Pregabalin Safety in Pregnancy: A Disproportionality Analysis of VigiBase Spontaneous Reporting System
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Reported Events
2.3. Disproportionality Analyses for Congenital Anomalies
3. Discussion
3.1. Key Results
3.2. Congenital Anomalies
3.3. Other Reported Events
3.4. Strengths and Limitations
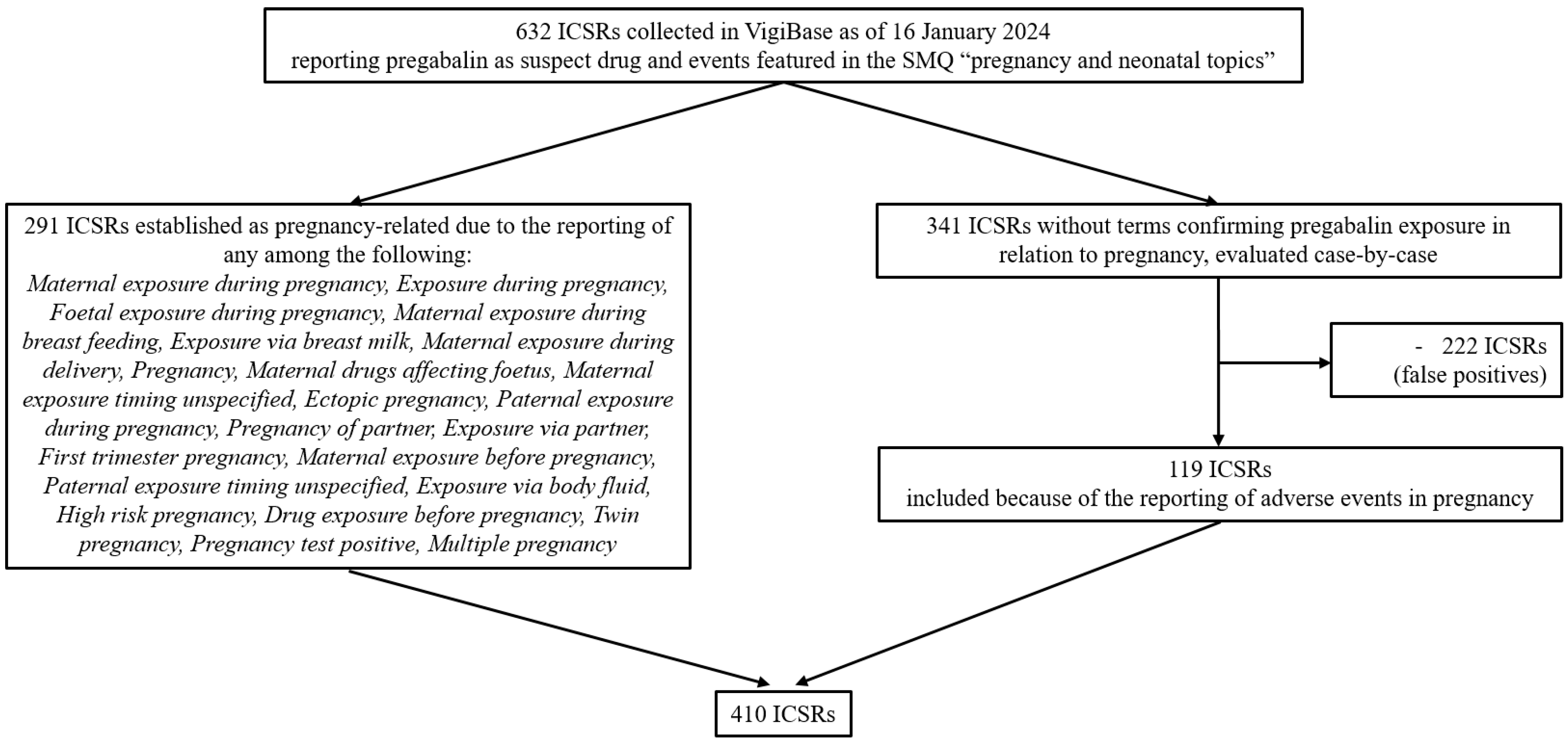
4. Materials and Methods
4.1. Study Design
4.2. Data Source
4.3. Study Population
4.4. Variables
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Congenital anomaly |
| ICSR | Individual case safety report |
| ROR | Reporting odds ratio |
| CI | Confidence interval |
| WHO | World Health Organization |
| MedDRA | Medical Dictionary for Regulatory Activities |
| SMQ | Standardized MedDRA Query |
| SDR | signal of disproportionate reporting |
| ADR | Adverse drug reaction |
References
- Spoendlin, J.; Blozik, E.; Graber, S.; Rauch, M.; Marxer, C.; Rüegg, S.; Meier, C.; Winterfeld, U.; Panchaud, A. Use of valproate in pregnancy and in women of childbearing age between 2014 and 2018 in Switzerland: A retrospective analysis of Swiss healthcare claims data. Swiss Med. Wkly. 2021, 151, w20386. [Google Scholar] [CrossRef] [PubMed]
- Hurault-Delarue, C.; Morris, J.K.; Charlton, R.; Gini, R.; Loane, M.; Pierini, A.; Puccini, A.; Neville, A.; Snowball, J.; Damase-Michel, C.; et al. Prescription of antiepileptic medicines including valproate in pregnant women: A study in three European countries. Pharmacoepidemiol. Drug Saf. 2019, 28, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Asomaning, K.; Abramsky, S.; Liu, Q.; Zhou, X.; Sobel, R.E.; Watt, S. Pregabalin prescriptions in the United Kingdom: A drug utilisation study of The Health Improvement Network (THIN) primary care database. Int. J. Clin. Pract. 2016, 70, 380–388. [Google Scholar] [CrossRef]
