Microbiota-Accessible Borates as Novel and Emerging Prebiotics for Healthy Longevity: Current Research Trends and Perspectives
Abstract
1. Introduction
2. World’s Healthiest Eating Habits
2.1. Nordic Diet
2.2. Mediterranean Diet
2.3. Green Mediterranean Diet
2.4. Japanese and Korean Diet
2.5. Thai Diet
2.6. West African Diet
2.7. “Microbiome” Diet
2.8. Dietary Approaches to Stop Hypertension Diet
2.9. Food Restriction as Key Factor in Longevity
2.10. Intermittent Fasting
3. Microbiome and Exceptional Longevity
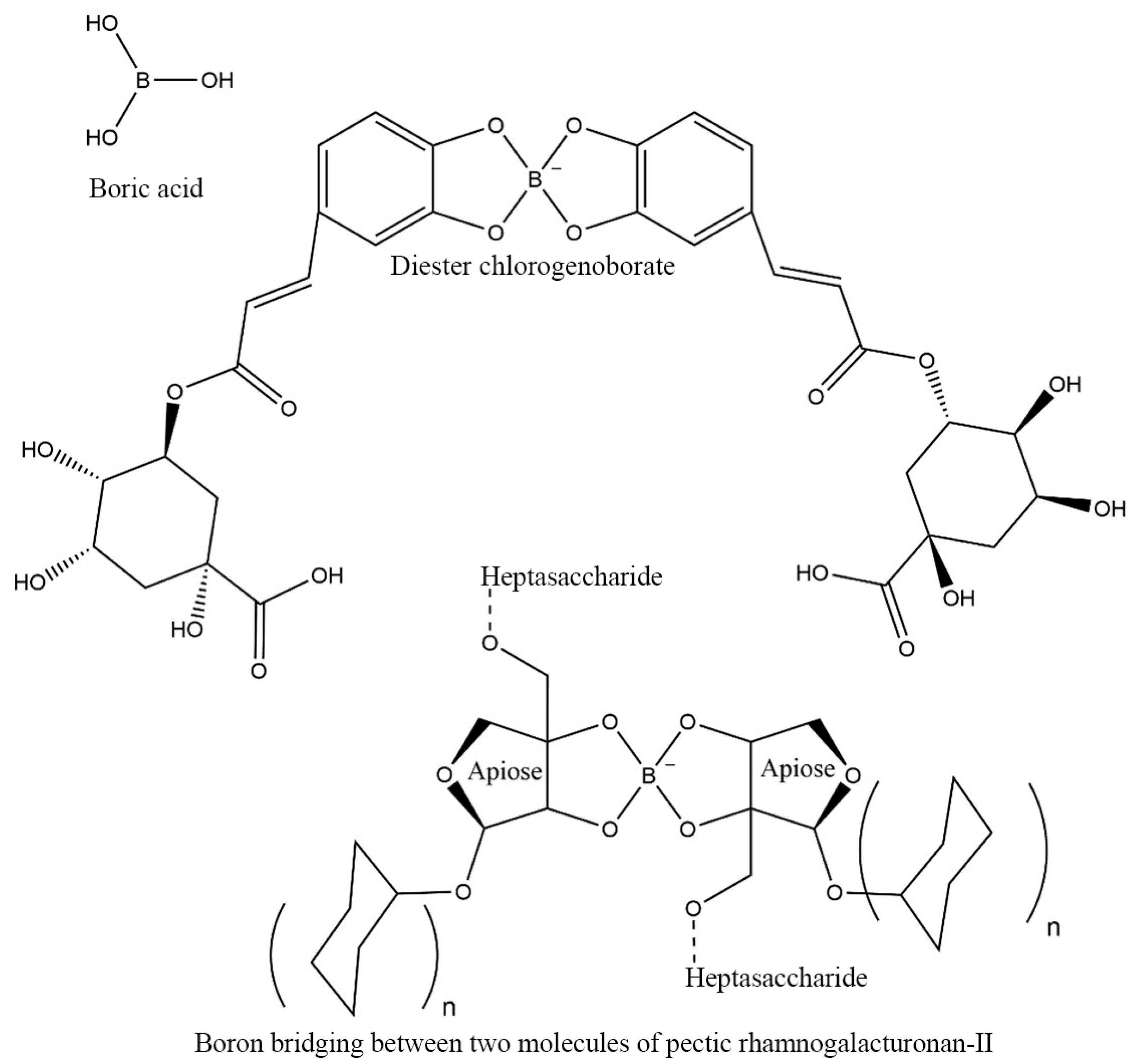
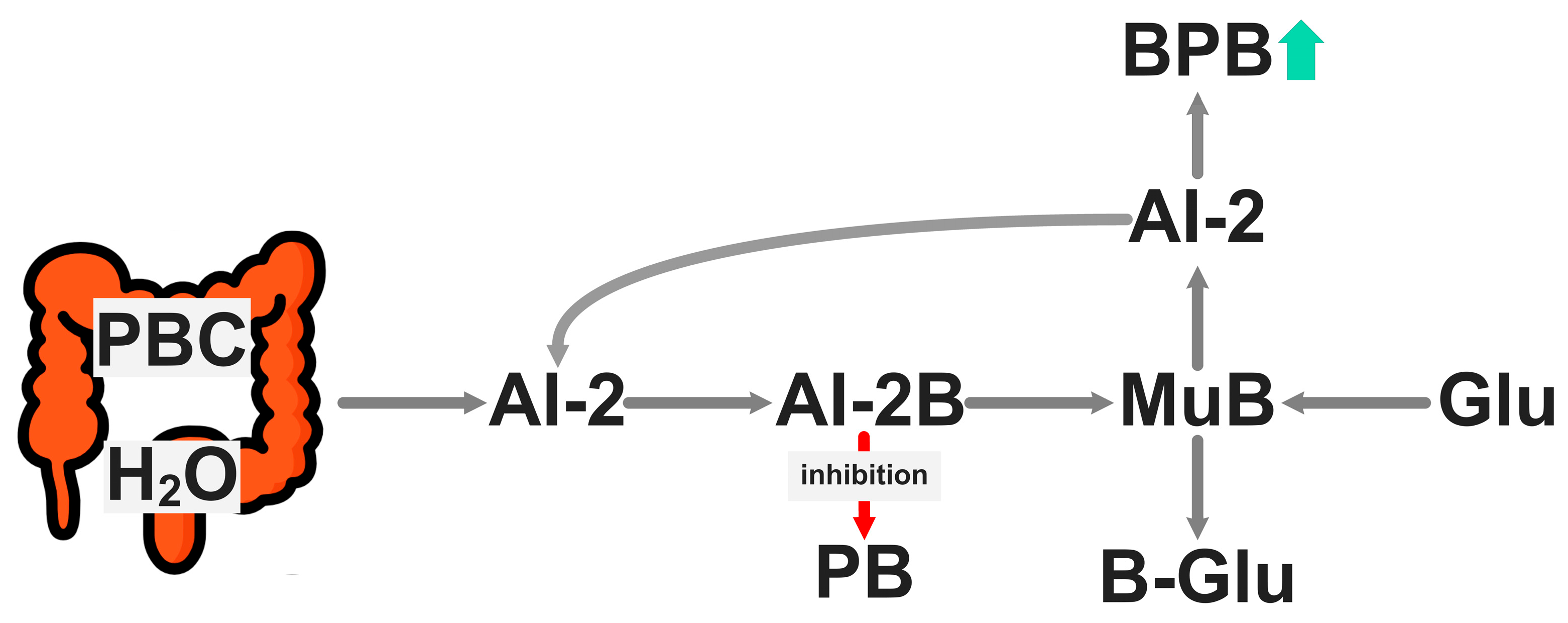
4. Microbiota-Accessible Boron Complexes to Longer Healthspan
5. Microbiota-Accessible Borate Diet: A Targeted and Precise Nutrition
6. Trends and Perspectives: Essentiality of Boron in Host–Microbiome Symbiosis and Targeted Precision Nutrition
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-CQA | 3-O-caffeoylquinic acid; |
| 4-CQA | 4-O-caffeoylquinic acid; |
| 5-CQA | 5-O-caffeoylquinic acid; |
| AI-2 | Autoinducer-2; |
| AI-2B | Autoinducer-2–borate; |
| AD | Alzheimer’s disease; |
| ADME | Absorption, distribution, metabolism, and excretion; |
| AMP | Adenosine monophosphate; |
| AMPK | Adenosine monophosphate-activated protein kinase; |
| B | Boron; |
| B-Glu | Monosaccharide–boron complex; |
| BA | Boric acid; |
| BBB | Blood–brain barrier; |
| BDNF | Brain-derived neurotrophic factor; |
| BMI | Body mass index; |
| BND | Boron nutrient density; |
| BNDAM | Accessible to microbiota boron nutrient density; |
| BNDT | Total boron nutrient density; |
| BP | Blood pressure; |
| BPB | Butyrate-producing bacteria; |
| BPE | Borate–phenolic ester; |
| BPP | Borate–pectic polysaccharide; |
| BUT | Butyrate; |
| Ca | Calcium; |
| CA | Chlorogenic acid; |
| CTX | C-terminal telopeptide of type I collagen; |
| Cu | Copper; |
| CV | Cardiovascular; |
| CVD | Cardiovascular disease; |
| DASH | Dietary approaches to stopping hypertension; |
| DCB | Diester chlorogenoborate; |
| DNA | Deoxyribonucleic acid; |
| DPD | 4,5-Dihydroxy-2,3-pentanedione; |
| DYS | Dysbiosis; |
| FAs | Fatty acids; |
| Fe | Iron; |
| FGF21 | Fibroblast growth factor 21; |
| FMD | Fasting-mimicking diet; |
| FOS | Fructooligosaccharides; |
| Glu | Monosaccharide; |
| GM | Gut microbiota; |
| GMD | Green Mediterranean diet; |
| HbA1C | Glycated hemoglobin; |
| HDL | High-density lipoprotein; |
| Hg | Mercury; |
| HipA | Hippuric acid; |
| HMS | Host–microbiota symbiosis; |
| IGF-1 | Insulin-like growth factor-1; |
| J-diet | Japanese diet; |
