1. Introduction
Gastric cancer represents a significant public health burden in China, contributing to 44.0% of global cases and 48.6% of related deaths in 2020 [
1]; the escalating incidence and mortality rates underscore the urgent need for effective interventions. Chronic atrophic gastritis (CAG), a chronic digestive disorder marked by gastric mucosal gland degeneration, is a well-established precursor to gastric cancer when accompanied by intestinal metaplasia and dysplasia [
2]. Early detection and intervention are critical to mitigating disease progression. However, the nonspecific clinical presentation of CAG has hindered the development of effective pharmacological therapies in Western medicine. Traditional Chinese medicine (TCM) offers a promising alternative, promoting gastric mucosal regeneration and potentially reversing atrophy, thereby reducing cancer risk with minimal adverse effects.
Bombyx Batryticatus (BB), a traditional medicine in Chinese pharmacopeia, is classified as a product of ‘blood, flesh, and emotions’. First documented in the
Shennong Bencao Jing, BB is also known as Bai Jiang Can or Tian Chong. BB is derived from the dried larvae of
Bombyx mori infected by the fungus
Beauveria bassiana, undergoing sclerotization during processing [
3]. According to TCM theory, BB exhibits a neutral nature, a salty flavor profile, and therapeutic effects on the liver, spleen, and stomach meridians. BB demonstrates diverse pharmacological activities, including wind-calming, convulsion-mitigating, pain-alleviating, phlegm-resolving, and nodule-dispersing properties [
4]. Clinical studies have validated BB’s efficacy in treating diverse conditions, such as lymphatic tuberculosis, lung cancer, nasopharyngeal carcinoma, bladder cancer, brain tumors, malignant lymphoma, and breast cancer.
Rooted in systems biology, network pharmacology employs multi-dimensional networks to map the complex interactions between TCM components, molecular targets, and biological pathways, offering a holistic understanding of therapeutic mechanisms. This approach aligns with the holistic philosophy of TCM, providing a systems-level perspective on pharmacological mechanisms [
5].
UPLC-QE-Orbitrap-MS/MS provides exceptional resolution, rapid scanning, and precise structural characterization. This technology enables simultaneous, high-throughput identification and quantification of multiple components without requiring reference standards. As a result, UPLC-QE-Orbitrap-MS/MS has been widely adopted for rapid screening and qualitative analysis of both single-ingredient and compound TCM formulations [
6].
In this study, we integrated UPLC-QE-Orbitrap-MS/MS, network pharmacology, molecular docking, and experimental validation to elucidate the pharmacological mechanisms underlying BB’s anti-PL-CAG effects. This integrative approach not only establishes a robust experimental foundation for BB’s clinical application in PL-CAG management but also paves the way for future mechanistic investigations.
3. Discussion
First proposed by Correa in 1975, the widely accepted multi-stage gastric carcinogenesis model delineates a progression sequence from normal gastric mucosa to chronic superficial gastritis, precancerous lesions, and ultimately invasive gastric cancer (GC) [
7]. The World Health Organization classifies CAG presenting with intestinal metaplasia (IM) and dysplasia (Dys) as gastric precancerous lesions (PLGC). In this work, we collectively term these pathological states—CAG with or without IM/Dys—as precancerous lesions of chronic atrophic gastritis (PL-CAG) to unify mechanistic investigations.
Current therapeutic strategies in modern medicine lack an effective oral pharmacotherapy specifically targeting PL-CAG. Clinical management predominantly relies on pharmacological interventions including proton pump inhibitors, antibiotics, acid suppressants, and gastric mucosal protectants. While these agents demonstrate efficacy in mitigating mucosal inflammation and relieving abdominal pain symptoms, prolonged administration may induce adverse effects such as hepatorenal toxicity, multidrug resistance development, and osteoporosis risk [
8]. In contrast, TCM presents a multi-target therapeutic approach with enhanced safety profiles [
9], offering distinct advantages for PL-CAG management.
The Chinese Pharmacopoeia documents BB’s pharmacological properties as pungent–salty flavor with thermodynamic neutrality, exhibiting meridian tropism towards hepatic, splenic, and gastric systems. BB has the effect of soothing hepatic wind and relieving spasm, expelling wind and relieving pain, eliminating phlegm, and dispersing knots. It is often used as a small compound imperial medicine [
10]. Modern research on BB suggests that it comprises a diverse array of chemical compounds and demonstrates a spectrum of pharmacological activities.
In the clinical treatment of CAG, especially PLGC with traditional Chinese medicine, BB is often used as an important component of Chinese herbal compound. Professor LiuXiang Huang, a distinguished expert in traditional Chinese medicine, posits that CAG with atypical hyperplasia is predominantly attributed to phlegm and blood stasis, as well as intense heat and toxicity. His clinical protocol advocates therapeutic formula modification through BB integration to address these pathophysiological characteristics [
11]. Grand Master Diangui Li posits that the primary pathogenesis of CAG is the accumulation of turbid toxins. His therapeutic strategy emphasizes BB-mediated detoxification and meridian regulation, particularly effective in intestinal metaplasia cases with turbid toxin manifestations [
12]. Shuwen Shen, a distinguished Chinese physician, posits that in the management of PLGC, particularly among patients with gastrointestinal polyps, the combination of BB and wu mei can be employed to effectively dissolve phlegm and disperse nodules [
13].
Therefore, this study initially validated the pharmacological efficacy of BB in treating CAG-PLGC through both in vivo and in vitro approaches.
