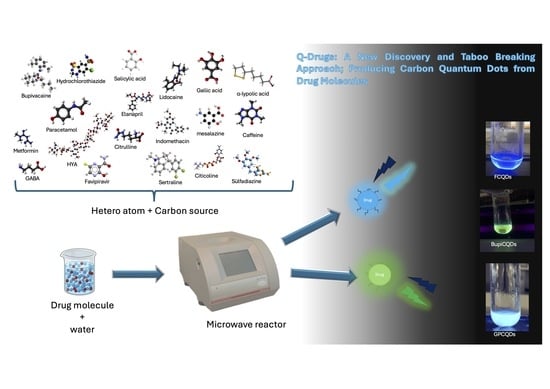
Quantum Drugs (Q-Drugs): A New Discovery and Taboo Breaking Approach; Producing Carbon Quantum Dots from Drug Molecules
Abstract
1. Introduction
2. Results
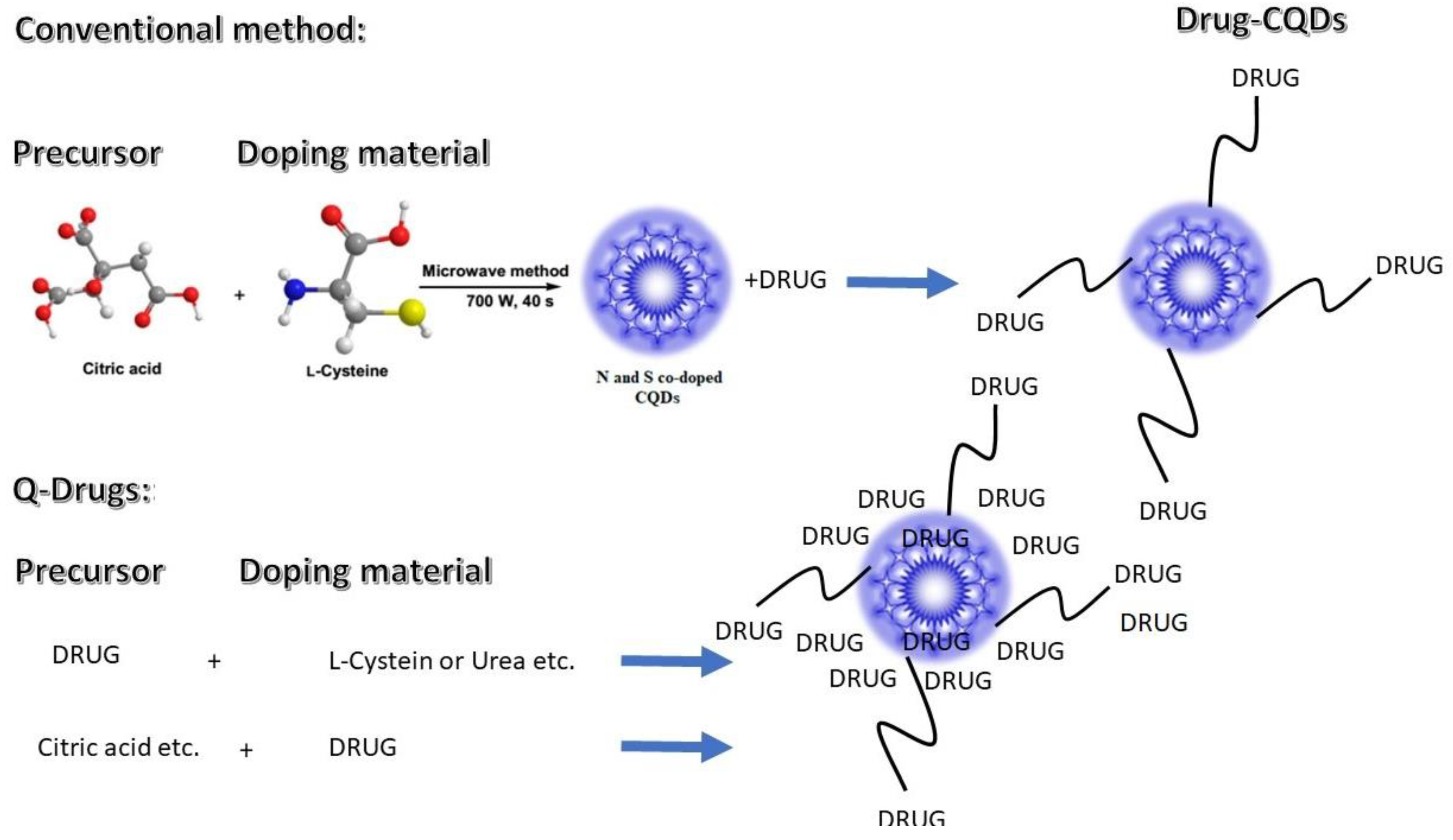
2.1. One-Pot Production Q-Drugs
2.2. Characterization of Q-Drugs
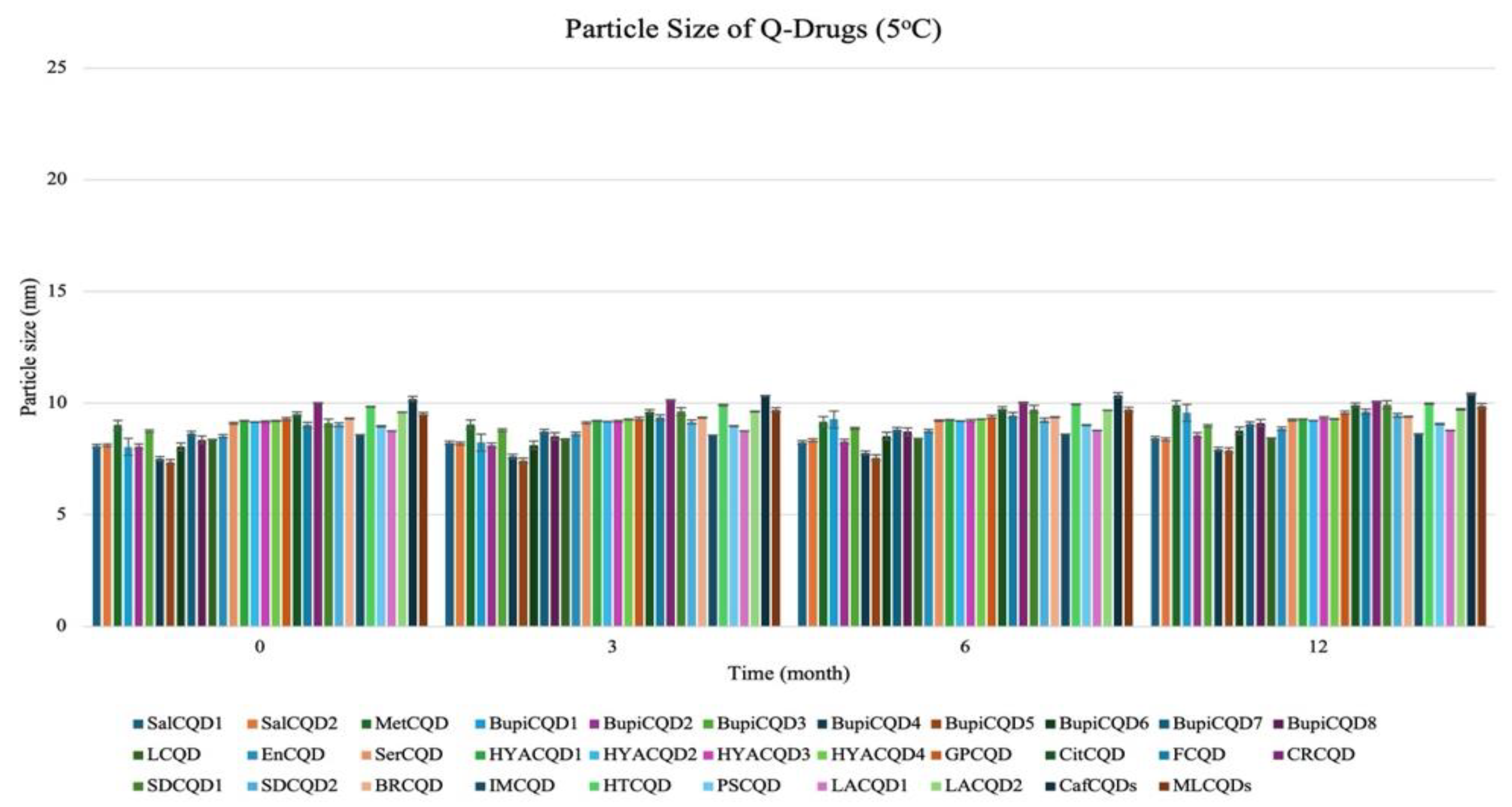
2.2.1. Particle Size
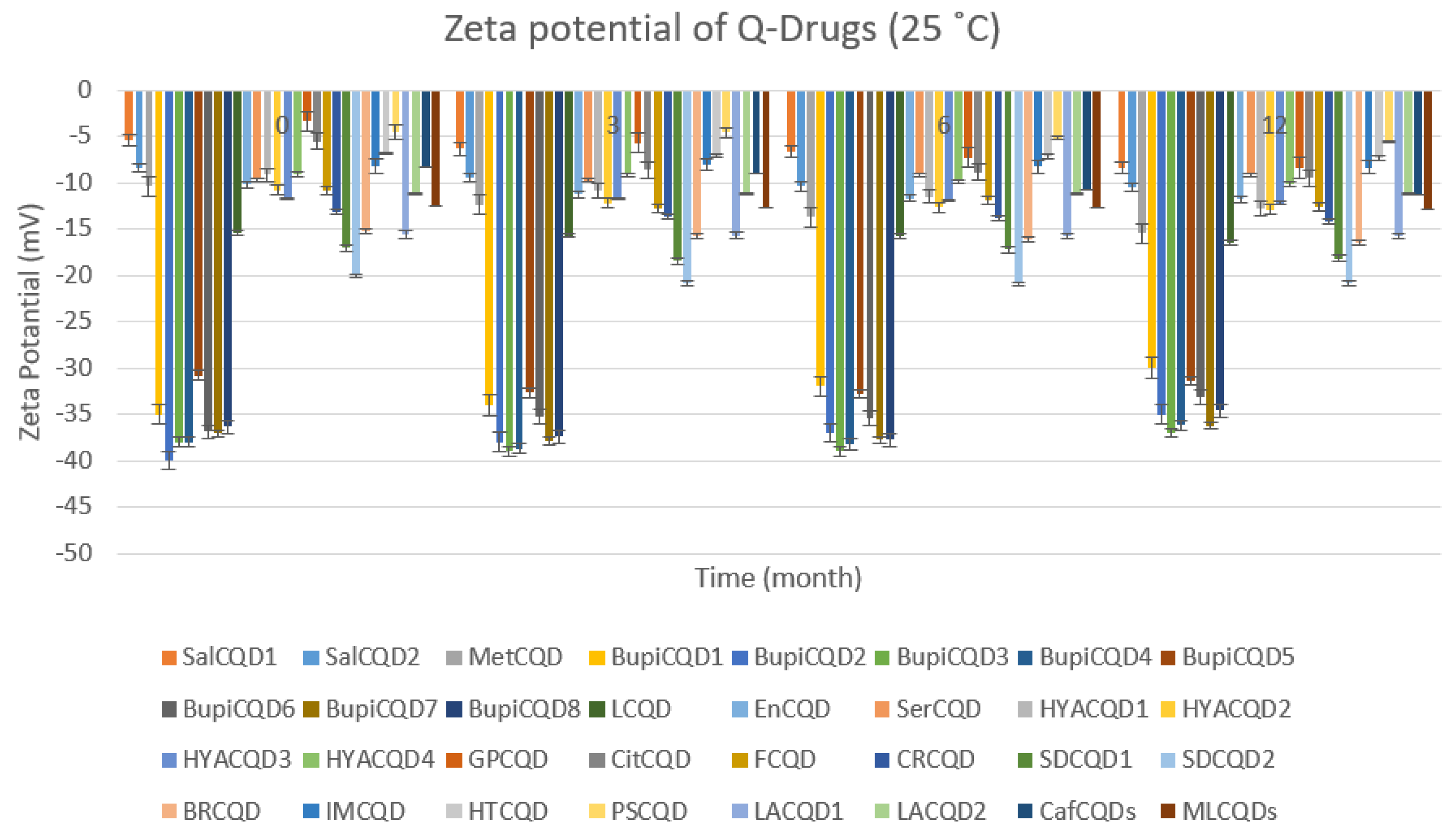
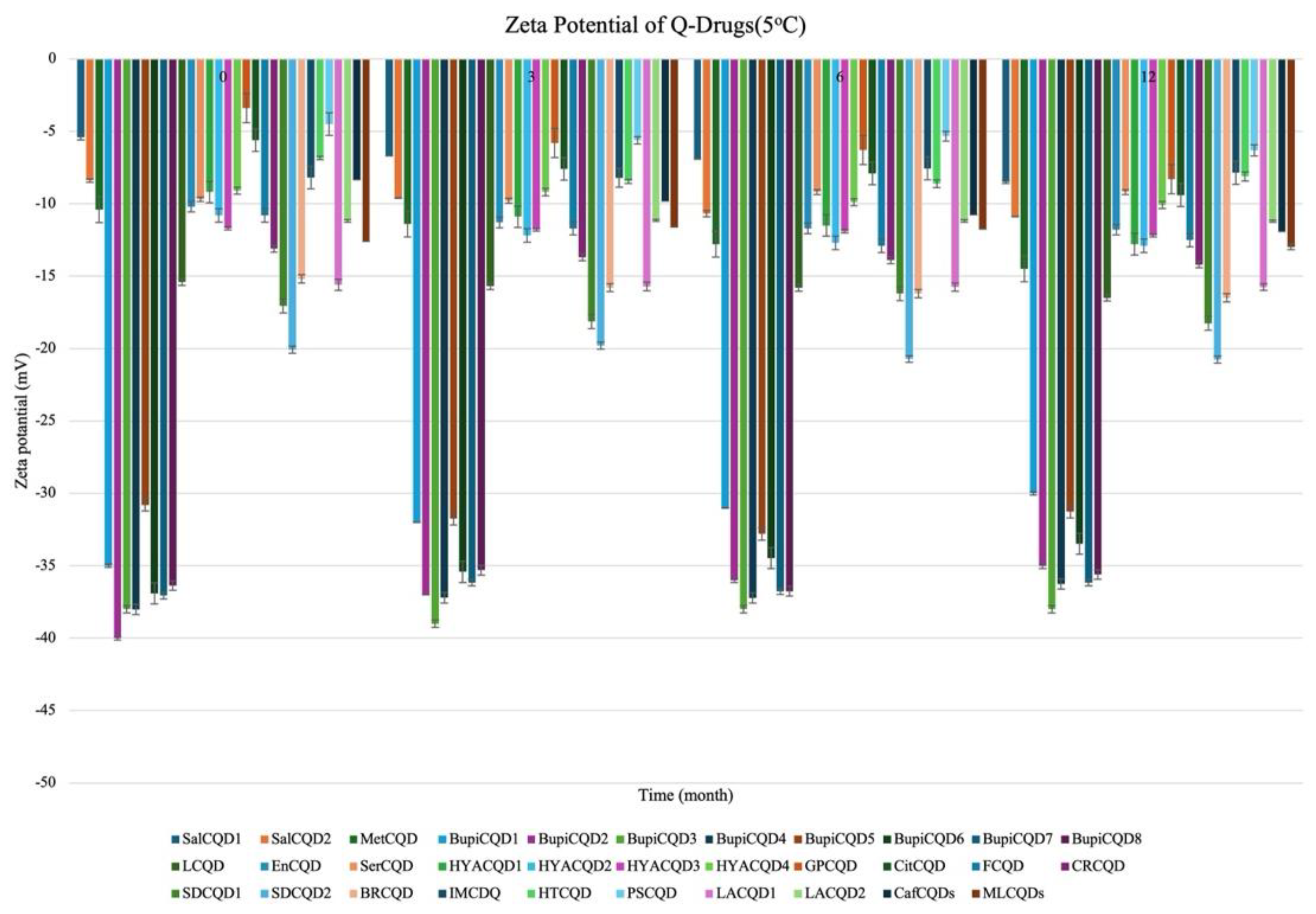
2.2.2. Stability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. One-Pot Production of Composite Q-Drugs
4.3. Characterization of Q-Drugs
4.4. Stability
4.5. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APIs | Active pharmaceutical ingredients |
| BRCQD | Bromelain carbon quantum dot |
| BupiCQD | Bupivacaine carbon quantum dot |
| CafCQD | Caffeine carbon quantum dot |
| CAMH | Citric acid monohydrate |
| CitCQD | Citicoline carbon quantum dot |
| CQD | Carbon quantum dots |
| CRCQD | Citrulline carbon quantum dot |
| EDA | Ethylenediamine |
| EnCQD | Enalapril carbon quantum dot |
| FCQD | Favipiravir carbon quantum dot |
| GPCQD | GABA-Pentin carbon quantum dot |
| GQDs | Graphene quantum dots |
| HTCQD | Hydrochlorothiazide carbon quantum dot |
| HYACQD | Hyaluronic acid carbon quantum dot |
| IMCQD | Indomethacin carbon quantum dot |
| LCQD | Lidocaine carbon quantum dot |
| LPCQD | α-Lipoic acid carbon quantum dot |
| MetCQD | Metformin carbon quantum dot |
| MLCQD | Mesalazine carbon quantum dot |
| Na-Borat | Sodium borate |
| PSCQD | Paracetamol carbon quantum dot |
| q-Drugs | Quantum Drugs |
| QDs | Quantum dots |
| SalCQD | Salicylic acid carbon quantum dot |
| SDCQD | Sulfadiazine carbon quantum dot |
| SerCQD | Sertraline carbon quantum dot |
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.-H.; Kumar, P. Current and Future Perspectives of Carbon and Graphene Quantum Dots: From Synthesis to Strategy for Building Optoelectronic and Energy Devices. Renew. Sustain. Energy Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Cao, W.; Mao, B.; Liu, Y.; Kang, Z. Advances in Carbon Dots: From the Perspective of Traditional Quantum Dots. Mater. Chem. Front. 2020, 4, 1586–1613. [Google Scholar] [CrossRef]