| K | Potassium; |
| K-diet | Korean diet; |
| LDL | Low-density lipoprotein; |
| MAB | Microbiota-accessible borate; |
| MCFAs | Medium-chain fatty acids; |
| MD | Mediterranean diet; |
| Mg | Magnesium; |
| MuB | Mucin gel–borate complex; |
| Na | Sodium; |
| ND | Nordic diet; |
| NSAIDs | Non-steroidal anti-inflammatory drugs; |
| PB | Bacterial pathogens; |
| PBC | Prebiotic boron complex; |
| PTH | Parathyroid hormone; |
| PUFAs | Polyunsaturated fatty acids; |
| QS | Quorum sensing; |
| (R)-THMF | (2R,4S)-2-Methyl-2,3,3,4-tetrahydroxytetrahydrofuran; |
| RG-II | Rhamnogalacturonan-II; |
| rRNA | Ribosomal ribonucleic acid; |
| S | Sulfur; |
| (S)-THMF-borate | (2S,4S)-2-Methyl-2,3,3,4-tetrahydroxytetrahydrofuran-borate; |
| SCFAs | Short-chain fatty acids; |
| SIRT1 | Sirtuin 1; |
| T2D | Type 2 diabetes; |
| TRE | Time-restricted eating; |
| UL | Tolerable upper intake level; |
| UroA | Urolithin A; |
| Zn | Zinc. |
References
- National Academy of Medicine; Commission for a Global Roadmap for Healthy Longevity. Global Roadmap for Healthy Longevity, 1st ed.; The National Academies Press: Washington, DC, USA, 2022; pp. 1–238. [Google Scholar] [CrossRef]
- Welsh, C.E.; Matthews, F.E.; Jagger, C. Trends in life expectancy and healthy life years at birth and age 65 in the UK, 2008–2016, and other countries of the EU28: An observational cross-sectional study. Lancet Reg. Health Eur. 2021, 2, 100023. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, R.A.C.; Pinto, A.C. Healthy longevity: A systematic review of nutrological and lifestyle aspects. Int. J. Nutrol. 2023, 16, 1–9. [Google Scholar] [CrossRef]
- Perls, T.T.; Tan, E.J. Healthy Longevity: An Introduction to the Special Issue. J. Gerontol. Ser. A 2019, 74, S1–S3. [Google Scholar] [CrossRef]
- Vera-Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Matoh, T.; Kobayashi, M. Boron and calcium, essential inorganic constituents of pectic polysaccharides in higher plant cell walls. J. Plant Res. 1998, 111, 179–190. [Google Scholar] [CrossRef]
- Pagliuso, D.; Grandis, A.; Igarashi, E.S.; Lam, E.; Buckeridge, M.S. Correlation of Apiose Levels and Growth Rates in Duckweeds. Front. Chem. 2018, 6, 291. [Google Scholar] [CrossRef]
- Patova, O.A.; Golovchenko, V.V.; Ovodov, Y.S. Pectic polysaccharides: Structure and properties. Russ. Chem. Bull. 2014, 63, 1901–1924. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Ying, X.; Ma, A.; Li, Z.; Liu, H.; Guo, Q. Fermentation properties and prebiotic potential of different pectins and their corresponding enzymatic hydrolysates. Food Hydrocoll. 2023, 143, 108878. [Google Scholar] [CrossRef]
