Cystoseira spinosa Polysaccharide: A Promising Natural Source for Antioxidant, Pro-Angiogenic, and Wound Healing Applications: In Silico Study
Abstract
1. Introduction
2. Results and Discussion
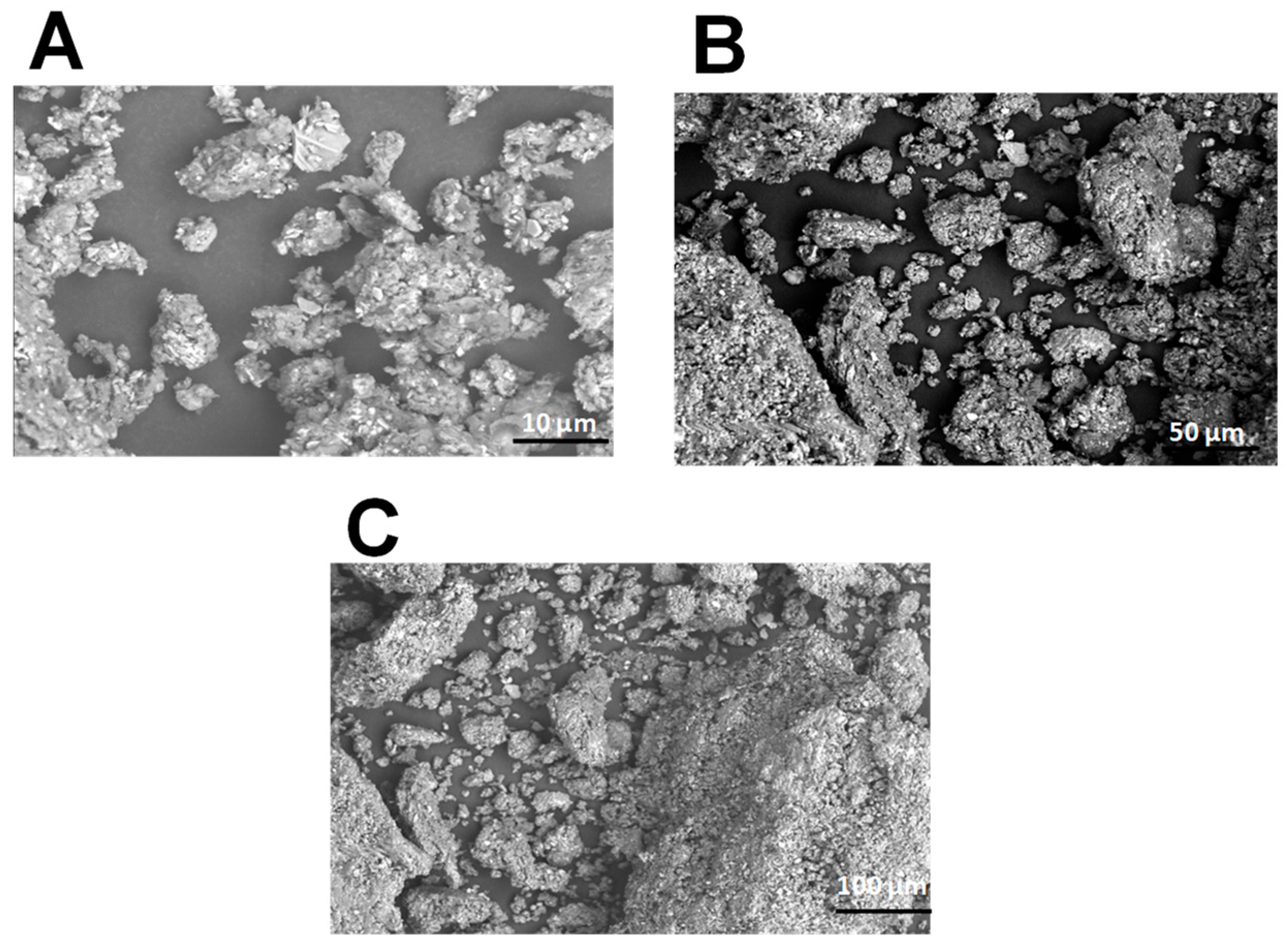
2.1. Scanning Electron Microscopy of PCS
2.2. X-Ray Diffraction of PCS
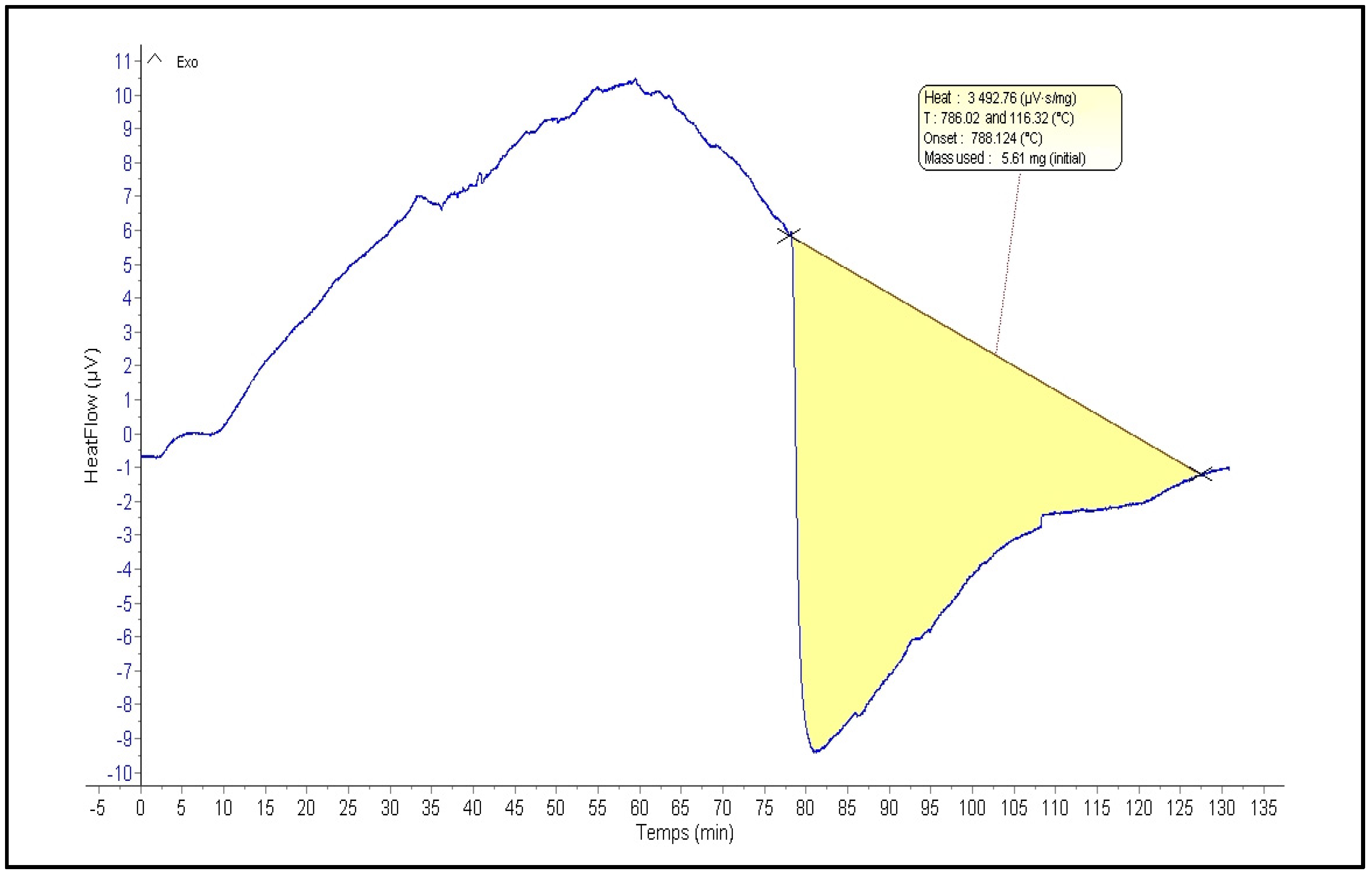
2.3. Thermogravimetric Analysis
2.4. Physical Analysis
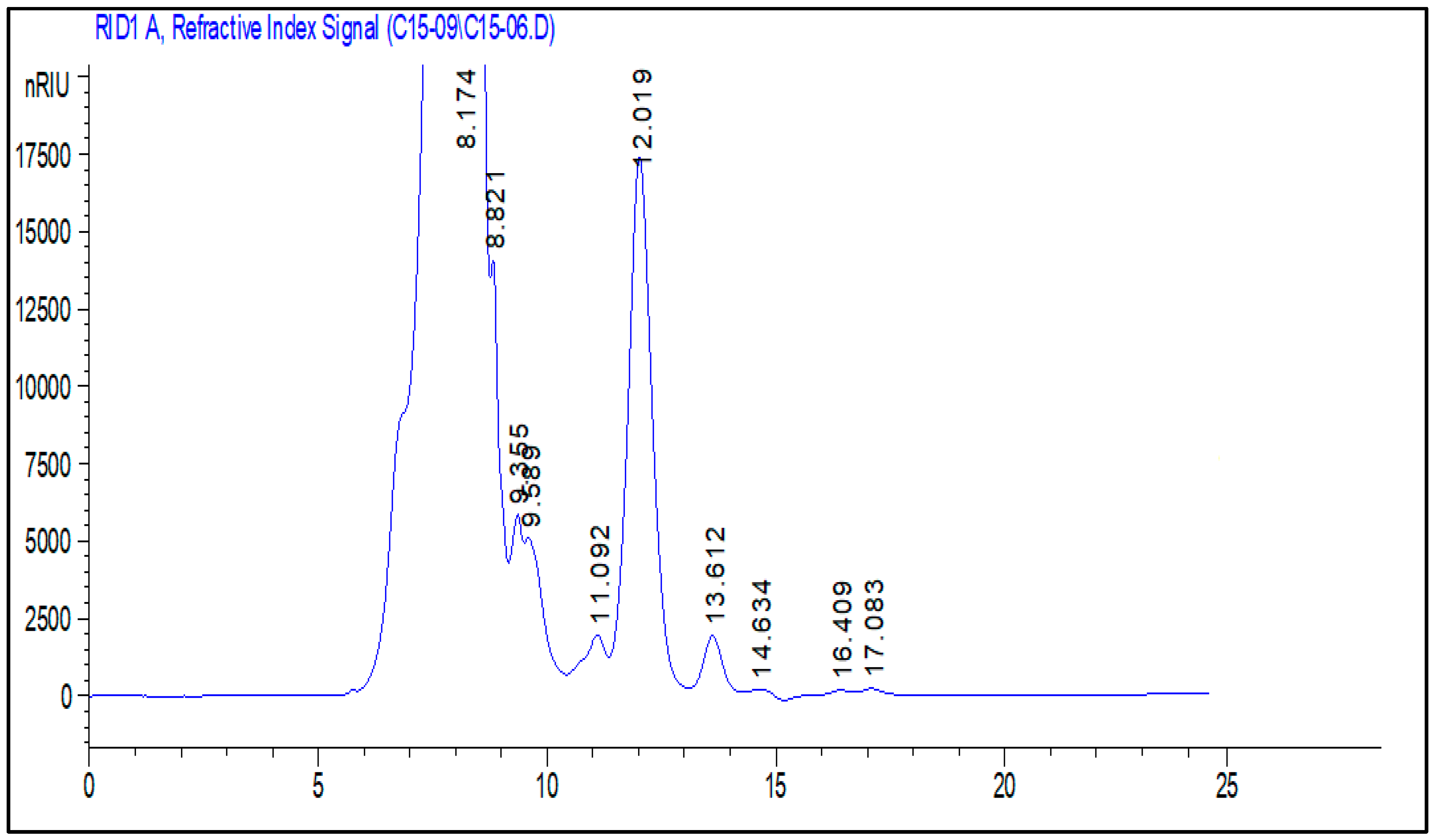
2.5. Polysaccharide Composition Analysis by High-Performance Liquid Chromatography-Refractive Index Detector
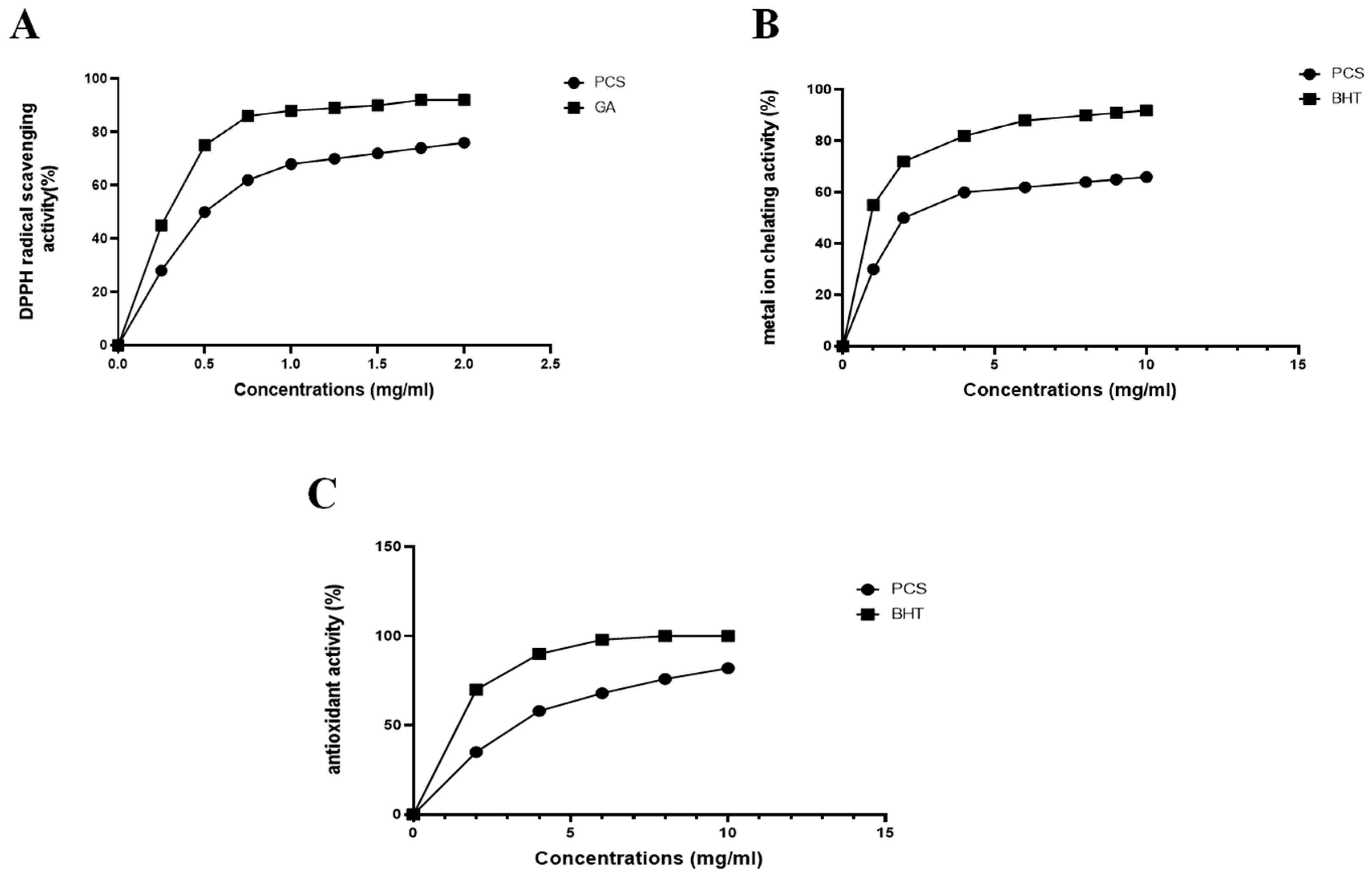
2.6. Determination of the In Vitro Antioxidant Properties of PCS
2.7. Evaluation of the Anti-Inflammatory Activity
2.8. Evaluation of the Pro-Angiogenic Effect of PCS
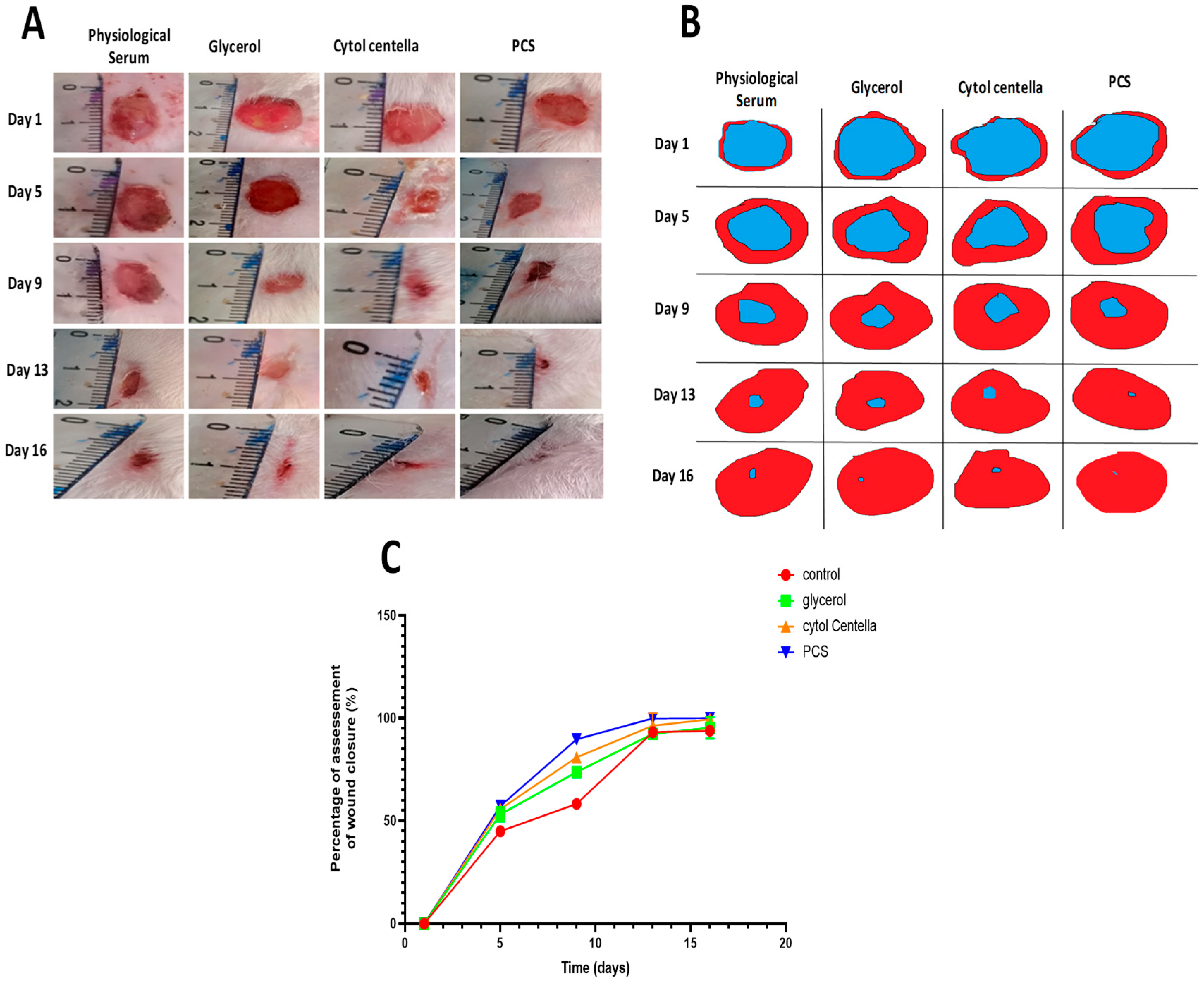
2.9. In Vivo Experimental Study
2.9.1. Morphological Evaluation
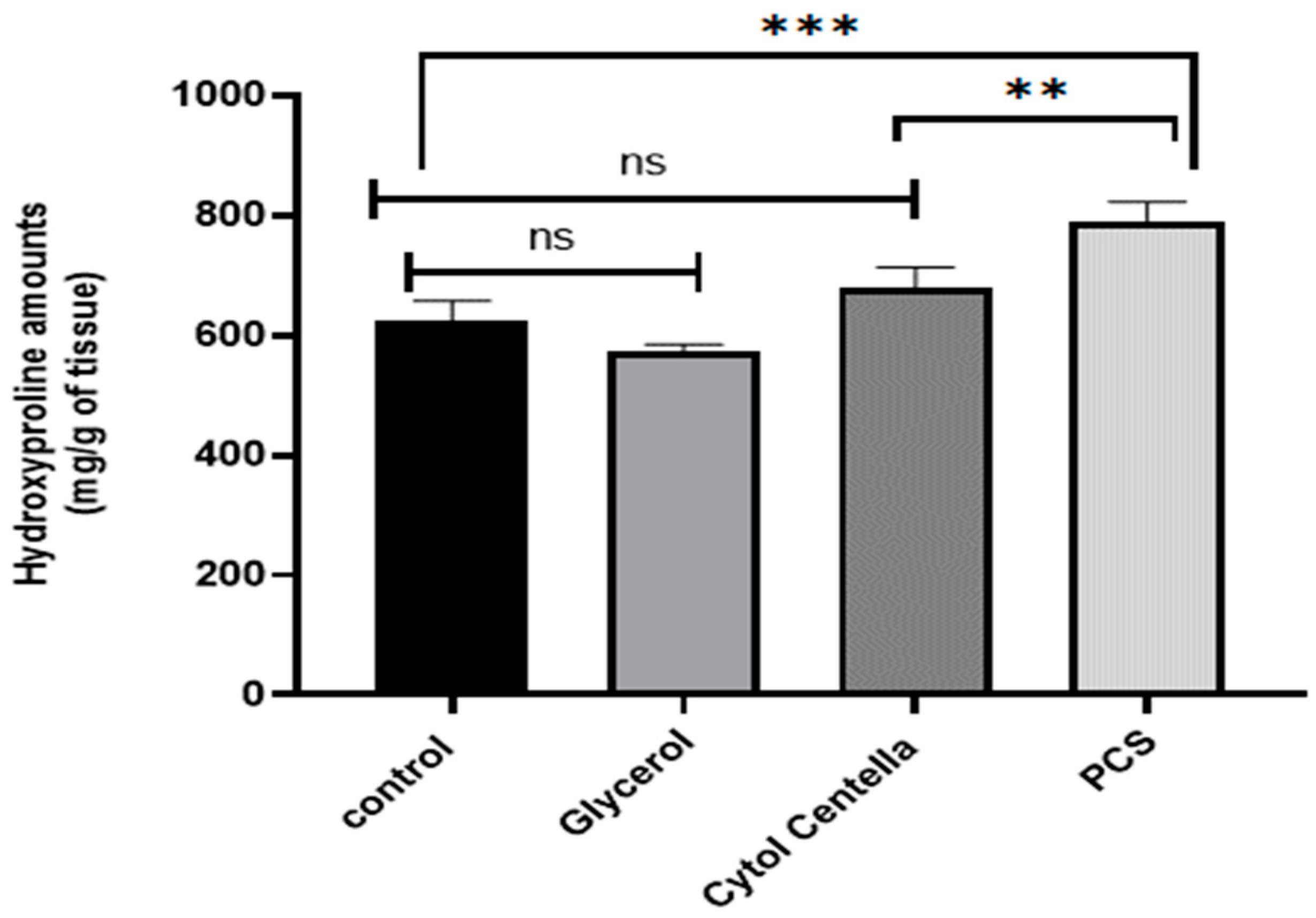
2.9.2. Calculating the Amount of Hydroxyproline
2.9.3. Histological Assessment
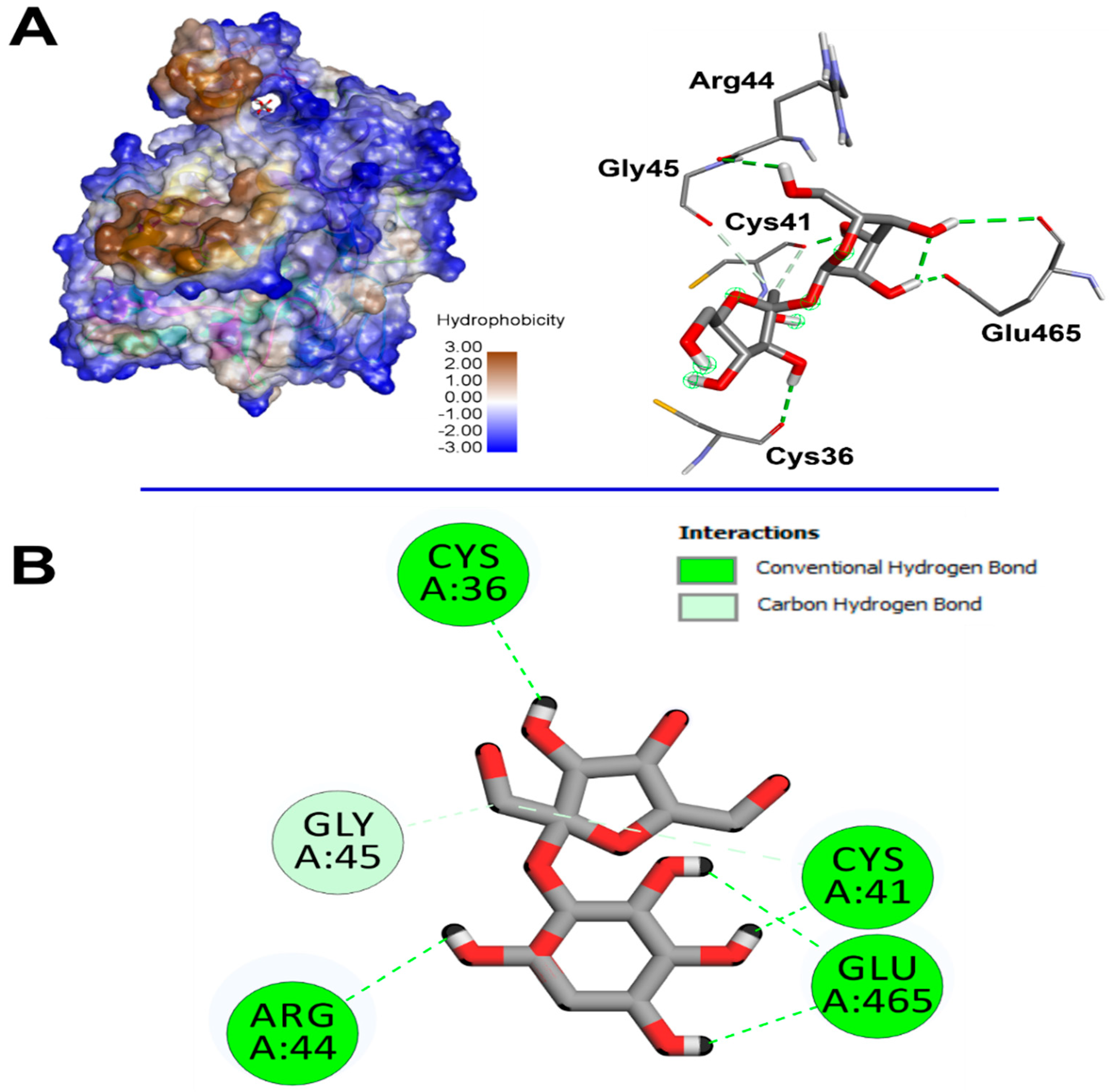
2.10. Computational Findings
3. Materials and Methods
3.1. Chemicals
3.2. Sampling and Preparation
3.3. Polysaccharides Extraction
3.4. Spectroscopic Analysis of PCS
3.4.1. Scanning Electron Microscopy
3.4.2. Monosaccharide Analysis by High Performance Liquid Chromatography-Refractive Index Detector
3.4.3. X-Ray Diffraction
3.4.4. Thermal Properties of PCS
3.4.5. Physical Analysis
3.5. In Vitro Antioxidant Properties of PCS
3.5.1. DPPH Free Radical Scavenging Assay
3.5.2. Ferrous Iron (Fe2+) Chelating Activity
3.5.3. Total Antioxidant Activity
3.6. Anti-Inflammatory Activity Assay
3.7. Angiogenic Potential of PCS
3.8. Experimental Protocol
3.8.1. Animal
3.8.2. Excision Wound Model
3.8.3. Experimental Design
- Group 1: treated with saline solution (0.9%), and used as a control group;
- Group 2: treated with standard drug “CYTOL CENTELLA” cream;
- Group 3: treated with glycerol;
- Group 4: treated with glycerol + PCS.
3.8.4. Measurement of Wound Area and Contraction Rate
3.8.5. Determination of the Content of Hydroxyproline
3.8.6. Histological Examination
3.9. Computational Assays
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Philippeos, C.; Telerman, S.B.; Oulès, B.; Pisco, A.O.; Shaw, T.J.; Elgueta, R.; Lombardi, G.; Driskell, R.R.; Soldin, M.; Lynch, M.D.; et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J. Investig. Dermatol. 2018, 138, 811–825. [Google Scholar] [CrossRef]