- Hassan Ahmed, H.E.; Soylak, M. Exploring the Potential of Carbon Quantum Dots (CQDs) as an Advanced Nanomaterial for Effective Sensing and Extraction of Toxic Pollutants. TrAC Trends Anal. Chem. 2024, 180, 117939. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
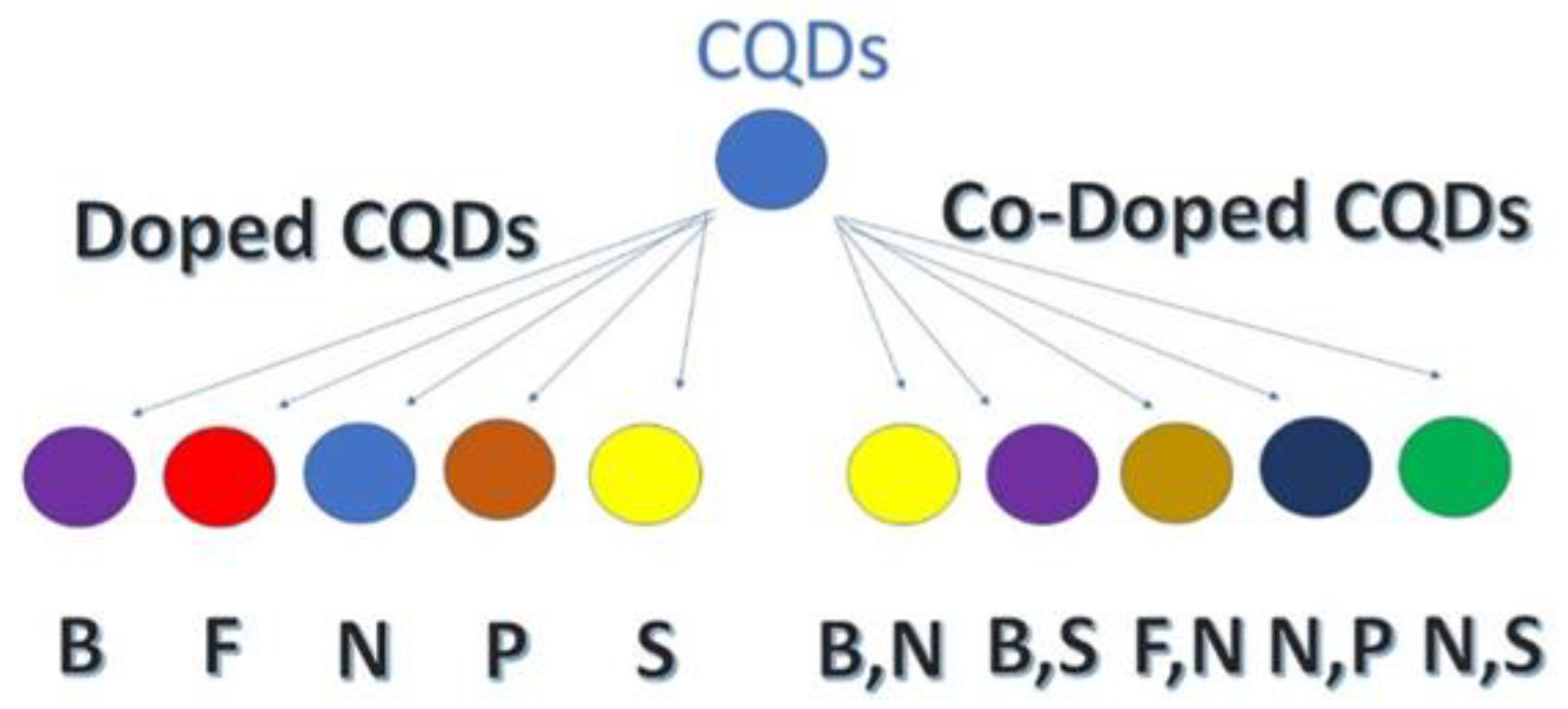
- Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C 2019, 5, 24. [Google Scholar] [CrossRef]
- Brkić, S. Applicability of Quantum Dots in Biomedical Science. In Ionizing Radiation Effects and Applications; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Sikiru, S.; Oladosu, T.L.; Kolawole, S.Y.; Mubarak, L.A.; Soleimani, H.; Afolabi, L.O.; Oluwafunke Toyin, A.-O. Advance and Prospect of Carbon Quantum Dots Synthesis for Energy Conversion and Storage Application: A Comprehensive Review. J. Energy Storage 2023, 60, 106556. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Camlik, G.; Kupeli Akkol, E.; Degim, Z.; Degim, I.T. Can Carbon Quantum Dots (CQDs) or Boron Compounds Be an Ultimate Solution for COVID-19 Therapy? Iran J. Pharm. Res. 2021, 20, 9–20. [Google Scholar] [PubMed]
- Song, Y.; Wu, Y.; Wang, H.; Liu, S.; Song, L.; Li, S.; Tan, M. Carbon Quantum Dots from Roasted Atlantic Salmon (Salmo Salar L.): Formation, Biodistribution and Cytotoxicity. Food Chem. 2019, 293, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and Surface Modification of Carbon Quantum Dots for Enhanced Functionalities and Related Applications. Part. Part. Syst. Charact. 2021, 38, 2100170. [Google Scholar] [CrossRef]
- Camlik, G.; Bilakaya, B.; Uyar, P.; Degim, Z.; Degim, I.T. New Generation of Composite Carbon Quantum Dots for Imaging, Diagnosing, and Treatment of Cancer. In Functionalized Nanomaterials for Cancer Research; Elsevier: Amsterdam, The Netherlands, 2024; pp. 543–557. [Google Scholar]
