Uncovering the Advantages of Foam Dressings with Active Ingredients
Abstract
1. Introduction
2. Results
2.1. Outcome Questionnaire Among Wound Care Conference Attendees
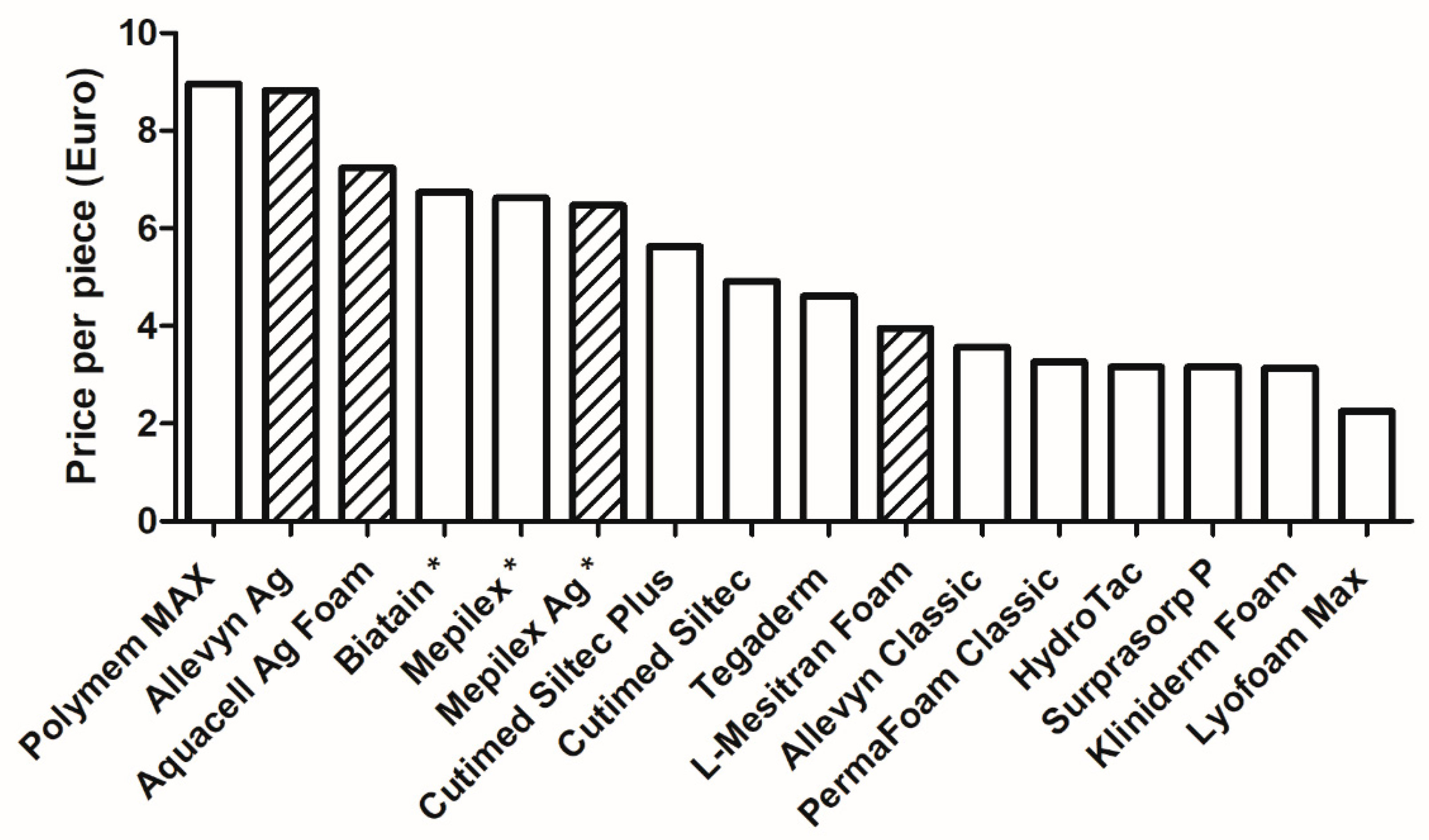
2.2. Prices per Foam Dressing
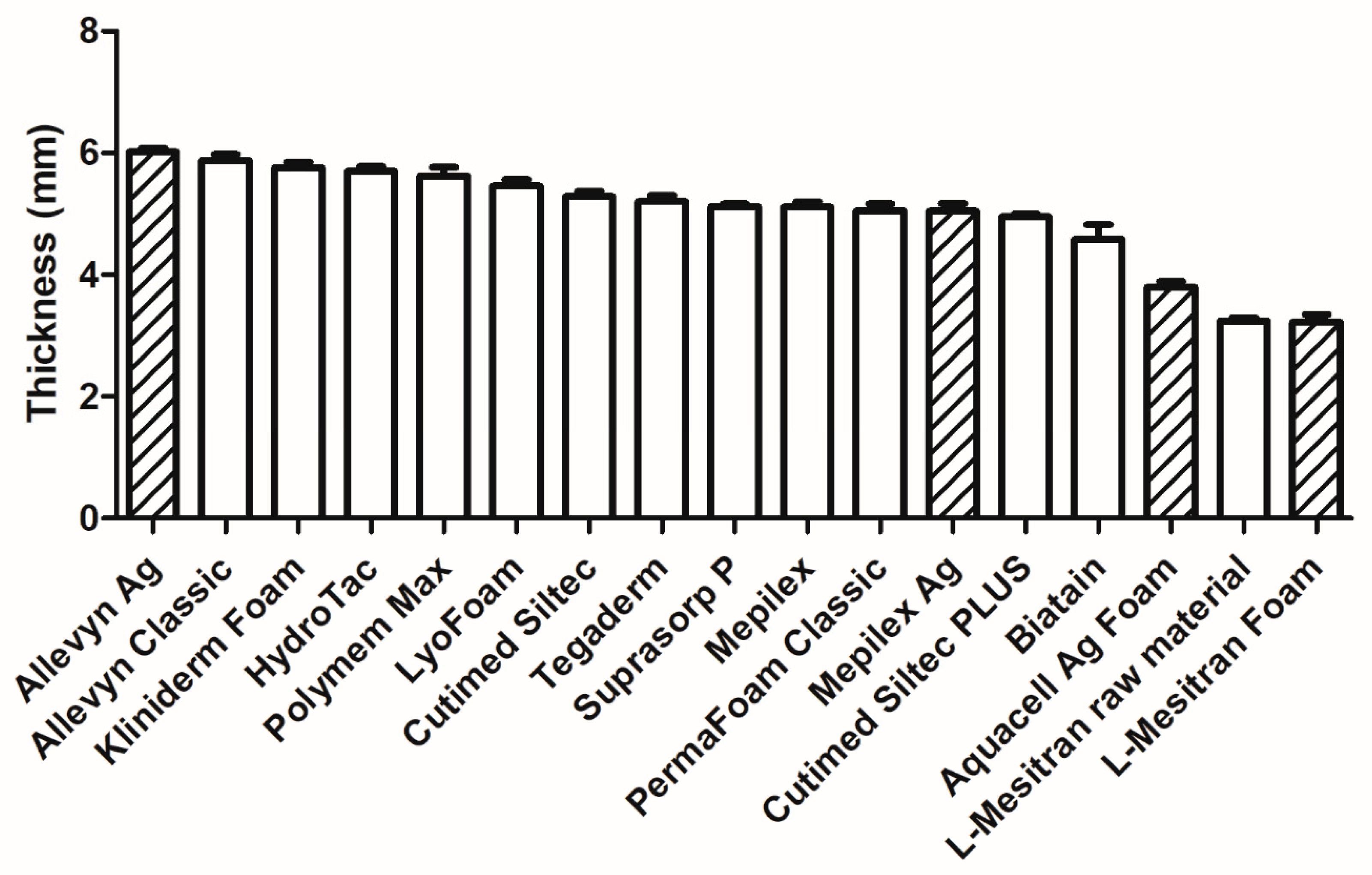
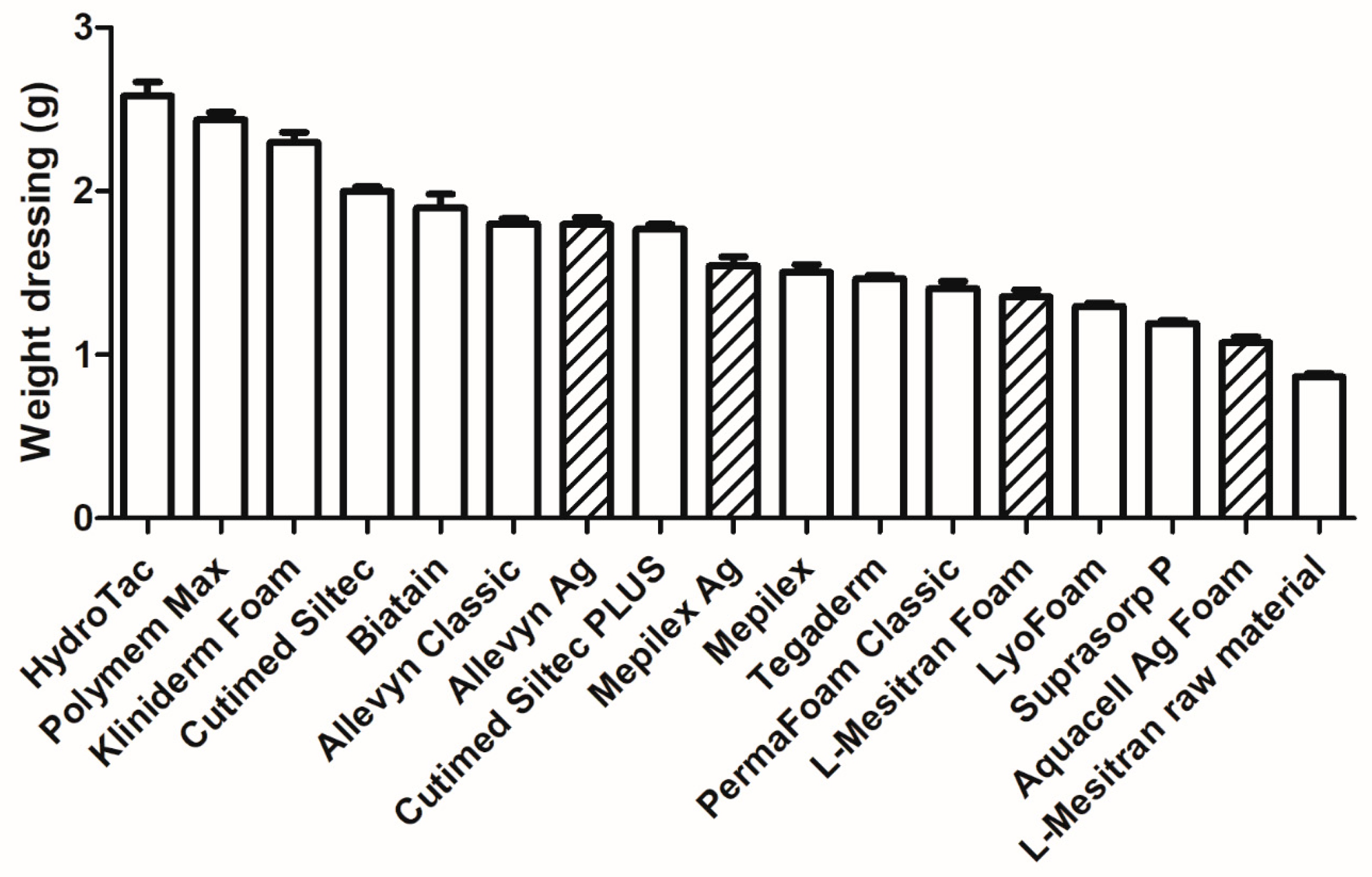
2.3. Thickness and Weight of the Dressings
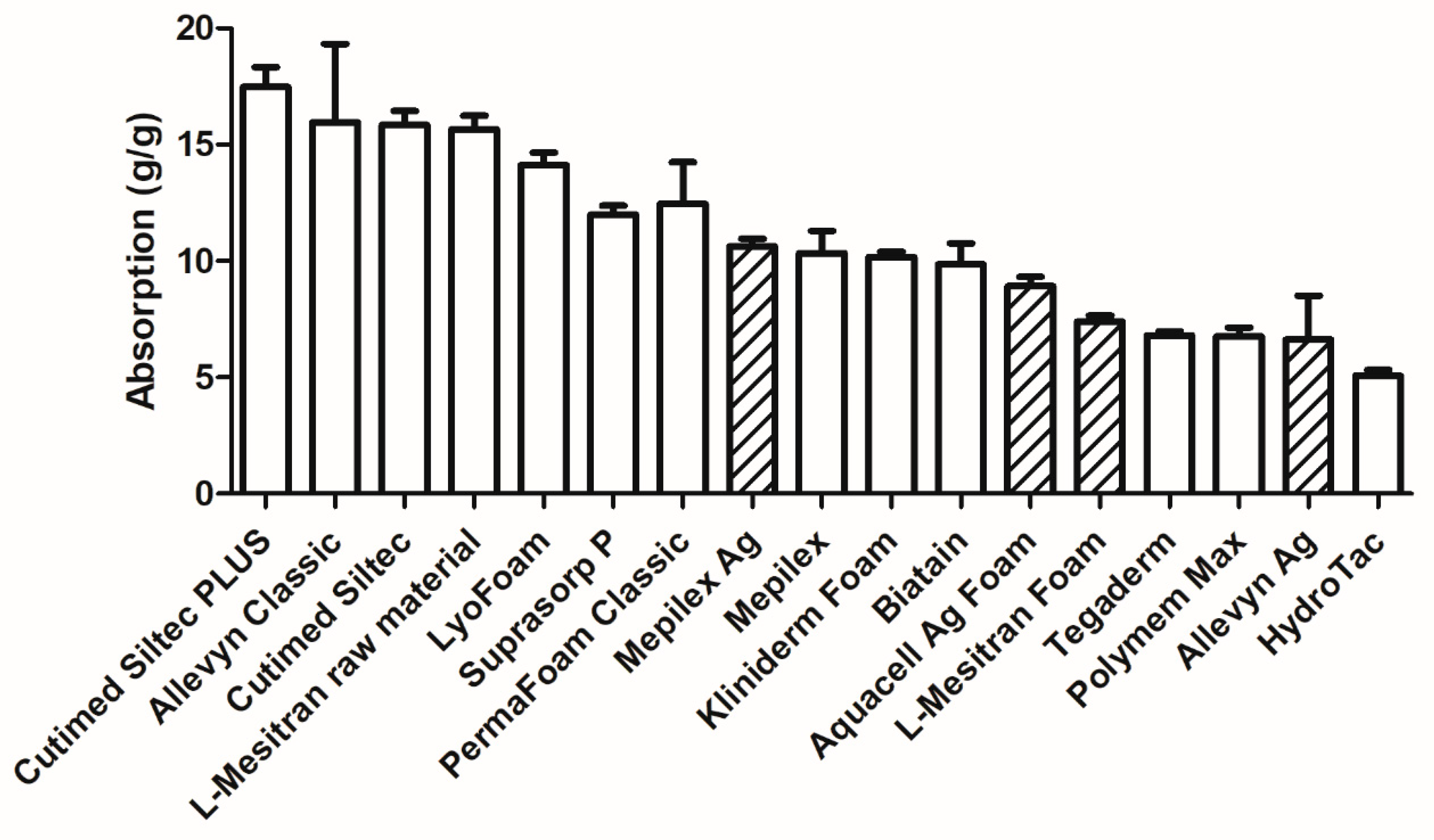
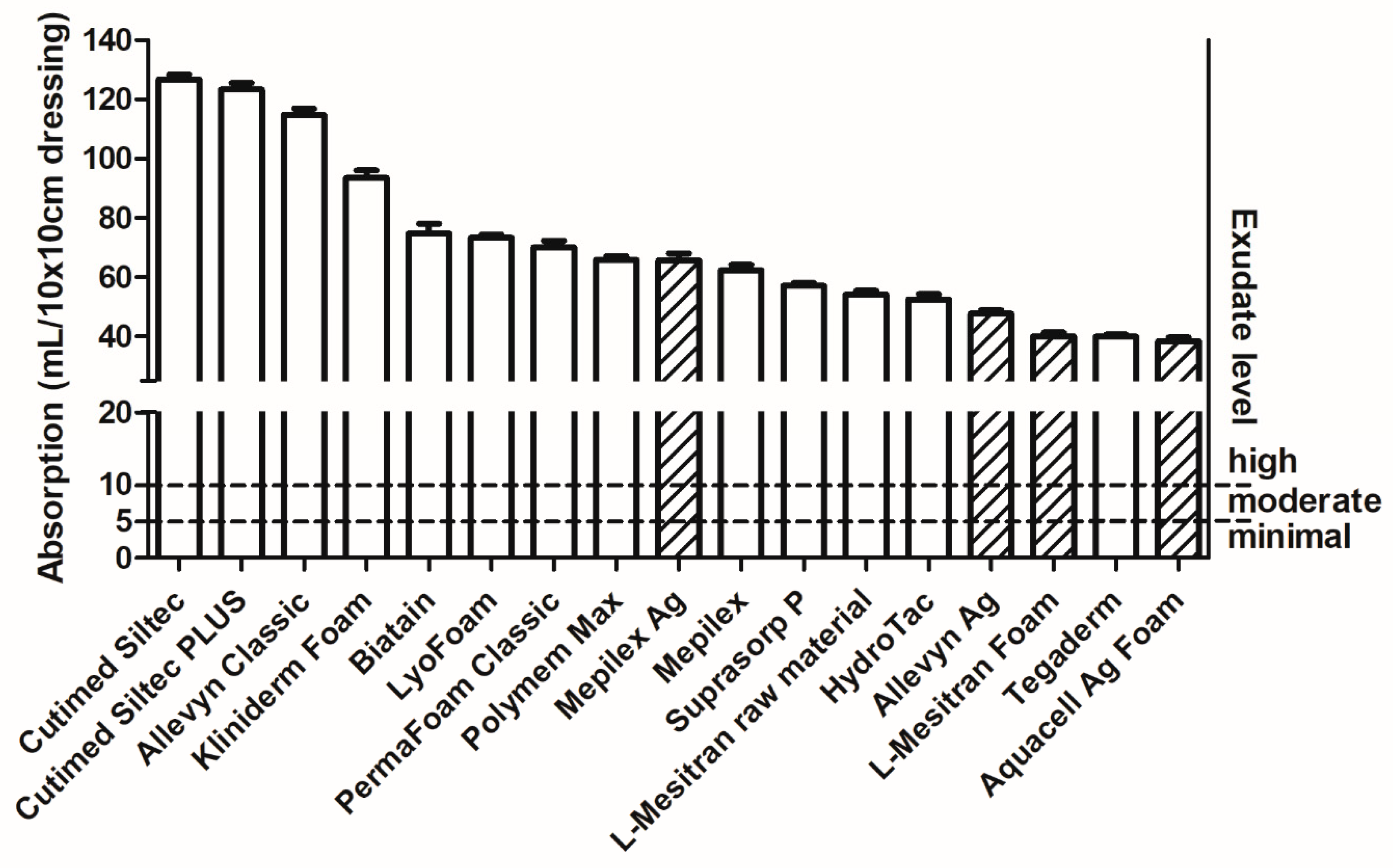
2.4. Absorption Capacity