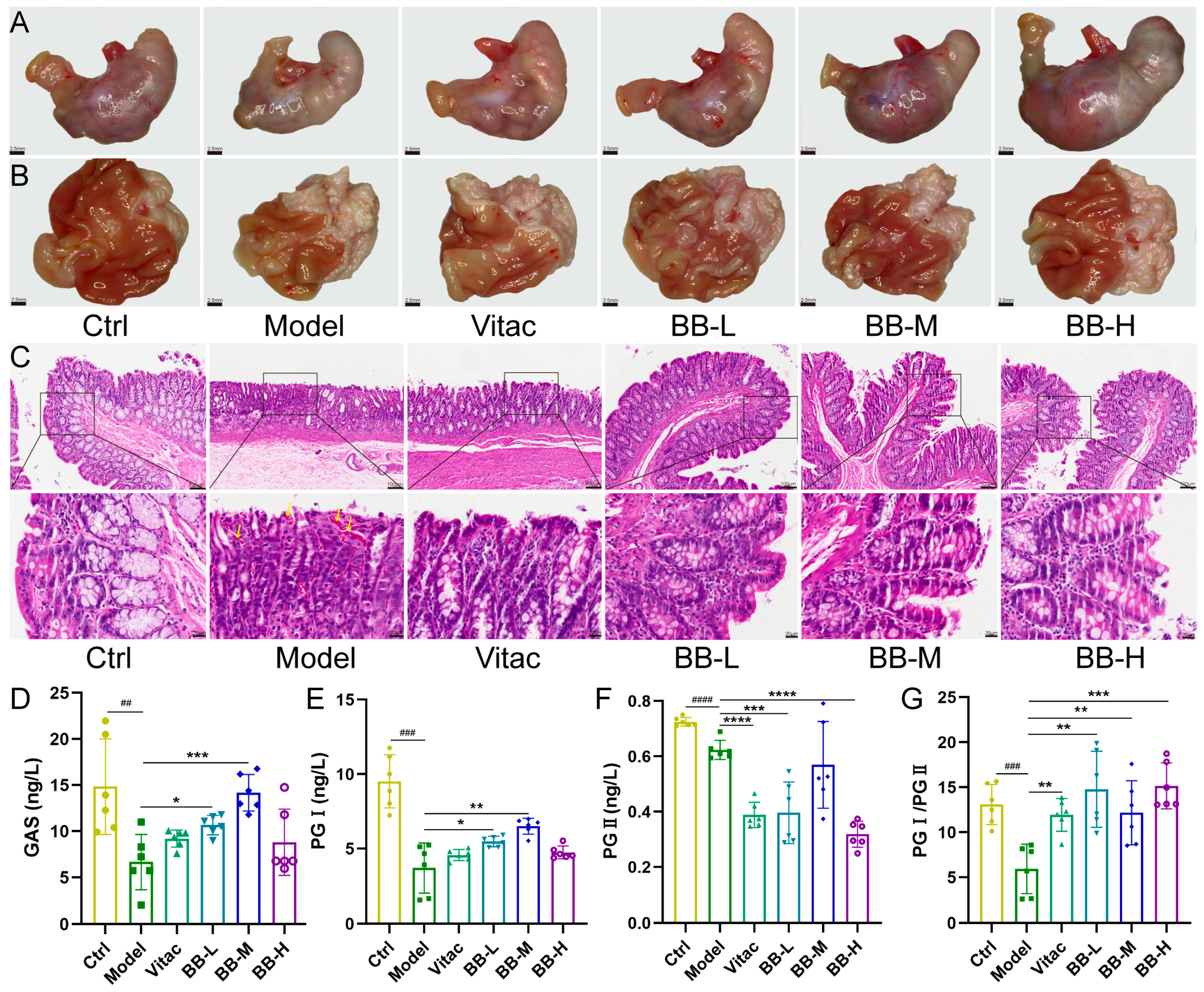
This investigation established a comprehensive evaluation system that encompasses morphological, histological, and molecular biomarkers to assess PL-CAG progression and therapeutic response, including gastric mucosal morphology, histopathological features, functional biomarkers, and inflammatory mediators. Gastric mucosal metaplasia often presents with a colorectal type, which is closely linked to the development of gastric cancer. The diagnostic criterion for this condition is the presence of goblet cells within the gastric mucosa. Gastric pepsinogen (PG) is secreted by chief cells, and its serum levels decrease in the context of PL-CAG; lower PG I/PG II levels are closely associated with the occurrence of gastric cancer [
14]. Gastrin (GAS), a polypeptide hormone, is produced by G cells situated in the gastric and duodenal mucosa adjacent to the stomach. Its primary role involves stimulating the secretion of gastric acid and pepsinogen while also supporting the functionality of parietal cells [
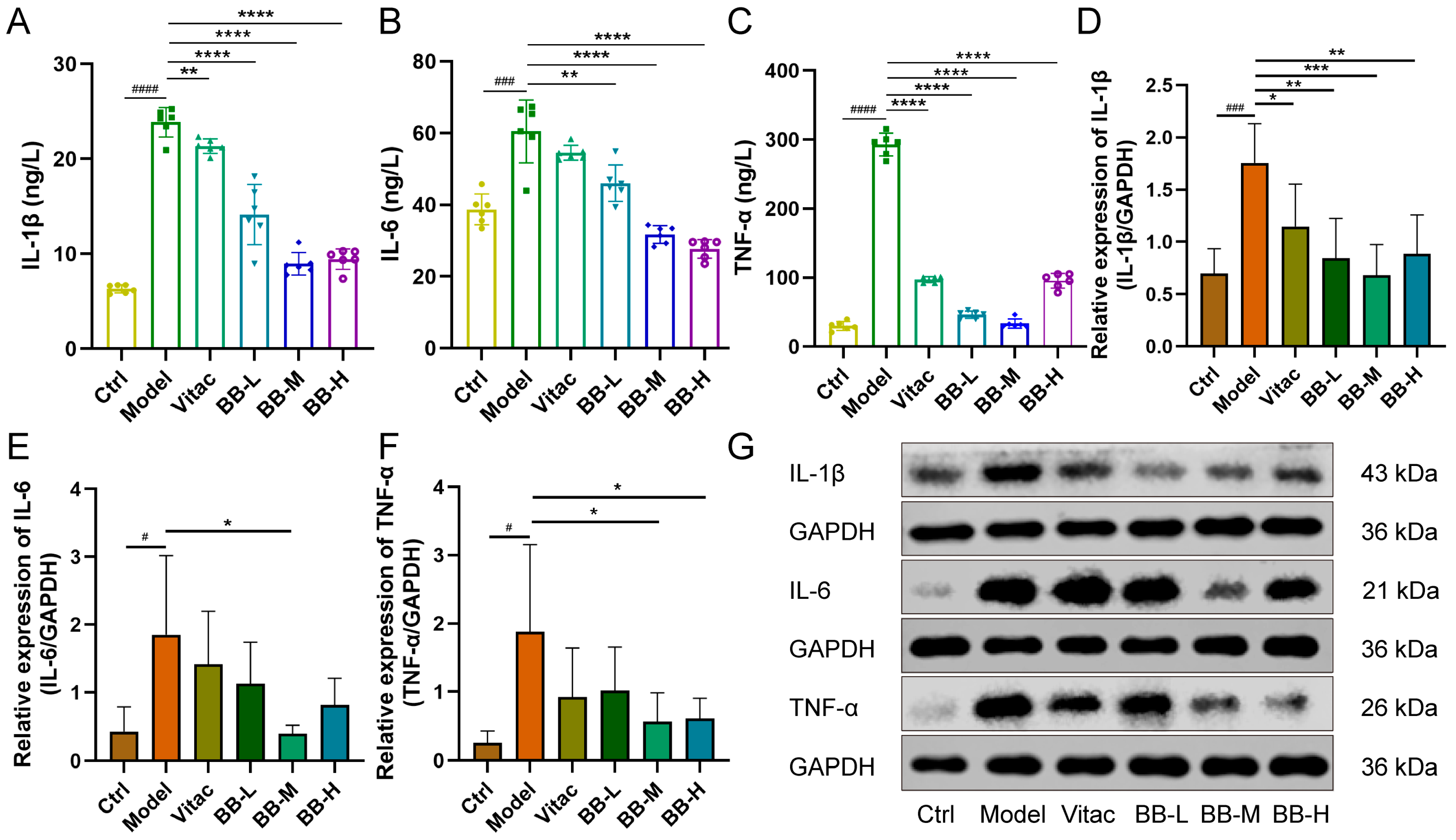
15]. Interleukin-1β (IL-1β) functions not only as a key pro-inflammatory mediator but also as a powerful inhibitor of gastric acid secretion, leading to a substantial decrease in both gastric juice volume and total acidity, which may result in delayed gastric emptying [
16]. Additionally, IL-1β promotes the synthesis of cyclooxygenase-2 (COX-2) [
17], thereby exacerbating damage to the gastric mucosa. Interleukin-1β has the potential to induce tumorigenesis through the prolonged activation of oncogenes [
18]. Tumor Necrosis Factor-alpha (TNF-α) has the potential to induce cellular immortality by modulating telomerase activity [
19]. It can also cause DNA damage and promote genetic mutations [
20]. Interleukin-6 (IL-6) is a widely recognized biomarker of inflammation. During an inflammatory response, the concentration of IL-6 in serum increases rapidly. Following the resolution of inflammation, its levels promptly return to baseline [
21]. Our study demonstrates that, compared with the normal control group, rats in the Model group exhibited weight loss. Gross observation revealed that the gastric mucosa appeared grayish-white with a granular texture and flattened folds. HE staining results indicated mucosal epithelial hyperemia, disordered arrangement of lamina propria glands, occasional goblet cells, and extensive infiltration of inflammatory cells into the submucosa. ELISA results showed decreased serum levels of GAS and PGI/PGII, along with elevated expression levels of pro-inflammatory factors. Western blot analysis further confirmed increased expression of pro-inflammatory factors in the tissue. And after BB treatment, these pathological conditions have been significantly improved, with the improvement effect exceeding that observed in the positive control group. These findings collectively confirm the effectiveness of BB in treating rats with PL-CAG. Certainly, in the present study, we primarily focused on the changes in levels of IL-1β, TNF-α, and IL-6. However, we acknowledge the importance of evaluating additional inflammatory markers such as COX-2 and NF-κB in future investigations. COX-2 is an inducible enzyme involved in prostaglandin synthesis, which plays a critical role in mediating inflammatory responses. NF-κB serves as a pivotal transcription factor that regulates the expression of multiple inflammation-related genes, including IL-1β, IL-6, and TNF-α. These factors will constitute key areas of focus in our future research on the anti-inflammatory mechanisms of BB.
