Comprehensive GC-MS Profiling and Multi-Modal Pharmacological Evaluations of Haloxylon griffithii: In Vitro and In Vivo Approaches
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis
2.2. In Silico Models
ADME Profiling of GC-MS-Identified Compounds of H. griffithii
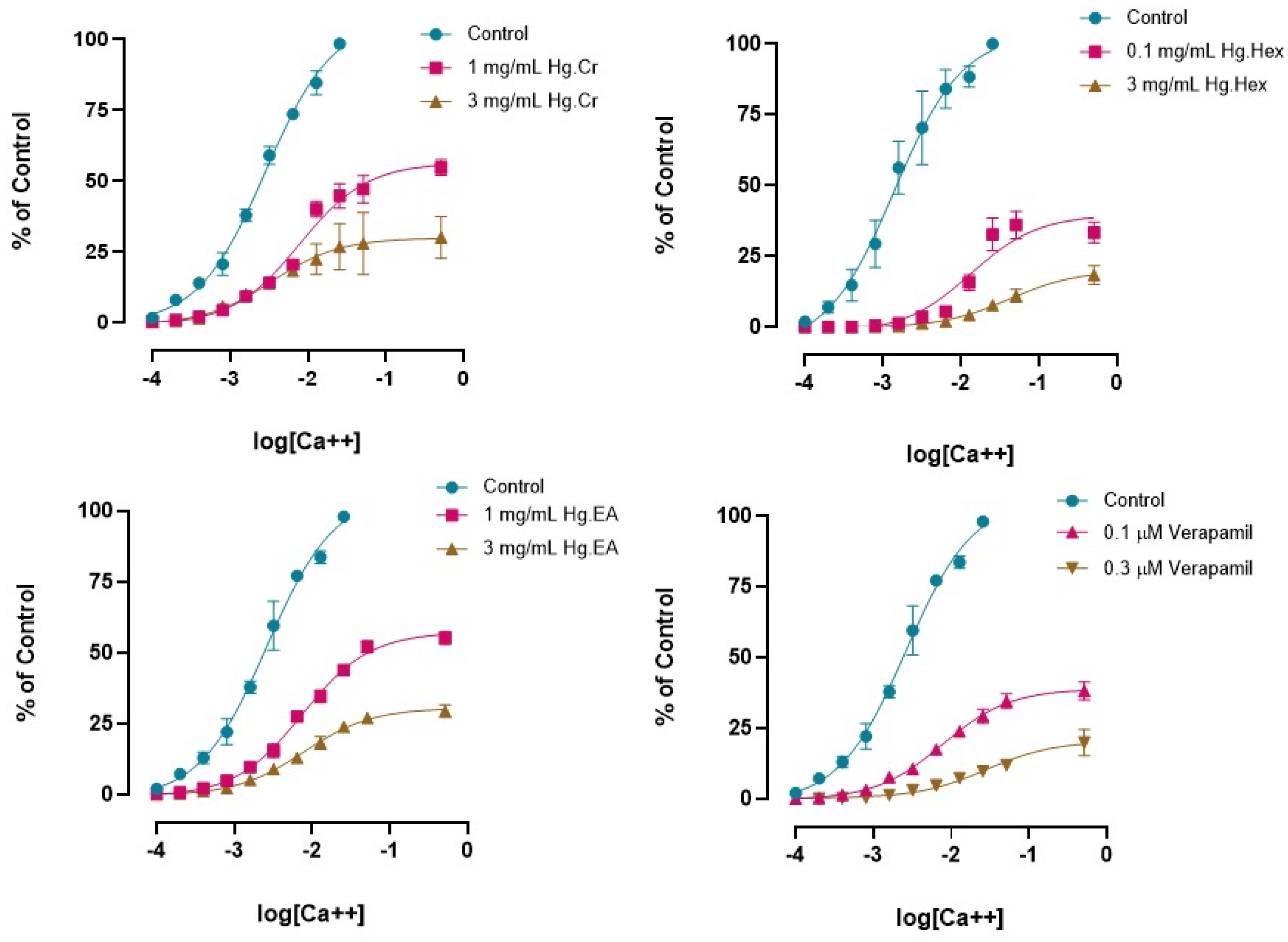
2.3. In Vitro Experiments
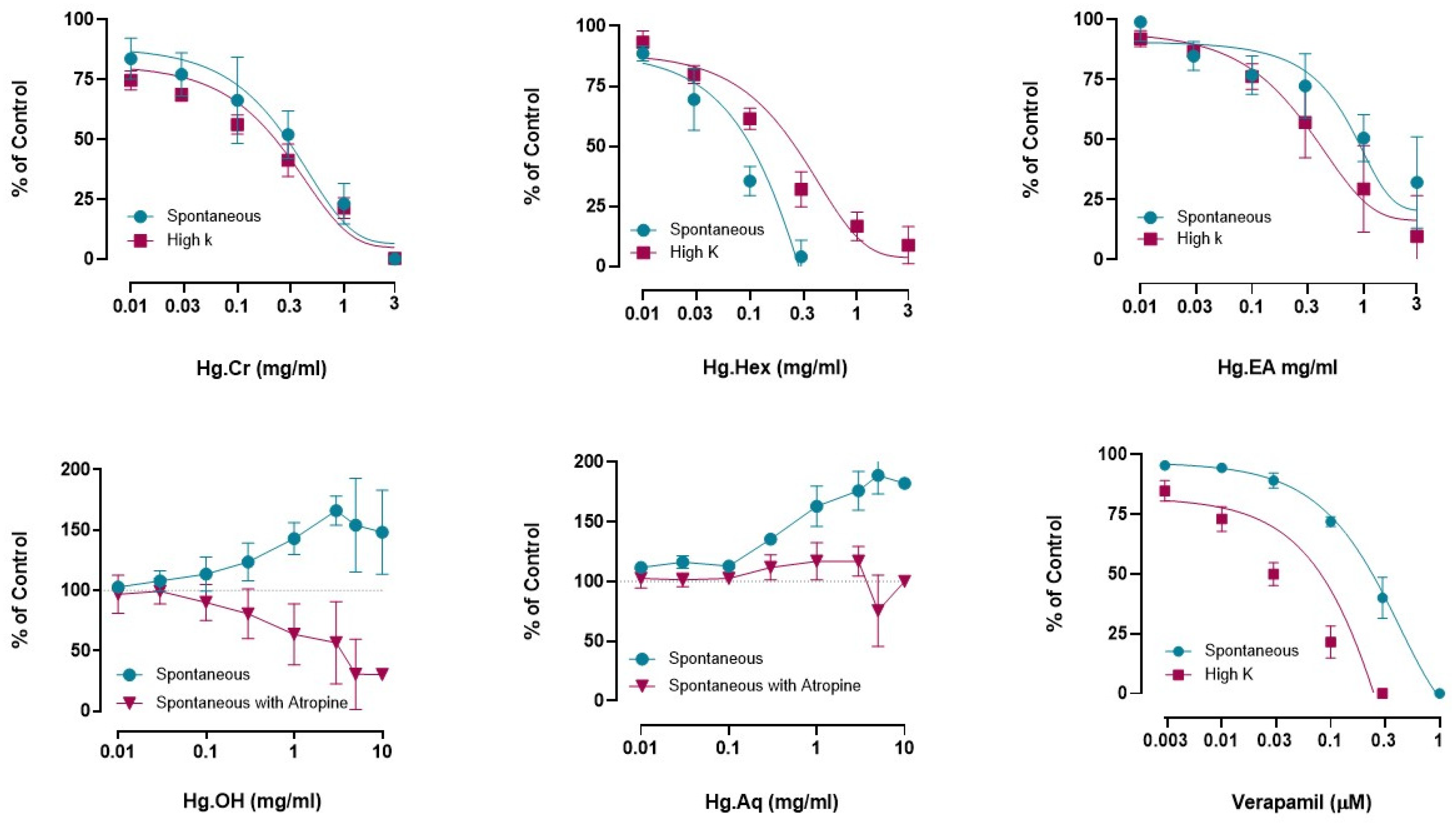
Effects of H. griffithii Extracts on Isolated Tissues of Rabbit Jejunum
2.4. In Vivo Experiments
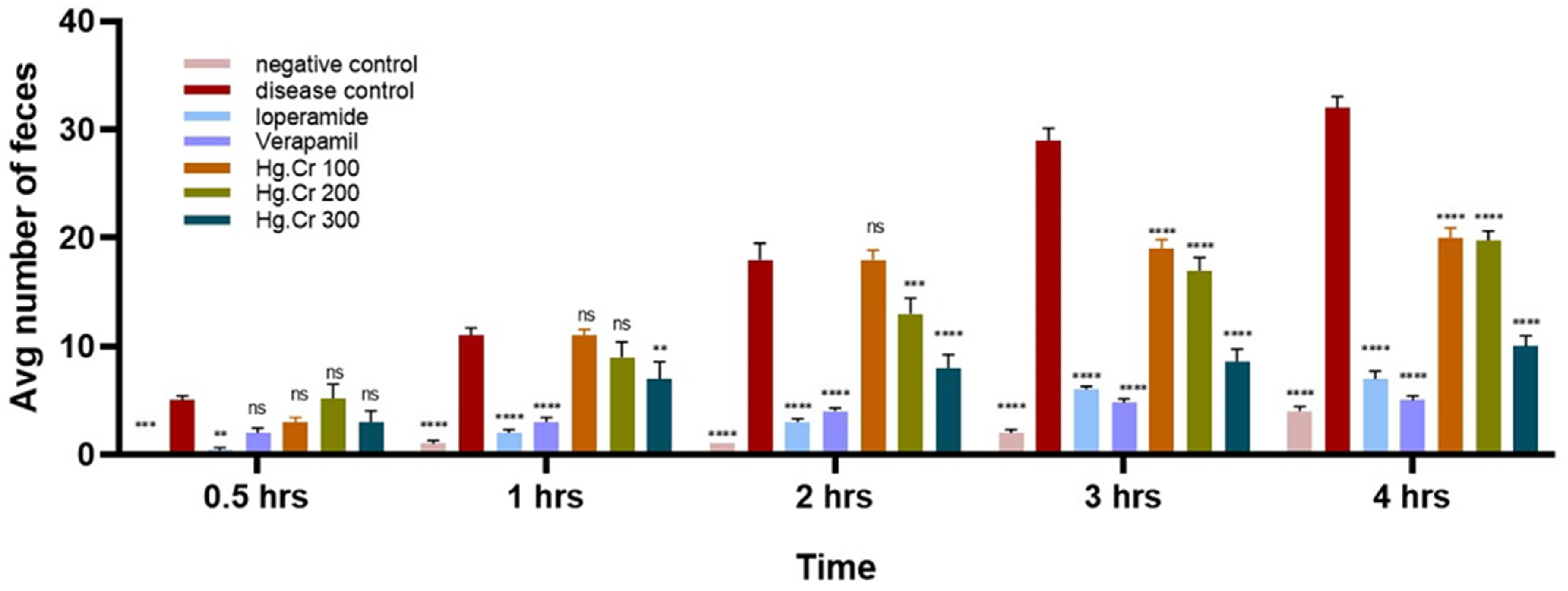
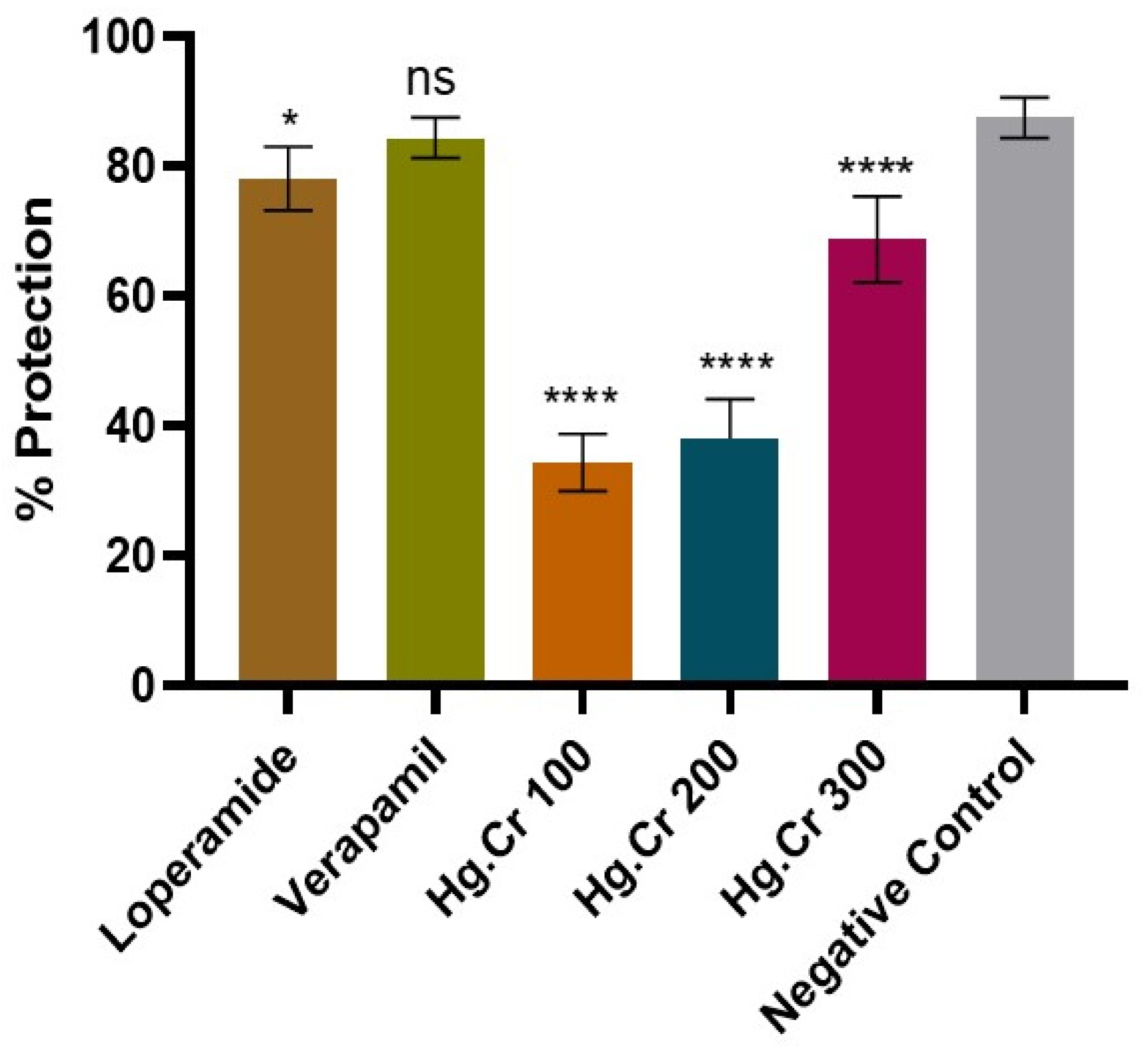
2.4.1. Antidiarrheal Activity
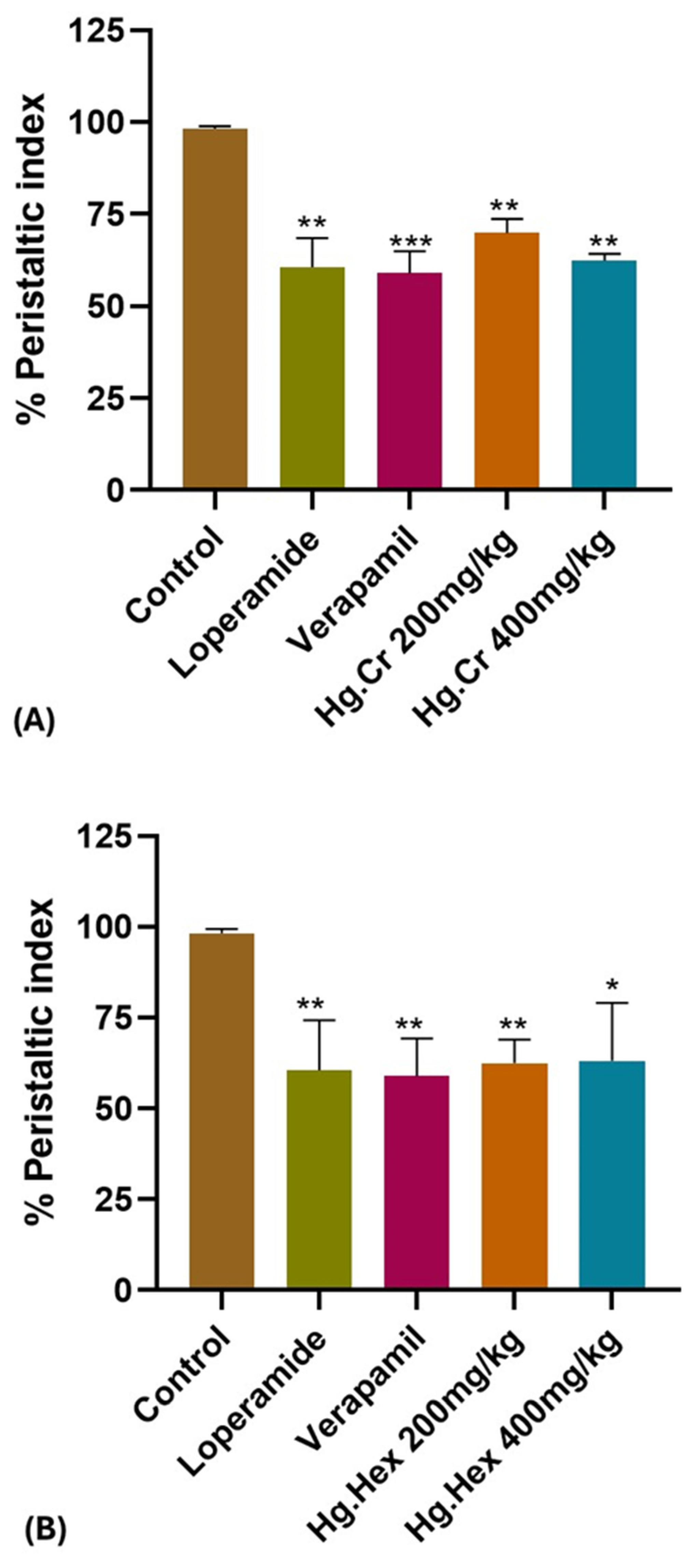
2.4.2. Effect of H. griffithii on Charcoal Meal GIT Transit Time
3. Discussion
4. Materials and Methods
4.1. Identification and Collection of Haloxylon griffithii
4.2. Preparation of Crude Extract and Fractionations
4.3. Drugs and Chemicals
4.4. Animals
4.5. GC-MS Analysis
4.6. In-Silico Models
ADME Profiling of GC-MS-Identified Compounds
4.7. In Vitro Investigation
Effect on Isolated Rabbit Jejunum
4.8. In Vivo Investigations
4.8.1. Castor Oil-Induced Diarrhea Model
4.8.2. Charcoal Meal GIT Transit Time
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| H. griffithii | Haloxylon griffithii |
| CRCs | Concentration response curves |
| Hg.Cr | Haloxylon griffithii crude extract |
| Hg.Hex | Haloxylon griffithii n-hexane fraction |
| Hg.EA | Haloxylon griffithii ethyl acetate fraction |
| Hg.OH | Haloxylon griffithii ethanolic fraction |
| Hg.Aq | Haloxylon griffithii Aqueous fraction |
| GIT | Gastrointestinal tract |
| VGCC | Voltage-gated calcium channels |
Appendix A
| Peak no. | RT | % Area | Compound Name | Hit Quality |
|---|---|---|---|---|
| 1 | 3.466 | 0.21 | 2-Butanone, 4-hydroxy-3-methyl- | 52 |
| 2 | 7.703 | 0.10 | o-Cymene | 95 |
| 3 | 10.480 | 0.14 | 4-Hydroxy-.beta.-thujone | 72 |
| 4 | 11.750 | 0.18 | Cyclohexasiloxane, dodecamethyl- | 93 |
| 5 | 13.215 | 0.12 | 1-(1-Butyny)cyclopentanol | 50 |
| 6 | 13.677 | 0.26 | Hexanoic acid, 3,7-dimethyl-6-octenyl ester | 49 |
| 7 | 13.958 | 0.41 | Cycloheptasiloxane, tetradecamethyl- | 93 |
| 8 | 14.126 | 0.15 | Carane, 4,5-epoxy-, trans | 46 |
| 9 | 14.29 | 0.15 | Pentacosane | 74 |
| 10 | 14.38 | 0.06 | Ledol | 38 |
| 11 | 14.541 | 0.04 | 2,4-Di-tert-butylphenol | 78 |
| 12 | 15.930 | 0.25 | Cyclooctasiloxane, hexadecamethyl- | 94 |
| 13 | 16.174 | 0.03 | Bicyclo [2.2.1]heptane, 1,3,3-trimethyl | 46 |
| 14 | 16.217 | 0.04 | 2-Heptenoic acid, methyl ester, (E)- | 44 |
| 15 | 16.499 | 0.11 | 2-Propenal, 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 59 |
| 16 | 16.808 | 0.17 | 2-Hexen-1-ol, 2-ethyl | 89 |
| 17 | 16.979 | 0.23 | (S,E)-6-Hydroxy-6-methyl-2-((2S,5R)-5-methyl-5-vinyltetrahydrofuran-2-yl) | 68 |
| 18 | 17.315 | 0.14 | 1H-Indene, 1-ethylideneoctahydro-7a-methyl-, (1Z,3a.alpha.,7a.beta.) | 87 |
| 19 | 17.631 | 0.11 | Cyclononasiloxane, octadecamethyl | 90 |
| 20 | 18.154 | 0.12 | Isopropyl myristate | 35 |
| 21 | 18.536 | 0.14 | 7,10,10-Trimethyl-4-oxa-3,5-diazatricyclo [5.2.1.0(2,6)]deca-2,5-dien-3-one | 35 |
| 22 | 18.959 | 0.14 | 2,6-Pyrazinedicarbonitrile, 3-amino-5-(diethylamino)- | 41 |
| 23 | 19.045 | 0.15 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 99 |
| 24 | 19.146 | 0.18 | Cyclodecasiloxane, eicosamethyl- | 52 |
