Exploring the Anticancer Properties of 1,2,3-Triazole-Substituted Andrographolide Derivatives
Abstract
1. Introduction
2. Results and Discussion
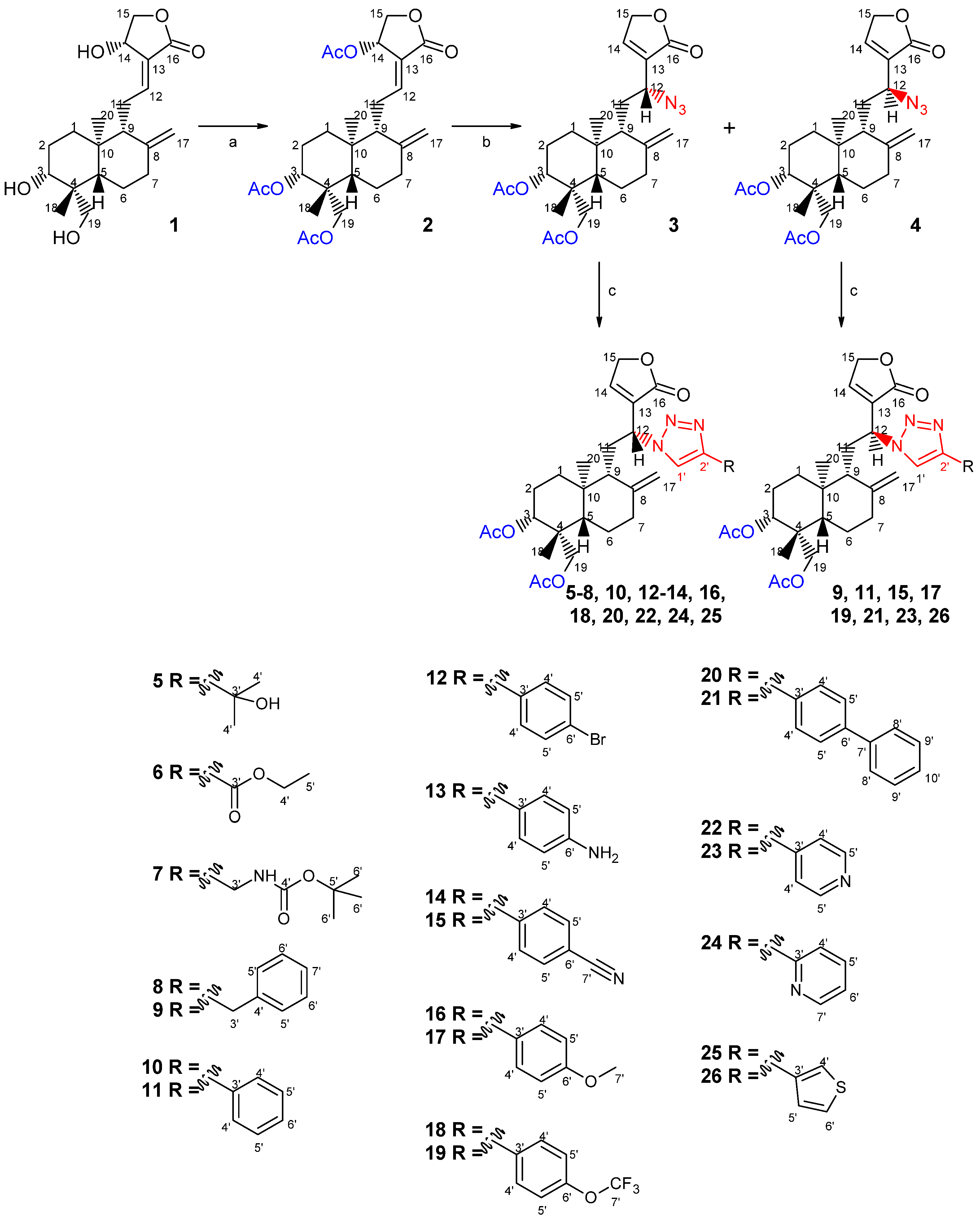
2.1. Chemistry
2.2. Biological Studies
2.2.1. In Vitro Antiproliferative Activity
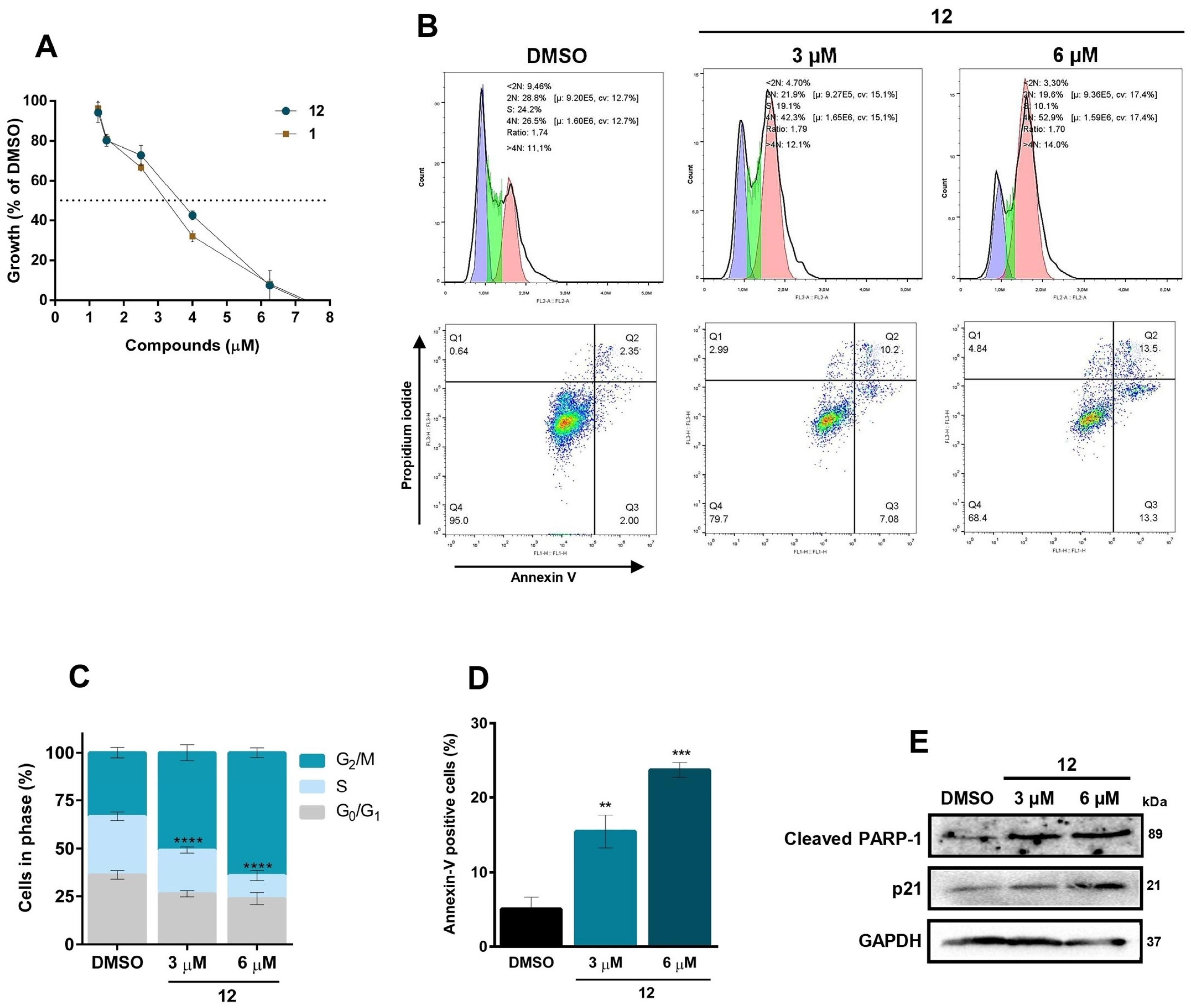
2.2.2. Cell Cycle Assay
2.2.3. Annexin V-FITC/PI Double-Staining Assay