- Rathna, R.P.; Kulandhaivel, M. Advancements in Wound Healing: Integrating Biomolecules, Drug Delivery Carriers, and Targeted Therapeutics for Enhanced Tissue Repair. Arch. Microbiol. 2024, 206, 199. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol. Pharm. 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, X.; Liu, Y.; Zhou, J.; Hu, H.; Yang, T.; Yuan, H.; Song, Q. A Polysaccharide/Chitin Hydrogel Wound Dressing from a Periplanattica Americana Residue: Coagulation, Antioxidant Activity, and Wound Healing Properties. J. Biomater. Sci. Polym. Ed. 2023, 34, 1579–1602. [Google Scholar] [CrossRef]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold Nanoparticles and Angiogenesis: Molecular Mechanisms and Biomedical Applications. Int. J. Nanomed. 2019, 14, 7643–7663. [Google Scholar] [CrossRef]
- Kazerounian, S.; Lawler, J. Integration of Pro- and Anti-Angiogenic Signals by Endothelial Cells. J. Cell Commun. Signal. 2018, 12, 171–179. [Google Scholar] [CrossRef]
- Bhat, G.P.; Maurizio, A.; Motta, A.; Podini, P.; Diprima, S.; Malpighi, C.; Brambilla, I.; Martins, L.; Badaloni, A.; Boselli, D.; et al. Structured Wound Angiogenesis Instructs Mesenchymal Barrier Compartments in the Regenerating Nerve. Neuron 2024, 112, 209–229.e11. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on Mechanisms of in Vitro Antioxidant, Antibacterial and Anticancer Activities of Water-Soluble Plant Polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea Antarctica. Mar. Drugs 2022, 20, 223. [Google Scholar] [CrossRef]
- Ersoydan, S.; Rustemeyer, T. Investigating the Anti-Inflammatory Activity of Various Brown Algae Species. Mar. Drugs 2024, 22, 457. [Google Scholar] [CrossRef]
- Feki, A.; Cherif, B.; Sellem, I.; Naifar, M.; Ben Amar, I.; Ben Azaza, Y.; Kallel, R.; Hariz, L.; Zeghal, S.; Makni Ayadi, F.; et al. Biomedical Applications of Polysaccharide Derived from Tetrasporophyte Tufts of Asparagopsis Armata (Falkenbergia rufolanosa): Focus on Antioxidant, Anti-Inflammatory, Anti-Coagulant and Hepato-Protective Activities. Algal Res. 2023, 69, 102958. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Chondrus Crispus Treated with Ultrasound as a Polysaccharides Source with Improved Antitumoral Potential. Carbohydr. Polym. 2021, 273, 118588. [Google Scholar] [CrossRef] [PubMed]
- Peipei, L.; Qinghong, Z.; Yin, C.; Pengfei, H.; Junjie, Z. Structure and Anticoagulant Activity of a Galactoarabinan Sulfate Polysaccharide and Its Oligosaccharide from the Green Algae, Codium fragile. Int. J. Biol. Macromol. 2024, 279, 135255. [Google Scholar] [CrossRef]