- Funakawa, H.; Miwa, K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015, 6, 223. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Plaza-Diaz, J. Dietary Polysaccharides as Modulators of the Gut Microbiota Ecosystem: An Update on Their Impact on Health. Nutrients 2022, 14, 4116. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, E.; Voragen, A.G.J.; Kort, R. The pectin metabolizing capacity of the human gut microbiota. Crit. Rev. Food Sci. Nutr. 2024, 12, 1–23. [Google Scholar] [CrossRef]
- Scorei, I.R.; Bita, A.; Dinca, L.; Mogosanu, G.D.; Rangavaila, N. Borate Complexes of Chlorogenic Acid and Uses Thereof. International Patent Application WO 2023/070074 A1. 27 April 2023. Available online: https://patents.google.com/patent/WO2023070074A1/en (accessed on 3 March 2025).
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Bolat, M.; Köse, D.A. Boron Ester compounds as daily food supplements. J. Mol. Struct. 2025, 1335, 142022. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Mogoşanu, G.D.; Bejenaru, L.E.; Biţă, C.E.; Dinescu, V.C.; Rău, G.; Ciocîlteu, M.V.; Bejenaru, C.; Croitoru, O. Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview. Inorganics 2024, 12, 308. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Araújo Sugizaki, C.S.; Lima, G.C.; Veloso Naves, M.M. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Li, Z.; Kanwal, R.; Yue, X.; Li, M.; Xie, A. Polyphenols and intestinal microorganisms: A review of their interactions and effects on human health. Food Biosci. 2024, 62, 105220. [Google Scholar] [CrossRef]
- Sentürk, N.B.; Kasapoglu, B.; Sahin, E.; Ozcan, O.; Ozansoy, M.; Ozansoy, M.B.; Siyah, P.; Sezerman, U.; Sahin, F. The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study. Pharmaceuticals 2024, 17, 1334. [Google Scholar] [CrossRef] [PubMed]
- Biţă, C.E.; Scorei, I.R.; Vreju, A.F.; Muşetescu, A.E.; Mogoşanu, G.D.; Biţă, A.; Dinescu, V.C.; Dinescu, Ş.C.; Criveanu, C.; Bărbulescu, A.L.; et al. Microbiota-Accessible Boron-Containing Compounds in Complex Regional Pain Syndrome. Medicina 2023, 59, 1965. [Google Scholar] [CrossRef]
- Scorei, I.R.; Bita, A.; Mogosanu, G.D.; Dinca, L.; Neamtu, J.; Rogoveanu, I.; Gheonea, D.I. Microbiota-Accessible Complexes for Healthspan Extension and Uses Thereof. United States Patent and Trademark Office (USPTO), Provisional Patent Application No. 63536442. 5 May 2025. Available online: https://www.uspto.gov/patents (accessed on 12 May 2025).