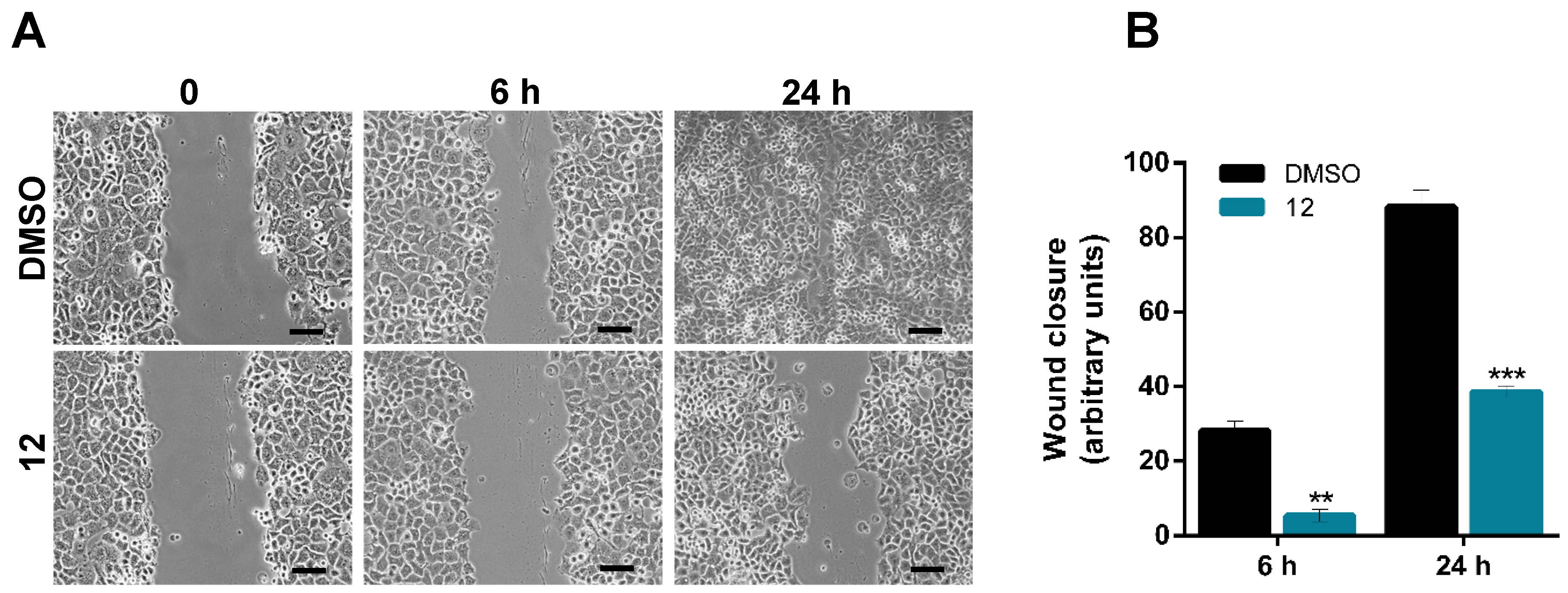
2.2.4. Scratch (Wound Healing) Assay
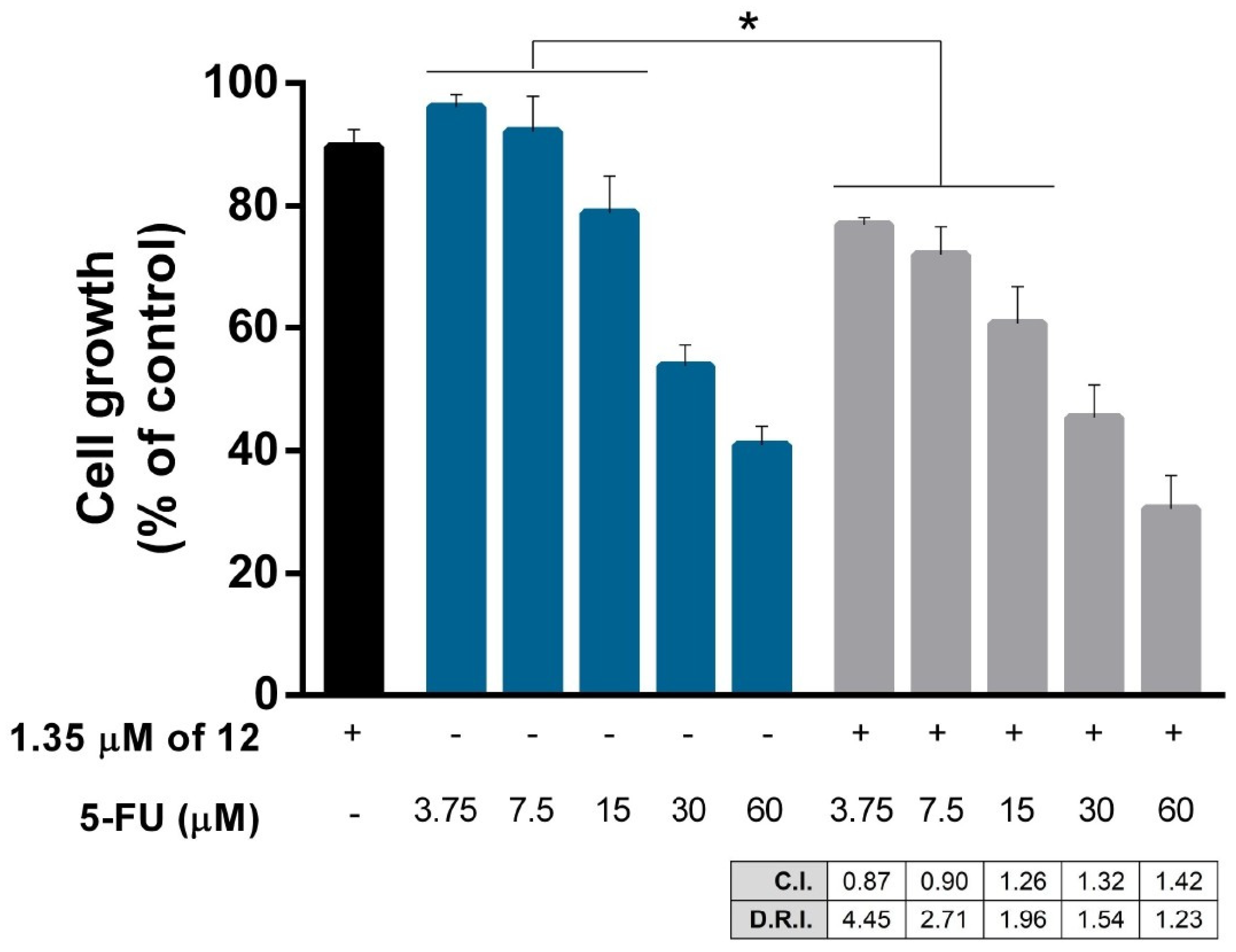
2.2.5. Drug Combination Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. General Remarks
3.1.2. NMR Data of Andrographolide (1)
3.1.3. Preparation of 3,14,19-Triacetyl Andrographolide (2)
3.1.4. Preparation of the Azide Andrographolide Derivatives