- Zhu, P.; Liu, Y.; Tang, Y.; Zhu, S.; Liu, X.; Yin, L.; Liu, Q.; Yu, Z.; Xu, Q.; Luo, D.; et al. Bi-Doped Carbon Quantum Dots Functionalized Liposomes with Fluorescence Visualization Imaging for Tumor Diagnosis and Treatment. Chin. Chem. Lett. 2024, 35, 108689. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, D. Carbon Quantum Dots in Bioimaging and Biomedicines. Front. Bioeng. Biotechnol. 2024, 11, 1333752. [Google Scholar] [CrossRef]
- Camlik, G.; Ozakca, I.; Bilakaya, B.; Ozcelikay, A.T.; Velaro, A.J.; Wasnik, S.; Degim, I.T. Development of Composite Carbon Quantum Dots-Insulin Formulation for Oral Administration. J. Drug Deliv. Sci. Technol. 2022, 76, 103833. [Google Scholar] [CrossRef]
- Yerlikaya, F.; Camlik, G.; Akkol, E.K.; Degim, Z.; Degim, I.T.; Sobarzo-Sánchez, E. Formation of Quantum Water in Nanoparticulate Systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102456. [Google Scholar] [CrossRef]
- Mandal, T.; Mishra, S.R.; Singh, K.; Agarwalla, H.; Masto, R.E.; Kumar, M.; Singh, V. Fluorescent Carbon Nanomaterials from Coal and Its Derivatives: Structure, Properties, and Applications. J. Nanopart. Res. 2023, 25, 125. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, Y.; Lu, W.; Liu, Y.; Han, H.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Carbon Dots with Red Emission as a Fluorescent and Colorimeteric Dual-Readout Probe for the Detection of Chromium and Cysteine and Its Logic Gate Operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef] [PubMed]
- Camlik, G.; Bilakaya, B.; Ozsoy, Y.; Degim, I.T. A New Approach for the Treatment of Alzheimer’s Disease: Insulin-Quantum Dots. J. Microencapsul. 2024, 41, 18–26. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene Quantum Dots: An Emerging Material for Energy-Related Applications and Beyond. Energy Environ. Sci. 2012, 5, 8869. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.