| 25 | 19.197 | 0.13 | Hexadecanoic acid, methyl ester | 96 |
| 26 | 19.419 | 0.10 | 5-(6-Methyl-7-oxooctyl)-2(5H)-furanone (isomer 2) | 47 |
| 27 | 19.533 | 0.34 | n-Hexadecanoic acid | 96 |
| 28 | 19.873 | 0.48 | Hexadecanoic acid, ethyl ester | 99 |
| 29 | 20.019 | 0.13 | (3aR,4R,7R)-1,4,9,9-Tetramethyl-3,4,5,6,7,8-hexahydro-2H-3a,7-methanoazulen-2-one | 78 |
| 30 | 20.216 | 1.89 | Lumisantonin | 95 |
| 31 | 20.326 | 0.92 | Propionic acid, 3-(2-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]diazepin-1-yl)- | 44 |
| 32 | 20.542 | 0.82 | Benzene, 1,2,4,5-tetrakis(1-methylethyl)- | 89 |
| 33 | 20.651 | 0.12 | 6-Hydroxy-5a,9-dimethyl-3-methylidene-4,5,6,7,9a,9b-hexahydro-3aH-benzo[g][1]benzofuran-2-one | 66 |
| 34 | 20.760 | 0.20 | 2-Butanone, 4-(2,6,6-trimethyl-2-cyclohexen-1-ylidene)- | 80 |
| 35 | 20.838 | 0.11 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 91 |
| 36 | 20.929 | 0.44 | Benzene, hexaethyl- | 89 |
| 37 | 20.990 | 0.33 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.alpha.,5.alpha | 43 |
| 38 | 21.182 | 0.36 | 9,12-Octadecadienoic acid (Z,Z)- | 95 |
| 39 | 21.235 | 1.55 | 4(1H)-Quinolinone,7-amino-3-carboxy-6-fluoro-1-ethyl | 44 |
| 40 | 21.460 | 0.90 | Linoleic acid ethyl ester | 99 |
| 41 | 21.515 | 0.67 | (E)-9-Octadecenoic acid ethyl ester | 95 |
| 42 | 21.707 | 0.18 | 3,6-Nonadien-5-one, 2,2,8,8-tetramethyl | 46 |
| 43 | 21.778 | 0.25 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 27 |
| 44 | 22.024 | 33.57 | α-Santonin | 99 |
| 45 | 22.123 | 1.22 | N-[1-Ethylpyridinylidene]-1,3,2-dioxaphospholan-2-amine 2-sulfide | 42 |
| 46 | 22.260 | 3.11 | Phenanthrene, 3,6-dimethoxy-9,10-dimethyl | 59 |
| 47 | 22.305 | 2.30 | Alantolactone, 4.alpha.,4A.alpha.-epoxy | 64 |
| 48 | 22.615 | 0.30 | (-)-Neoclovene-(II), dihydro | 90 |
| 49 | 22.668 | 0.34 | Verrucarol | 62 |
| 50 | 22.941 | 0.31 | Oxazepam, 2TMS derivative | 56 |
| 51 | 23.039 | 0.23 | 2H-3,9a-Methano-1-benzoxepin, octahydro-2,2,5a,9-tetramethyl-, [3R-(3.alpha.,5a.alpha.,9.alpha.,9a.alpha.)]- | 56 |
| 52 | 23.203 | 0.13 | Spiro[2.5]octane, 5,5-dimethyl-4-(3-oxobutyl)- | 30 |
| 53 | 23.351 | 0.17 | Ethanone, 1-(1,2,3,4,7,7a-hexahydro-1,4,4,5-tetramethyl-1,3a-ethano-3aH-inden-6-yl)- | 47 |
| 54 | 23.545 | 0.26 | Benzothiophene-3-carboxylic acid, 4,5,6,7-tetrahydro-2-amino-6-ethyl-, ethyl ester | 38 |
| 55 | 23.850 | 0.11 | 2-Pyridinamine, N-(4,5-dihydro-5-methyl-2-thiazolyl)-3-methyl | 47 |
| 56 | 24.383 | 0.19 | Bis(2-ethylhexyl) phthalate | 68 |
| 57 | 24.563 | 0.19 | 2-N,2-N,4-N,4-N,8-Pentamethylquinoline-2,4,5-triamine | 70 |
| 58 | 24.963 | 43.93 | Bis(2-ethylhexyl) phthalate | 90 |
| 59 | 27.919 | 0.09 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 94 |
| S. No. | Peak no. | Compound Name | Mol. Weightg/mol | Mol. Formula | Structure |
|---|---|---|---|---|---|
| 1 | 2 | o-Cymene | 134.2182 | C10H14 | 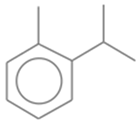 |
| 2 | 4 | Cyclohexasiloxane, dodecamethyl- | 444.9236 | C12H36O6Si6 | 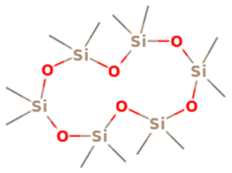 |
| 3 | 7 | Cycloheptasiloxane, tetradecamethyl- | 519.0776 | C14H42O7Si7 | 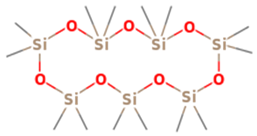 |
| 4 | 9 | Pentacosane | 352.6804 | C25H52 | 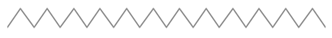 |
| 5 | 11 | 2,4-Di-tert-butylphenol | 206.3239 | C14H22O | 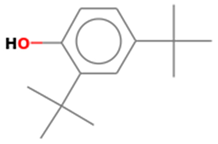 |