- (12R)-azido-3,19-diacetoxy-14-deoxy-andrographolide (3): 1H-NMR (300 MHz, CDCl3) δ = 7.41 (1H, q, J = 1.6 Hz, H-14), 5.00 (1H, s, H-17a), 4.90 (1H, s, H-17b), 4.88 (2H, m, H-15 a,b), 4.64 (1H, dd, J = 11.5, 4.7 Hz, H-3), 4.38* (1H, m, H-12), 4.38 (1H, d, J = 11.7 Hz, H-19a), 4.11 (1H, d, J = 11.7 Hz, H-19b), 2.05 (6H, s, H-3OCOCH3 and H-19OCOCH3), 1.54 (1H, m, H-9), 1.05 (3H, s, H-18), 0.76 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.1 (C-16), 171.0 (C-19OCOCH3), 170.6 (C-3OCOCH3), 145.9 (C-14), 145.8 (C-8), 134.2 (C-13), 108.6 (C-17), 79.8 (C-3), 70.5 (C-15), 64.7 (C-19), 56.8 (C-12), 55.2 (C-5), 52.3 (C-9), 41.2 (C-4), 38.9 (C-10), 38.2 (C-7), 36.7 (C-1), 28.8 (C-11), 24.8 (C-6), 24.2 (C-2), 22.6 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 460 [M + H]+. *Overlapped with H-19a.