2.5. Exudate Absorption Capacity of Foam Dressings
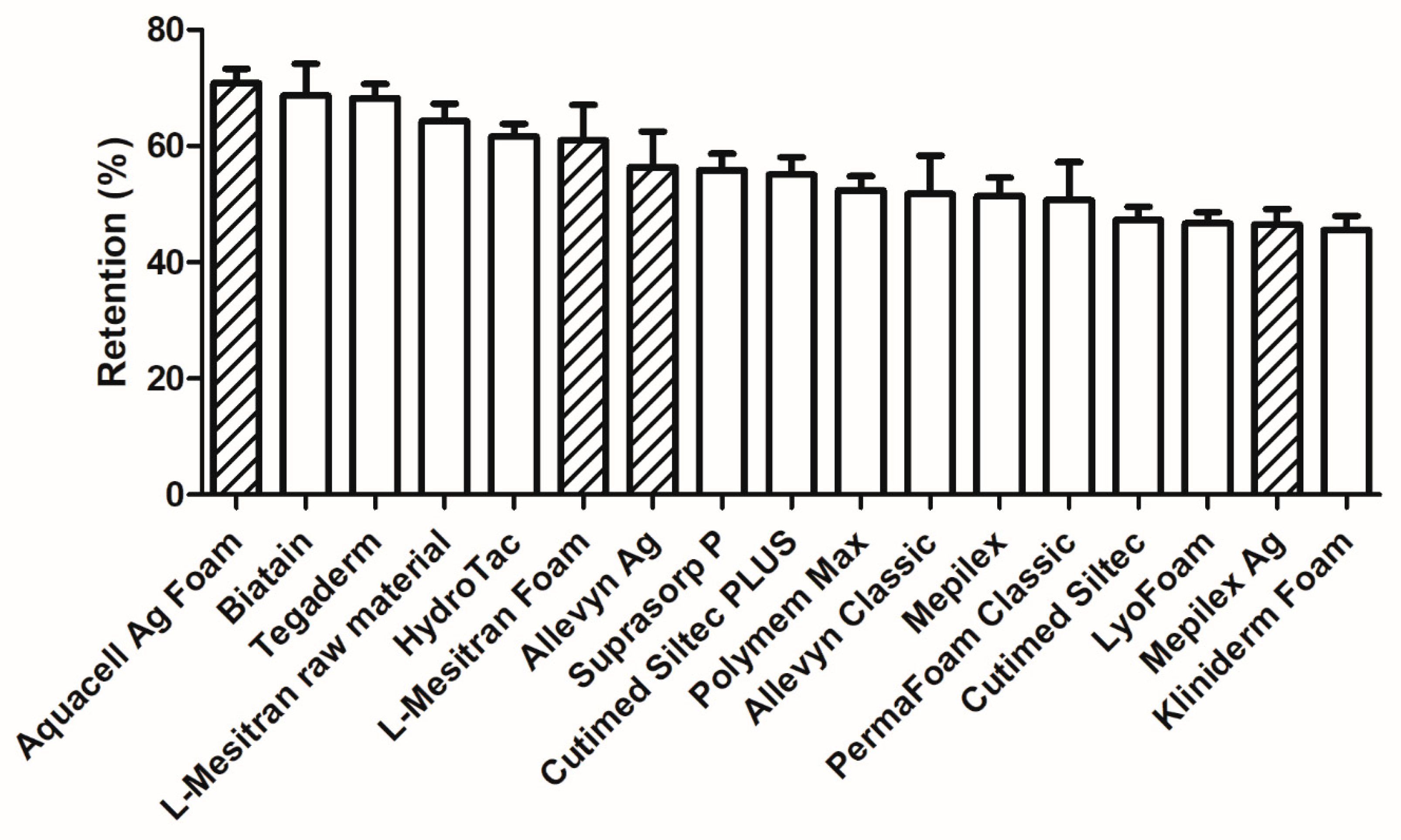
2.6. Retention of Fluid
2.7. Clinical Evaluation of L-Mesitran Foam by Wound Care Specialists
2.8. Clinical Cases Supporting the Cost-Effectiveness of L-Mesitran Foam
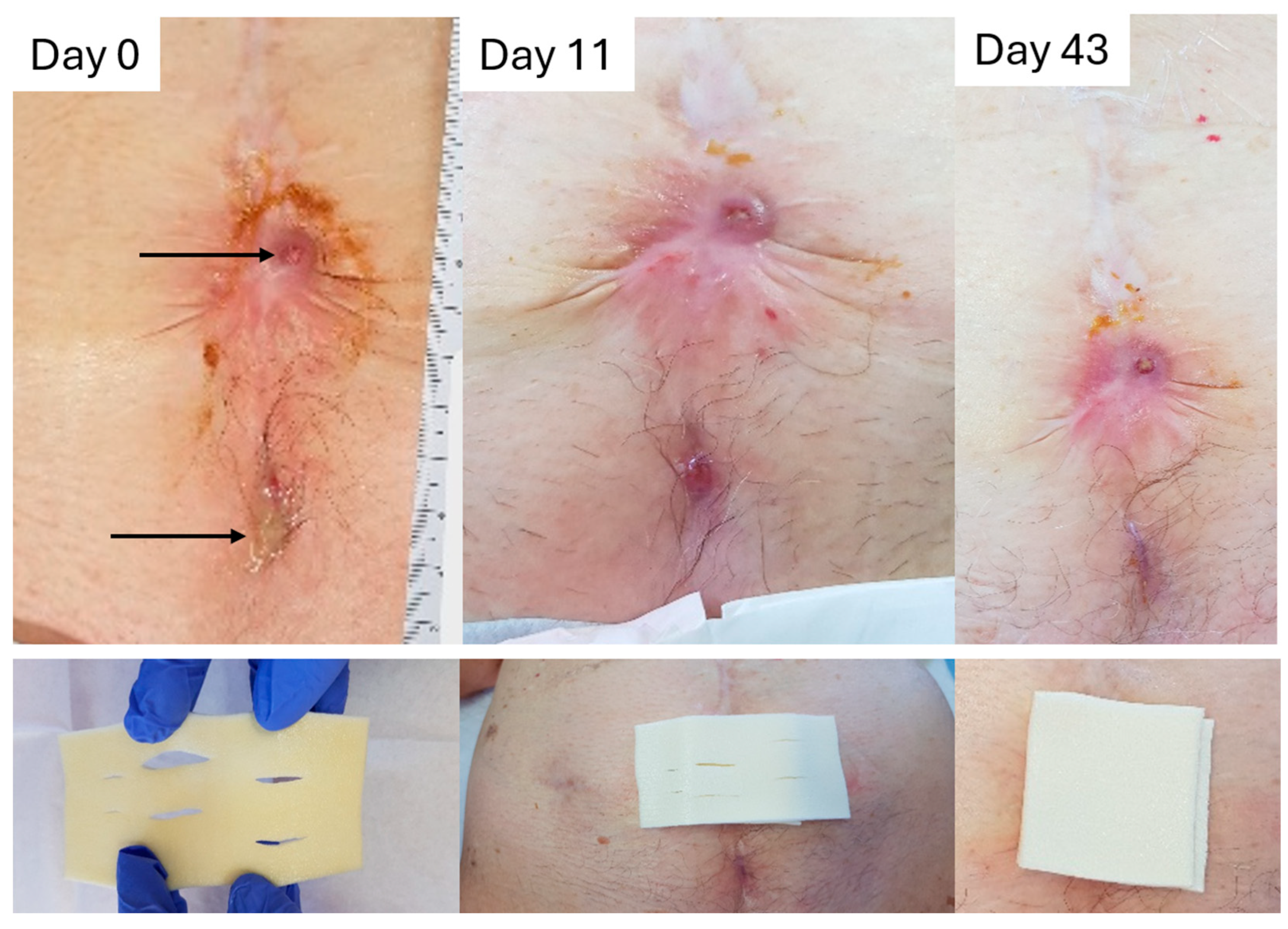
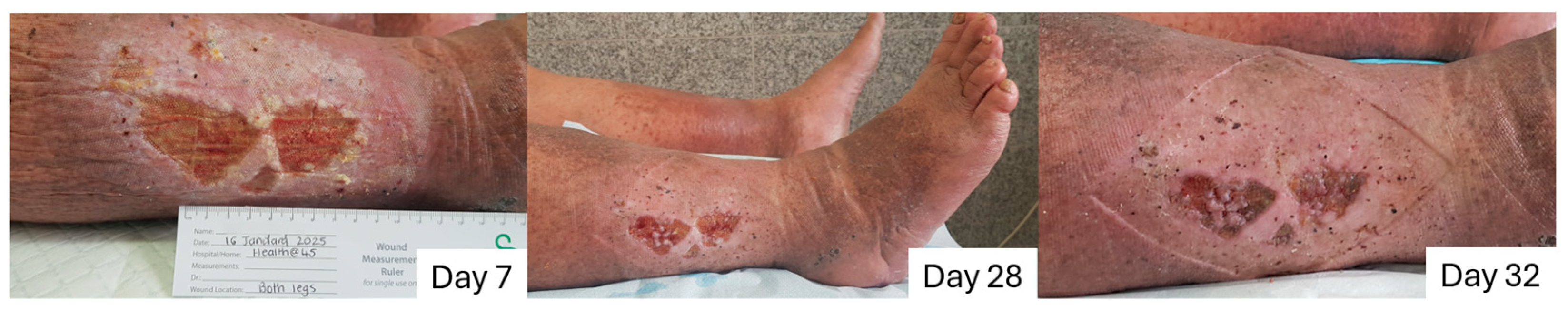
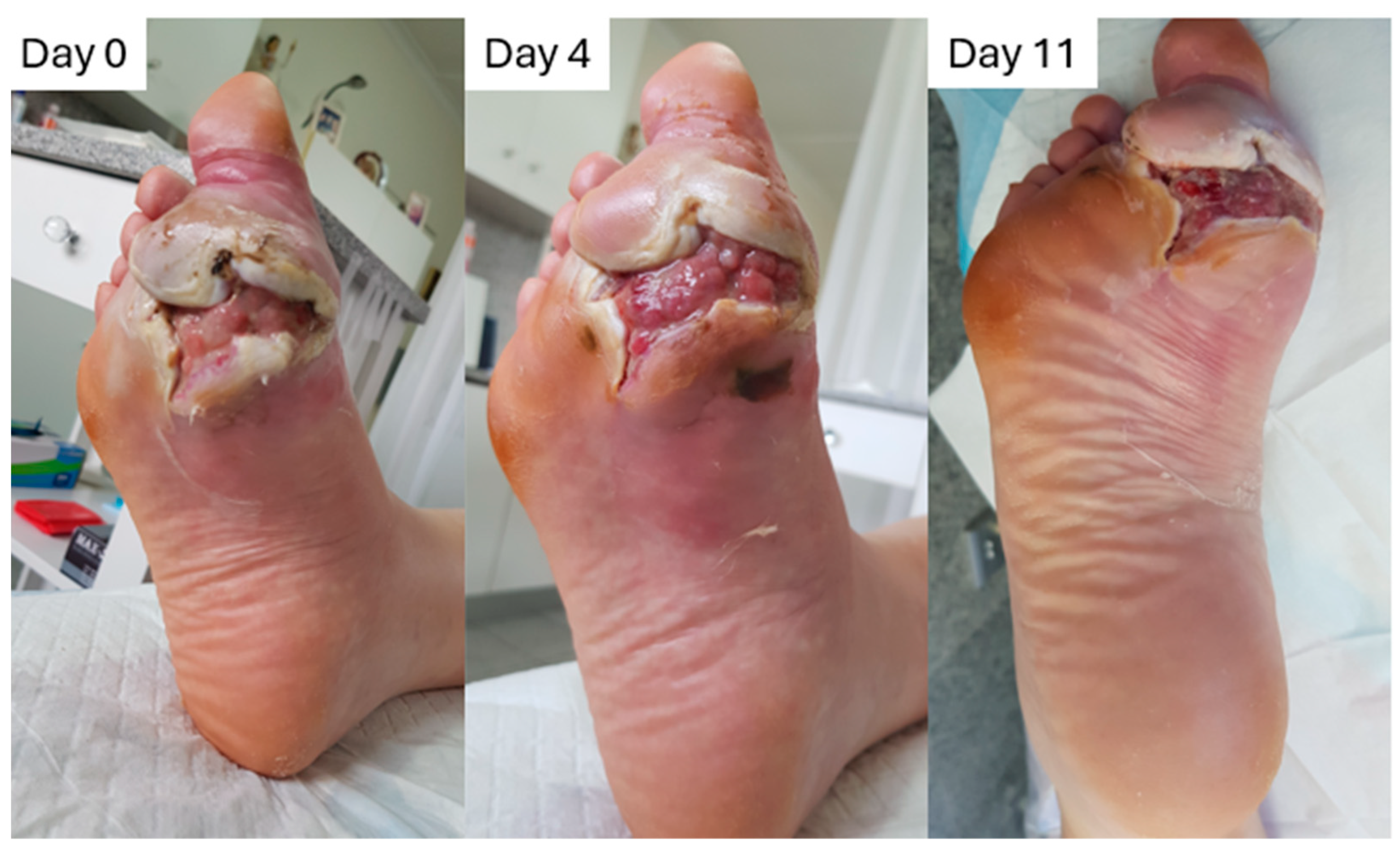
2.8.1. Case to Support the Absorption/Retention Capacity Is Enough for Heavily Exuding Wounds