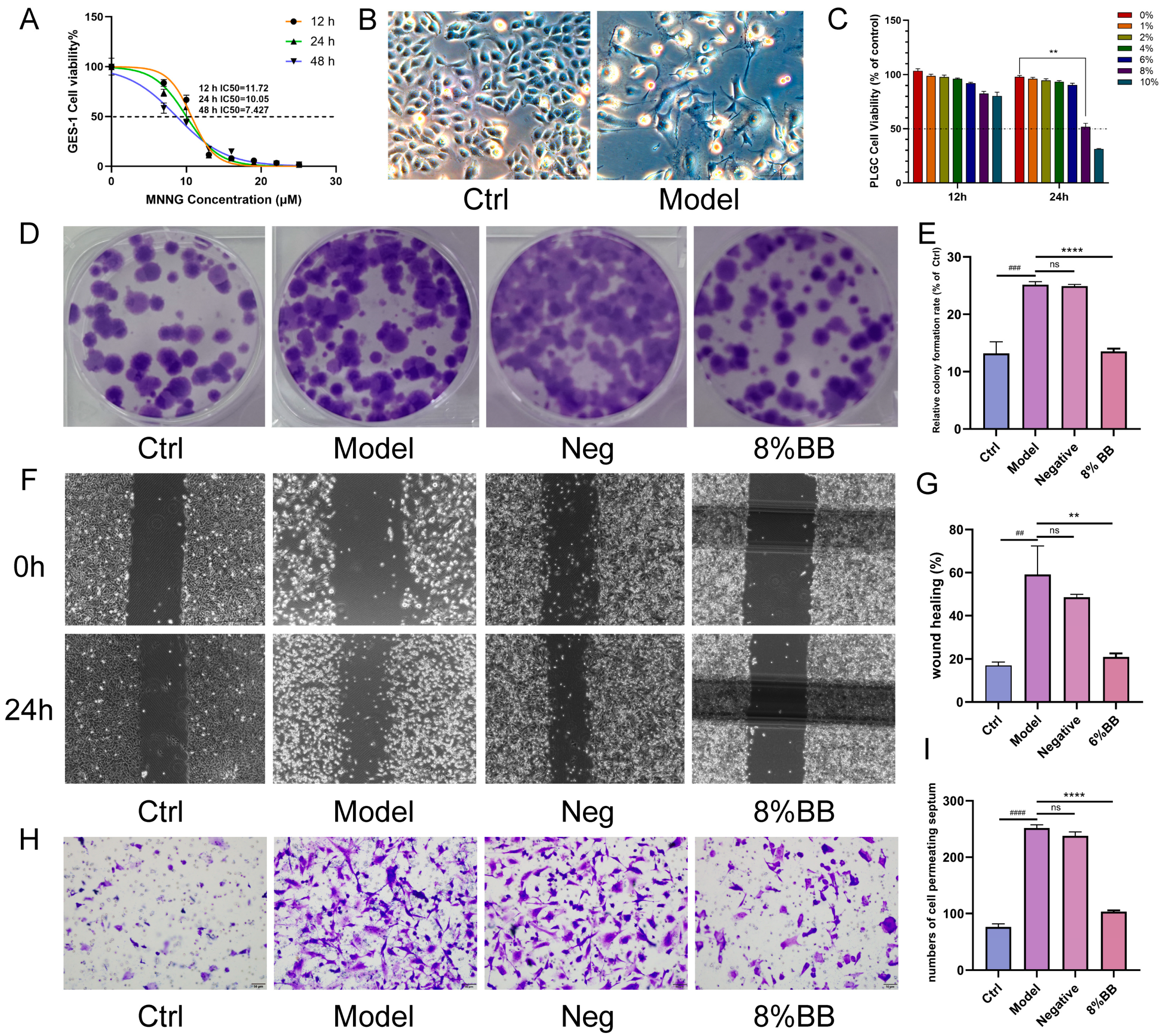
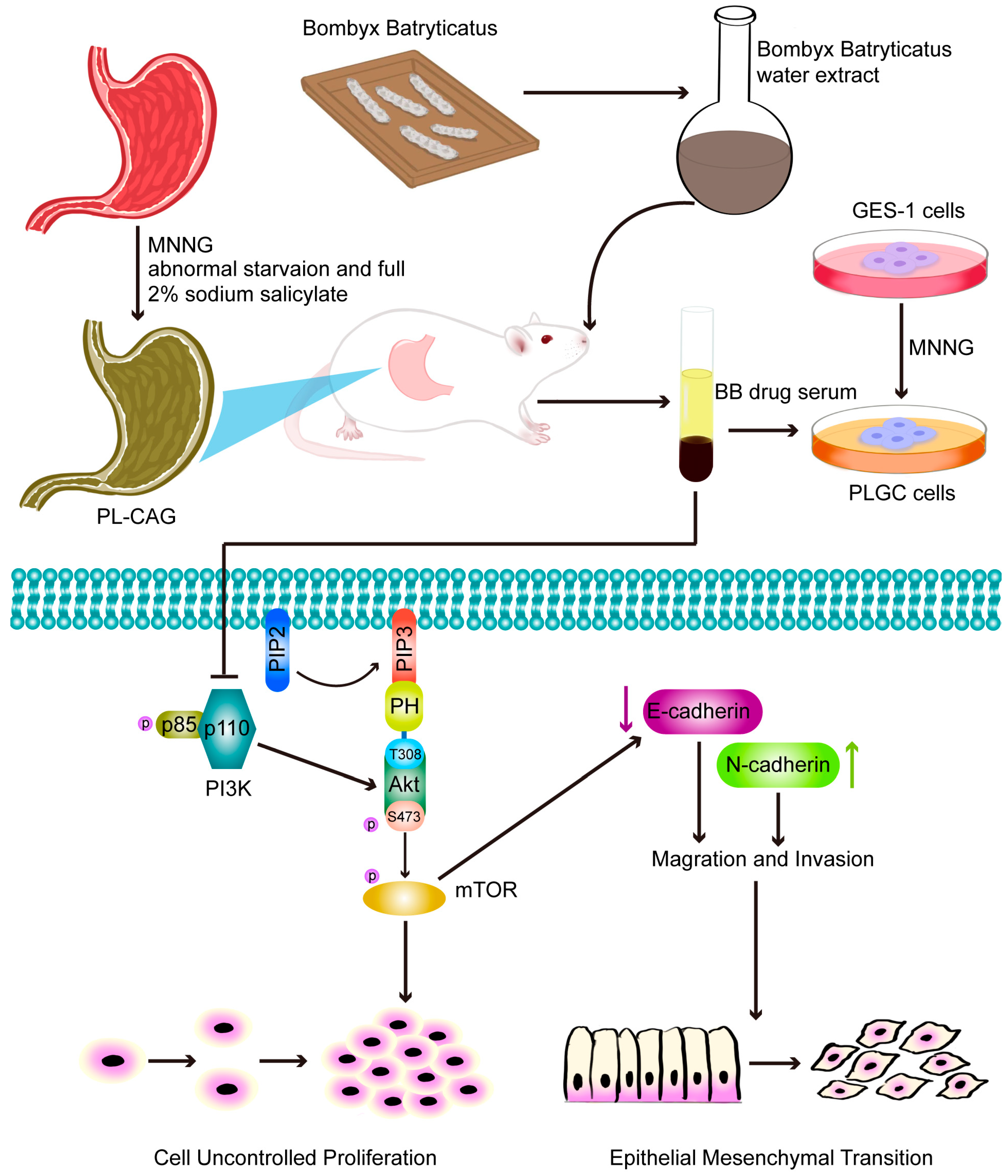
To elucidate the mechanism of drug action, we conducted pharmacodynamic validation at the cellular level. This study employed MNNG to treat GES-1 cells for a duration of 24 h. Subsequently, the normal culture medium was replaced, and the cells were cultured for an additional week to establish a PL-CAG cell model, hereafter referred to as the PLGC cell. Studies have shown that MNNG is widely used in the construction of gastric cancer precursor cell models. Following treatment with MNNG, the GES-1 cells exhibit morphological and growth characteristics typical of tumor cells. This study demonstrated that an 8% concentration of drug-containing serum significantly suppressed the proliferation, migration, and invasion capabilities of PLGC cells. These results suggest that the BB drug-containing serum exhibits therapeutic potential at the cellular level for treating PL-CAG.
