanotubes for Thermal Therapy CNTs: Effect of Nanotube Structure and Doping on Photothermal Properties, Anticancer Efficacy of CNT-Enhanced PTT and Systemic Delivery and Biocompatibility of CNTs for PTT. Curr. Clin. Med. Educ. 2024, 2, 29–47. [Google Scholar]
- Ghosh, S.; Dave, V.; Wal, P. Therapeutic approach of carbon nanotube: Revolutionize nanomaterial in biomedical and pharmaceutical sector. J. Food Drug Anal. 2024, 32, 412. [Google Scholar] [CrossRef]
- Biovia, D.S. Materials Studio, R2; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2017. [Google Scholar]
- Froudakis, G.E. Hydrogen interaction with single-walled carbon nanotubes: A combined quantum-mechanics/molecular-mechanics study. Nano Lett. 2001, 1, 179–182. [Google Scholar] [CrossRef]
- Han, S.S.; Lee, H.M. Adsorption properties of hydrogen on (10, 0) single-walled carbon nanotube through density functional theory. Carbon N. Y. 2004, 42, 2169–2177. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Delley, B. DMol3 DFT studies: From molecules and molecular environments to surfaces and solids. Comput. Mater. Sci. 2000, 17, 122–126. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef]
- Van Lenthe, J.; Faas, S.; Snijders, J. Gradients in the ab initio scalar zeroth-order regular approximation (ZORA) approach. Chem. Phys. Lett. 2000, 328, 107–112. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- QIAGEN Digital Insights. Latest Improvements—Molegro Virtual Docker 6.0. 2013. Available online: https://digitalinsights.qiagen.com/products/molegro-virtual-docker/latest-improvements/ (accessed on 21 May 2025).
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, M.; Olsson, M.H.; Søndergaard, C.R.; Jensen, J.H. Graphical analysis of pH-dependent properties of proteins predicted using PROPKA. Bmc Struct. Biol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Teriete, P.; Banerji, S.; Noble, M.; Blundell, C.D.; Wright, A.J.; Pickford, A.R.; Lowe, E.; Mahoney, D.J.; Tammi, M.I.; Kahmann, J.D.; et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol. Cell 2004, 13, 483–496. [Google Scholar] [CrossRef] [PubMed]
| Complex | DDS1 | DDS2 | ||
|---|---|---|---|---|
| (Ha) | (eV) | (Ha) | (eV) | |
| CP | ||||
| CBP | ||||
| LBP | ||||
| Complex | DDS1 | DDS2 |
|---|---|---|
| CP/DDS | 12 | 16 |
| CBP/DDS | 14 | 18 |
| LBP/DDS | 15 | 20 |
| Drug | DDS1 | DDS2 | ||||
|---|---|---|---|---|---|---|
| CP | ||||||
| CBP | ||||||
| LBP | ||||||
| Compound | HOMO (eV) | LUMO (eV) | (eV) |
|---|---|---|---|
| Cisplatin (CP) | −4.451 | −2.376 | 2.075 |
| Carboplatin (CBP) | −4.455 | −2.388 | 2.067 |
| Lobaplatin (LBP) | −3.477 | −1.415 | 2.062 |
| DDS1 | −2.931 | −2.684 | 0.247 |
| DDS2 | −3.667 | −3.356 | 0.311 |
| Ligand | 1POZ (HSA) | 4LRH (AR) | 4MRD (MMP-9) | 4PZ3 (CD44) |
|---|---|---|---|---|
| CNT-NH2 | ||||
| CNT-NH2–HA (DDS2) |
| Complex | MolDock Score (kcal/mol) |
|---|---|
| DDS1-CD44 | −106.68 |
| DDS2-CD44 | −171.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.U.; Jabeen, I.; Althagafi, A.; Farooq, M.U.; Harb, M.; Arkook, B. DFT and Molecular Docking Study of HA-Conjugated SWCNTs for CD44-Targeted Delivery of Platinum-Based Chemotherapeutics. Pharmaceuticals 2025, 18, 805. https://doi.org/10.3390/ph18060805
Khan MU, Jabeen I, Althagafi A, Farooq MU, Harb M, Arkook B. DFT and Molecular Docking Study of HA-Conjugated SWCNTs for CD44-Targeted Delivery of Platinum-Based Chemotherapeutics. Pharmaceuticals. 2025; 18(6):805. https://doi.org/10.3390/ph18060805
Chicago/Turabian StyleKhan, Muhammad Uzair, Ishrat Jabeen, Abdulhamid Althagafi, Muhammad Umar Farooq, Moussab Harb, and Bassim Arkook. 2025. "DFT and Molecular Docking Study of HA-Conjugated SWCNTs for CD44-Targeted Delivery of Platinum-Based Chemotherapeutics" Pharmaceuticals 18, no. 6: 805. https://doi.org/10.3390/ph18060805
APA StyleKhan, M. U., Jabeen, I., Althagafi, A., Farooq, M. U., Harb, M., & Arkook, B. (2025). DFT and Molecular Docking Study of HA-Conjugated SWCNTs for CD44-Targeted Delivery of Platinum-Based Chemotherapeutics. Pharmaceuticals, 18(6), 805. https://doi.org/10.3390/ph18060805