- (12S)-azido-3,19-diacetoxy-14-deoxy-andrographolide (4): 1H-NMR (300 MHz, CDCl3) δ = 7.37 (1H, br s, H-14), 4.92 (1H, s, H-17a), 4.89 (2H, br s, H-15 a,b), 4.63 (1H, s, H-17b), 4.55 (1H, dd, J = 11.3, 5.1 Hz, H3), 4.36* (1H, dd, J = 8,4, 3,8 Hz, H-12), 4.33 (1H, d, J = 11.7 Hz, H-19a), 4.08 (1H, d, J = 11.7 Hz, H-19b), 2.02 (6H, s, H-3OCOCH3 and H-19OCOCH3), 1.51 (1H, m, H-9), 0.98 (3H, s, H-18), 0.71 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.9 (C-19OCOCH3), 170.6 (C-3OCOCH3), 147.4 (C-14), 146.5 (C-8), 132.9 (C-13), 107.6 (C-17), 79.7 (C-3), 70.3 (C-15), 64.7 (C-19), 56.2 (C-12), 55.3 (C-5), 52.9 (C-9), 41.3 (C-4), 39.3 (C-10), 38.1 (C-7), 36.8 (C-1), 28.0 (C-11), 24.8 (C-6), 24.2 (C-2), 22.6 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 460[M + H]+. *Overlapped with H-19a.
3.1.5. Preparation of Triazole-Containing Andrographolide Derivatives
- (12R)-4-(2-methyl-2-ol)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (5): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 2-methyl-3-butyn-2-ol (7.6 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 5 (14 mg, 38%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.49 (1H, s, H-1′), 7.26 (1H, q, J = 1.5 Hz, H-14), 5.45 (1H, br d, J = 11.9 Hz, H-12), 5.03 (1H, s, H-17a), 4.94 (1H, s, H-17b), 4.80* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.69* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.5, 5.0 Hz, H-3), 4.31 (1H, d, J = 11.8 Hz, H-19a), 4.06 (1H, d, J = 11.8 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.65 (6H, s, H-4′), 1.11 (1H, m, H-9), 0.94 (3H, s, H-18), 0.73 (3H, s, H20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.9 (C-19OCOCH3), 170.4 (C-3OCOCH3), 155.4 (C-2′), 147.9 (C-14), 145.8 (C-8), 133.6 (C-13), 120.2 (C-1′), 108.5 (C-17), 79.5 (C-3), 70.5 (C-15), 68.5 (C-3′), 64.7 (C-19), 55.7 (C-12), 55.1 (C-5), 51.9 (C-9), 41.2 (C-4), 38.9 (C-10), 38.2 (C-7), 36.4 (C-1), 30.4 (C-4′), 28.9 (C-11), 24.8 (C-6), 24.1 (C-2), 22.3 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 544 [M + H]+.
- (12R)-4-propriolate-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (6): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 2-ethynylpyridine (7.9 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 6 (11 mg, 31%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.14 (1H, s, H-1′), 7.37 (1H, q, J = 1.5 Hz, H-14), 5.55 (1H, br d, J = 11.6 Hz, H-12), 5.04 (1H, s, H-17a), 4.91 (1H, s, H-17b), 4.92* (1H, dt, J = 18.6, 1.5 Hz, H-15a), 4.77* (1H, dt, J = 18.6, 1.7 Hz, H-15b), 4.47 (1H, dd, J = 11.7, 4.9 Hz, H-3), 4.44 (2H, q, J = 7.1 Hz, H-4′), 4.30 (1H, d, J = 11.8 Hz, H-19a), 4.06 (1H, d, J = 11.8 Hz, H-19b), 2.02 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.43 (3H, t, J = 7.1 Hz, H-5′), 1.14 (1H, m, H-9), 0.94 (3H, s, H-18), 0.73 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.7 (C-16), 170.9 (C-19OCOCH3), 170.3 (C-3OCOCH3), 160.5 (C-3′), 148.1 (C-14), 145.6 (C-8), 140.2 (C-2′), 132.9 (C-13), 128.2 (C-1′), 118.6 (C-7′), 108.6 (C-17), 79.3 (C-3), 70.6 (C-15), 64.7 (C-19), 61.6 (C-4′), 56.4 (C-12), 54.9 (C-5), 51.7 (C-9), 41.2 (C-4), 38.9 (C-10), 38.1 (C-7), 36.4 (C-1), 28.8 (C-11), 24.8 (C-6), 24.0 (C-2), 22.2 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20), 14.3 (C-5′) ppm. ESIMS m/z 558 [M + H]+.