- Gordon, S. Elie Metchnikoff, the Man and the Myth. J. Innate Immun. 2016, 8, 223–227. [Google Scholar] [CrossRef]
- Salazar, J.; Durán, P.; Díaz, M.P.; Chacín, M.; Santeliz, R.; Mengual, E.; Gutiérrez, E.; León, X.; Díaz, A.; Bernal, M.; et al. Exploring the Relationship between the Gut Microbiota and Ageing: A Possible Age Modulator. Int. J. Environ. Res. Public Health 2023, 20, 5845. [Google Scholar] [CrossRef]
- Conway, J.; Duggal, N.A. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- El Maï, M.; Bird, M.; Allouche, A.; Targen, S.; Şerifoğlu, N.; Lopes-Bastos, B.; Guigonis, J.M.; Kang, D.; Pourcher, T.; Yue, J.X.; et al. Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish. Nat. Aging 2023, 3, 567–584. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Rainey, C.J.; Nyquist, L.A.; Christensen, R.E.; Strong, P.L.; Culver, B.D.; Coughlin, J.R. Daily Boron Intake from the American Diet. J. Am. Diet. Assoc. 1999, 99, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kan, F.; Kucukkurt, I. The effects of boron on some biochemical parameters: A review. J. Trace Elem. Med. Biol. 2023, 79, 127249. [Google Scholar] [CrossRef] [PubMed]
- Kucukkurt, I.; Ince, S.; Eryavuz, A.; Demirel, H.H.; Arslan-Acaroz, D.; Zemheri-Navruz, F.; Durmus, I. The effects of boron-supplemented diets on adipogenesis-related gene expressions, anti-inflammatory, and antioxidative response in high-fat fed rats. J. Biochem. Mol. Toxicol. 2023, 37, e23257. [Google Scholar] [CrossRef] [PubMed]
- Kuru, R.; Yilmaz, S.; Balan, G.; Tuzuner, B.A.; Tasli, P.N.; Akyuz, S.; Yener Ozturk, F.; Altuntas, Y.; Yarat, A.; Sahin, F. Boron-rich diet may regulate blood lipid profile and prevent obesity: A non-drug and self-controlled clinical trial. J. Trace Elem. Med. Biol. 2019, 54, 191–198. [Google Scholar] [CrossRef]
- Ramezani-Jolfaie, N.; Mohammadi, M.; Salehi-Abargouei, A. Effects of a healthy Nordic diet on weight loss in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Eat. Weight Disord. 2020, 25, 1141–1150. [Google Scholar] [CrossRef]
- Pétursson, J.Þ.; Hafstein, V.T. Stirring Up Skyr: From Live Cultures to Cultural Heritage. J. Am. Folk. 2022, 135, 49–74. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Abrahamsen, R.K. Traditional and modern Nordic fermented milk products: A review. Int. Dairy J. 2023, 142, 105641. [Google Scholar] [CrossRef]
- Massara, P.; Zurbau, A.; Glenn, A.J.; Chiavaroli, L.; Khan, T.A.; Viguiliouk, E.; Mejia, S.B.; Comelli, E.M.; Chen, V.; Schwab, U.; et al. Nordic dietary patterns and cardiometabolic outcomes: A systematic review and meta-analysis of prospective cohort studies and randomised controlled trials. Diabetologia 2022, 65, 2011–2031. [Google Scholar] [CrossRef]
- Jafari, R.S.; Behrouz, V. Nordic diet and its benefits in neurological function: A systematic review of observational and intervention studies. Front. Nutr. 2023, 10, 1215358. [Google Scholar] [CrossRef]
- Landberg, R.; Hanhineva, K. Biomarkers of a Healthy Nordic Diet—From Dietary Exposure Biomarkers to Microbiota Signatures in the Metabolome. Nutrients 2020, 12, 27. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Baldivia, A.S.; Ibarra, G.R.; Barra, J.D.E. Will boron be essential for human nutrition? Arch. Latinoam. Nutr. 2016, 66, 82–83. Available online: https://www.alanrevista.org/ediciones/2016/1/art-10/ (accessed on 6 March 2025).
- Vázquez-Cuesta, S.; Lozano García, N.; Rodríguez-Fernández, S.; Fernández-Avila, A.I.; Bermejo, J.; Fernández-Avilés, F.; Muñoz, P.; Bouza, E.; Reigadas, E. Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients 2024, 16, 793. [Google Scholar] [CrossRef]
- Martinez-Lacoba, R.; Pardo-Garcia, I.; Amo-Saus, E.; Escribano-Sotos, F. Mediterranean diet and health outcomes: A systematic meta-review. Eur. J. Public Health 2018, 28, 955–961. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Yaskolka Meir, A.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L.; et al. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
- Pachter, D.; Kaplan, A.; Tsaban, G.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Levakov, G.; Salti, M.; Yovell, Y.; Huhn, S.; et al. Glycemic control contributes to the neuroprotective effects of Mediterranean and green-Mediterranean diets on brain age: The DIRECT PLUS brain-magnetic resonance imaging randomized controlled trial. Am. J. Clin. Nutr. 2024, 120, 1029–1036. [Google Scholar] [CrossRef]
- Farfán-García, E.D.; Abad-García, A.; Alatorre, A.; Pérez-Capistran, T.; Querejeta, E.; Soriano-Ursúa, M.A. Olive oil limited motor disruption and neuronal damage in parkinsonism induced by MPTP administration. Toxicol. Res. Appl. 2020, 4, 2397847320922939. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The effect of a high-polyphenol Mediterranean diet (Green-MED) combined with physical activity on age-related brain atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef]
- Rinott, E.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Alufer, L.; Tsaban, G.; Rinott, E.; Kaplan, A.; Yaskolka Meir, A.; Zelicha, H.; Ceglarek, U.; Isermann, B.; Blüher, M.; Stumvoll, M.; et al. Long-term green-Mediterranean diet may favor fasting morning cortisol stress hormone; the DIRECT-PLUS clinical trial. Front. Endocrinol. 2023, 14, 1243910. [Google Scholar] [CrossRef] [PubMed]
- Zelicha, H.; Kloting, N.; Kaplan, A.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Chassidim, Y.; Bluher, M.; Ceglarek, U.; Isermann, B.; et al. The effect of high-polyphenol Mediterranean diet on visceral adiposity: The DIRECT PLUS randomized controlled trial. BMC Med. 2022, 20, 327. [Google Scholar] [CrossRef]
- Kim, J.; Jo, I.; Joung, H. A rice-based traditional dietary pattern is associated with obesity in Korean adults. J. Acad. Nutr. Diet. 2012, 112, 246–253. [Google Scholar] [CrossRef]
- Saeki, T. Comparative Study on ’Dietary Education’ in Japan and Korea: From the Latest Nutritional Knowledge Perspective. Int. J. Glob. Health 2024, 2, 1–17. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, M.S.; Park, Y.S.; Lee, H.J.; Kang, S.; Lee, H.S.; Lee, K.E.; Yang, H.J.; Kim, M.J.; et al. Korean diet: Characteristics and historical background. J. Ethn. Foods 2016, 3, 26–31. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Soon-Hee, K.; Chung, K.R.; Daily, J.W.; Park, S. Science and philosophy of Korea traditional foods (K-food). J. Ethn. Foods 2023, 10, 26. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary Exposure of the Japanese General Population to Elements: Total Diet Study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef]
- Choi, M.K.; Jun, Y.S. Analysis of Boron Content in Frequently Consumed Foods in Korea. Biol. Trace Elem. Res. 2008, 126, 13–26. [Google Scholar] [CrossRef]
- Sirichakwal, P.P.; Sranacharoenpong, K.; Tontisirin, K. Food based dietary guidelines (FBDGs) development and promotion in Thailand. Asia Pac. J. Clin. Nutr. 2011, 20, 477–483. Available online: http://apjcn.nhri.org.tw/server/APJCN/20/3/477.pdf (accessed on 10 March 2025).