| 6 | 12 | Cyclooctasiloxane, hexadecamethyl- | 593.2315 | C16H48O8Si8 | 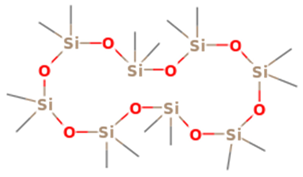 |
| 7 | 15 | 2-Propenal, 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 178.2707 | C12H18O | 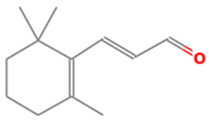 |
| 8 | 16 | 2-Hexen-1-ol, 2-ethyl | 128.2120 | C8H16O |  |
| 9 | 19 | Cyclononasiloxane, octadecamethyl | 667.3855 | C18H54O9Si9 | 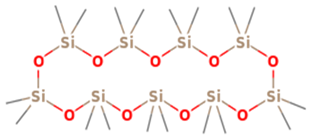 |
| 10 | 23 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 276.3707 | C17H24O3 | 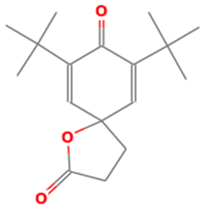 |
| 11 | 24 | Cyclodecasiloxane, eicosamethyl- | 741.5394 | C20H60O10Si10 | 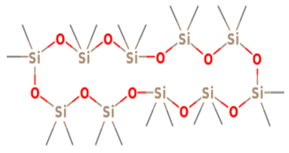 |
| 12 | 25 | Hexadecanoic acid, methyl ester | 270.4507 | C17H34O2 | 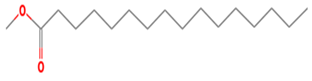 |
| 13 | 27 | n-Hexadecanoic acid | 256.4241 | C16H32O2 |  |
| 14 | 28 | Hexadecanoic acid, ethyl ester | 284.4772 | C18H36O2 | 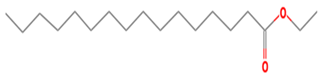 |
| 15 | 29 | (3aR,4R,7R)-1,4,9,9-Tetramethyl-3,4,5,6,7,8-hexahydro-2H-3a,7-methanoazulen-2-one | 218.3346 | C15H22O |  |
| 16 | 32 | Benzene, 1,2,4,5-tetrakis(1-methylethyl)- | 246.4308 | C18H30 | 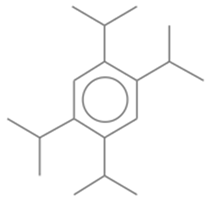 |
| 17 | 35 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 294.48 | C19H34O2 |  |
| 18 | 36 | Benzene, hexaethyl- | 246.4308 | C18H30 | 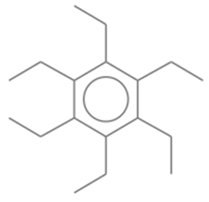 |
| 19 | 38 | 9,12-Octadecadienoic acid (Z,Z)- | 280.4455 | C18H32O2 | 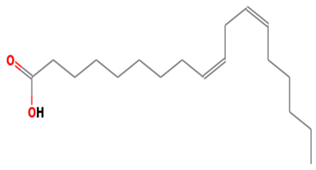 |
| 20 | 40 | Linoleic acid ethyl ester | 308.4986 | C20H36O2 | 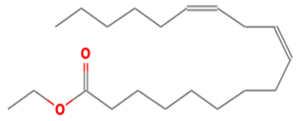 |
| 21 | 41 | (E)-9-Octadecenoic acid ethyl ester | 310.5145 | C20H38O2 |  |
| 22 | 44 | α-Santonin | 246.3016 | C15H18O3 |  |
| 23 | 58 | Bis(2-ethylhexyl) phthalate | 390.5561 | C24H38O4 |  |
References
- Temml, V.; Schuster, D. Molecular docking for natural product investigations: Pitfalls and ways to overcome them. In Molecular Docking for Computer-Aided Drug Design; Elsevier: Amsterdam, The Netherlands, 2021; pp. 391–405. [Google Scholar]
- Singh, H.; Bharadvaja, N. Treasuring the computational approach in medicinal plant research. Prog. Biophys. Mol. Biol. 2021, 164, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elbadry, A.M.M.; Doghish, A.S.; El-Nashar, H.A.S. Unveiling the pharmacological potential of plant triterpenoids in breast cancer management: An updated review. Naunyn Schmiedebergs Arch. Pharmacology 2024, 397, 5571–5596. [Google Scholar]
- Askar, M.A.; El-Nashar, H.A.; Al-Azzawi, M.A.; Rahman, S.S.A.; Elshawi, O.E. Synergistic Effect of Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of Breast Cancer. Breast Cancer 2022, 16, 11782234221086728. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Adel, M.; El-Shazly, M.; Yahia, I.S.; El Sheshtawy, H.S.; Almalki, A.A.; Ibrahim, N. Chemical Composition, Antiaging Activities and Molecular Docking Studies of Essential Oils from Acca sellowiana (Feijoa). Chem. Biodivers 2022, 19, e202200272. [Google Scholar]
- Rahman, M.M.; Islam, M.R.; Rahman, F.; Rahaman, M.S.; Khan, M.S.; Abrar, S.; Ray, T.K.; Uddin, M.B.; Kali, M.S.K.; Dua, K. Emerging promise of computational techniques in anti-cancer research: At a glance. Bioengineering 2022, 9, 335. [Google Scholar] [CrossRef]