- (12R)-4-(tert-butyl methyl carbamate)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (7): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with N-boc-propargylamine (12 mg, 0.78 mmol). The residue was purified by column chromatography to afford compound 7 (21 mg, 52%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.53 (1H, s, H-1′), 7.28 (1H, q, J = 1.5 Hz, H-14), 5.45 (1H, br d, J = 11.5 Hz, H-12), 5.03 (1H, s, H-17a), 4.96 (1H, s, H-17b), 4.85* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.76* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.3, 4.9 Hz, H-3), 4.42 (2H, d, J = 6.0 Hz, H-3′), 4.30 (1H, d, J = 11.7 Hz, H-19a), 4.05 (1H, d, J = 11.7 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.43 (9H, s, H-6′), 1.11 (1H, m, H-9), 0.92 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.8 (C-16), 170.9 (C-19OCOCH3), 170.6 (C-3OCOCH3), 147.7 (C-14), 145.7 (C-8), 145.3 (C-4′), 130.2 (C-2′), 122.8 (C-1′), 133.5 (C-13), 108.6 (C-17), 79.9 (C-5′), 79.4 (C-3), 70.5 (C-15), 64.7 (C-19), 55.8 (C-12), 55.0 (C-5), 51.8 (C-9), 41.2 (C-4), 38.9 (C-10), 38.2 (C-7), 36.4 (C-1), 36.1 (C-3′), 28.9 (C-11), 28.4 (C-6′), 24.8 (C-6), 24.1 (C-2), 22.3 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 615 [M + H]+.
- (12R)-4-benzyl-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (8): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 3-phenyl-1-propyne (9.7 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 8 (17 mg, 46%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.35–7.18 (7H, m, H-14, H-1′, H-5′, H-6′ and H-7′), 5.41 (1H, br d, J = 11.6 Hz, H-12), 4.98 (1H, s, H-17a), 4.91 (1H, s, H-17b), 4.84* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.76* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.3, 4.7 Hz, H-3), 4.30 (1H, d, J = 11.8 Hz, H-19a), 4.11 (2H, br s, H-3′), 4.05 (1H, d, J = 11.8 Hz, H-19b), 2.02 (3H, s, H-3OCOCH3), 2.01 (3H, s, H-19OCOCH3), 1.04 (1H, m, H-9), 0.95 (3H, s, H-18), 0.70 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.9 (C-19OCOCH3), 170.4 (C-3OCOCH3), 147.9 (C-14), 147.7 (C-2′), 145.7 (C-8), 139.0 (C-4′), 133.4 (C-13), 128.7 (C-5′), 128.6 (C-6′), 126.7 (C-7′), 123.0 (C-1′), 108.5 (C-17), 79.5 (C-3), 70.5 (C-15), 64.7 (C-19), 55.7 (C-12), 55.1 (C-5), 51.8 (C-9), 41.2 (C-4), 38.8 (C-10), 38.1 (C-7), 36.8 (C-1), 32.3 (C-3′), 29.7 (C-11), 24.8 (C-6), 24.1 (C-2), 22.3 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 576 [M + H]+.