2.8.2. Case to Support the Additional Benefit of Honey in Enhancing Debridement and Proliferation, and Why L-Mesitran Foam Is the First Choice to Combine with Compression Therapy
2.8.3. Case to Support the Additional Benefits of Honey in Resolving Infections and Protecting Against External Trauma
2.8.4. Case to Support the Dressing Can Stay in Place for 7 Days
3. Discussion
3.1. Clinical Relevance—Strengths and Limitations
3.2. Practical Considerations
3.3. Influence of Dressing Thickness and Patient Comfort
3.4. Cost-Effectiveness Considerations
3.5. Shortcomings and Future Directions
4. Materials and Methods
4.1. Questionnaire for Wound Care Specialists
4.2. Patients and Treatments
4.3. Materials
4.4. Laboratory Measurements to Assess the Physical Properties of the Dressings
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Hinman, C.D.; Maibach, H. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nature 1963, 200, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Swezey, L. Moist Wound healing. Wound Educators. 2014. Available online: https://woundeducators.com/wound-moisture-balance/ (accessed on 13 March 2025).
- Moore, Z.; Strapp, H. Managing the problem of excess exudate. Br. J. Nurs. 2015, 24, S14–S17. [Google Scholar] [CrossRef]
- Jones, M.L. An introduction to absorbent dressings. Br. J. Community Nurs. 2014, 19, S28–S30. [Google Scholar] [CrossRef]
- Dowsett, C. Moisture in wound healing: Exudate management. Br. J. Community Nurs. 2013, 16 (Suppl. S6), S6–S12. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS) Consensus Document. Wound exudate: Effective assessment and management Wounds International. 2019. Available online: https://woundsinternational.com/world-union-resources/wuwhs-consensus-document-wound-exudate-effective-assessment-and-management/ (accessed on 13 March 2025).