-J. Focusing on Luminescent Graphene Quantum Dots: Current Status and Future Perspectives. Nanoscale 2013, 5, 4015. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene Quantum Dots: Emergent Nanolights for Bioimaging, Sensors, Catalysis and Photovoltaic Devices. Chem. Commun. 2012, 48, 3686. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Luo, P.G.; Sahu, S.; Yang, S.-T.; Sonkar, S.K.; Wang, J.; Wang, H.; LeCroy, G.E.; Cao, L.; Sun, Y.-P. Carbon “Quantum” Dots for Optical Bioimaging. J. Mater. Chem. B 2013, 1, 2116. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Li, X.; Wang, W.; Wang, L.; Gu, M.; Li, Q. Upconversion Fluorescent Carbon Nanodots Enriched with Nitrogen for Light Harvesting. J. Mater. Chem. 2012, 22, 15522. [Google Scholar] [CrossRef]
- Zhou, W.; Zhuang, J.; Li, W.; Hu, C.; Lei, B.; Liu, Y. Towards Efficient Dual-Emissive Carbon Dots through Sulfur and Nitrogen Co-Doped. J. Mater. Chem. C Mater. 2017, 5, 8014–8021. [Google Scholar] [CrossRef]
- Luo, M.; Hua, Y.; Liang, Y.; Han, J.; Liu, D.; Zhao, W.; Wang, P. Synthesis of Novel β-Cyclodextrin Functionalized S, N Codoped Carbon Dots for Selective Detection of Testosterone. Biosens. Bioelectron. 2017, 98, 195–201. [Google Scholar] [CrossRef]
- Olsen, C.; Wiborg, E.; Lundanes, E.; Abadpour, S.; Scholz, H.; Wilson, S.R. On-line Reduction of Insulin Disulfide Bonds with Photoinduced Radical Reactions, Upstream to Nano Liquid Chromatography-mass Spectrometry. Sep. Sci. Plus 2022, 5, 220–227. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Zaini, M.S.; Jian, L.Y.; Liew, J.Y.C.; Kamarudin, M.A. Impact of Carbon Concentration on Optical and Zeta Potential Properties of Carbon Quantum Dots. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 1039–1049. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and Surface Charge Characterization of Nanoparticles with a Salt Gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Li, Y.; Cao, M.-J.; Dai, C.-L.; He, L.; Yang, Y.