- Taechangam, S.; Pinitchun, U.; Pachotikarn, C. Development of nutrition education tool: Healthy eating index in Thailand. Asia Pac. J. Clin. Nutr. 2008, 17, 365–367. Available online: http://apjcn.nhri.org.tw/server/APJCN/17%20Suppl%201//365.pdf (accessed on 10 March 2025). [PubMed]
- Suvarnakich, K. The Thai diet. Nutr. Rev. 1950, 8, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, R.A.I.; de Silva, P.G.S.M.; Ekanayaka, M.K.I.; Jayathilake, W.M.M.; Pathirana, R.P.M.M.R.; Amaratunga, Y.N.; De Silva, P.J.D.; Perera, B. Effect of different forms of coconut on the lipid profile in normal free-living healthy subjects: A randomized controlled trial (Phase II). Glob. Epidemiol. 2024, 7, 100138. [Google Scholar] [CrossRef] [PubMed]
- Chavasit, V.; Kasemsup, V.; Tontisirin, K. Thailand conquered under-nutrition very successfully but has not slowed obesity. Obes. Rev. 2013, 14, 96–105. [Google Scholar] [CrossRef][Green Version]
- Chadare, F.J.; Affonfere, M.; Sacla Aidé, E.; Fassinou, F.K.; Salako, K.V.; Pereko, K.; Deme, B.; Failler, P.; Glèlè Kakaï, R.L.; Assogbadjo, A.E. Current state of nutrition in West Africa and projections to 2030. Glob. Food Secur. 2022, 32, 100602. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Osuagwu, C.G. Forest West African Indigenous diet and modernization diseases. Funct. Foods Health Dis. 2019, 9, 772–787. [Google Scholar] [CrossRef]
- Ramaboli, M.C.; Ocvirk, S.; Khan Mirzaei, M.; Eberhart, B.L.; Valdivia-Garcia, M.; Metwaly, A.; Neuhaus, K.; Barker, G.; Ru, J.; Nesengani, L.T.; et al. Diet changes due to urbanization in South Africa are linked to microbiome and metabolome signatures of Westernization and colorectal cancer. Nat. Commun. 2024, 15, 3379. [Google Scholar] [CrossRef]
- Kellman, R. The Microbiome Diet: The Scientifically Proven Way to Restore Your Gut Health and Achieve Permanent Weight Loss, 1st ed.; Da Capo Lifelong Books: Boston, MA, USA, 2014; pp. 3–232. Available online: https://archive.org/details/microbiomedietsc0000kell (accessed on 10 March 2025).
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Kellman, R. Your Microbiome Superfoods. Available online: http://richardhruby.com/wp-content/uploads/2018/11/Microbiome-Protocol-Raphael-Kellman-MD.pdf (accessed on 10 March 2025).
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Babcock, M.C.; Robinson, A.T.; Migdal, K.U.; Watso, J.C.; Wenner, M.M.; Stocker, S.D.; Farquhar, W.B. Reducing Dietary Sodium to 1000 mg per Day Reduces Neurovascular Transduction Without Stimulating Sympathetic Outflow. Hypertension 2019, 73, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Svetkey, L.P.; Sacks, F.M.; Obarzanek, E.; Vollmer, W.M.; Appel, L.J.; Lin, P.H.; Karanja, N.M.; Harsha, D.W.; Bray, G.A.; Aickin, M.; et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-Sodium): Rationale and Design. DASH-Sodium Collaborative Research Group. J. Am. Diet. Assoc. 1999, 99, S96–S104. [Google Scholar] [CrossRef]
- Shirani, F.; Salehi-Abargouei, A.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: A systematic review and meta-analysis on controlled clinical trials. Nutrition 2013, 29, 939–947. [Google Scholar] [CrossRef]
- Ziaee, A.; Afaghi, A.; Sarreshtehdari, M. Effect of low glycemic load diet on glycated hemoglobin (HbA1c) in poorly-controlled diabetes patients. Glob. J. Health Sci. 2011, 4, 211–216. [Google Scholar] [CrossRef][Green Version]
- Shoaibinobarian, N.; Danehchin, L.; Mozafarinia, M.; Hekmatdoost, A.; Eghtesad, S.; Masoudi, S.; Mohammadi, Z.; Mard, A.; Paridar, Y.; Abolnezhadian, F.; et al. The Association between DASH Diet Adherence and Cardiovascular Risk Factors. Int. J. Prev. Med. 2023, 14, 24. [Google Scholar] [CrossRef]
- Onwuzo, C.; Olukorode, J.O.; Omokore, O.A.; Odunaike, O.S.; Omiko, R.; Osaghae, O.W.; Sange, W.; Orimoloye, D.A.; Kristilere, H.O.; Addeh, E.; et al. DASH Diet: A Review of Its Scientifically Proven Hypertension Reduction and Health Benefits. Cureus 2023, 15, e44692. [Google Scholar] [CrossRef]
- Dietary Approaches to Stop Hypertension (DASH) Protocol: Version 4.0. 9 November 1995. Available online: https://biolincc.nhlbi.nih.gov/media/studies/dash/Protocol.pdf?link_time=2019-07-01_01:34:12.714420 (accessed on 10 March 2025).