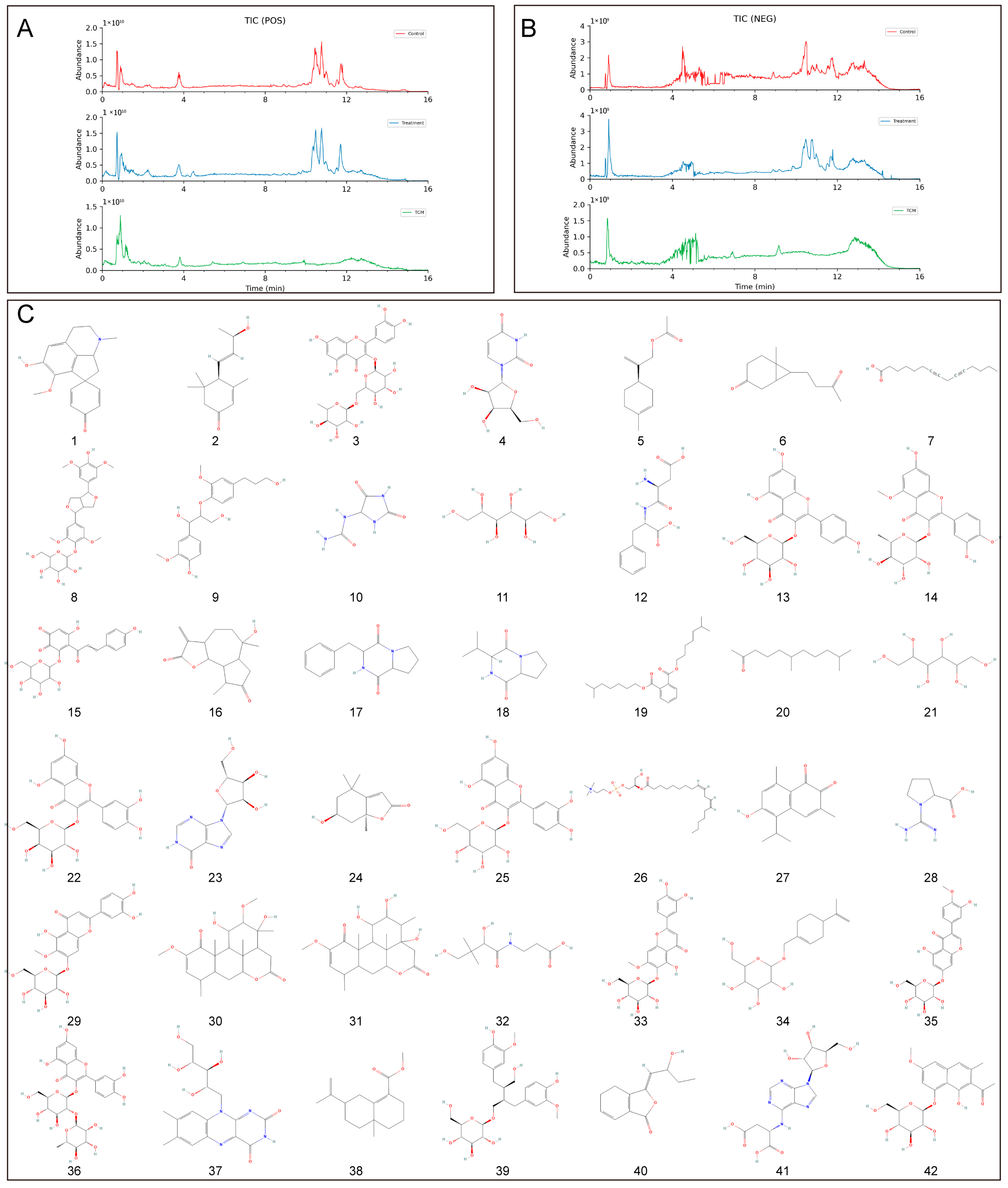
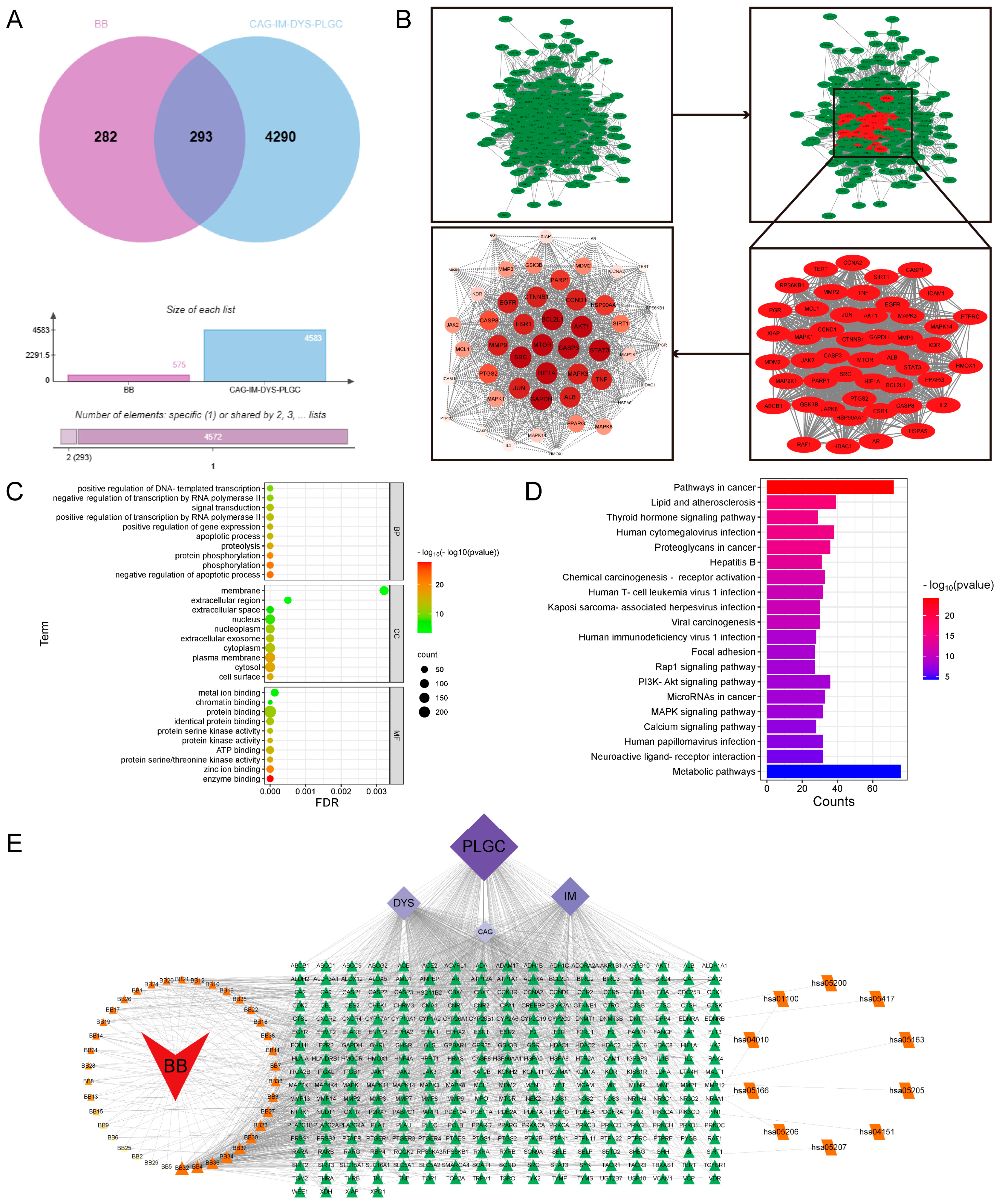
According to the principles of serum pharmacochemistry in TCM, the pharmacological effects of these medicinal substances are genuinely manifested by their serum-translocating constituents. To further elucidate the pharmacological basis and mechanism of action of BB in treating PL-CAG, this study utilized UPLC-QE-Orbitrap-MS/MS technology to analyze and identify the key components of BB that enter systemic circulation. This analysis provides a scientific foundation for understanding the pharmacologically active constituents of BB and guiding mechanistic investigations into its role in PL-CAG treatment. This study analyzed and determined the prototype compounds of 42 BB components that enter the bloodstream. By employing network pharmacology and molecular docking