Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review
Abstract
1. Zombie-like State
1.1. Inflammation and Senescent Hepatocytes
1.2. Hepatocyte Senescence vs. Hepatocyte Senescence-Associated Secretory Phenotypes
2. Hepatocyte Senescence-Associated Secretory Phenotype and Tissue Remodeling
3. Scoping Review
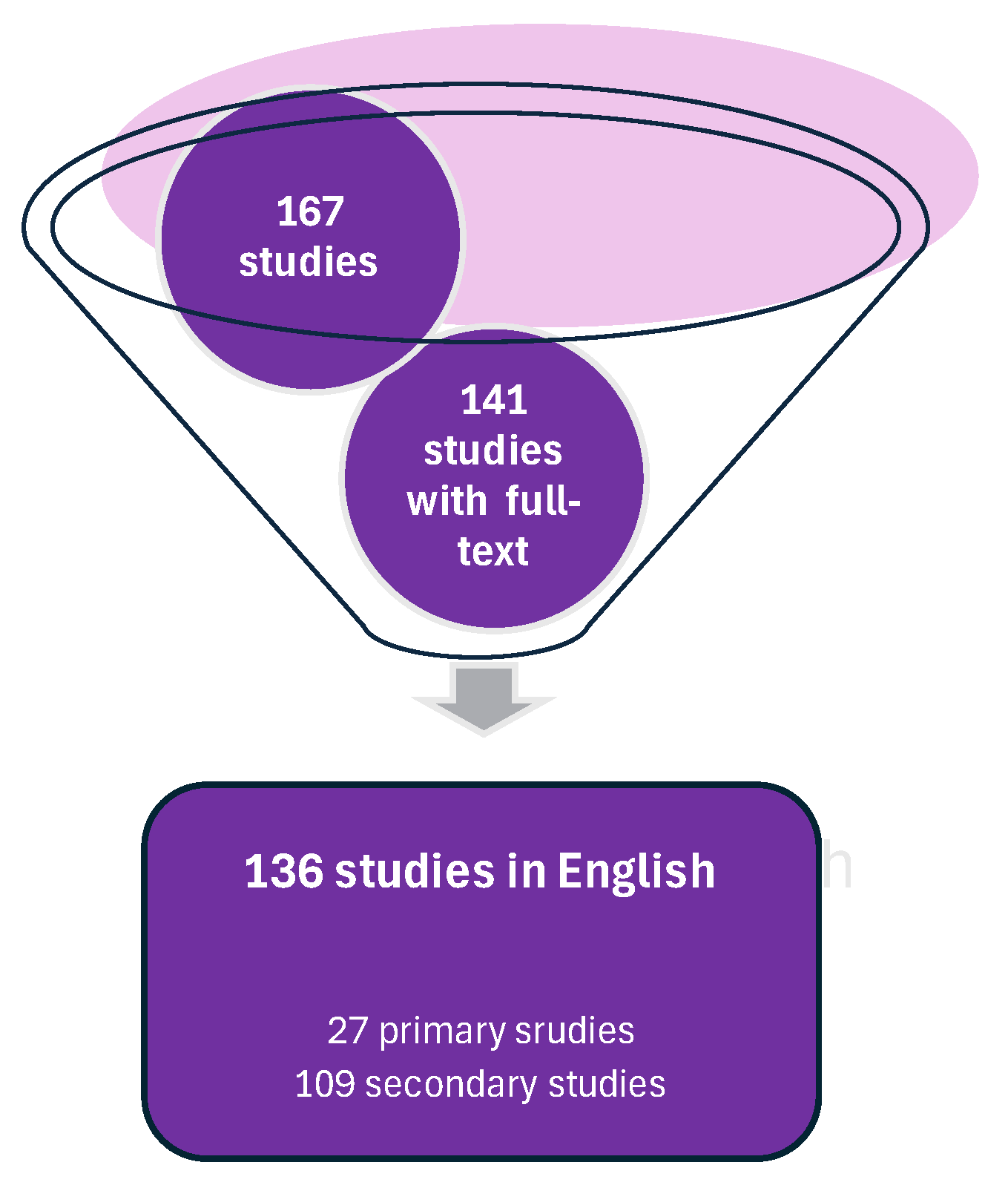
3.1. Methodology
3.2. Primary Studies
3.3. Secondary Publications and Non-Peer-Reviewed Material
4. Clinical Implications
5. Pharmaceuticals and Senescent Hepatocytes
Substance Abuse
6. Supporting Evidence-Based Expertise
Molecular Testing
- Cyclin-dependent kinase inhibitor2A (CDKN2A, p16);
- Cyclin-dependent kinase inhibitor1A (CDKN1A, p21);
- IL-6 (Figure 2).