- Daugaard, C.A.; Sun, Y.; Dreier, J.W.; Christensen, J. Use of antiepileptic drugs in women of fertile age. Dan. Med. J. 2019, 66, A5563. [Google Scholar]
- Blotière, P.-O.; Raguideau, F.; Weill, A.; Elefant, E.; Perthus, I.; Goulet, V.; Rouget, F.; Zureik, M.; Coste, J.; Dray-Spira, R. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 2019, 93, e167–e180. [Google Scholar] [CrossRef]
- Margulis, A.V.; Hernandez-Diaz, S.; McElrath, T.; Rothman, K.J.; Plana, E.; Almqvist, C.; D’onofrio, B.M.; Oberg, A.S. Relation of in-utero exposure to antiepileptic drugs to pregnancy duration and size at birth. PLoS ONE 2019, 14, e0214180. [Google Scholar] [CrossRef]
- Patorno, E.; Bateman, B.T.; Huybrechts, K.F.; MacDonald, S.C.; Cohen, J.M.; Desai, R.J.; Panchaud, A.; Mogun, H.; Pennell, P.B.; Hernandez-Diaz, S. Pregabalin use early in pregnancy and the risk of major congenital malformations. Neurology 2017, 88, 2020–2025. [Google Scholar] [CrossRef]
- Richardson, J.L.; Damkier, P.; Diav-Citrin, O.; George, N.; Greenall, A.J.; Oliver, A.M.; Stephens, S.; Hodson, K.K. A critical appraisal of controlled studies investigating malformation risks following pregabalin use in early pregnancy. Br. J. Clin. Pharmacol. 2023, 89, 630–640. [Google Scholar] [CrossRef]
- Upjohn UK Limited. Summary of Product Characteristics: Lyrica 100 mg Hard Capsules. Available online: www.medicines.org.uk/emc/product/10303/smpc (accessed on 9 May 2025).
- Food and Drug Adminstration (FDA). Pharmacology Review Lyrica® Pregabalin Capsules. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2006/021446s002,s003lbl.pdf (accessed on 9 May 2025).