- Parikh, A.; Lipsitz, S.R.; Natarajan, S. Association between a DASH-like diet and mortality in adults with hypertension: Findings from a population-based follow-up study. Am. J. Hypertens. 2009, 22, 409–416. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- O’Leary, J.; Georgeaux-Healy, C.; Serpell, L. The impact of continuous calorie restriction and fasting on cognition in adults without eating disorders. Nutr. Rev. 2025, 83, 146–159. [Google Scholar] [CrossRef]
- Di Francesco, A.; Deighan, A.G.; Litichevskiy, L.; Chen, Z.; Luciano, A.; Robinson, L.; Garland, G.; Donato, H.; Vincent, M.; Schott, W.; et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 2024, 634, 684–692. [Google Scholar] [CrossRef]
- Nye, K.; Cherrin, C.; Meires, J. Intermittent Fasting: Exploring Approaches, Benefits, and Implications for Health and Weight Management. J. Nurse Pract. 2024, 20, 104893. [Google Scholar] [CrossRef]
- Teker, H.T.; Ceylani, T. Intermittent fasting supports the balance of the gut microbiota composition. Int. Microbiol. 2023, 26, 51–57. [Google Scholar] [CrossRef]
- Brandhorst, S.; Levine, M.E.; Wei, M.; Shelehchi, M.; Morgan, T.E.; Nayak, K.S.; Dorff, T.; Hong, K.; Crimmins, E.M.; Cohen, P.; et al. Fasting-mimicking diet causes hepatic and blood markers changes indicating reduced biological age and disease risk. Nat. Commun. 2024, 15, 1309. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Ianiro, G.; Laterza, L.; Lopetuso, L.R.; Ponziani, F.R.; Gasbarrini, A.; Mele, M.C. Gut Microbiota during Dietary Restrictions: New Insights in Non-Communicable Diseases. Microorganisms 2020, 8, 1140. [Google Scholar] [CrossRef]
- Angoorani, P.; Ejtahed, H.S.; Hasani-Ranjbar, S.; Siadat, S.D.; Soroush, A.R.; Larijani, B. Gut microbiota modulation as a possible mediating mechanism for fasting-induced alleviation of metabolic complications: A systematic review. Nutr. Metab. 2021, 18, 105. [Google Scholar] [CrossRef]
- Nordic Nutrition Recommendations 2023: Integrating Environmental Aspects; Nordic Council of Ministers: Copenhagen, Denmark, 2023; Available online: https://www.norden.org/en/publication/nordic-nutrition-recommendations-2023 (accessed on 12 March 2025).
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2023, 23, 75–89. [Google Scholar] [CrossRef]
- Liao, P.; Yan, B.; Wang, C.; Lei, P. Telomeres: Dysfunction, Maintenance, Aging and Cancer. Aging Dis. 2023, 15, 2595–2631. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Molinuevo, J.L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10, S296–S303. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Rău, G.; Ciocîlteu, M.V.; Mogoşanu, G.D. Zinc-Boron Complex-Based Dietary Supplements for Longevity and Healthy Life. Curr. Health Sci. J. 2023, 49, 381–387. [Google Scholar] [CrossRef]
- Nielsen, F.H. Boron in Aging and Longevity. In Trace Elements and Minerals in Health and Longevity, 1st ed.; Malavolta, M., Mocchegiani, E., Eds.; Healthy Ageing and Longevity (HAL); Springer: Cham, Switzerland, 2018; Volume 8, pp. 163–177. [Google Scholar] [CrossRef]
- Yazbeck, C.; Kloppmann, W.; Cottier, R.; Sahuquillo, J.; Debotte, G.; Huel, G. Health Impact Evaluation of Boron in Drinking Water: A Geographical Risk Assessment in Northern France. Environ. Geochem. Health 2005, 27, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sepp, E.; Smidt, I.; Rööp, T.; Štšepetova, J.; Kõljalg, S.; Mikelsaar, M.; Soidla, I.; Ainsaar, M.; Kolk, H.; Vallas, M.; et al. Comparative Analysis of Gut Microbiota in Centenarians and Young People: Impact of Eating Habits and Childhood Living Environment. Front. Cell Infect. Microbiol. 2022, 12, 851404. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving healthy aging through gut microbiota-directed dietary intervention: Focusing on microbial biomarkers and host mechanisms. J. Adv. Res. 2024, 68, 179–200. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.X.Y.; Kim, H.; Faylon, L.E.; Martin, K.A.; Purbojati, R.; Drautz-Moses, D.I.; Ghosh, S.; et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Shin, J.; Noh, J.R.; Choe, D.; Lee, N.; Song, Y.; Cho, S.; Kang, E.J.; Go, M.J.; Ha, S.K.; Chang, D.H.; et al. Ageing and rejuvenation models reveal changes in key microbial communities associated with healthy ageing. Microbiome 2021, 9, 240. [Google Scholar] [CrossRef]
- Han, K.; Jin, W.; Mao, Z.; Dong, S.; Zhang, Q.; Yang, Y.; Chen, B.; Wu, H.; Zeng, M. Microbiome and butyrate production are altered in the gut of rats fed a glycated fish protein diet. J. Funct. Foods 2018, 47, 423–433. [Google Scholar] [CrossRef]
- Di Rosa, C.; Di Francesco, L.; Spiezia, C.; Khazrai, Y.M. Effects of Animal and Vegetable Proteins on Gut Microbiota in Subjects with Overweight or Obesity. Nutrients 2023, 15, 2675. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Sun, Z.; Grimm, V.; Riedel, C.U. AI-2 to the rescue against antibiotic-induced intestinal dysbiosis? Trends Microbiol. 2015, 23, 327–328. [Google Scholar] [CrossRef]