techniques, we investigated the target sites and associated pathways linked to these bloodstream components in their therapeutic effects on CAG, IM, Dys, and PLGC.
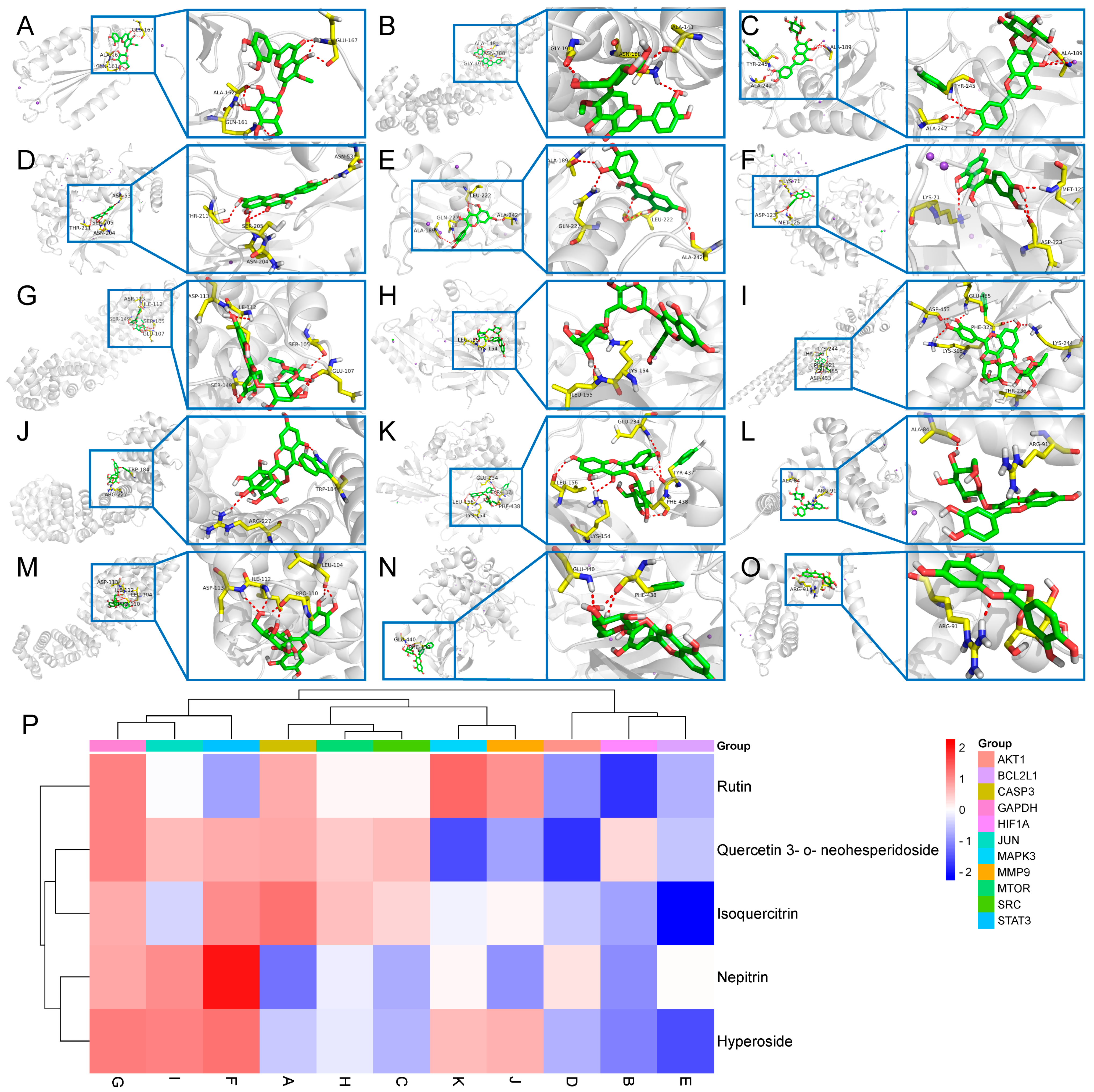
Through the construction of a component–target network and PPI analysis, the key active components of BB in treating PL-CAG were identified as Nepitrin, quercetin-3-O-neohesperidoside, Rutin, Hyperoside, and Isoquercitrin. The core targets associated with these components include CASP3, HIF1A, SRC, AKT1, BCL2L1, STAT3, GAPDH, MTOR, JUN, MMP9, and MAPK3. GO and KEGG enrichment analyses revealed that the therapeutic effects of BB on PL-CAG are involved in cellular biological processes such as protein phosphorylation and enzyme binding, as well as pathways related to cancer progression, PI3K-Akt signaling pathway, and lipid metabolism disorders.