- Saeed, K.; Rahkama, V.; Eldfors, S.; Bychkov, D.; Mpindi, J.P.; Yadav, B.; Paavolainen, L.; Aittokallio, T.; Heckman, C.; Wennerberg, K. Comprehensive drug testing of patient-derived conditionally reprogrammed cells from castration-resistant prostate cancer. Eur. Urol. 2017, 71, 319–327. [Google Scholar] [CrossRef]
- Bappi, M.H.; Mia, M.N.; Ansari, S.A.; Ansari, I.A.; Prottay, A.A.S.; Akbor, M.S.; El-Nashar, H.A.S.; El-Shazly, M.; Mubarak, M.S.; Torequl Islam, M. Quercetin increases the antidepressant-like effects of sclareol and antagonizes diazepam in thiopental sodium-induced sleeping mice: A possible GABAergic transmission intervention. Phytother. Res. 2024, 38, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Asif, R.; Ali, M.; Mazhar, M.; Mustafa, M.; Yasmin, R. Evaluation of Phytochemical Contents, Antimicrobial, and antioxidant Potential of Haloxylon Griffithii Collected From Northern Region of Balochistan, Pakistan. Dose-Response 2023, 21, 15593258231163389. [Google Scholar] [CrossRef]
- Kamal, S.; Bibi, I.; Rehman, K.; Zahoor, A.F.; Kamal, A.; Aslam, F.; Alasmary, F.A.; Almutairi, T.M.; Alhajri, H.M.; Alissa, S.A. Biological activities of in-house developed Haloxylon griffithii plant extract formulations. Plants 2021, 10, 1427. [Google Scholar] [CrossRef]
- Mengal, A.; Samiullah, N.K.; Baqi, A. Antibacterial and antifungal activities of extracts of Convolvulus leiocalycinus and Haloxylon griffithii of Balochistan, Pakistan. Pure Appl. Bio. 2019, 8, 2286–2294. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Al-Azzawi, M.A.; Al-Kazzaz, H.H.; Alghanimi, Y.K.; Kocaebli, S.M.; Alhmammi, M.; Asad, A.; Salam, T.; El-Shazly, M.; Ali, M.A.M. HPLC-ESI/MS-MS metabolic profiling of white pitaya fruit and cytotoxic potential against cervical cancer: Comparative studies, synergistic effects, and molecular mechanistic approaches. J. Pharm. Biomed. Anal. 2024, 244, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Gouranmohit, G.; Naimi-Jamal, M.R.; Neysani, E.; El-Nashar, H.A.S.; El-Shazly, M.; Khoshnevisan, K. The potential role of Hypericum perforatum in wound healing: A literature review on the phytochemicals, pharmacological approaches, and mechanistic perspectives. Phytother. Res. 2024, 38, 3271–3295. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, M.P.Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological survey on medicinal plants used in herbal drinks among the traditional communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Eldahshan, O.A.; Fattah, N.F.A.; Loutfy, S.A.; Abdel-Salam, I.M. HPLC-ESI/MS-MS characterization of compounds in Dolomiaea costus extract and evaluation of cytotoxic and antiviral properties: Molecular mechanisms underlying apoptosis-inducing effect on breast cancer. BMC Complement. Med. Ther. 2023, 23, 354. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell 2018, 34, 186–195. [Google Scholar] [CrossRef]
- Hochman, J.H. Adapting ADME and pharmacokinetic analysis to the next generation of therapeutic modalities. J. Pharm. Sci. 2021, 110, 35–41. [Google Scholar] [CrossRef]
- Saqib, F.; Janbaz, K.H. Ethnopharmacological basis for folkloric claims of Anagallis arvensis Linn. (Scarlet Pimpernel) as prokinetic, spasmolytic and hypotensive in province of Punjab, Pakistan. J. Ethnopharmacol. 2021, 267, 113634. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M. Past, present and future of Carotenoids Research. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 827–854. [Google Scholar]