-P. Preparation and Stabilization Mechanism of Carbon Dots Nanofluids for Drag Reduction. Pet. Sci. 2020, 17, 1717–1725. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P.P. Permeation of Topically Applied Caffeine from a Food by—Product in Cosmetic Formulations: Is Nanoscale in Vitro Approach an Option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef]
- Demirbolat, M.; Değim, Z.; Değim, İ.T. Zeta Potential Determination of Targeted Nanoparticles. In Drug Delivery with Targeted Nanoparticles, 1st ed.; Çapan, Y., Sahin, A., Tonbul, H., Eds.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; pp. 29–56. [Google Scholar]

| Code | Physical Appearances | Color |
|---|---|---|
| SalCQD1 |  | Blue |
| SalCQD2 |  | Bright Green |
| MetCQD |  | Blue-Green |
| BupiCQD1 |  | Yellowish Green |
| BupiCQD2 |  | Blue |
| BupiCQD3 |  | Bright Green |
| BupiCQD4 |  | Bright Green |
| BupiCQD5 |  | Yellowish Green |
| BupiCQD6 |  | Green Blue |
| BupiCQD7 |  | Bright Turquoise-Green |
| BupiCQD8 |  | Yellowish Blue |
| LCQD |  | Blue |
| EnCQD |  | Blue |
| SerCQD |  | Blue |
| HYACQD1 |  | Green |
| HYACQD2 |  | Blue |
| HYACQD3 |  | Bright Blue-Turquoise |
| HYACQD4 |  | Blue |
| GPCQD |  | Bright Blue |
| CitCQD |  | Bright Blue |
| FCQD |  | Bright Blue |
| CRCQD |  | Bright Blue |
| SDCQD1 |  | Transparent Blue |
| SDCQD2 |  | Light Transparent Blue |
| BRCQD |  | Blue |
| HTCQD |  | Blue |
| IMCQD |  | Yellowish Green |
| PSCQD |  | Blue |
| LPCQD1 |  | Green |
| LPCQD2 |  | Blue |
| CafCQD |  | Bright Blue |
| MLCQD | 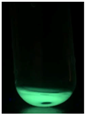 | Bright Green |
| Codes | Particle Size (nm) | Polydispersity Index % (PDI %) | Zeta Potential (mV) |
|---|---|---|---|
| SalCQD1 | 8.040 ± 0.031 | 18.050 ± 0.102 | −35.000 ± 0.124 |
| SalCQD2 | 8.060 ± 0.213 | 20.000 ± 0.102 | −40.000 ± 0.142 |
| MetCQD | 8.750 ± 0.302 | 19.200 ± 0.102 | −38.000 ± 0.109 |
| BupiCQD1 | 7.510 ± 0.221 | 20.300 ± 0.312 | −38.020 ± 0.032 |