- Raposo, M.F.d.J.; de Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Matin, M.; Koszarska, M.; Atanasov, A.G.; Król-Szmajda, K.; Jóźwik, A.; Stelmasiak, A.; Hejna, M. Bioactive Potential of Algae and Algae-Derived Compounds: Focus on Anti-Inflammatory, Antimicrobial, and Antioxidant Effects. Molecules 2024, 29, 4695. [Google Scholar] [CrossRef]
- Gangadoo, S.; Owen, S.; Rajapaksha, P.; Plaisted, K.; Cheeseman, S.; Haddara, H.; Truong, V.K.; Ngo, S.T.; Vu, V.V.; Cozzolino, D.; et al. Nano-Plastics and Their Analytical Characterisation and Fate in the Marine Environment: From Source to Sea. Sci. Total Environ. 2020, 732, 138792. [Google Scholar] [CrossRef]
- Fonseca, I.; Guarda, I.; Mourato, M.; Martins, L.L.; Gomes, R.; Matos, J.; Gomes-Bispo, A.; Bandarra, N.M.; Cardoso, C.; Afonso, C. Undervalued Atlantic Brown Seaweed Species (Cystoseira abies-marina and Zonaria tournefortii): Influence of Treatment on Their Nutritional and Bioactive Potential and Bioaccessibility. Eur. Food Res. Technol. 2021, 247, 221–232. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. IJPR 2014, 13, 207–220. [Google Scholar]
- Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Appl. Sci. 2021, 11, 11261. [Google Scholar] [CrossRef]
- Abourriche, A.; Charrouf, M.; Berrada, M.; Bennamara, A.; Chaib, N.; Francisco, C. Antimicrobial Activities and Cytotoxicity of the Brown Alga Cystoseira tamariscifolia. Fitoterapia 1999, 70, 611–614. [Google Scholar] [CrossRef]
- Obeidi, N.; Mousavi, M.J.; Hosseinpouri, A.; Malekhayati, H.; Ehsandoost, E. Anticoagulative Effect of Two Species of Brown Algae; Sargassum Angustifolium and Cystoseira Indica. Res. Mol. Med. 2020, 8, 125–132. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Shan, S.; Song, C.; Baranenko, D.; Li, Y.; Lu, W. A Novel Polysaccharide Isolated from Ulva pertusa: Structure and Physicochemical Property. Carbohydr. Polym. 2020, 233, 115849. [Google Scholar] [CrossRef] [PubMed]
- Kolsi, R.B.A.; Jardak, N.; Hajkacem, F.; Chaaben, R.; Jribi, I.; Feki, A.E.; Rebai, T.; Jamoussi, K.; Fki, L.; Belghith, H.; et al. Anti-Obesity Effect and Protection of Liver-Kidney Functions by Codium fragile Sulphated Polysaccharide on High Fat Diet Induced Obese Rats. Int. J. Biol. Macromol. 2017, 102, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Effect of Season on the Composition of Bioactive Polysaccharides from the Brown Seaweed Saccharina longicruris. Phytochemistry 2009, 70, 1069–1075. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological Activities of Sulfated Polysaccharides from Tropical Seaweeds. Biomed. Pharmacother. Biomedecine Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Ktari, N.; Trabelsi, I.; Bardaa, S.; Triki, M.; Bkhairia, I.; Ben Slama-Ben Salem, R.; Nasri, M.; Ben Salah, R. Antioxidant and Hemolytic Activities, and Effects in Rat Cutaneous Wound Healing of a Novel Polysaccharide from Fenugreek (Trigonella foenum-graecum) Seeds. Int. J. Biol. Macromol. 2017, 95, 625–634. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In Vitro Antioxidant and in Vivo Anti-Inflammatory Potential of Crude Polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef]
- Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. [Google Scholar] [CrossRef]
- Duan, W.; Jin, Y.; Cui, Y.; Xi, F.; Liu, X.; Wo, F.; Wu, J. A Co-Delivery Platform for Synergistic Promotion of Angiogenesis Based on Biodegradable, Therapeutic and Self-Reporting Luminescent Porous Silicon Microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Trujillo, J.I.; Jandeleit, B.; Chibale, K.; Rosenfeld, M.; Diefenbach, B.; Cheresh, D.A.; Goodman, S.L. Design, Synthesis and Biological Evaluation of Nonpeptide Integrin Antagonists. Bioorg. Med. Chem. 1998, 6, 1185–1208. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A Comparative Study of the Anti-Inflammatory, Anticoagulant, Antiangiogenic, and Antiadhesive Activities of Nine Different Fucoidans from Brown Seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira barbata Seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Ayoub, A.; Pereira, J.M.; Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M.; Moulin, V.J. Role of Seaweed Laminaran from Saccharina longicruris on Matrix Deposition during Dermal Tissue-Engineered Production. Int. J. Biol. Macromol. 2015, 75, 13–20. [Google Scholar] [CrossRef]
- Feki, A.; Bardaa, S.; Hajji, S.; Ktari, N.; Hamdi, M.; Chabchoub, N.; Kallel, R.; Boudawara, T.; Nasri, M.; Ben Amara, I. Falkenbergia rufolanosa Polysaccharide—Poly(Vinyl Alcohol) Composite Films: A Promising Wound Healing Agent against Dermal Laser Burns in Rats. Int. J. Biol. Macromol. 2020, 144, 954–966. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ktari, N.; Ben Slima, S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben Salah, R. Evaluation of Dermal Wound Healing Activity and in Vitro Antibacterial and Antioxidant Activities of a New Exopolysaccharide Produced by Lactobacillus Sp.Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef]
- Li, Q.; Kao, H.; Matros, E.; Peng, C.; Murphy, G.F.; Guo, L. Pulsed Radiofrequency Energy Accelerates Wound Healing in Diabetic Mice. Plast. Reconstr. Surg. 2011, 127, 2255–2262. [Google Scholar] [CrossRef]