- Gilani, A.H.; Khan, A.-U.; Ghayur, M.N. Ca2+ antagonist and cholinergic activities explain the medicinal use of olive in gut disorders. Nutr. Res. 2006, 26, 277–283. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Ahmedah, H.T.; Gavris, C.M.; De Feo, V.; Hogea, M.; Moga, M.; Chicea, R. Cucumis sativus L. seeds ameliorate muscular spasm-induced gastrointestinal and respiratory disorders by simultaneously inhibiting calcium mediated signaling pathway. Pharmaceuticals 2021, 14, 1197. [Google Scholar] [CrossRef]
- Iqbal, I.; Saqib, F.; Latif, M.F.; Shahzad, H.; Dima, L.; Sajer, B.; Manea, R.; Pojala, C.; Necula, R. Pharmacological Basis for Antispasmodic, Bronchodilator, and Antidiarrheal Potential of Dryopteris ramosa (Hope) C. via In Vitro, In Vivo, and In Silico Studies. ACS Omega 2023, 8, 26982–27001. [Google Scholar] [CrossRef] [PubMed]
- Saqib, F.; Janbaz, K.H. Rationalizing ethnopharmacological uses of Alternanthera sessilis: A folk medicinal plant of Pakistan to manage diarrhea, asthma and hypertension. J. Ethnopharm. 2016, 182, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Wilairatana, P.; Mubarak, M.S. Possible mechanisms underlying the antispasmodic, bronchodilator, and antidiarrheal activities of polarity–based extracts of cucumis sativus L. Seeds in in silico, in vitro, and in vivo studies. Pharmaceuticals 2022, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Ghayur, M.N.; Saify, Z.S.; Ahmed, S.P.; Choudhary, M.I.; Khalid, A. Presence of cholinomimetic and acetylcholinesterase inhibitory constituents in betel nut. Life Sci. 2004, 75, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Ali, A. Calcium Channel Inhibitory Effect of Marjoram (Origanum majorana L.): Its Med. Use Diarrhea Gut Hyperactivity. Front. Biosci. 2024, 29, 47. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Gould, R.J.; Snyder, S.H. Loperamide: Blockade of calcium channels as a mechanism for antidiarrheal effects. J. Pharmacol. Exper. Therap. 1984, 231, 628–632. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Zhang, Y.; Huang, Z.; Yan, W.; Zhou, T.; Wang, Z.; Liao, L.; Cao, H.; Tan, B. Therapeutic effects of berberine hydrochloride on stress-induced diarrhea-predominant irritable bowel syndrome rats by inhibiting neurotransmission in colonic smooth muscle. Front. Pharmacol. 2021, 12, 596686. [Google Scholar] [CrossRef]
- Na’Allah, A.; Bashir, S.; Alabi, M.; Kareem, F.A.; Olaifa, G.O.; Afolabi, F.J.; Aransiola, S.A.; Seth, C.S.; Maddela, N.R.; Prasad, R. Anti-diarrhoea properties of ethanol extract of Citrullus colocynthis fruit pulp: In vivo and in silico studies. Adv. Tradit. Med. 2025, 25, 269–285. [Google Scholar] [CrossRef]
- Woldeyohannes, M.G.; Eshete, G.T.; Abiye, A.A.; Hailu, A.E.; Huluka, S.A.; Tadesse, W.T. Antidiarrheal and antisecretory effect of 80% hydromethanolic leaf extract of Moringa stenopetala baker f. in mice. Biochem. Res. Int. 2022, 2022, 5768805. [Google Scholar] [CrossRef]
- Gilani, A.U.H.; Shah, A.J.; Ahmad, M.; Shaheen, F. Antispasmodic effect of Acorus calamus Linn. is mediated through calcium channel blockade. Phytother. Res. 2006, 20, 1080–1084. [Google Scholar] [CrossRef]