- European Medicines Agency. Summary of the European Public Assessment Report: Lyrica Pregabalin. Available online: www.ema.europa.eu/en/medicines/human/EPAR/lyrica (accessed on 9 May 2025).
- Morse, D.C. Embryo-Fetal Developmental Toxicity Studies with Pregabalin in Mice and Rabbits. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2016, 107, 85–93. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, K. Teratogenic Effects of Third-Generation Antiepileptic Drug, Pregabalin: An In vivo Study. Curr. Drug Saf. 2018, 13, 113–121. [Google Scholar] [CrossRef]
- Bergman, J.E.H.; Perraud, A.; Barišić, I.; Kinsner-Ovaskainen, A.; Morris, J.K.; Tucker, D.; Wellesley, D.; Garne, E. Updated EUROCAT guidelines for classification of cases with congenital anomalies. Birth Defects Res. 2024, 116, e2314. [Google Scholar] [CrossRef]
- Re: Product Literature (SmPC and PIL) Pregnancy Section Updates for Pregabalin. 2022. Available online: https://www.entis-org.eu/wp-content/uploads/2022/06/ENTIS-pregabalin-SmPC-updates.pdf (accessed on 9 May 2025).
- Dudukina, E.; Szépligeti, S.K.; Karlsson, P.; Asomaning, K.; Daltveit, A.K.; Hakkarainen, K.; Hoti, F.; Kieler, H.; Lunde, A.; Odsbu, I.; et al. Prenatal exposure to pregabalin, birth outcomes and neurodevelopment—A population-based cohort study in four Nordic countries. Drug Saf. 2023, 46, 661–675. [Google Scholar] [CrossRef]
- Winterfeld, U.; Merlob, P.; Baud, D.; Rousson, V.; Panchaud, A.; Rothuizen, L.E.; Bernard, N.; Vial, T.; Yates, L.M.; Pistelli, A.; et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology 2016, 86, 2251–2257. [Google Scholar] [CrossRef]
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Evoy, K.E.; Sadrameli, S.; Contreras, J.; Covvey, J.R.; Peckham, A.M.; Morrison, M.D. Abuse and Misuse of Pregabalin and Gabapentin: A Systematic Review Update. Drugs 2021, 81, 125–156. [Google Scholar] [CrossRef]
- Benassayag Kaduri, N.; Dressler, R.; Abu Ahmad, W.; Rotshild, V. Trends in Pregabalin Use and Prescribing Patterns in the Adult Population: A 10-Year Pharmacoepidemiologic Study. CNS Drugs 2024, 38, 153–162. [Google Scholar] [CrossRef]
- Zaccaria, C.; Piccolo, L.; Gordillo-Marañón, M.; Touraille, G.; de Vries, C. Identification of Pregnancy Adverse Drug Reactions in Pharmacovigilance Reporting Systems: A Novel Algorithm Developed in EudraVigilance. Drug Saf. 2024, 47, 1127–1136. [Google Scholar] [CrossRef]
- Noseda, R.; Müller, L.; Bedussi, F.; Fusaroli, M.; Raschi, E.; Ceschi, A. Immune checkpoint inhibitors and pregnancy: Analysis of the VigiBase® Spontaneous Reporting System. Cancers 2022, 15, 173. [Google Scholar] [CrossRef]
- Noseda, R.; Bedussi, F.; Gobbi, C.; Ceschi, A.; Zecca, C. Calcitonin gene-related peptide antagonists in pregnancy: A disproportionality analysis in VigiBase®. J. Headache Pain. 2024, 25, 10. [Google Scholar] [CrossRef]
- Noseda, R.; Bedussi, F.; Panchaud, A.; Ceschi, A. Safety of Monoclonal Antibodies Inhibiting PCSK9 in Pregnancy: Disproportionality Analysis in VigiBase®. Clin. Pharmacol. Ther. 2024, 116, 346–350. [Google Scholar] [CrossRef]