| BupiCQD2 | 7.360 ± 0.030 | 20.000 ± 0.102 | −30.810 ± 0.035 |
| BupiCQD3 | 8.050 ± 0.209 | 20.000 ± 0.102 | −36.910 ± 0.129 |
| BupiCQD4 | 8.650 ± 0.310 | 20.000 ± 0.102 | −37.060 ± 0.042 |
| BupiCQD5 | 8.360 ± 0.014 | 20.000 ± 0.102 | −36.370 ± 0.253 |
| BupiCQD6 | 8.360 ± 0.024 | 15.300 ± 0.012 | −15.400 ± 0.032 |
| BupiCQD7 | 8.520 ± 0.032 | 18.500 ± 0.038 | −9.200 ± 0.042 |
| BupiCQD8 | 9.100 ± 0.042 | 19.100 ± 0.016 | −9.700 ± 0.023 |
| LCQD | 9.200 ± 0.012 | 17.500 ± 0.018 | −9.200 ± 0.022 |
| EnCQD | 9.150 ± 0.122 | 16.700 ± 0.035 | −10.800 ± 0.012 |
| SerCQD | 9.170 ± 0.045 | 16.900 ± 0.078 | −11.700 ± 0.502 |
| HYACQD1 | 9.210 ± 0.042 | 17.300 ± 0.033 | −9.100 ± 0.102 |
| HYACQD2 | 9.130 ± 0.072 | 18.500 ± 0.038 | −9.500 ± 0.021 |
| HYACQD3 | 9.290 ± 0.051 | 14.400 ± 0.006 | −3.400 ± 0.054 |
| HYACQD4 | 9.520 ± 0.151 | 10.500 ± 1.230 | −5.600 ± 0.084 |
| GPCQD | 10.000 ± 0.022 | 10.700 ± 1.024 | −13.100 ± 0.132 |
| CitCQD | 9.120 ± 0.042 | 35.500 ± 1.340 | −17.060 ± 1.025 |
| FCQD | 9.040 ± 0.320 | 32.610 ± 1.401 | −20.080 ± 1.241 |
| CRCQD | 9.310 ± 0.048 | 20.500 ± 0.029 | −15.200 ± 0.104 |
| SDCQD1 | 8.040 ± 0.031 | 18.050 ± 0.102 | −35.000 ± 0.124 |
| SDCQD2 | 8.060 ± 0.213 | 20.000 ± 0.102 | −40.000 ± 0.142 |
| BRCQD | 8.750 ± 0.302 | 19.200 ± 0.102 | −38.000 ± 0.109 |
| HTCQD | 9.840 ± 0.054 | 20.400 ± 0.121 | −6.850 ± 0.110 |
| IMCQD | 8.580 ± 0.081 | 19.490 ± 0.550 | −8.200 ± 0.781 |
| PSCQD | 8.960 ± 0.024 | 22.350 ± 0.025 | −4.500 ± 0.301 |
| LPCQD1 | 8.750 ± 0.060 | 18.130 ± 0.050 | −15.610 ± 1.382 |
| LPCQD2 | 9.600 ± 0.015 | 16.700 ± 1.067 | −11.150 ± 0.084 |
| CafCQD | 10.190 ± 0.107 | 16.200 ± 1.021 | −8360 ± 0.104 |
| MLCQD | 9.520 ± 0.162 | 17.240 ± 0.035 | −12.600 ± 0.054 |
| Code | Contents | Process/Settings |
|---|---|---|
| SalCQD1 | 0.1 g salicylic acid, 0.05 g urea 1 mL distilled water | 180 °C—20 min |
| SalCQD2 | 0.1 g salicylic acid, 100 µL EDA, 2 mL distilled water | 180 °C—20 min |