- Munir Ahmad, M.A.; Attiq-ur-Rehman, A.-u.-R.; Samiullah, S.; Rasool Bakhsh Tareen, R.B.T.; Naqeebullah Khan, N.K.; Abdul Baqi, A.B.; Abdul Manan, A.M. Qualitative and quantitative determination of phytochemicals in Convolvulus leiocalycinus and Haloxylon griffithii. Pure Appl. Bio. 2019, 8, 733–741. [Google Scholar]
- Amtaghri, S.; Eddouks, M. Comprehensive Review on the Genus Haloxylon: Pharmacological and Phytochemical Properties. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; He, Y.; Zhang, L.; Liu, L.; Qu, C.; Zheng, Z.; Miao, J. Conjugated linoleic acid ameliorates hepatic steatosis by modulating intestinal permeability and gut microbiota in ob/ob mice. Food Nutr. Res. 2022, 66, 29210–29219. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.N.; Majinda, R.R.T. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Agha, N.F.S.; Aziz, P.Y.; Thabit, M.A.; Agha, D.N.F. Gastroprophylactic effects of p-cymene in ethanol-induced gastric ulcer in rats. Processeses 2022, 10, 1314. [Google Scholar] [CrossRef]
- Micucci, M.; Protti, M.; Aldini, R.; Frosini, M.; Corazza, I.; Marzetti, C.; Mattioli, L.B.; Tocci, G.; Chiarini, A.; Mercolini, L. Thymus vulgaris L. essential oil solid formulation: Chem. Profile Spasmolytic Antimicrob. effects. Biomolecules 2020, 10, 860. [Google Scholar] [CrossRef]
- Ammar, S.; Edziri, H.; Mahjoub, M.A.; Chatter, R.; Bouraoui, A.; Mighri, Z. Spasmolytic and anti-inflammatory effects of constituents from Hertia cheirifolia. Phytomedicine 2009, 16, 1156–1161. [Google Scholar] [CrossRef]
- Council, N. Committee for the Update of the Guide for the, C. & Use of Laboratory, A. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Vijh, D.; Gupta, P. GC-MS analysis, molecular docking, and pharmacokinetic studies on Dalbergia sissoo barks extracts for compounds with anti-diabetic potential. Sci. Rep. 2024, 14, 24936. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Zaeem Ahsan, M.; Saqib, F.; Imran, I.; Zia-Ul-Haq, M.; Abid Rashid, M.; Jaafar, H.Z.; Moga, M. Scientific basis for use of Pyrus pashia Buch.-Ham. ex D. Don. fruit in gastrointestinal, respiratory and cardiovascular ailments. PLoS ONE 2015, 10, e0118605. [Google Scholar] [CrossRef] [PubMed]
- Saqib, F.; Usman, F.; Malik, S.; Bano, N.; Ur-Rahman, N.; Riaz, M.; Mureşan, C.C. Antidiarrheal and Cardio-Depressant Effects of Himalaiella heteromalla (D. Don) Raab-Straube: In Vitro, In Vivo, and In Silico Studies. Plants 2022, 11, 78. [Google Scholar] [CrossRef]
- Zarei, M.; Mohammadi, S.; Komaki, A. Antinociceptive activity of Inula britannica L. and patuletin: Vivo Possible Mech. studies. J. Ethnopharmacol. 2018, 219, 351–358. [Google Scholar] [CrossRef] [PubMed]
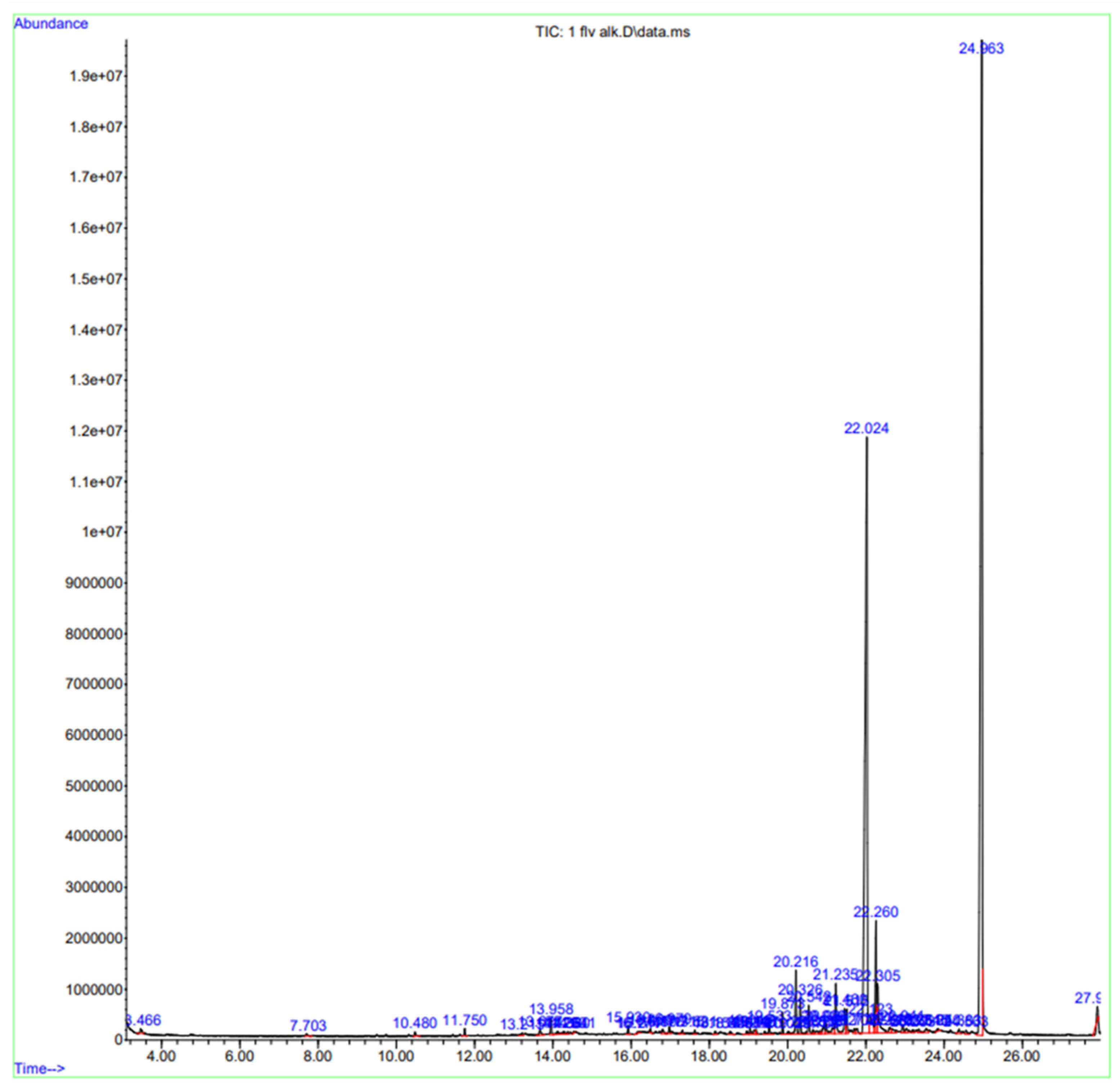


| Sr. No. | Peak no. | RT | % Area | Compound Name | Mol. Weight g/mol | Mol. Formula |
|---|---|---|---|---|---|---|
| 1 | 2 | 7.703 | 0.10 | o-Cymene | 134.2182 | C10H14 |
| 2 | 4 | 11.750 | 0.18 | Cyclohexasiloxane, dodecamethyl- | 444.9236 | C12H36O6Si6 |
| 3 | 7 | 13.958 | 0.41 | Cycloheptasiloxane, tetradecamethyl- | 519.0776 | C14H42O7Si7 |
| 4 | 9 | 14.29 | 0.15 | Pentacosane | 352.6804 | C25H52 |
| 5 | 11 | 14.541 | 0.04 | 2,4-Di-tert-butylphenol | 206.3239 | C14H22O |
| 6 | 12 | 15.930 | 0.25 | Cyclooctasiloxane, hexadecamethyl- | 593.2315 | C16H48O8Si8 |