- Kumar, S.; Marrero-Berrios, I.; Kabat, M.; Berthiaume, F. Recent Advances in the Use of Algal Polysaccharides for Skin Wound Healing. Curr. Pharm. Des. 2019, 25, 1236–1248. [Google Scholar] [CrossRef]
- Ben Slima, S.; Ktari, N.; Chouikhi, A.; Hzami, A.; Bardaa, S.; Trabelsi, I.; Ben Salah, B.; Ben Salah, R. Extraction, Characterization, and Structure of a Novel Heteropolysaccharide from Lepidium Sativum and Its Effects on Wound Healing in Diabetic Rats. BioMed Res. Int. 2022, 2022, 7858865. [Google Scholar] [CrossRef]
- Almuzaini, N.A.M.; Sulieman, A.M.E.; Alanazi, N.A.; Badraoui, R.; Abdallah, E.M. Mass Spectrometric Based Metabolomics of the Saudi Cultivar of Fenugreek (Trigonella foenum-graecum L.): A Combined GC-MS, Antimicrobial and Computational Approach. Pharmaceuticals 2024, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Radwan, H.A.; Badraoui, R.; Aouadi, K.; Snoussi, M.; Kadri, A. Synthesis, Structure-Activity Relationship and in Silico Studies of Novel Pyrazolothiazole and Thiazolopyridine Derivatives as Prospective Antimicrobial and Anticancer Agents. ChemistrySelect 2021, 6, 7860–7872. [Google Scholar] [CrossRef]
- Boudjema, K.; Chouala, K.; Khelef, Y.; Chenna, H.; Badraoui, R.; Boumendjel, M.; Boumendjel, A.; Messarah, M. Antioxidant Effects of Moringa Oleifera Against Abamectin-Induced Oxidative Stress in the Brain and Erythrocytes of Rats. Chem. Biodivers. 2024, e202402709. [Google Scholar] [CrossRef] [PubMed]
- Hamrita, B.; Emira, N.; Papetti, A.; Badraoui, R.; Bouslama, L.; Ben Tekfa, M.-I.; Hamdi, A.; Patel, M.; Elasbali, A.M.; Adnan, M.; et al. Phytochemical Analysis, Antioxidant, Antimicrobial, and Anti-Swarming Properties of Hibiscus Sabdariffa L. Calyx Extracts: In Vitro and In Silico Modelling Approaches. Evid.-Based Complement. Altern. Med. ECAM 2022, 2022, 1252672. [Google Scholar] [CrossRef]
- Mzid, M.; Ben Khedir, S.; Bardaa, S.; Sahnoun, Z.; Rebai, T. Chemical Composition, Phytochemical Constituents, Antioxidant and Anti-Inflammatory Activities of Urtica urens L. Leaves. Arch. Physiol. Biochem. 2017, 123, 93–104. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A Review of Bioactive Plant Polysaccharides: Biological Activities, Functionalization, and Biomedical Applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and Characterization of Three Polysaccharides Extracted from Opuntia Ficus Indica Cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of Chemical Composition, Antimicrobial and Antioxidant Activities of Artemisia Essential Oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric Determination of Serum Iron at the Submicrogram Level with a New Reagent (Ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The Nitric Oxide-Scavenging Properties of Ginkgo Biloba Extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-Angiogenic and Osteogenic Composite Scaffolds of Fibrin, Alginate and Calcium Phosphate for Bone Tissue Engineering. J. Tissue Eng. 2021, 12, 20417314211005610. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Orkesterjournalen L. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 20 February 2025).
- Sene, M.; Barboza, F.S.; Top, B.; Ndiaye, M.; Sarr, A.; Fall, A.D.; Sy, G.Y. Activité cicatrisante de l’extrait aqueux des feuilles de Elaeis guineensis Jacq. (Arecaceae). Int. J. Biol. Chem. Sci. 2020, 14, 674–684. [Google Scholar] [CrossRef]
- Edwards, C.A.; O’Brien, W.D. Modified Assay for Determination of Hydroxyproline in a Tissue Hydrolyzate. Clin. Chim. Acta Int. J. Clin. Chem. 1980, 104, 161–167. [Google Scholar] [CrossRef]



 Ulceration
Ulceration  Neutrophil
Neutrophil  Collagen formation.
Collagen formation.
 Ulceration
Ulceration  Neutrophil
Neutrophil  Collagen formation.
Collagen formation.

| Color | PCS |
|---|---|
| L* (lightness) | 71.2 ± 0.05 |
| a* (redness) | 0.5 ± 0.01 |
| b* (yellowness) | 5 ± 0.05 |
| 0.5 g/L | 4.6 ± 0.05 |
| 1 g/L | 6.50 ± 0.45 |
| 1.5 g/L | 8.5 ± 0.2 |
| pH | 7.1 ± 0.1 |
| RT | Monosaccharide |
|---|---|
| 8.17 | Glucuronic acid |
| 9.35 | Saccharose |
| 11.09 | Glucose |
| 12.01 | Xylose |
| 13.61 | Fructose |
| 14.63 | Galactose |
| 16.40 | Arabinose |
| 17.08 | Rhamnose |
| Feature Graded | Grade | Description |
|---|---|---|
| Inflammatory infiltrate | 1 | Profound (>50%) |
| 2 | Scanty (10–50%) | |
| 3 | A few (10%) | |
| 4 | Absent | |
| Fibroblast proliferation | 1 | Mild |
| 2 | Moderate | |
| 3 | Marked | |
| Collagen formation | 1 | Mild |
| 2 | Moderate | |
| 3 | Marked | |
| New vessels | 1 | Mild |
| 2 | Moderate | |
| 3 | Marked | |
| Epithelium | 1 | Epithelial necrosis |
| 2 | Epithelial proliferation on the edges of the ulcer | |
| 3 | Partial re-epithelialization | |
| 4 | Complete re-epithelialization | |
| Epidermal differentiation | 1 | Basal cells |
| 2 | Spinous epidermal differentiation (early) | |
| 3 | Granular epidermal differentiation (late) | |
| 4 | Complete |
| Groups | Inflammatory Infiltrate | Fibroblast Proliferation | Collagen Formation | Epithelium | Epidermal Differentiation | Total Score |
|---|---|---|---|---|---|---|
| Control | 2 | 2 | 3 | 3 | 4 | 14 |