- IL-8;
- Vascular endothelial growth factor (VEGF);
- MMPs.
- Analysis of microarrays of gene expression profiles to identify senescence-associated genes and pathways [112];
- High-throughput RNA sequencing (RNA-seq) to analyze gene expression and identify senescence-associated transcripts [113];
- DNA methylation levels in senescence-associated gene promoters, such as CDKN2A and CDKN1A [114];
- Histone modification analysis, such as H3K9me3 and H3K27me3, in senescence-associated gene promoters [115];
- Telomere length measurement using techniques such as quantitative PCR (qPCR) or fluorescence in situ hybridization (FISH) [116];
- Cytokine and growth factor analysis of levels of SASP-associated cytokines and growth factors, such as IL-6, IL-8, and VEGF, using ELISA or multiplex assays [26];
- Proteomic analysis to identify SASP-associated proteins [84];
- Senescence-associated beta-galactosidase (SA-β-Gal) staining—a biochemical stain that detects senescent cells [117];
- Immunostaining of p16 and p21—markers of cellular senescence [118].
7. Identifying a Reliable Biomarker
8. Limitations of Using the “Zombie-like State” of the Liver in Legal Medicine
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Xue, B. New insights into the role of cellular senescence and chronic wounds. Front. Endocrinol. 2024, 15, 1400462. [Google Scholar] [CrossRef] [PubMed]
- Lavarti, R.; Cai, L.; Alvarez-Diaz, T.; Medina-Rodriguez, T.; Bombin, S.; Raju, R.P. Senescence landscape in the liver following sepsis and senolytics as potential therapeutics. Aging Cell 2025, 24, e14354. [Google Scholar] [CrossRef]
- Sladky, V.C.; Eichin, F.; Reiberger, T.; Villunger, A. Polyploidy control in hepatic health and disease. J. Hepatol. 2021, 75, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L. Navigating the complex role of senescence in liver disease. Chin. Med. J. 2024, 137, 3061–3072. [Google Scholar] [CrossRef]
- Bonnet, L.; Alexandersson, I.; Baboota, R.K.; Kroon, T.; Oscarsson, J.; Smith, U.; Boucher, J. Cellular senescence in hepatocytes contributes to metabolic disturbances in NASH. Front. Endocrinol. 2022, 13, 957616. [Google Scholar] [CrossRef]
- Liu, B.; Meng, Q.; Gao, X.; Sun, H.; Xu, Z.; Wang, Y.; Zhou, H. Lipid and glucose metabolism in senescence. Front. Nutr. 2023, 10, 1157352. [Google Scholar] [CrossRef]
- Du, K.; Umbaugh, D.S.; Liuyang, W.; Jun, J.H.; Dutta, R.K.; Oh, S.H.; Ren, N.; Zhang, Q.; Ko, D.C.; Ferreira, A.; et al. Targeting senescent hepatocytes for treatment of metabolic dysfunction-associated steatotic liver disease and multi-organ dysfunction. Nat. Commun. 2025, 16, 3038. [Google Scholar] [CrossRef]
- Schwarz, S.; Nientiedt, C.; Prigge, E.S.; Kaczorowski, A.; Geisler, C.; Lucena Porcel, C.; von Knebel Doeberitz, M.; Hohenfellner, M.; Duensing, S. Senescent Tumor Cells Are Frequently Present at the Invasion Front: Implications for Improving Disease Control in Patients with Locally Advanced Prostate Cancer. Pathobiology 2023, 90, 312–321. [Google Scholar] [CrossRef]
- Irvine, K.M.; Skoien, R.; Bokil, N.J.; Melino, M.; Thomas, G.P.; Loo, D.; Gabrielli, B.; Hill, M.M.; Sweet, M.J.; Clouston, A.D.; et al. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J. Gastroenterol. 2014, 20, 17851–17862. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, A.; Lv, L.; Zheng, Z.; Liu, P.; Min, J.; Wei, J. Exosomal miRNA-146a-5p Derived from Senescent Hepatocellular Carcinoma Cells Promotes Aging and Inhibits Aerobic Glycolysis in Liver Cells via Targeting IRF7. J. Cancer 2024, 15, 4448–4466. [Google Scholar] [CrossRef] [PubMed]