To further investigate the specific binding sites where BB exerts its aforementioned effects, we performed molecular docking validation. Generally, the results of molecular docking are primarily assessed based on the minimum binding free energy [
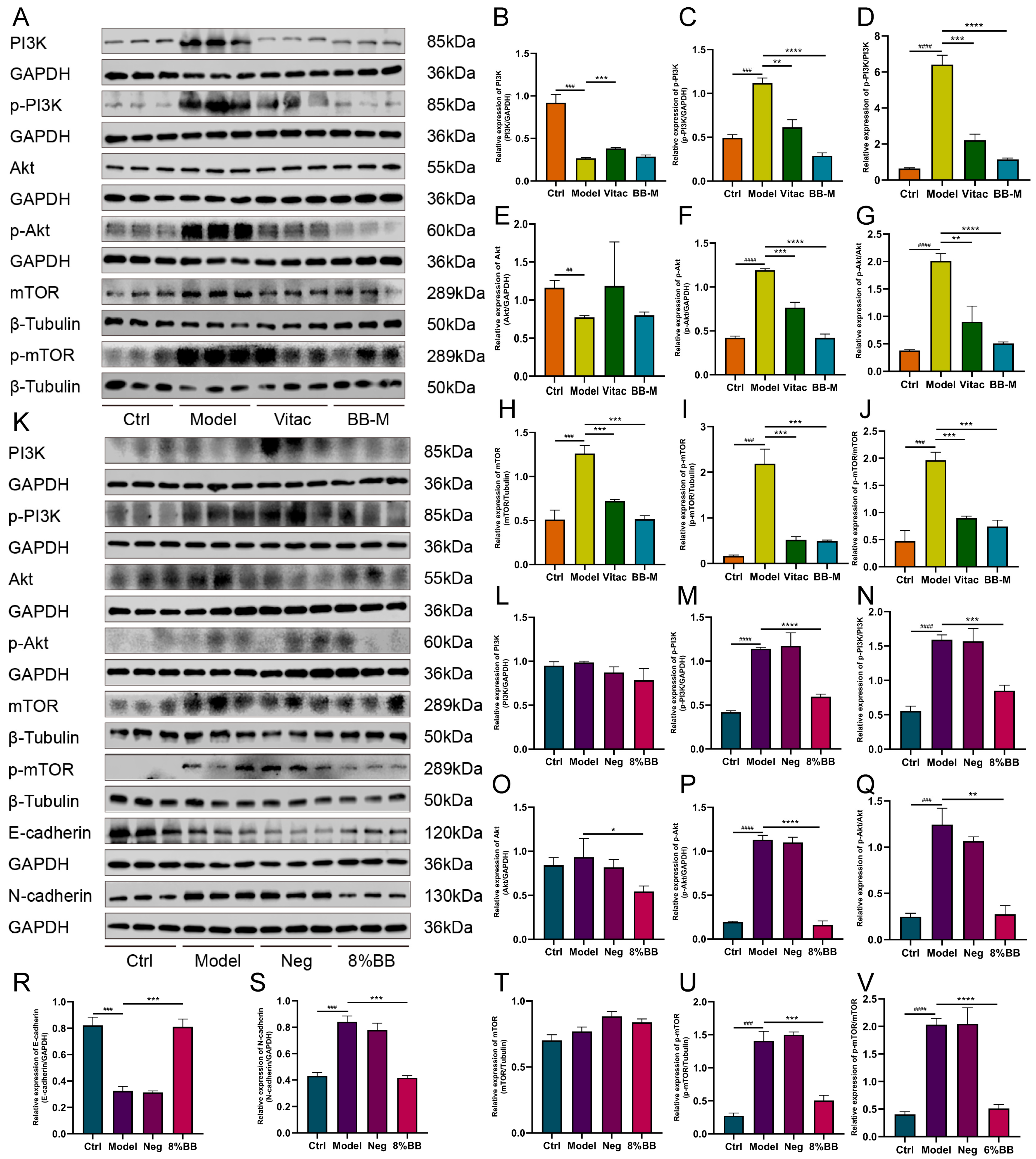
22]. Through molecular docking analysis, we discovered that among the top 11 targets exhibiting stronger affinity for the main component, core effector molecules AKT1 and mTOR within the PI3K/AKT/mTOR signaling pathway were identified.
AKT is a serine/threonine protein kinase categorized into three subtypes: Akt1, Akt2, and Akt3, and it functions as a downstream effector influenced by PI3K signaling. Among these, Akt1 is ubiquitously expressed across all tissue types, including the stomach. By contrast, Akt2 and Akt3 are scarcely detected in gastric tissues. As a critical regulatory kinase, Akt1 orchestrates cell growth and survival through the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway [
23]. The activation of PI3K promotes the translocation of Akt to the cell membrane, where it is phosphorylated by PIP3, leading to its activation. The phosphorylated form of Akt, denoted as p-Akt, acts as a critical marker for evaluating the degree of Akt activation and can further activate multiple downstream effectors. Both PI3K and Akt are frequently overexpressed in various cancer cell types and play significant roles in cancer metastasis [
24]. Furthermore, a substantial body of evidence has shown that the activation of the Akt signaling pathway is frequently correlated with increased tumor invasiveness in various human cancers [
25].
mTOR is a serine/threonine kinase, which is a downstream effector molecule of the PI3K/AKT signaling pathway. The activation of mTOR is typically achieved through phosphorylation, a process that is regulated by various signals [
26]. mTOR, as a crucial role in intracellular key signal transduction pathways, is involved in regulating cell growth, proliferation, and metabolism. Studies have shown that the abnormal activation of the mTOR signaling pathway is closely related to the occurrence and development of tumors in various cancers. For instance, in pancreatic cancer, the inhibition of the mTOR signaling pathway can effectively promote the phenotypic transformation of cancer stem cells, thereby inhibiting their metastatic ability [
27]. In renal cell carcinoma, overexpressed exogenous proteins can enhance epithelial–mesenchymal transition (EMT) through the mTOR signaling pathway, thereby promoting the migration and invasion capabilities of tumor cells [
28]. Furthermore, the mTOR signaling pathway is also involved in regulating the cell’s response to external stimuli. In breast cancer cells, treatment strategies targeting mTOR have been found to effectively inhibit the EMT process, thereby improving the effectiveness of chemotherapy [
29]. In pancreatic cancer, mTOR affects cell proliferation and metastatic ability by regulating the phosphorylation of YBX1, further promoting the occurrence of EMT [
30]. Therefore, by modulating the mTOR signaling pathway, it is possible to effectively intervene in the changes in the extracellular matrix, thereby affecting the invasiveness and metastatic ability of tumors.
In this study, we discovered that BB inhibited the activation of the PI3K/AKT/mTOR signaling pathway both in vitro and in vivo. Then, we examined the effect of BB-containing serum on the EMT in PLGC cells. During the occurrence of EMT, the expression level of E-cadherin, a calcium-dependent adhesion molecule associated with EMT, decreases, whereas the expression level of N-cadherin, another type of cadherin molecule, increases [
31]. E-cadherin is a transmembrane glycoprotein primarily expressed in epithelial cells and plays an essential role in maintaining cell-to-cell adhesion [
32]. N-cadherin is predominantly expressed in the interstitial tissue, and its upregulated expression serves as a biomarker for EMT, which confers enhanced migratory and invasive capabilities on cells [
33]. This study demonstrated that BB-containing serum causes an upregulation of E-cadherin expression and a downregulation of N-cadherin expression in PLGC cells. These findings suggest that BB may inhibit the EMT of gastric mucosal epithelial cells by modulating the PI3K/AKT/mTOR signaling pathway, thereby reversing the progression of PL-CAG. Although our current study primarily focused on the expression changes of E-cadherin and N-cadherin, we acknowledge the necessity of evaluating other EMT markers to comprehensively understand the underlying mechanisms. In future studies, we plan to systematically investigate additional markers, including vimentin and Snail, thereby providing more robust evidence for BB’s inhibitory effects on EMT.