| Glycerol | 2 | 2 | 2 | 3 | 4 | 13 |
| Cytol Centella | 2 | 3 | 3 | 3 | 4 | 15 |
| PCS | 3 | 3 | 3 | 4 | 4 | 17 |
| Entry/Compound Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Arabinose | Fructose | Galactose | Glucose | Glucoronic Acid | Rhamnose | Saccharose | Xylose | ||
| Lipophilicity/ Druglikeness | Molecular weight | 150.13 | 180.16 | 180.16 | 180.16 | 397.17 | 164.16 | 342.3 | 150.13 |
| TPSA (Å2) | 90.15 | 110.38 | 110.38 | 110.38 | 114.43 | 97.99 | 189.53 | 90.15 | |
| Consensus Log Po/w | −1.85 | −2.04 | −2.33 | −2.16 | 0.63 | −1.5 | −3.29 | −2 | |
| Lipinski’s Rule | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.17 | 0.55 | |
| Pharmacokinetics/ Medicinal Chemistry | GI absorption | Low | Low | Low | Low | High | Low | Low | Low |
| BBB permeant | No | No | No | No | No | No | No | No | |
| P-gp substrate | No | No | Yes | Yes | No | No | Yes | No | |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | |
| Log Kp (cm/s) | −9.36 | −9.42 | −9.7 | −9.7 | −8.3 | −8.99 | −11.02 | −9.36 | |
| Synthetic accessibility | 3.8 | 3.96 | 4.08 | 4.08 | 4.82 | 3.11 | 5.16 | 3.8 | |
| Monosaccharide | Receptor | Binding Affinity (kcal/mol) | Closest Interacting Residues | |
|---|---|---|---|---|
| Bond Category/Interacting Residues | Residues (Distance, Å) | |||
| Arabinose | COX-2 | −5.8 | Conventional H-Bonds: Gln461, Cys41, Asn39, Gln461, Cys41, Gly45 | Gln461 (2.01) |
| VEGF | −4.5 | Conventional H-Bonds: Cys61, Cys61, Cys61, Asp64 Carbon H-Bonds: Leu66 | Cys61 (2.319) | |
| Fructose | COX-2 | −6.4 | Conventional H-Bonds: Lys511, Tyr475, Glu520, Tyr475, Glu510 | Lys511 (2.089) |
| VEGF | −4.5 | Conventional H-Bonds: Leu47, Leu74, Leu35, Leu47, Lys45 Carbon H-Bonds: Ala44, Ser50 | Leu74 (2.004) | |
| Galactose | COX-2 | −6.6 | Conventional H-Bonds: Arg120, Ser471, Glu524, Phe470, Glu524, Glu524 | Glu524 (2.079) |
| VEGF | −4.6 | Conventional H-Bonds: Cys61, Cys61, Gly65, Asp64, Leu66, Cys61 Carbon H-Bonds: Asp64, Leu66 | Leu66 (1.852) | |
| Glucose | COX-2 | −6.3 | Conventional H-Bonds: Asn34, Cys47, Ala156, Cys47, Asn39, Cys36, Cys37 | Asn34 (2.096) |
| VEGF | −4.6 | Conventional H-Bonds: Thr42, Val43, Ala44, Val37, Leu39, Lys45, Leu39 | Leu39 (2.218) | |
| Glucoronic acid | COX-2 | −6.5 | Conventional H-Bonds: His207, His207, Thr212, Thr212, Asn382, His388 Carbon H-Bonds: His207, Thr212 | His388 (1.973) |
| VEGF | −4.4 | Conventional H-Bonds: Val37, Gln46, Leu47, Leu47 | Leu47 (2.095) | |
| Rhamnose | COX-2 | −5.2 | Conventional H-Bonds: Thr394, Asn396, Asn396, Gln429, Thr394, Thr394, Glu401 Pi-Sigma: Phe187 | Glu401 (2.055) |