- Wong, C. How to Kill the ‘Zombie’ Cells that Make You Age. Nature, 15 May 2024. [Google Scholar]
- Kumar, P.; Hassan, M.; Tacke, F.; Engelmann, C. Delineating the heterogeneity of senescence-induced-functional alterations in hepatocytes. Cell. Mol. Life Sci. 2024, 81, 200. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, K.; Vasilieva, M.; Minskaia, E.; Rybtsov, S.; Shevyrev, D. T-cell immunity against senescence: Potential role and perspectives. Front. Immunol. 2024, 15, 1360109. [Google Scholar] [CrossRef]
- Cherayil, B.J. The Logic of Immunity: Deciphering an Enigma; JHU Press: Baltimore, MD, USA, 2024. [Google Scholar]
- Ma, X.; Huang, T.; Chen, X.; Li, Q.; Liao, M.; Fu, L.; Huang, J.; Yuan, K.; Wang, Z.; Zeng, Y. Molecular mechanisms in liver repair and regeneration: From physiology to therapeutics. Signal Transduct. Target. Ther. 2025, 10, 63. [Google Scholar] [CrossRef]
- Choi, B.; Lee, C.; Yu, J.W. Distinctive role of inflammation in tissue repair and regeneration. Arch. Pharm. Res. 2023, 46, 78–89. [Google Scholar] [CrossRef]
- Eze, U.O.; Ojifinni, K.A. Trauma Forensics in Blunt and Sharp Force Injuries. J. West Afr. Coll. Surg. 2022, 12, 94–101. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, T.; Wang, F.Y.; Yao, X.; Bai, Q.X.; Su, H.W.; Liu, J.; Wang, L.; Tan, R.Z. The Dual Role of Cellular Senescence in Macrophages: Unveiling the Hidden Driver of Age-Related Inflammation in Kidney Disease. Int. J. Biol. Sci. 2025, 21, 632–657. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Varela-Eirin, M.; Demaria, M. Cellular senescence. Curr. Biol. 2022, 32, R448–R452. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The role of the dynamic epigenetic landscape in senescence: Orchestrating SASP expression. NPJ Aging 2024, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Du, Y.; Hao, S.; Ni, T. The Regulation of Cellular Senescence in Cancer. Biomolecules 2025, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Yoshida, Y.; Ohtani, N. Cellular senescence and the tumour microenvironment. Mol. Oncol. 2022, 16, 3333–3351. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londono-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): A complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 2021, 15, 3242–3255. [Google Scholar] [CrossRef]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef]
- Samiminemati, A.; Aprile, D.; Siniscalco, D.; Di Bernardo, G. Methods to Investigate the Secretome of Senescent Cells. Methods Protoc. 2024, 7, 52. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, M.; Hou, H.; Fang, S.; Chen, L.; Yang, J.; Yao, W.; Zhang, Q.; Hei, Z. Cellular senescence in ischemia/reperfusion injury. Cell Death Discov. 2022, 8, 420. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Cellular Senescence in Acute and Chronic Wound Repair. Cold Spring Harb. Perspect. Biol. 2022, 14, a041221. [Google Scholar] [CrossRef]
- Kim, K.H.; Chen, C.C.; Monzon, R.I.; Lau, L.F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell. Biol. 2013, 33, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, M.; Li, J.; Danielson, P.; Yang, L.; Zhou, Q. Induction of Fibroblast Senescence During Mouse Corneal Wound Healing. Investig. Opthalmol. Vis. Sci. 2019, 60, 3669–3679. [Google Scholar] [CrossRef]