- (12S)-4-benzyl-1,2,3-triazole-3,19-diacetoxy-14-deoxy andrographolide (9): Obtained from reaction of compound 4 (20 mg, 0.044 mmol) with 3-phenyl-1-propyne (6.5 µL, 0.52 mmol). The residue was purified by column chromatography to afford compound 9 (12 mg, 49%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.58 (1H, br s, H-14), 7.45 (1H, s, H-1′), 7.34–7.18 (5H, m, H-5′, H-6′ and H-7′), 5.50 (1H, dd, J = 11.1, 3.7 Hz, H-12), 4.94 (1H, s, H-17a), 4.88 (2H, br s, H-15), 4.59 (1H, s, H-17b), 4.57 (1H, dd, J = 11.4, 5.0 Hz, H-3), 4.33 (1H, d, J = 11.8 Hz, H-19a), 4.08 (1H, d, J = 11.8 Hz, H-19b), 4.05 (2H, s, H-3′), 2.04 (3H, s, H-3OCOCH3), 2.03 (3H, s, H-19OCOCH3), 1.52 (1H, m, H-9), 0.99 (3H, s, H-18), 0.72 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.3 (C-14), 147.5 (C-2′), 145.9 (C-8), 138.8 (C-4′), 131.7 (C-13), 128.7 (C-5′), 128.6 (C-6′), 126.5 (C-7′), 121.2 (C-1′), 108.3 (C-17), 79.6 (C-3), 70.5 (C-15), 64.7 (C-19), 55.2 (C-5 and C-12), 52.4 (C-9), 42.2 (C-4), 39.3 (C-10), 38.1 (C-7), 36.5 (C-1), 32.8 (C-3′), 28.9 (C-11), 24.7 (C-6), 24.1 (C-2), 22.3 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 576 [M + H]+.
- (12R)-4-phenyl-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (10): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with phenylacetylene (8.6 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 10 (11 mg, 37%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.89–7.85 (2H, m, H-4′), 7.81 (1H, s, H-1′), 7.51–7.30 (3H, m, H14, H-5′ and H-6′), 5.54 (1H, br d, J = 11.8 Hz, H-12), 5.06 (1H, s, H-17a), 5.00 (1H, s, H-17b), 4.88* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.79* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.6, 4.8 Hz, H-3), 4.31 (1H, d, J = 11.8 Hz, H-19a), 4.07 (1H, d, J = 11.8 Hz, H-19b), 2.02 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.22 (1H, m, H-9), 0.92 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.9 (C-19OCOCH3), 170.3 (C-3OCOCH3), 147.9 (C-14), 147.5 (C-2′), 145.9 (C-8), 133.6 (C-13), 130.1 (C-3′), 128.9 (C-6′), 128.4 (C-5′), 125.8 (C-4′), 120.5 (C-1′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 55.9 (C-12), 54.9 (C-5), 51.7 (C-9), 41.2 (C-4), 38.9 (C-10), 38.1 (C-7), 36.3 (C-1), 28.6 (C-11), 25.1 (C-6), 24.1 (C-2), 22.1 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 563 [M + H]+.
- (12S)-4-phenyl-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (11): Obtained from reaction of compound 4 (30 mg, 0.065 mmol) with phenylacetylene (8.6 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 11 (11 mg, 31%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.04 (1H, s, H-1′), 7.82 (2H, dd, J = 7.0, 1.7 Hz, H-4′), 7.65 (1H, br s H-14), 7.46–7.29 (3H, m, H-5′ and H-6′), 5.53 (1H, dd, J = 11.2, 3.6 Hz, H-12), 5.00 (1H, s, H-17a), 4.91 (2H, br s, H-15), 4.66 (1H, s, H-17b), 4.59 (1H, dd, J = 11.5, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.10 (1H, d, J = 11.8 Hz, H-19b), 2.05 (3H, s, H-3OCOCH3), 2.03 (3H, s, H-19OCOCH3), 1.59 (1H, m, H-9), 1.01 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.5 (C-14), 147.9 (C-2′), 145.9 (C-8), 131.5 (C-13), 130.4 (C-3′), 128.3 (C-6′), 128.3 (C-5′), 125.8 (C-4′), 119.4 (C-1′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.7 (C-19), 55.4 (C-12), 52.9 (C-5), 51.9 (C-9), 41.2 (C-4), 39.4 (C-10), 38.2 (C-7), 36.9 (C-1), 28.8 (C-11), 24.9 (C-6), 24.2 (C-2), 22.3 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.5 (C-20) ppm. ESIMS m/z 563 [M + H]+.