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Britto, E.J.; Nezwek, T.A.; Popowicz, P.; Robins, M. Wound Dressings. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- ConvaTec Aquacel Ag Foam Adhesive Dressing Product Information. Available online: https://medisa.com.au/products/wound-care-basic-dressings-first-aid-dressing-convatec-aquacel-ag-foam-adhesive-dressing-all-sizes (accessed on 13 March 2025).
- Choi, H.; Castillo, B.; Seminario-Vidal, L. Silver absorption in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis treated with silver-impregnated dressings. A case series. Int. Wound J. 2018, 15, 1049–1051. [Google Scholar] [CrossRef]
- Allevyn Ag Product Information. Available online: https://www.smith-nephew.com/nl-be/health-care-professionals/products/advanced-wound-management/allevyn-ag-range (accessed on 13 March 2025).
- Shih, T.; Park, S.; Thorlacius, L.R.; Daveluy, S.; Garg, A.; Goegji, S.D.; Kirby, J.S.; McGrath, B.M.; Riis, P.T.; Villumsen, B.; et al. Wound drainage measurements: A narrative review. Arch. Dermatol. Res. 2023, 315, 1863–1874. [Google Scholar] [CrossRef]
- Mulder, G.D. Quantifying wound fluids for the clinician and researcher. Ostomy Wound Manag. 1994, 40, 66–69. [Google Scholar]
- Dealey, C.; Cameron, J.; Arrowsmith, M. A study comparing two objective methods of quantifying the production of wound exudate. J. Wound Care 2006, 15, 149–153. [Google Scholar] [CrossRef]
- Cutting, K.F.; White, R.J. Avoidance and management of peri-wound maceration of the skin. Prof. Nurse 2002, 18, 35–36. [Google Scholar]
- Romanelli, M.; Vowden, K.; Weir, D. Exudate management made easy. Wounds Int. 2010, 1, 1–6. [Google Scholar]
- Dowsett, C.; Ayello, E. TIME principles of chronic wound bed preparation and treatment. Br. J. Nurs. 2004, 13, S16–S23. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Cullen, B.; Lazaro-Martinez, J.L.; Lev-Tov, H.; Santamaria, N.; Swanson, T.; Woo, K.; Soderstrom, B.; et al. Fluid handling by foam wound dressings: From engineering theory to advanced laboratory performance evaluations. Int. Wound J. 2024, 21, e14674. [Google Scholar] [CrossRef]
- Nygren, E.; Malone, M.; Gefen, A. Laboratory Evaluations of Wound Dressings: Key Advances to Reflect Clinical Reality. Int. Wound J. 2025, 22 (Suppl. S1). [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Wagener, F.; de Rooster, H.; Cremers, N.A.J. Revolutionizing non-conventional wound healing using honey by simultaneously targeting multiple molecular mechanisms. Drug Resist. Update 2022, 62, 100834. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Spear, M. Wound exudate--the good, the bad, and the ugly. Plast. Surg. Nurs. 2012, 32, 77–79. [Google Scholar] [CrossRef]
- Vermeulen, H.; Ubbink, D.T.; Goossens, A.; de Vos, R.; Legemate, D.A. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br. J. Surg. 2005, 92, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. 2), 5–15. [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Yildiz Karadeniz, E.; Kaplan Serin, E. Use of honey in diabetic foot ulcer: Systematic review and meta-analysis. J. Tissue Viability 2023, 32, 270–278. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef]
- Bocoum, A.; Riel, S.; Traore, S.O.; Ngo Oum Ii, E.F.; Traore, Y.; Thera, A.T.; Fane, S.; Dembele, B.T.; Cremers, N.A.J. Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections-A Prospective Cohort Study. Antibiotics 2023, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Tatz, A.J.; Slavin, R.A.; Sutton, G.A.; Dahan, R.; Abu Ahmad, W.; Kelmer, G. Intra-incisional medical grade honey decreases the prevalence of incisional infection in horses undergoing colic surgery: A prospective randomised controlled study. Equine Vet. J. 2020, 53, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Mandel, H.H.; Sutton, G.A.; Abu, E.; Kelmer, G. Intralesional application of medical grade honey improves healing of surgically treated lacerations in horses. Equine Vet. J. 2020, 52, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.K.; Pool, L.; Ulrich, M.M. The effect of a honey based gel and silver sulphadiazine on bacterial infections of in vitro burn wounds. Burns 2013, 39, 754–759. [Google Scholar] [CrossRef]
- Boekema, B.; Chrysostomou, D.; Ciprandi, G.; Elgersma, A.; Vlig, M.; Pokorna, A.; Peters, L.J.F.; Cremers, N.A.J. Comparing the antibacterial and healing properties of medical-grade honey and silver-based wound care products in burns. Burns 2024, 50, 597–610. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- NEN Standard 13726-2023; Test Methods for Wound Dressings—Aspects of Absorption, Moisture Vapour Transmission, Waterproofness and Extensibility. Royal Dutch Standardisation Institute (NEN): Delft, the Netherlands, 1 September 2023. Available online: https://www.nen.nl/nen-en-13726-2023-en-315671 (accessed on 10 February 2025).