- Trujillo Cubillo, L.; Gurdal, M.; Zeugolis, D.I. Corneal fibrosis: From in vitro models to current and upcoming drug and gene medicines. Adv. Drug Deliv. Rev. 2024, 209, 115317. [Google Scholar] [CrossRef]
- Ring, N.A.R.; Valdivieso, K.; Grillari, J.; Redl, H.; Ogrodnik, M. The role of senescence in cellular plasticity: Lessons from regeneration and development and implications for age-related diseases. Dev. Cell 2022, 57, 1083–1101. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; He, D. Diverse effects of platelet-derived growth factor-BB on cell signaling pathways. Cytokine 2019, 113, 13–20. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.H.; Lee, D.H.; Lee, S.H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Papadopoulou, A.; Pratsinis, H.; Kletsas, D. Senescence-associated alterations in the extracellular matrix: Deciphering their role in the regulation of cellular function. Am. J. Physiol. Cell Physiol. 2023, 325, C633–C647. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Role of Senescent Astrocytes in Health and Disease. Int. J. Mol. Sci. 2023, 24, 8498. [Google Scholar] [CrossRef]
- Bang, M.; Ryu, O.; Kim, D.G.; Mabunga, D.F.; Cho, K.S.; Kim, Y.; Han, S.H.; Kwon, K.J.; Shin, C.Y. Tenovin-1 Induces Senescence and Decreases Wound-Healing Activity in Cultured Rat Primary Astrocytes. Biomol. Ther. 2019, 27, 283–289. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Ding, H.; Wang, Y.; Xie, Y.; Zhang, X. Cellular Senescence in Cardiovascular Diseases: From Pathogenesis to Therapeutic Challenges. J. Cardiovasc. Dev. Dis. 2023, 10, 439. [Google Scholar] [CrossRef]
- Haddad, D.; Al Madhoun, A.; Nizam, R.; Al-Mulla, F. Role of Caveolin-1 in Diabetes and Its Complications. Oxid. Med. Cell Longev. 2020, 2020, 9761539. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; O’Shea, P.; Wrench, C.; Mattsson, J.; Paulin, R.; Overed-Sayer, C.; Rosenberg, L.; Olsson, H.; Gianni, D. A secretome screen in primary human lung fibroblasts identifies FGF9 as a novel regulator of cellular senescence. SLAS Discov. 2025, 32, 100223. [Google Scholar] [CrossRef] [PubMed]
- Blokland, K.E.C.; Waters, D.W.; Schuliga, M.; Read, J.; Pouwels, S.D.; Grainge, C.L.; Jaffar, J.; Westall, G.; Mutsaers, S.E.; Prele, C.M.; et al. Senescence of IPF Lung Fibroblasts Disrupt Alveolar Epithelial Cell Proliferation and Promote Migration in Wound Healing. Pharmaceutics 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Yang, Y.; Cho, W.C.; Flynn, R.J.; Harandi, M.F.; Song, H.; Luo, X.; Zheng, Y. Interplays of liver fibrosis-associated microRNAs: Molecular mechanisms and implications in diagnosis and therapy. Genes Dis. 2023, 10, 1457–1469. [Google Scholar] [CrossRef]
- Wu, N.; Zhou, T.; Carpino, G.; Baiocchi, L.; Kyritsi, K.; Kennedy, L.; Ceci, L.; Chen, L.; Wu, C.; Kundu, D. Prolonged administration of a secretin receptor antagonist inhibits biliary senescence and liver fibrosis in Mdr2−/− mice. Hepatology 2023, 77, 1849–1865. [Google Scholar] [CrossRef]
- Andrade, A.M.; Sun, M.; Gasek, N.S.; Hargis, G.R.; Sharafieh, R.; Xu, M. Role of Senescent Cells in Cutaneous Wound Healing. Biology 2022, 11, 1731. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Šoša, I. Liver Senescence and its Medico-legal Implications. 2025. Available online: https://osf.io/2gyzc/ (accessed on 15 April 2025).