| VEGF | −4.2 | Conventional H-Bonds: Leu47, Lys45, Ala44, Thr42, Ala44, Leu35 | Leu47 (1.924) | |
| Saccharose | COX-2 | −7.1 | Conventional H-Bonds: Cys36, Arg44, Glu465, Cys41, Glu465 Carbon H-Bonds: Cys41, Gly45 | Cys41 (1.816) |
| VEGF | −5.3 | Conventional H-Bonds: Leu47, Val37, Leu39, Thr42, Ala44, Lys45, Leu35 Carbon H-Bonds: Thr36 | Leu39 (1.924) | |
| Xylose | COX-2 | −6.0 | Conventional H-Bonds: Gln461, Glu465, Glu465, Arg44, Gly45 | Glu465 (2.010) |
| VEGF | −4.1 | Conventional H-Bonds: Leu39, Leu35, Thr36, Lys45 | Lys45 (2.125) | |
| Score | Evaluation of the Healing Process |
|---|---|
| 0 | Healing is complete, and tissue repair is complete |
| 1 | Tissue healing is almost complete |
| 2 | Remains of the crust, the size of the lesion decreases (skin reconstruction) |
| 3 | All dead tissue (crusts) are removed, wounds, oozing |
| 4 | Necrotic skin is partially removed, ulceration, oozing |
| 5 | Necrotic skin completely covers the burned part |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berfad, M.A.; Kammoun, I.; Lakhrem, M.; Boujhoud, Z.; Eleroui, M.; Mellouli, M.; Makni, S.; Kammoun, M.; Badraoui, R.; Pujo, J.M.; et al. Cystoseira spinosa Polysaccharide: A Promising Natural Source for Antioxidant, Pro-Angiogenic, and Wound Healing Applications: In Silico Study. Pharmaceuticals 2025, 18, 774. https://doi.org/10.3390/ph18060774
Berfad MA, Kammoun I, Lakhrem M, Boujhoud Z, Eleroui M, Mellouli M, Makni S, Kammoun M, Badraoui R, Pujo JM, et al. Cystoseira spinosa Polysaccharide: A Promising Natural Source for Antioxidant, Pro-Angiogenic, and Wound Healing Applications: In Silico Study. Pharmaceuticals. 2025; 18(6):774. https://doi.org/10.3390/ph18060774
Chicago/Turabian StyleBerfad, Mouhamed Ayad, Intissar Kammoun, Marwa Lakhrem, Zakaria Boujhoud, Malek Eleroui, Manel Mellouli, Saadia Makni, Majed Kammoun, Riadh Badraoui, Jean Marc Pujo, and et al. 2025. "Cystoseira spinosa Polysaccharide: A Promising Natural Source for Antioxidant, Pro-Angiogenic, and Wound Healing Applications: In Silico Study" Pharmaceuticals 18, no. 6: 774. https://doi.org/10.3390/ph18060774
APA StyleBerfad, M. A., Kammoun, I., Lakhrem, M., Boujhoud, Z., Eleroui, M., Mellouli, M., Makni, S., Kammoun, M., Badraoui, R., Pujo, J. M., Kallel, H., & Ben Amara, I. (2025). Cystoseira spinosa Polysaccharide: A Promising Natural Source for Antioxidant, Pro-Angiogenic, and Wound Healing Applications: In Silico Study. Pharmaceuticals, 18(6), 774. https://doi.org/10.3390/ph18060774