- Lagerlund, O.; Strese, S.; Fladvad, M.; Lindquist, M. WHODrug: A global, validated and updated dictionary for medicinal information. Ther. Innov. Regul. Sci. 2020, 54, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, P.M.; Sartori, D.; Tuccori, M.; Crisafulli, S.; Battini, V.; Carnovale, C.; Rafaniello, C.; Capuano, A.; Poluzzi, E.; Moretti, U.; et al. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Front. Drug Saf. Regul. 2024, 3, 1323057. [Google Scholar] [CrossRef]
- Fusaroli, M.; Simonsen, A.; Borrie, S.A.; Low, D.M.; Parola, A.; Raschi, E.; Poluzzi, E.; Fusaroli, R. Identifying Medications Underlying Communication Atypicalities in Psychotic and Affective Disorders: A Pharmacovigilance Study Within the FDA Adverse Event Reporting System. J. Speech Lang. Hear. Res. 2023, 66, 3242–3259. [Google Scholar] [CrossRef]
- Sultana, J.; Scondotto, G.; Cutroneo, P.M.; Morgante, F.; Trifirò, G. Intravitreal Anti-VEGF Drugs and Signals of Dementia and Parkinson-Like Events: Analysis of the VigiBase Database of Spontaneous Reports. Front. Pharmacol. 2020, 11, 315. [Google Scholar] [CrossRef]
- Tian, X.; Chen, L.; Gai, D.; He, S.; Jiang, X.; Zhang, N. Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Front. Pharmacol. 2022, 13, 851246. [Google Scholar] [CrossRef]
- Sandberg, L.; Taavola, H.; Aoki, Y.; Chandler, R.; Norén, G.N. Risk Factor Considerations in Statistical Signal Detection: Using Subgroup Disproportionality to Uncover Risk Groups for Adverse Drug Reactions in VigiBase. Drug Saf. 2020, 43, 999–1009. [Google Scholar] [CrossRef]
- Mahaux, O.; Powell, G.; Haguinet, F.; Sobczak, P.; Saini, N.; Barry, A.; Mustafa, A.; Bate, A. Identifying Safety Subgroups at Risk: Assessing the Agreement Between Statistical Alerting and Patient Subgroup Risk. Drug Saf. 2023, 46, 601–614. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The Reporting of a Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Saf. 2024, 47, 575–584. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024, 47, 585–599. [Google Scholar] [CrossRef]
| Characteristic | n (%), N = 410 ICSRs |
|---|---|
| Country | |
| Europe | 266 (64.9) |
| North America | 109 (26.6) |
| Asia | 13 (3.2) |
| Australia | 13 (3.2) |
| South America | 5 (1.2) |
| Africa | 4 (0.9) |
| Reporting year | |
| 2006–2010 | 52 (12.7) |
| 2011–2015 | 122 (29.8) |
| 2016–2020 | 170 (41.5) |
| 2021–2024 (as of 16 January 2024) | 66 (16.0) |
| Reporter | |
| Physician | 210 (51.2) |
| Other health professional | 103 (25.1) |
| Pharmacist | 14 (3.4) |
| Patient | 63 (15.4) |
| Lawyer | 2 (0.5) |
| Not reported | 18 (4.4) |
| Patient sex | |
| Female | 248 (60.5) |
| Male | 57 (13.9) |
| Not reported | 105 (25.6) |
| Patient age | |
| Adult | |
| Reported | 130 (31.7) |
| Median [IQR], years | 33 [28–38] |
| Not reported (age group: adult) | 28 (6.8) |
| Neonate (<28 days) | 78 (19.0) |
| Infant (≥28 days to ≤1 year) | 27 (6.6) |
| Child (>1 year) | 8 (2.0) |
| Not reported (no age information) | 139 (33.9) |
| Exposure to pregabalin in relation to pregnancy | |
| Before pregnancy | 2 (0.5) |