- Anastasopoulos, N.A.; Charchanti, A.V.; Barbouti, A.; Mastoridou, E.M.; Goussia, A.C.; Karampa, A.D.; Christodoulou, D.; Glantzounis, G.K. The Role of Oxidative Stress and Cellular Senescence in the Pathogenesis of Metabolic Associated Fatty Liver Disease and Related Hepatocellular Carcinoma. Antioxidants 2023, 12, 1269. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Patten, D.; Gough, S.; de Barros Goncalves, S.; Chan, A.; Olan, I.; Cassidy, L.; Poblocka, M.; Zhu, H.; Lun, A.; et al. Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance. Genes Dev. 2022, 36, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiao, Z.; Pfeifer, R.; Pape, H.C.; Mao, K.; Tang, H.; Meng, B.; Chen, S.; Liu, H. Modulation of fracture healing by senescence-associated secretory phenotype (SASP): A narrative review of the current literature. Eur. J. Med. Res. 2024, 29, 38. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Zhang, Y. Editorial: New basic and translational perspectives on skin repair. Front. Physiol. 2024, 15, 1469900. [Google Scholar] [CrossRef] [PubMed]
- Wijayasiri, P.; Astbury, S.; Needham, G.; Kaye, P.; Bhat, M.; Piccinini, A.M.; Aravinthan, A.D. Role of hepatocellular senescence in the development of hepatocellular carcinoma and the potential for therapeutic manipulation. Hum. Cell 2025, 38, 70. [Google Scholar] [CrossRef]
- Huda, N.; Liu, G.; Hong, H.; Yan, S.; Khambu, B.; Yin, X.M. Hepatic senescence, the good and the bad. World J. Gastroenterol. 2019, 25, 5069–5081. [Google Scholar] [CrossRef]
- Chen, J.; Ou, L.; Hillman, K. Measuring Complex and Macro Research in Rapid Response Systems. In Textbook of Rapid Response Systems: Concept and Implementation; Springer: Berlin/Heidelberg, Germany, 2025; pp. 293–306. [Google Scholar]
- Chalasani, N.; Hayashi, P.H.; Luffer-Atlas, D.; Regev, A.; Watkins, P.B. Assessment of liver injury potential of investigational medicines in drug development. Hepatology 2025. [Google Scholar] [CrossRef]
- Kiourtis, C.; Terradas-Terradas, M.; Gee, L.M.; May, S.; Georgakopoulou, A.; Collins, A.L.; O’Sullivan, E.D.; Baird, D.P.; Hassan, M.; Shaw, R.; et al. Hepatocellular senescence induces multi-organ senescence and dysfunction via TGFbeta. Nat. Cell Biol. 2024, 26, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Neri, F.; Takajjart, S.N.; Lerner, C.A.; Desprez, P.-Y.; Schilling, B.; Campisi, J.; Gerencser, A.A. A Fully-Automated Senescence Test (FAST) for the high-throughput quantification of senescence-associated markers. GeroScience 2024, 46, 4185–4202. [Google Scholar] [CrossRef]
- Wang, X.; Rao, J.; Tan, Z.; Xun, T.; Zhao, J.; Yang, X. Inflammatory signaling on cytochrome P450-mediated drug metabolism in hepatocytes. Front. Pharmacol. 2022, 13, 1043836. [Google Scholar] [CrossRef]
- Armani, S.; Geier, A.; Forst, T.; Merle, U.; Alpers, D.H.; Lunnon, M.W. Effect of changes in metabolic enzymes and transporters on drug metabolism in the context of liver disease: Impact on pharmacokinetics and drug–drug interactions. Br. J. Clin. Pharmacol. 2024, 90, 942–958. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, D.; Pu, Z.; Chen, S.; Shen, Y.; Zhao, S.; Qian, X.; Hu, Q.; Wu, X.; Xie, Z.; et al. Cellular Senescence in Acute Liver Injury: What Happens to the Young Liver? Aging Dis. 2024, 16, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, X.; Meng, Y.; Shao, C.; Liao, J.; Li, F.; Li, R.; Jing, Y.; Huang, A. The hepatic senescence-associated secretory phenotype promotes hepatocarcinogenesis through Bcl3-dependent activation of macrophages. Cell Biosci. 2021, 11, 173. [Google Scholar] [CrossRef]