- McKenney, E.S.; Kendall, M.M. Microbiota and pathogen ’pas de deux’: Setting up and breaking down barriers to intestinal infection. Pathog. Dis. 2016, 74, ftw051. [Google Scholar] [CrossRef]
- Ji, Y.C.; Sun, Q.; Fu, C.Y.; She, X.; Liu, X.C.; He, Y.; Ai, Q.; Li, L.Q.; Wang, Z.L. Exogenous Autoinducer-2 Rescues Intestinal Dysbiosis and Intestinal Inflammation in a Neonatal Mouse Necrotizing Enterocolitis Model. Front. Cell. Infect. Microbiol. 2021, 11, 694395. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Seidel, J.; Valenzano, D.R. The role of the gut microbiome during host ageing. F1000Research 2018, 7, 1086. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Dugan, B.; Conway, J.; Duggal, N.A. Inflammaging as a target for healthy ageing. Age Ageing 2023, 52, afac328. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarães, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Cuadra, G.A.; Frantellizzi, A.J.; Gaesser, K.M.; Tammariello, S.P.; Ahmed, A. Autoinducer-2 detection among commensal oral streptococci is dependent on pH and boric acid. J. Microbiol. 2016, 54, 492–502. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N. Symbiotic Interactions of Archaea in Animal and Human Microbiomes. Curr. Clin. Microbiol. Rep. 2023, 10, 161–173. [Google Scholar] [CrossRef]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Hu, R.; Yang, T.; Ai, Q.; Shi, Y.; Ji, Y.; Sun, Q.; Tong, B.; Chen, J.; Wang, Z. Autoinducer-2 promotes the colonization of Lactobacillus rhamnosus GG to improve the intestinal barrier function in a neonatal mouse model of antibiotic-induced intestinal dysbiosis. J. Transl. Med. 2024, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015, 10, 1861–1871. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Kremer, D.; Post, A.; Seidel, U.; Huebbe, P.; van der Veen, Y.; Groothof, D.; Gomes-Neto, A.W.; Knobbe, T.J.; Lüersen, K.; Eisenga, M.F.; et al. Boron Intake and decreased risk of mortality in kidney transplant recipients. Eur. J. Nutr. 2022, 61, 973–984. [Google Scholar] [CrossRef]
- Hsiao, A.; Ahmed, A.M.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A., Jr.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef]
- Sizmaz, O.; Koksal, B.H.; Yildiz, G. Rumen microbial fermentation, protozoan abundance and boron availability in yearling rams fed diets with different boron concentrations. J. Anim. Feed Sci. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Mitruţ, I.; Cojocaru, M.O.; Scorei, I.R.; Biţă, A.; Mogoşanu, G.D.; Popescu, M.; Olimid, D.A.; Manolea, H.O. Preclinical and histological study of boron-containing compounds hydrogels on experimental model of periodontal disease. Rom. J. Morphol. Embryol. 2021, 62, 219–226. [Google Scholar] [CrossRef]
- Becker, W.; Brasseur, D.; Bresson, J.L.; Flynn, A.; Jackson, A.A.; Lagiou, P.; Mingrone, G.; Moseley, B.; Palou, A.; Przyrembel, H.; et al. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Boron (Sodium Borate and Boric Acid) (Request No. EFSA-Q-2003-018). EFSA J. 2004, 80, 1–22. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Fan, S.; Ma, J.; Yan, Y.; Wang, M.; Tang, X.; Lv, P.; Zhang, Y. Targeted delivery of food functional ingredients in precise nutrition: Design strategy and application of nutritional intervention. Crit. Rev. Food Sci. Nutr. 2024, 64, 7854–7877. [Google Scholar] [CrossRef]
- Kalache, A.; Bazinet, R.P.; Carlson, S.; Evans, W.J.; Kim, C.H.; Lanham-New, S.; Visioli, F.; Griffiths, J.C. Science-based policy: Targeted nutrition for all ages and the role of bioactives. Eur. J. Nutr. 2021, 60, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.P.; Luo, Y.; Wu, X.T.; Ansari, A.R.; Wang, J.; Yang, K.L.; Xiao, K.; Peng, K.M. Effects of Supplemental Boron on Intestinal Proliferation and Apoptosis in African Ostrich Chicks [Efectos del Boro Suplementario sobre la Proliferación Intestinal y Apoptosis en Polluelos de Avestruz Africana]. Int. J. Morphol. 2016, 34, 830–835. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-Improving Effects of Polyphenols on the Human Intestinal Microbiota: A Review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef] [PubMed]

| Dietary Pattern | Main Features | References |
|---|---|---|
| Nordic diet | ▪ vegetables and fruits (cabbage, berries, root vegetables and legumes), wild-gathered herbs and mushrooms, potatoes, fresh herbs, nuts, whole-grain cereals, whole grains, oily fish (salmon, herring, mackerel), rapeseed oil, shellfish, seaweed, white and low-fat meat, low-fat dairy products, game (Nordic Nutrition Recommendations 2023 suggest 10 mg/day of B); ▪ avoiding products sweetened with sugar. | [38,39,40,93] |