| During pregnancy | 248 (60.5) |
| During pregnancy and during lactation | 10 (2.4) |
| During lactation | 3 (0.7) |
| Paternal exposure and transmission of drug via semen | 11 (2.7) |
| Unknown | 136 (33.2) |
| Indication | |
| Neuropathic pain | 65 (15.8) |
| Other pain disorder | 51 (12.4) |
| Anxiety/Depression | 49 (12.0) |
| Epilepsy | 40 (9.8) |
| Fibromyalgia | 27 (6.6) |
| Not Reported | 178 (43.4) |
| Co-reporting of additional drugs besides pregabalin | 281 (68.5) |
| Adverse Events in Pregnancy, Coded as MedDRA PTs | n (%), N = 315 ICSRs |
|---|---|
| Pregnancy complications | 35 (11.1) |
| - reported in ≥10% of ICSRs: | |
| Abortion induced | 9 |
| Caesarean section | 7 |
| Premature rupture of membranes | 4 |
| Abortion spontaneous | 104 (33.0) |
| Foetal outcomes | 25 (7.9) |
| - reported in ≥10% of ICSRs: | |
| Foetal growth restriction | 8 |
| Foetal death/Stillbirth | 6 |
| Congenital anomalies § | 82 (26.0) |
| Congenital heart defects | 30 |
| Other neonatal malformations Δ | 20 |
| Nervous system congenital anomalies | 18 |
| Limb congenital anomalies | 12 |
| Genital congenital anomalies | 7 |
| Oro-facial clefts | 6 |
| Chromosomal disorder | 6 |
| Renal congenital anomalies | 5 |
| Gastrointestinal congenital anomalies | 3 |
| Skeletal congenital anomalies | 2 |
| Skin congenital anomalies | 2 |
| Respiratory congenital anomalies | 2 |
| Urinary congenital anomalies | 1 |
| Congenital eye disorder | 1 |
| Abdominal wall defects | 1 |
| Neonatal outcomes | 113 (35.9) |
| - reported in ≥10% of ICSRs: | |
| Premature baby | 33 |
| Small for dates baby | 24 |
| Drug withdrawal syndrome | 17 |
| Neonatal respiratory distress | 12 |
| a | b | c | d | ROR [95% CI] | |
|---|---|---|---|---|---|
| Heart congenital anomalies | 30 | 150,375 | 12,378 | 36,393,041 | 0.587 [0.410–0.839] |
| Nervous system congenital anomalies | 18 | 150,387 | 7414 | 36,398,005 | 0.588 [0.370–0.933] |
| Limb congenital anomalies | 12 | 150,393 | 4327 | 36,401,092 | 0.671 [0.381–1.183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondada, S.; Bedussi, F.; Richardson, J.L.; Noseda, R.; Ceschi, A. Pregabalin Safety in Pregnancy: A Disproportionality Analysis of VigiBase Spontaneous Reporting System. Pharmaceuticals 2025, 18, 759. https://doi.org/10.3390/ph18050759
Mondada S, Bedussi F, Richardson JL, Noseda R, Ceschi A. Pregabalin Safety in Pregnancy: A Disproportionality Analysis of VigiBase Spontaneous Reporting System. Pharmaceuticals. 2025; 18(5):759. https://doi.org/10.3390/ph18050759
Chicago/Turabian StyleMondada, Sarah, Francesca Bedussi, Jonathan L. Richardson, Roberta Noseda, and Alessandro Ceschi. 2025. "Pregabalin Safety in Pregnancy: A Disproportionality Analysis of VigiBase Spontaneous Reporting System" Pharmaceuticals 18, no. 5: 759. https://doi.org/10.3390/ph18050759
APA StyleMondada, S., Bedussi, F., Richardson, J. L., Noseda, R., & Ceschi, A. (2025). Pregabalin Safety in Pregnancy: A Disproportionality Analysis of VigiBase Spontaneous Reporting System. Pharmaceuticals, 18(5), 759. https://doi.org/10.3390/ph18050759