| MetCQD | 0.2 g CAMH, 0.5 g metformin, 1 mL distilled water | 160 °C—20 min |
| BupiCQD1 | 0.1 g Bupivacaine, 1 mL distilled water | 180 °C—20 min |
| BupiCQD2 | 0.05 g Bupivacaine, 0.2 g CAMH, 2 mL distilled water | 180 °C—20 min |
| BupiCQD3 | 0.03 g Bupivacaine, 0.03 g salicylic acid, 100 µL EDA, 0.03 g Na-borate, 4 mL distilled water | 185 °C—20 min |
| BupiCQD4 | 0.3 g Bupivacaine, 0.03 g salicylic acid, 100 µL EDA, 4 mL distilled water | 185 °C—20 min |
| BupiCQD5 | 0.3 g Bupivacaine, 0.03 g salicylic acid, 100 µL EDA, 2 mL distilled water | 185 °C—20 min |
| BupiCQD6 | 0.1 g Bupivacaine, 0.03 g salicylic acid, 100 µL EDA, 2 mL distilled water | 185 °C—20 min |
| BupiCQD7 | 0.1 g Bupivacaine, 0.05 g salicylic acid, 100 µL EDA, 2 mL distilled water | 185 °C—20 min |
| BupiCQD8 | 0.1 g Bupivacaine, 0.1 g salicylic acid, 100 µL EDA, 2 mL distilled water | 185 °C—20 min |
| LCQD | 0.05 g Lidocaine, 0.2 g CAMH, 1 mL distilled water | 160 °C—20 min |
| EnCQD | 0.2 g Enalapril, 1 mL distilled water | 160 °C—30 min |
| SerCQD | 0.1 g Sertraline, 0.03 g urea, 1 mL distilled water | 150 °C—20 min |
| HYACQD1 | 500 µL HYA solution, 0.05 g caffeine, 0.005 g L-cysteine, 0.05 g Na-Borate, 1 mL distilled water | 140 °C—20 min |
| HYACQD2 | 500 µL HYA solution, 0.005 g caffeine, 0.005 g L-cysteine, 0.05 g Na-Borate, 1 mL distilled water | 140 °C—20 min |
| HYACQD3 | 500 µL HYA solution, 0.005 g caffeine, 0.005 g L-cysteine, 0.025 g Na-Borate, 1 mL distilled water | 140 °C—20 min |
| HYACQD4 | 500 µL HYA solution, 0.05 g caffeine, 0.005 g L-cysteine, 0.05 g boric acid, 1 mL distilled water | 140 °C—20 min |
| GPCQD | 0.2 g GABA-Pentin, 0.005 g L-cysteine, 2 mL distilled water | 140 °C—20 min |
| CitCQD | 0.1 g Citicoline, 1 mL distilled water | 140 °C—20 min |
| FCQD | 0.01 g Favipiravir, 1 mL distilled water | 140 °C—20 min |
| CRCQD | 0.2 g Citrulline, 1 mL distilled water | 140 °C—20 min |
| SDCQD1 | 0.1 g Sulfadiazine, 0.005 g L-cysteine, 1 mL distilled water | 140 °C—20 min |