| Mediterranean diet | ▪ whole grains and unprocessed cereals (such as whole wheat bread, whole wheat pasta, and brown rice), along with fresh fruits, vegetables, nuts, and low-fat dairy products; ▪ olive oil as the primary fat source; ▪ moderate intake of alcohol, ideally red wine, consumed with meals; ▪ balanced consumption of fish, poultry, potatoes, eggs, and sweets; ▪ limited intake of red meat, typically on a monthly basis; ▪ engaging in regular physical activity. | [44,46] |
| Green Mediterranean diet | ▪ more plant-based foods and more polyphenols (Mankai plant, green tea, coffee, cocoa, berries, raisins, red wine, red onions, herbs and spices, olives, olive oil, flaxseed); ▪ sources of animal protein, such as dairy and fish; ▪ no red meat. | [50,51,64] |
| Japanese and Korean diet | ▪ various rice- and grain-based recipes; ▪ more fermented foods (“natto”, “kimchi”, “jang”); ▪ more vegetables from wild landscapes and the seas; ▪ more legumes and fish; ▪ more medicinal herbs (green tea, garlic, green onions, red peppers, ginger); ▪ more sesame oil and Japanese basil oil (perilla); ▪ more home-cooked meals (below 100 °C without using oil); ▪ less red meat and limited deep-fried cooking with fat. | [57,58,59,60] |
| Thai diet | ▪ rice and starch (rice, bread, cereals, pasta); ▪ vegetables, fruits, dried beans, and nuts; ▪ dairy (milk, yogurt, cheese, coconut milk); ▪ meat (poultry, fish, eggs) and seafood; ▪ spices (lemongrass, papaya, chili peppers, Thai basil, turmeric). | [64,65,77] |
| West African diet | ▪ low content of proteins; ▪ high content of starchy carbohydrates (yams, cassava, tubers, rice, sorghum); ▪ spices (garlic, melon seeds, African nutmeg). | [68,70] |
| “Microbiome” diet | ▪ organic, plant-based diet and foods rich in prebiotics (asparagus, garlic, onions, leeks); ▪ fermented foods rich in probiotics (sauerkraut, “kimchi”, kefir, yogurt); ▪ certain supplements (probiotics, Zn, vitamin D, berberine, grapefruit seed extract, wormwood, oregano oil); ▪ dairy, free-range eggs, gluten-free grains, and legumes; ▪ non-starchy fruits and vegetables (mangoes, melons, peaches, pears, sweet potatoes, yams); ▪ grass-fed meats and low-Hg wild-caught fish. | [72] |
| DASH diet | ▪ vegetables, fruits, and whole grains; ▪ fat-free or low-fat dairy products, fish, poultry, beans, and nuts; ▪ less foods that are high in salt, added sugar, and saturated fat (such as in fatty meats and full-fat dairy products). | [77,78] |
| Calorie restricted diet | ▪ reduced consumption of prebiotic B, omega-3 FAs, polyphenols, SCFAs (primarily BUT), MCFAs (such as caproic acid), and probiotic-rich fermented foods (including yogurt, cheese, and pickles); ▪ limiting certain nutrients (such as sulfur, iron, and gluten) for better health and longevity. | [86,87] |
| Intermittent fasting | ▪ daily eating window of 8–10 h, varying in duration from 4 to 12 weeks; ▪ plant-based, low-calorie, low-protein five-day dietary intervention. | [88,89] |
| Food Group | Calories/100 g | B Content (mg/100 g) | BNDT (mg B/1000 Calories) | BNDAM (mg B/1000 Calories) |
|---|---|---|---|---|
| Fruits | 40 | 0.5 | 12.5 | 1.25 |
| Vegetables | 30 | 0.3 | 10 | 1 |
| Seeds | 600 | 1.5 | 2.5 | 0.25 |
| Fermented foods | 70 | 0.15 | 2.1 | 0.21 |
| Marine fish | 150 | 0.12 | 0.8 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biţă, A.; Scorei, I.R.; Soriano-Ursúa, M.A.; Mogoşanu, G.D.; Belu, I.; Ciocîlteu, M.V.; Biţă, C.E.; Rău, G.; Pisoschi, C.G.; Racu, M.-V.; et al. Microbiota-Accessible Borates as Novel and Emerging Prebiotics for Healthy Longevity: Current Research Trends and Perspectives. Pharmaceuticals 2025, 18, 766. https://doi.org/10.3390/ph18060766
Biţă A, Scorei IR, Soriano-Ursúa MA, Mogoşanu GD, Belu I, Ciocîlteu MV, Biţă CE, Rău G, Pisoschi CG, Racu M-V, et al. Microbiota-Accessible Borates as Novel and Emerging Prebiotics for Healthy Longevity: Current Research Trends and Perspectives. Pharmaceuticals. 2025; 18(6):766. https://doi.org/10.3390/ph18060766
Chicago/Turabian StyleBiţă, Andrei, Ion Romulus Scorei, Marvin A. Soriano-Ursúa, George Dan Mogoşanu, Ionela Belu, Maria Viorica Ciocîlteu, Cristina Elena Biţă, Gabriela Rău, Cătălina Gabriela Pisoschi, Maria-Victoria Racu, and et al. 2025. "Microbiota-Accessible Borates as Novel and Emerging Prebiotics for Healthy Longevity: Current Research Trends and Perspectives" Pharmaceuticals 18, no. 6: 766. https://doi.org/10.3390/ph18060766
APA StyleBiţă, A., Scorei, I. R., Soriano-Ursúa, M. A., Mogoşanu, G. D., Belu, I., Ciocîlteu, M. V., Biţă, C. E., Rău, G., Pisoschi, C. G., Racu, M.-V., Pinzaru, I., Contreras-Ramos, A., Kostici, R., Neamţu, J., Biciuşcă, V., & Gheonea, D. I. (2025). Microbiota-Accessible Borates as Novel and Emerging Prebiotics for Healthy Longevity: Current Research Trends and Perspectives. Pharmaceuticals, 18(6), 766. https://doi.org/10.3390/ph18060766