- Gadd, V.L.; Ferreira-Gonzalez, S.; Man, T.Y.; Kilpatrick, A.M.; Aird, R.E.; Smith, I.P.; Rodrigo-Torres, D.; Kurian, D.; Hallett, J.M.; Ashmore-Harris, C.; et al. Host hepatocyte senescence determines the success of hepatocyte transplantation in a mouse model of liver injury. J. Hepatol. 2025, 16, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Takeda, F.; Oda, M.; Terasaki, M.; Kubota, A.; Asada, K.; Ichimura, Y.; Kojima, H.; Saitoh, H. Downregulated expression of organic anion transporting polypeptide (Oatp) 2b1 in the small intestine of rats with acute kidney injury. Drug Metab. Pharmacokinet. 2021, 40, 100411. [Google Scholar] [CrossRef]
- Li, W.; Iusuf, D.; Sparidans, R.W.; Wagenaar, E.; Wang, Y.; de Waart, D.R.; Martins, M.L.F.; van Hoppe, S.; Lebre, M.C.; van Tellingen, O.; et al. Organic anion-transporting polypeptide 2B1 knockout and humanized mice; insights into the handling of bilirubin and drugs. Pharmacol. Res. 2023, 190, 106724. [Google Scholar] [CrossRef]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Hahn, M.; Roll, S.C. The Influence of Pharmacogenetics on the Clinical Relevance of Pharmacokinetic Drug–Drug Interactions: Drug–Gene, Drug–Gene–Gene and Drug–Drug–Gene Interactions. Pharmaceuticals 2021, 14, 487. [Google Scholar] [CrossRef]
- Negi, R.; Yadav, R.; Upadhye, V.J.; Jain, B.; Berdimurodov, E. Design and Movements of Drug in Human Metabolism by Multifunctional Magnetic Nanoparticles. In Multifunctional Magnetic Nanoparticles in Therapy, Biology, and Pharmacy; CRC Press: Boca Raton, FL, USA, 2025; pp. 92–113. [Google Scholar]
- Abudahab, S.; Slattum, P.W.; Price, E.T.; McClay, J.L. Epigenetic regulation of drug metabolism in aging: Utilizing epigenetics to optimize geriatric pharmacotherapy. Pharmacogenomics 2024, 25, 41–54. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretić, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Guaraldi, G.; Erlandson, K.M.; Milic, J.; Landay, A.L.; Montano, M.A. Can statin preventative treatment inform geroscience-guided therapeutics? Aging Cell 2023, 22, e13998. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Ma, J.; Wang, X.; Liu, J. Correlation between Metabolic Parameters and Warfarin Dose in Patients with Heart Valve Replacement of Different Genotypes. Rev. Cardiovasc. Med. 2024, 25, 128. [Google Scholar] [CrossRef] [PubMed]
- Hailu, B.Y.; Berhe, D.F.; Gudina, E.K.; Gidey, K.; Getachew, M. Drug related problems in admitted geriatric patients: The impact of clinical pharmacist interventions. BMC Geriatr. 2020, 20, 13. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wang, Y.; Jin, W.; Zhang, Z.; Jin, L.; Qian, J.; Zheng, L. CYP3A4 and CYP3A5: The crucial roles in clinical drug metabolism and the significant implications of genetic polymorphisms. PeerJ 2024, 12, e18636. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Numa, K.; Patel, S.K.; King, C.D.; Matsumoto, A.; Sotozono, C.; Desprez, P.Y.; Schilling, B.; Campisi, J. Cellular senescence exacerbates features of aging in the eyes. Aging Biol. 2023, 1, 20230014. [Google Scholar] [CrossRef]
- Evans, D.S.; Young, D.; Tanaka, T.; Basisty, N.; Bandinelli, S.; Ferrucci, L.; Campisi, J.; Schilling, B. Proteomic Analysis of the Senescence-Associated Secretory Phenotype: GDF-15, IGFBP-2, and Cystatin-C Are Associated With Multiple Aging Traits. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad265. [Google Scholar] [CrossRef]
- Quintás, G.; Castell, J.V.; Moreno-Torres, M. The assessment of the potential hepatotoxicity of new drugs by in vitro metabolomics. Front. Pharmacol. 2023, 14, 1155271. [Google Scholar] [CrossRef]