This study has preliminarily explored the mechanism by which BB inhibits the EMT of gastric mucosal epithelial cells by suppressing the PI3K/AKT/mTOR signaling pathway, thereby potentially reversing the PL-CAG.
Although we have endeavored to ensure the scientific rigor of the study design and the reliability of the results, several limitations of this research warrant acknowledgment. First, the potential pharmacological relevance between active compounds and core targets necessitates further investigation. Second, although in vitro experiments and rat models offer valuable initial insights into drug mechanisms, they cannot fully simulate the intricate physiological and pathological conditions of humans. Consequently, this may result in discrepancies between the drug effects observed in these models and those encountered in clinical settings. Third, our study predominantly examined the inhibitory effects of BB on the PI3K/AKT signaling pathway but did not explore activators of this pathway in depth. As a result, our comprehension of the regulatory mechanisms governing this pathway remains limited, which hinders a thorough elucidation of BB’s complete pharmacological profile. Fourth, while the BB extract comprises multiple compounds, we did not conduct quantitative analysis of specific components. This limitation restricts our capacity to accurately pinpoint the primary compounds responsible for the observed effects or to exclude potential interference from other constituents.
In future research, we aim to compare the binding energy of components with their respective targets against that of known target inhibitors, thereby assessing pharmacological relevance. Additionally, we are actively planning collaborations with clinical research institutions to carry out pharmacokinetic and toxicological studies, which will provide more comprehensive data support for subsequent clinical trials. Activators of the PI3K/AKT signaling pathway will be introduced to investigate BB’s regulatory effects on this pathway more comprehensively via both activation and inhibition approaches. In vitro cell experiments and animal models will be employed to screen specific compounds with significant therapeutic efficacy, followed by an in-depth investigation into their mechanisms of action. Furthermore, human trials and combination therapy studies can be conducted to validate the safety and efficacy of BB in humans, thereby providing robust evidence for its clinical application. Through combination therapy research, the practical feasibility and advantages of BB in real-world clinical settings will be evaluated, aiming to enhance therapeutic outcomes while minimizing side effects.
4. Materials and Methods
4.1. Drugs and Reagents
The BB was obtained from Hebei Meiwei Pharmaceutical Co. (Anguo, China), and methanol, acetonitrile, and formic acid were sourced from Thermo Fisher Scientific (Waltham, MA, USA). N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) and sodium salicylate were obtained from Aladdin. Vitac tablets were acquired from Beihai Sunshine Pharmaceutical Co. Rat GAS, PGI, PGII, IL-6, TNF-α, and IL-1β ELISA kits were sourced from Shanghai Thrive Color Biotechnology Co. The 1640 medium, fetal bovine serum, 0.25% trypsin-EDTA, and PBS were obtained from Gibco (New York, CA, USA). CCK-8 reagents were acquired from Abbkine. The serum-free freezing solution was obtained from Suzhou Xinsaimei Biotechnology Co. (Suzhou, China). GAPDH, β-Tubulin, E-Cadherin, and N-Cadherin antibodies were acquired from Wuhan Proteintech Co. (Wuhan, China). IL-6, IL-1β, and TNF-α antibodies were obtained from SANTA CRUZ BIOTECHNOLOGY, INC. Akt, p-Akt, PI3K, and p-PI3K antibodies were sourced from Affinity. mTOR and p-mTOR antibodies were acquired from ZenBio (Chengdu, China). The HRP-goat anti-rabbit antibody was purchased from Cohesion, and the HRP-goat anti-mouse antibody was obtained from Tianjin Simu Biotechnology Co. (Tianjin, China).
4.2. Preparation of BB
BB was crushed, passed through an 800-mesh sieve, and subjected to reflux extraction. A total of 150 g of the sample was weighed into a round-bottomed flask, soaked immersed in water equivalent to five times its weight in water for 30 min, and subsequently extracted by heated reflux three times, each for 2 h. The filtrate was filtered, combined, and centrifuged at 3000 r/min for 10 min to remove the residue. The supernatant was then concentrated under vacuum to a concentration of 2 g/mL of raw drug and stored in a −80 °C refrigerator. In in vitro experiments, drug-containing serum was utilized. The preparation procedure for the drug-containing serum was as follows: Rats were orally administered the water extract of BB for three consecutive days. Subsequently, blood was collected from the abdominal aorta and placed into a coagulant tube. The samples were kept on ice for 30 min, then centrifuged at 3000 rpm for 15 min, and finally stored at −80 °C for subsequent use. Under the same conditions, the rat blood was placed in the anticoagulant tube and the above operation was repeated to obtain the drug-containing plasma of BB.
4.3. Chromatographic and Mass Spectrometric Conditions
The UPLC-QE-Orbitrap-MS/MS was utilized to analyze samples from both the blank plasma group and the drug-containing plasma group of BB. The data collected were then imported into the UNIFI platform for rapid matching and compared against the confidential retention times and secondary mass spectrometry fragment ion information for each component in the chemical composition analysis of BB. Chromatographic conditions: the chromatographic column was ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 μm), the column temperature was set at 45 °C, and the gradient elution was carried out using A (0.1% formic acid-water) -B (acetonitrile) as the mobile phase. The elution program was as follows: 0–2 min, 95–95%A; 2–4 min, 95–70%A; 4–8 min. 70–50%A; 8–10 min, 50–20%A; 10–14 min, 20–0%A; 14–15 min, 0–0%A; 15–15.1 min, 0–95%A; 15.1–16 min, 95– 95%A; the flow rate was 0.35 mL/min, and the injection volume was 5 μL. Mass spectrometry (MS) conditions: electrospray ion source (ESI source), TurboV ion source, source temperature of 550.0 °C. The source injection voltage was −4500.0 V. The atomization gas (gas1) and heating gas (gas2) were both 55.0 psi, the air curtain gas was 25.0 psi, and the negative ions were monitored by the multi-reaction monitoring (MRM) mode.
4.4. Animals and Model Establishment
Specific Pathogen Free (SPF)-grade 5-week-old Wistar male rats (200 ± 20 g), provided by Specific Pathogen Free (Beijing) Biotechnology Co. (Beijing, China). The relevant experiments were performed after one week of adaptive rearing in a constant temperature and humidity environment with a temperature of 22 °C and a humidity of 50%. Animal experiment operation license number: SCXK (Ji) 2022-010. The experimental procedures involving the animals adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were granted approval by the Animal Care and Use Committee of Medical Ethics of the Hebei University of Chinese Medicine (approval number DWLL202304006). In total, 15 rats were randomly selected from 100 Wistar male rats as the control (Ctrl) group, and the remaining rats were modeled by a three-factor method with reference to the literature [
34]: (i) MNNG was prepared as an aqueous solution of 180 μg/mL protected from light for free drinking, and was changed daily; (ii) starvation and satiety disorders: 2 days were for full feeding and 1 day was for fasting; (iii) after fasting, 0.5 mL of 2% sodium salicylate was administered orally to rats at a concentration of 0.5 mL/100 g. Starting from the 12th week of modeling, blood was taken from the inner canthus of the eye every two weeks, and the serum expression levels of GAS, PGI, PGII, IL-6, IL-1β, and TNF-α were detected in each group of rats using ELISA kits (Zhuocai Biotechnology Co., Shanghai, China). After the differences in serum indexes appeared, two rats in the modeling group were randomly selected and killed every two weeks. The histopathological damage of the stomach in each group was observed by HE staining until both rats showed a reduction in the number of intrinsic glands atrophy or disappearance in the gastric mucosal epithelial cells, which was regarded as the success of the modeling. The general condition of the rats was observed during the period and recorded by weekly weighing at regular intervals. The treatment was started on the next day after successful modeling. The administered dose was converted according to the equivalent human clinical dose and the rat body surface area coefficient [
35]: 0.32 g/kg for the Vitac group, 0.63 g/kg, 1.26 g/kg and 2.52 g/kg for the BB low-dose group (BB-L), BB medium-dose group (BB-M), and BB high-dose group (BB-H), respectively, and was administered once a day for 12 weeks, while the Ctrl group and the Model group were given a normal diet every day. The rats were euthanized under anesthesia at the end of the experiment after a 12 h fast with access to water. The blood was removed from the abdominal aorta and centrifuged at 3000 r/15 min after being placed on ice for 30 min, then the supernatant was stored at −80 °C for measurement. The gastric tissue was dissected along the greater curvature of the stomach to observe the general changes of the gastric mucosa and photographed, part of the gastric tissue was frozen in liquid nitrogen for spare use and the remaining tissue was kept in paraformaldehyde for fixation.