| 7 | 15 | 16.499 | 0.11 | 2-Propenal, 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 178.2707 | C12H18O |
| 8 | 16 | 16.808 | 0.17 | 2-Hexen-1-ol, 2-ethyl | 128.2120 | C8H16O |
| 9 | 19 | 17.631 | 0.11 | Cyclononasiloxane, octadecamethyl | 667.3855 | C18H54O9Si9 |
| 10 | 23 | 19.045 | 0.15 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 276.3707 | C17H24O3 |
| 11 | 24 | 19.146 | 0.18 | Cyclodecasiloxane, eicosamethyl- | 741.5394 | C20H60O10Si10 |
| 12 | 25 | 19.197 | 0.13 | Hexadecanoic acid, methyl ester | 270.4507 | C17H34O2 |
| 13 | 27 | 19.533 | 0.34 | n-Hexadecanoic acid | 256.4241 | C16H32O2 |
| 14 | 28 | 19.873 | 0.48 | Hexadecanoic acid, ethyl ester | 284.4772 | C18H36O2 |
| 15 | 29 | 20.019 | 0.13 | (3aR,4R,7R)-1,4,9,9-Tetramethyl-3,4,5,6,7,8-hexahydro-2H-3a,7-methanoazulen-2-one | 218.3346 | C15H22O |
| 16 | 32 | 20.542 | 0.82 | Benzene, 1,2,4,5-tetrakis(1-methylethyl)- | 246.4308 | C18H30 |
| 17 | 35 | 20.838 | 0.11 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 294.48 | C19H34O2 |
| 18 | 36 | 20.929 | 0.44 | Benzene, hexaethyl- | 246.4308 | C18H30 |
| 19 | 38 | 21.182 | 0.36 | 9,12-Octadecadienoic acid (Z,Z)- | 280.4455 | C18H32O2 |
| 20 | 40 | 21.460 | 0.90 | Linoleic acid ethyl ester | 308.4986 | C20H36O2 |
| 21 | 41 | 21.515 | 0.67 | (E)-9-Octadecenoic acid ethyl ester | 310.5145 | C20H38O2 |
| 22 | 44 | 22.024 | 33.57 | α-Santonin | 246.3016 | C15H18O3 |
| 23 | 58 | 24.963 | 43.93 | Bis(2-ethylhexyl) phthalate | 390.5561 | C24H38O4 |
| Molecule | Verapamil | Alpha Santonin | O-Cymene | Hexadecanoic Acid, Ethyl Ester | Hexadecanoic Acid, Methyl Ester | n-Hexadecanoic Acid | (E)-9-Octadecenoic Acid Ethyl Ester | Bis(2-ethylhexyl) Phthalate | Benzene, Hexaethyl- | Cyclodecasiloxane, Eicosamethyl- | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic properties | Formula | C27H38N2O4 | C15H18O3 | C10H14 | C18H36O2 | C17H34O2 | C16H32O2 | C20H38O2 | C24H38O4 | C18H30 | C20H60O10Si10 |
| MW (g/mol) | 454.6 | 246.3 | 134.22 | 284.48 | 270.45 | 256.42 | 310.51 | 390.56 | 246.43 | 741.54 | |
| iLOGP | 4.5 | 2.25 | 2.43 | 4.65 | 4.41 | 3.85 | 5.03 | 4.77 | 3.85 | 6.55 | |
| XLOGP3 | 3.79 | 2.29 | 4.38 | 7.88 | 7.38 | 7.17 | 8.03 | 7.45 | 6.7 | 10.06 | |
| WLOGP | 5.09 | 2.42 | 3.12 | 6.03 | 5.64 | 5.55 | 6.59 | 6.43 | 5.06 | 7.18 | |
| MLOGP | 2.96 | 2.38 | 4.47 | 4.67 | 4.44 | 4.19 | 5.03 | 5.24 | 6.57 | –2.43 | |
| Absorption | GI absorption | High | High | Low | High | High | High | Low | High | Low | Low |
| Pgp substrate | Yes | No | No | No | No | No | No | Yes | No | Yes | |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.85 | 0.55 | 0.55 | 0.55 | 0.55 | |
| Skin Permeability log Kp (cm/s) | –6.38 | –6.18 | –4.01 | –2.44 | –2.71 | –2.77 | –2.49 | –3.39 | –3.05 | –3.68 | |
| Distribution | BBB permeant | Yes | Yes | Yes | No | Yes | Yes | No | No | No | No |
| BBB permeability log BB | –0.647 | 0.347 | 0.48 | 0.759 | 0.749 | –0.111 | 0.786 | –0.175 | 0.741 | –2.905 | |
| VDss (human) log L/kg | 0.931 | 0.205 | 0.744 | 0.373 | 0.334 | –0.543 | 0.332 | 0.36 | 1.334 | –0.106 | |
| Metabolism | CYP1A2 inhibitor | No | No | No | Yes | Yes | Yes | Yes | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | Yes | No | Yes | No | No | |
| CYP2D6 inhibitor | Yes | No | Yes | No | No | No | No | No | No | No | |
| CYP3A4 inhibitor | Yes | No | No | No | No | No | No | Yes | No | No | |