- Stephenson, L.; Van Den Heuvel, C.; Scott, T.; Byard, R.W. Difficulties associated with the interpretation of postmortem toxicology. J. Anal. Toxicol. 2024, 48, 405–412. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism-A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quant, E.; Richter, M.L.; Colome-Tatche, M.; Martinez-Jimenez, C.P. Single-cell metabolic profiling reveals subgroups of primary human hepatocytes with heterogeneous responses to drug challenge. Genome Biol. 2023, 24, 234. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Krawczyk, M.; Frühbeck, G.; Portincasa, P. Synergistic and Detrimental Effects of Alcohol Intake on Progression of Liver Steatosis. Int. J. Mol. Sci. 2022, 23, 2636. [Google Scholar] [CrossRef]
- Argo, A.; Pitingaro, W.; Puntarello, M.; Buscemi, R.; Malta, G.; D’Anna, T.; Albano, G.D.; Zerbo, S. A Comprehensive Review on Alcohol Abuse Disorder Fatality, from Alcohol Binges to Alcoholic Cardiomyopathy. Diagnostics 2024, 14, 1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, X.; Shao, Y.; Jiang, Y.; Zhou, Y.; Lu, C. NFATc4 mediates ethanol-triggered hepatocyte senescence. Toxicol. Lett. 2021, 350, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Centner, A.M.; Bhide, P.G.; Salazar, G. Nicotine in Senescence and Atherosclerosis. Cells 2020, 9, 1035. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Feng, Z.; Chen, H. Cigarette Smoke Contributes to the Progression of MASLD: From the Molecular Mechanisms to Therapy. Cells 2025, 14, 221. [Google Scholar] [CrossRef]
- Zhu, J.; Peltekian, K.M. Cannabis and the liver: Things you wanted to know but were afraid to ask. Can. Liver J. 2019, 2, 51–57. [Google Scholar] [CrossRef]
- Mboumba Bouassa, R.-S.; Sebastiani, G.; Di Marzo, V.; Jenabian, M.-A.; Costiniuk, C.T. Cannabinoids and Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 9423. [Google Scholar] [CrossRef]
- Araújo, M.; Almeida, M.B.; Araújo, L.L.N. The cannabinoids mechanism of action: An overview. BrJP 2023, 6, 109–113. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Liu, W.-Q.; Zhang, L.-W.; Li, S.-F. Revealing the anti-senescence effects and related mechanisms of flavonoid extracts from the buds of Wikstroemia chamaedaphne Meisn on D-galactose-induced PC12 cells based on network pharmacology and transcriptomics. Future J. Pharm. Sci. 2025, 11, 32. [Google Scholar] [CrossRef]
- da Silva Joaquim, L.; da Rosa, L.R.; Strickert, Y.; Machado, R.S.; Lanzzarin, E.; Bernardes, G.; de Souza Ramos, S.; de Novais, L.R.; Steiner, B.; Farias, B.; et al. Ayahuasca reverses ischemic stroke-induced neuroinflammation and oxidative stress. Behav. Brain Res. 2025, 485, 115521. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- Fearn, K.; Bhattacharyya, K.K. Is Use of Psychedelic Drugs a Risk or Protective Factor for Late-Life Cognitive Decline? Gerontol. Geriatr. Med. 2024, 10, 23337214241250108. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Durán, I.; Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug Discov. 2025, 24, 57–71. [Google Scholar] [CrossRef]
- Balloni, A.; Tini, A.; Prospero, E.; Busardo, F.P.; Huestis, M.A.; Lo Faro, A.F. Exposure to Synthetic Psychoactive Substances: A Potential Cause for Increased Human Hepatotoxicity Markers. Clin. Chem. 2024, 70, 597–628. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
- Wen, H.; Deng, H.; Li, B.; Chen, J.; Zhu, J.; Zhang, X.; Yoshida, S.; Zhou, Y. Mitochondrial diseases: From molecular mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 9. [Google Scholar] [CrossRef]
- Dolkar, T.; Hamad, A.M.; Han, M.M.; Thu, M.B.; Gayam, V.R. Cocaine and Opioid-Induced Acute Liver Injury: A Rare Case Report. Cureus 2022, 14, e23630. [Google Scholar] [CrossRef]