| SDCQD2 | 0.2 g Sulfadiazine, 1 mL distilled water | 140 °C—20 min |
| BRCQD | 0.2 g Bromelain, 1 mL distilled water | 150 °C—20 min |
| HTCQD | 0.01 g Hydrochlorothiazide, 0.005 g urea, 1 mL distilled water | 150 °C—20 min |
| IMCQD | 0.01 g Indomethacin, 0.005 g urea, 1 mL distilled water | 150 °C—20 min |
| PSCQD | 0.01 g Paracetamol, 0.005 g urea, 1 mL distilled water | 140 °C—20 min |
| LPCQD1 | 0.01 g α-Lipoic acid, 0.005 g urea, 1 mL distilled water | 150 °C—20 min |
| LPCQD2 | 0.01 g α-Lipoic acid, 1 mL distilled water | 150 °C—20 min |
| CafCQD | 0.2 g CAMH, 0.1 g caffeine, 1 mL distilled water | 180 °C—20 min |
| MLCQD | 0.01 g Mesalazine, 1 mL distilled water | 100 °C—20 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camlik, G.; Bilakaya, B.; Güven, G.K.; Akkol, E.K.; Degim, Z.; Sobarzo-Sánchez, E.; Degim, I.T. Quantum Drugs (Q-Drugs): A New Discovery and Taboo Breaking Approach; Producing Carbon Quantum Dots from Drug Molecules. Pharmaceuticals 2025, 18, 767. https://doi.org/10.3390/ph18060767
Camlik G, Bilakaya B, Güven GK, Akkol EK, Degim Z, Sobarzo-Sánchez E, Degim IT. Quantum Drugs (Q-Drugs): A New Discovery and Taboo Breaking Approach; Producing Carbon Quantum Dots from Drug Molecules. Pharmaceuticals. 2025; 18(6):767. https://doi.org/10.3390/ph18060767
Chicago/Turabian StyleCamlik, Gamze, Besa Bilakaya, Gökçe Karaotmarlı Güven, Esra Küpeli Akkol, Zelihagül Degim, Eduardo Sobarzo-Sánchez, and Ismail Tuncer Degim. 2025. "Quantum Drugs (Q-Drugs): A New Discovery and Taboo Breaking Approach; Producing Carbon Quantum Dots from Drug Molecules" Pharmaceuticals 18, no. 6: 767. https://doi.org/10.3390/ph18060767
APA StyleCamlik, G., Bilakaya, B., Güven, G. K., Akkol, E. K., Degim, Z., Sobarzo-Sánchez, E., & Degim, I. T. (2025). Quantum Drugs (Q-Drugs): A New Discovery and Taboo Breaking Approach; Producing Carbon Quantum Dots from Drug Molecules. Pharmaceuticals, 18(6), 767. https://doi.org/10.3390/ph18060767