4.5. Histological Analysis
Fix the gastric tissue in 10% neutral formalin for a duration of 24 h. Following dehydration, embed the tissue in paraffin wax and section it to a thickness of 5 μm. Dewax using xylene, perform gradient ethanol hydration, and apply hematoxylin and eosin staining. Subsequently, dehydrate with ethanol, clear with xylene, and mount using neutral balsam. Finally, examine the histological sections under an optical microscope.
4.6. Enzyme-Linked Immunosorbent Assay
In accordance with the provided instructions, serum levels of GAS, PGI, PGII, IL-1β, IL-6, and TNF-α were quantified utilizing ELISA kits.
4.7. Network Pharmacology Analysis
Taking 42 prototype compounds of blood-entering components in BB as the research objects, the SMILES numbers of the components were entered into the Swiss Target Prediction database (
http://swisstargetprediction.ch, accessed on 6 August 2024), and the targets with ‘probability’ values greater than 0 were combined and the duplicates were deleted to obtain the targets of the components. The targets were analyzed in the OMIM (
https://www.omim.org, accessed on 7 August 2024), Genecards (
https://www.genecards.org, accessed on 7 August 2024), and DisGeNET (
https://www.disgenet.org, accessed on 7 August 2024) databases under the names ‘Chronic atrophic gastritis’, ‘atrophic gastritis’, ‘intestinal metaplasia’, ‘dysplasia’, and ‘precancerous lesions of gastric cancer’, which were searched for disease targets. The components were intersected with the disease targets to obtain potential targets for the treatment of precancerous lesions of chronic atrophic gastritis by BB. Potential targets were inputted into the STRING platform (
https://string-db.org, accessed on 8 August 2024) for visualization, and core targets were screened according to MCODE using Cytoscape 3.9.1 software. The key targets were imported into the DAVID database (
https://davidbioinformatics.nih.gov, accessed on 9 August 2024), limited the study species to human, and subjected to molecular function (MF), biological process (BP) and cellular component (CC) analysis. Additionally, pathway enrichment analysis was performed using KEGG, followed by visualization of the results. using KEGG, and visualization of the results. The composite drug–components–diseases–targets–pathway network was constructed by importing BB, ingredients, potential targets, and pathways into Cytoscape 3.9.1 software for visualization, and the network parameters were analyzed and screened to identify the potential active ingredients of BB that could play a therapeutic role in the treatment of PL-CAG. The potential active ingredients were molecularly docked with the top 11 core targets. The structural formulae of the ligand small molecules were downloaded from the PubChem database (
https://pubchem.ncbi.nlm.nih.gov, accessed on 11 August 2024) in SDF format, and the structural formulae of the components were converted to PDB format using Open Babel 3.1.1 and processed for structure optimization. The crystal structures of the receptor proteins were obtained from the RCSB PDB database (
https://www.rcsb.org, accessed on 11 August 2024) and operations such as dehydration and hydrogenation were performed by PyMol 1.8.0.0. The receptor protein and ligand small molecules were semiflexible docked in AutoDockTools-1.5.6 to obtain the corresponding binding energies.
4.8. Cell Culture
GES-1 cells were cultured in 1640 medium containing 10% fetal bovine serum at 37 °C in a 5% CO
2 incubator. When the cells grew to 80–90% fusion, 10 μM MNNG was added to induce GES-1 cells to become PLGC model cells [
36].
4.9. Cell Cloning Assay
Cells from each experimental group were spread in 6-well plates with 200 cells/well, and placed in the incubator for 1 week, during which the liquid was changed normally. The cultured cells were washed twice with PBS, fixed with 4% paraformaldehyde for 15 min, then stained with crystal violet for 15 min, and the residual crystal violet was washed with purified water, then photographed and counted.
4.10. Wound Healing Assay
The cells were evenly spread in 6-well plates, the density of which was suitable for overnight fusion of 90–100%. After making scratches with a 200 µL tip, the width of the scratches in the same position was observed under a microscope at 6 h, 12 h, 24 h, and 48 h, and pictures were taken and quantitatively processed by using the software of ImageJ-win64.
4.11. Invasion Assays
We put the Transwell into a 24-well plate, diluted Matrigel gel with serum-free 1640 medium at 8:1 in the upper well, discarded the excess liquid in the upper layer after solidification, added 100 μL of serum-free 1640 medium, and continued to incubate for 30 min for basement membrane hydration. The upper well of Transwell after basement membrane hydration was taken out and the liquid was discarded. A suspension containing 2 × 103 cells in 200 μL was added to the upper well, and it was left to stand for 15 min. A total of 500 μL of 1640 medium containing 20% fetal bovine serum was added to the lower well. The 24-well plate was incubated in an incubator at 37 °C, 5% CO2 for 48 h. The culture medium was removed from the upper and lower wells and washed twice with PBS, and the matrix glue inside the upper chamber was carefully wiped away with a swab. Then, it was fixed with paraformaldehyde, stained with crystal violet, and photographed for counting.
4.12. Western Blot
Total proteins were extracted from tissues and cells. The total protein concentration was determined by BCA method. The protein sample is mixed with 5 × loading buffer at a ratio of 1:4, and denatured at 100 °C for 10 min. The electrophoresis voltages of the polyacrylamide gels were set at S1: 75 V, 35 min, and S2: 110 V, 95 min. The membrane was transferred with 0.45 μm PVDF membrane, closed with 5% skimmed milk powder prepared using 1× TBST for 2 h, incubated with diluted primary antibody at 4 °C overnight, and then washed 3 times with TBST for 10 min each time. After that, the membrane was reacted with horseradish peroxidase-coupled secondary antibody at room temperature for 1 h, washed 3 times with TBST, and ECL was added to develop and expose. The protein bands were quantified using ImageJ-win64, and the relative protein expression was calculated using GAPDH and β-Tubulin as internal references.
4.13. Statistical Analysis
Statistical analysis of the data was performed using GraphPad Prism 10.1 software. All the data were represented as mean ± SD. One-way ANOVA was used to compare the data between groups with normal distribution and homogeneity of variance. Pairwise comparison between groups was performed by the least significant difference method (LSD method). If the variance was not uniform, the rank sum test was used. p < 0.05 meant the difference was statistically significant, and p < 0.01 meant the difference was statistically significant.