- Robinson, K.; Coraluzzi, L.M.; Navarro, V.J. Liver injury in patients with substance use disorder. Clin. Liver Dis. 2024, 23, e0220. [Google Scholar] [CrossRef]
- Tarantino, G.; Cataldi, M.; Citro, V. Could chronic opioid use be an additional risk of hepatic damage in patients with previous liver diseases, and what is the role of microbiome? Front. Microbiol. 2024, 15, 1319897. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. NPJ Aging 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, D.; Wan, R.; Liu, J.; Zhou, Q.; Zhou, Z.; Wang, W.; Tao, C.; Liu, T. Gene Expression Microarray Data Identify Hub Genes Involved in Osteoarthritis. Front. Genet. 2022, 13, 870590. [Google Scholar] [CrossRef]
- Deshpande, D.; Chhugani, K.; Chang, Y.; Karlsberg, A.; Loeffler, C.; Zhang, J.; Muszynska, A.; Munteanu, V.; Yang, H.; Rotman, J.; et al. RNA-seq data science: From raw data to effective interpretation. Front. Genet. 2023, 14, 997383. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef]
- Hao, X.; Wang, C.; Zhang, R. Chromatin basis of the senescence-associated secretory phenotype. Trends Cell Biol. 2022, 32, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Wu, X.; Williams, M.; Verhulst, S.; Lin, J.; Takahashi, Y.; Ma, J.X.; Wang, Y. High-throughput single telomere analysis using DNA microarray and fluorescent in situ hybridization. Nucleic Acids Res. 2024, 52, e96. [Google Scholar] [CrossRef]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated beta-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Wagner, K.D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Consortium, A.B.; Jiang, M.; Zheng, Z.; Wang, X.; Chen, Y.; Qu, J.; Ding, Q.; Zhang, W.; Liu, Y.-S.; Yang, J. A biomarker framework for liver aging: The Aging Biomarker Consortium consensus statement. Life Med. 2024, 3, lnae004. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef] [PubMed]

| Type of Study | Search Term | Forensic | Medico-Legal | Expert Witness |
|---|---|---|---|---|
| Primary | Senescence | 21 | 0 | |
| Human/non-human | 5 | 16 | ||
| Hepatocyte senescence | 2 | 2 | ||
| Hepatocyte SASP | 0 | 0 | ||
| Secondary | Senescence | 121 | 121 | |
| Hepatocyte senescence | 7 | 1 | ||
| Hepatocyte SASP | 0 | 0 | ||
| 1. Cellular and Molecular Markers | |
| Cell Cycle Inhibitors | |
| Senescence-Associated Beta-Galactosidase (SA-\beta-gal) | |
| Telomere Shortening | |
| DNA Damage Markers | |
| Changes in Nuclear Morphology | |
| 2. Senescence-Associated Secretory Phenotype (SASP) | |
| Pro-inflammatory Cytokines and Chemokines | Such as Interleukin-6 (IL-6), Interleukin-8 (IL-8), and CC motif chemokine ligand 2 (CCL2). |
| Growth Factors | Including growth differentiation factor 15 (GDF15). |
| Matrix Metalloproteinases (MMPs) | Enzymes involved extracellular matrix remodeling. |
| 3. Functional Biomarkers | |
| 4. Imaging Biomarkers | |
| 5. Humoral Biomarkers | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I. Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review. Pharmaceuticals 2025, 18, 787. https://doi.org/10.3390/ph18060787
Šoša I. Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review. Pharmaceuticals. 2025; 18(6):787. https://doi.org/10.3390/ph18060787
Chicago/Turabian StyleŠoša, Ivan. 2025. "Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review" Pharmaceuticals 18, no. 6: 787. https://doi.org/10.3390/ph18060787
APA StyleŠoša, I. (2025). Influence of a Zombie-like State of the Liver on Drugs and Its Medico-Legal Implications: A Scoping Review. Pharmaceuticals, 18(6), 787. https://doi.org/10.3390/ph18060787