| Excretion | Total Clearance logml/min/kg | 1.072 | 0.197 | 0.259 | 1.912 | 1.861 | 1.763 | 2.03 | 1.898 | 2.03 | −0.484 |
| Toxicity | Hepatotoxicity | No | No | No | No | No | No | No | No | Yes | No |
| Skin Sensitisation | No | No | Yes | Yes | Yes | Yes | yes | No | yes | No | |
| Drug Likeness | Lipinski #violations | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ghose #violations | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 4 | |
| Veber #violations | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| Egan #violations | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | |
| Muegge #violations | 0 | 0 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 |
| Groups | Dose | No. of Wet Feces | ||||
|---|---|---|---|---|---|---|
| 0–0.5 h | 0–1 h | 0–2 h | 0–3 h | 0–4 h | ||
| Group I (Negative control) | 10 mL/kg (NS) | 0.2 | 1.0 | 1.0 | 2.0 | 4.0 |
| Group II (Disease control) | 10 mL/kg (NS) | 5.0 | 11.0 | 18.0 | 29.0 | 32.0 |
| Group III (standard) | 10 mg/kg (Loperamide) | 0.4 | 2.0 | 3.0 | 6.0 | 7.0 |
| Group IV (standard) | 10 mg/kg (verapamil) | 2.0 | 3.0 | 4.0 | 4.8 | 5.0 |
| Group V (Treatment) | 100 mg/kg (Hg.Cr) | 5.4 | 11.0 | 18.0 | 19.6 | 21.0 |
| 0Group VI (Treatment) | 200 mg/kg (Hg.Cr) | 5.2 | 9.0 | 13.0 | 17.0 | 19.8 |
| Group VII (Treatment) | 400 mg/kg (Hg.Cr) | 3.0 | 7.0 | 8.0 | 8.6 | 10.0 |
| Groups | Dose | Total Number of Feces | %Age Protection |
|---|---|---|---|
| Group I (Negative control) | 10 mL/kg (NS) | 4.0 ± 0.45 | 87.5 |
| Group II (Disease control) | 10 mL/kg (NS) | 32.0 ± 1.05 | 00.0 |
| Group III (standard) | 10 mg/kg (Loperamide) | 7.0 ± 0.71 | 78.1 |
| Group IV (standard) | 10 mg/kg (Verapamil) | 5.0 ± 0.45 | 84.4 |
| Group V (Treatment) | 100 mg/kg (Hg.Cr) | 21.0 ± 0.63 | 34.4 |
| Group VI (Treatment) | 200 mg/kg (Hg.Cr) | 19.8 ± 0.86 | 38.1 |
| Group VII (Treatment) | 400 mg/kg (Hg.Cr) | 10.0 ± 0.95 | 68.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, I.; Ali, M.A.M.; Saqib, F.; Alamgir, K.; Mubarak, M.S.; Chaudhary, A.A.; El-Shazly, M.; El-Nashar, H.A.S. Comprehensive GC-MS Profiling and Multi-Modal Pharmacological Evaluations of Haloxylon griffithii: In Vitro and In Vivo Approaches. Pharmaceuticals 2025, 18, 770. https://doi.org/10.3390/ph18060770
Iqbal I, Ali MAM, Saqib F, Alamgir K, Mubarak MS, Chaudhary AA, El-Shazly M, El-Nashar HAS. Comprehensive GC-MS Profiling and Multi-Modal Pharmacological Evaluations of Haloxylon griffithii: In Vitro and In Vivo Approaches. Pharmaceuticals. 2025; 18(6):770. https://doi.org/10.3390/ph18060770
Chicago/Turabian StyleIqbal, Iram, Mohamed A. M. Ali, Fatima Saqib, Kinza Alamgir, Mohammad S. Mubarak, Anis Ahmad Chaudhary, Mohamed El-Shazly, and Heba A. S. El-Nashar. 2025. "Comprehensive GC-MS Profiling and Multi-Modal Pharmacological Evaluations of Haloxylon griffithii: In Vitro and In Vivo Approaches" Pharmaceuticals 18, no. 6: 770. https://doi.org/10.3390/ph18060770
APA StyleIqbal, I., Ali, M. A. M., Saqib, F., Alamgir, K., Mubarak, M. S., Chaudhary, A. A., El-Shazly, M., & El-Nashar, H. A. S. (2025). Comprehensive GC-MS Profiling and Multi-Modal Pharmacological Evaluations of Haloxylon griffithii: In Vitro and In Vivo Approaches. Pharmaceuticals, 18(6), 770. https://doi.org/10.3390/ph18060770