| Description | Absorptive, sponge-like polymer dressings (with or without adhesive borders) |
| Composition | Polyurethane and other components |
| Key Properties |
|
| Uses | Primary or secondary dressings on wounds (flat or cavity) with minimal to high exudate, where a non-adherent surface is important. |
| # | Product Name | Description | Manufacturer (Reference #) | Lot# | Manufacturing/ Expiration Date | Size in cm (Number of Pieces Per Box) | Price Per Box in Euro (Price Per Piece/and, i.e., Corrected Per 100 cm2) |
|---|---|---|---|---|---|---|---|
| PLAIN FOAM DRESSINGS | |||||||
| 1 | Tegaderm | High-Performance Foam Non-Adhesive Dressing | 3M (90601) | 33W66H | 2023-12-16/ 2026-12-15 | 10 × 10 (10 pcs) | 46,06 (4.61) |
| 2 | HydroTac | Foam dressing impregnated with gel | Paul Hartmann AG (685832) | 300112113 | 2023-03-21/ 2026-03-01 | 10 × 10 (10 pcs) | 31.60 (3.16) |
| 3 | Allevyn Classic | Non-adhesive hydrocellular foam dressing | Smith and Nephew (66800022) | 202351 | 2023-12-01/ 2026-12-01 | 10 × 10 (10 pcs) | 35.64 (3.56) |
| 4 | PermaFoam Classic | Foam dressing | Paul Hartmann AG (882000) | 23061555 | 2023-10-20/ 2026-10-19 | 10 × 10 (10 pcs) | 32.74 (3.27) |
| 5 | Cutimed Siltec Plus | Soft tack silicone foam dressing with superabsorbers | Essity (73288-01) | 40720744 | 2024-02-13/ 2027-01-12 | 10 × 10 (10 pcs) | 56.29 (5.63) |
| 6 | Cutimed Siltec | Silicone Foam dressing with super-absorbers | Essity (73285-01) | 33630744 | 2023-09-06/ 2026-08-12 | 10 × 10 (10 pcs) | 49.05 (4.91) |
| 7 | Lyofoam Max | Absorbent foam dressing | Mölnlyke Health Care AB (603201) | 07332551952433 | NA/ 2025-01-28 | 10 × 10 (10 pcs) | 22.50 (2.25) |
| 8 | Biatain | Non-adhesive foam dressing—for wounds with extra fragile skin | Coloplast (33412) | 9553318 | 2024-01-05/ 2027-01-4 | 10 × 20 (5 pcs) | 33.69 (6.74/3.37) |
| 9 | Polymem MAX | Non-Adhesive Dressing | Ferris Mfg. Corp. (5045) | 04423E2 | NA/ 2028-02 | 11 × 11 (10 pcs) | 89.55 (8.96/7.40) |
| 10 | Kliniderm Foam | Foam dressing for the treatment of chronic and acute wounds | Klinion (4017 4811) | 321251-009 | 2023-11-30/ 2026-11-30 | 10 × 10 (10 pcs) | 31.40 (3.14) |
| 11 | Surprasorb P | PU Foam Dressing, non-adhesive | Lohmann and Rauscher (20407) | 2403312216 | 2024-01-07/ 2026-12-31 | 10 × 10 (10 pcs) | 31.60 (3.16) |
| 12 | Mepilex | Soft silicone foam dressing | Mölnlycke Health Care AB (294200) | 24154587 | NA/ 2027-03-28 | 10 × 20 (5 pcs) | 33.10 (6.62/3.31) |
| 13 | Raw material of L-Mesitran Foam | Polyurethane foam dressing without impregnated honey formulation | NA | NA | NA | Diverse (10 cm width, 100 m long) | NA |
| ACTIVE FOAM DRESSINGS (additional properties) | |||||||
| 14 | Mepilex Ag | Antimicrobial soft silicone foam dressing | Mölnlycke Health Care AB (287221) | 23332417 | NA/ 2026-07-28 | 10 × 21 (5pcs) | 32.37 (6.47/3.08) |
| 15 | Allevyn Ag | Non-adhesive antimicrobial hydrocellular foam dressing with a minimum of 1.99 mg/cm2 silver sulfadiazine | Smith and Nephew (66800084) | 202405 | 2024-01-01/ 2026-01-01 | 10 × 10 (10 pcs) | 88.27 (8.83) |
| 16 | Aquacell Ag Foam | Non-adhesive Hydrofiber Foam Dressing with Silver | Convatec (420642) | 3K00197 | 10-2023/ 2026-10-01 | 10 × 10 (10 pcs) | 72.43 (7.24) |
| 17 | L-Mesitran Foam | Non-adhering foam dressing | Theo Manufacturing BV (712.10) | 202305X | 2023-05/ 2026-05 | 10 × 10 (10 pcs) | 39.48 (3.95) |
| Most Suitable Product |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysostomou, D.; Papanikolaou, G.E.; Boshoff, L.; Mbele, T.; Pokorná, A.; Holubová, A.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Uncovering the Advantages of Foam Dressings with Active Ingredients. Pharmaceuticals 2025, 18, 768. https://doi.org/10.3390/ph18060768
Chrysostomou D, Papanikolaou GE, Boshoff L, Mbele T, Pokorná A, Holubová A, Wagener FADTG, Cremers NAJ. Uncovering the Advantages of Foam Dressings with Active Ingredients. Pharmaceuticals. 2025; 18(6):768. https://doi.org/10.3390/ph18060768
Chicago/Turabian StyleChrysostomou, Daniela, Georgios E. Papanikolaou, Lorraine Boshoff, Thandazi Mbele, Andrea Pokorná, Adéla Holubová, Frank A. D. T. G. Wagener, and Niels A. J. Cremers. 2025. "Uncovering the Advantages of Foam Dressings with Active Ingredients" Pharmaceuticals 18, no. 6: 768. https://doi.org/10.3390/ph18060768
APA StyleChrysostomou, D., Papanikolaou, G. E., Boshoff, L., Mbele, T., Pokorná, A., Holubová, A., Wagener, F. A. D. T. G., & Cremers, N. A. J. (2025). Uncovering the Advantages of Foam Dressings with Active Ingredients. Pharmaceuticals, 18(6), 768. https://doi.org/10.3390/ph18060768