- (12R)-4-bromophenyl-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (12): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 2-ethynylpyridine (7.9 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 12 (35 mg, 83%) as a yellow amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.83 (1H, s, H-1′), 7.72 (2H, d, J = 8.5 Hz, H-4′), 7.55 (2H, d, J = 8.5 Hz, H-5′), 7.38 (1H, q, J = 1.5 Hz, H-14), 5.52 (1H, br d, J = 11.6 Hz, H-12), 5.03 (1H, s, H-17a), 4.94 (1H, s, H-17b), 4.87* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.78* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.46 (1H, dd, J = 11.5, 4.9 Hz, H-3), 4.29 (1H, d, J = 11.7 Hz, H-19a), 4.04 (1H, d, J = 11.7 Hz, H-19b), 2.00 (3H, s, H-3OCOCH3), 1.98 (3H, s, H-19OCOCH3), 1.15 (1H, m, H9), 0.90 (3H, s, H-18), 0.73 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.9 (C-19OCOCH3), 170.4 (C-3OCOCH3), 148.1 (C-14), 146.5 (C-2′), 145.8 (C-8), 133.3 (C-13), 132.2 (C-5′), 129.1 (C-3′), 127.3 (C-4′), 122.3 (C-6′), 120.6 (C-1′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 55.9 (C-12), 54.9 (C-5), 51.9 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.4 (C-1), 28.7 (C-11), 24.8 (C-6), 24.1 (C-2), 22.2 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 564 [M + H]+.
- (12R)-4-aniline-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (13): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 4-ethynylaniline (9.8 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 13 (3 mg, 8%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.66 (2H, d, J = 8.4 Hz, H-4′), 7.35 (1H, q, J = 1,5 Hz H-14), 6.75 (2H, d, J = 8.4 Hz, H-5′), 5.50 (1H, br d, J = 11.8 Hz, H-12), 5.05 (1H, s, H-17a), 5.01 (1H, s, H-17b), 4.87* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.74* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.7, 4.8 Hz, H-3), 4.31 (1H, d, J = 11.8 Hz, H-19a), 4.07 (1H, d, J = 11.8 Hz, H-19b), 2.02 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 0.93 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. ESIMS m/z 577 [M + H]+.
- (12R)-4-benzonitrile-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (14): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 4-ethynylbenzonitrile (10.7 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 14 (18 mg, 47%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.98 (2H, d, J = 8.5 Hz, H-4′), 7.96 (1H, s, H-1′), 7.73 (2H, d, J = 8.5 Hz, H-5′), 7.44 (1H, q J = 1.5 Hz, H-14), 5.56 (1H, br d, J = 11.6 Hz, H-12), 5.05 (1H, s, H-17a), 4.92 (1H, s, H-17b), 4.89* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.81* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.46 (1H, dd, J = 11.4, 5.0 Hz, H-3), 4.30 (1H, d, J = 11.8 Hz, H-19a), 4.06 (1H, d, J = 11.8 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.14 (1H, m, H-9), 0.91 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.9 (C-19OCOCH3), 170.4 (C-3OCOCH3), 149.0 (C-2′), 148.2 (C-14), 145.8 (C-8), 134.6 (C-3′), 133.1 (C-13), 132.8 (C-5′), 126.2 (C-4′), 121.7 (C-1′), 118.7 (C-7′), 111.8 (C-6′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 56.0 (C-12), 55.0 (C-5), 52.0 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.5 (C-1), 28.7 (C-11), 24.8 (C-6), 24.1 (C-2), 22.2 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 587 [M + H]+.
- (12S)-4-benzonitrile-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (15): Obtained from reaction of compound 4 (20 mg, 0.044 mmol) with 4-ethynylbenzonitrile (7.1 µL, 0.52 mmol). The residue was purified by column chromatography to afford compound 15 (9 mg, 36%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.19 (1H, s, H-1′), 7.94 (2H, d, J = 8.6 Hz, H-4′), 7.70 (2H, d, J = 8.6 Hz, H-5′), 7.67 (1H, br s, Hz, H-14), 5.67 (1H, dd, J = 11.4, 3.5 Hz, H-12), 5.01 (1H, s, H-17a), 4.93 (2H, br s, H-15), 4.65 (1H, s, H-17b), 4.59 (1H, dd, J = 11.6, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.10 (1H, d, J = 11.8 Hz, H-19b), 2.05 (3H, s, H-3OCOCH3), 2.03 (3H, s, H-19OCOCH3), 1.50 (1H, m, H-9), 1.01 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.9 (C-14), 146.1 (C-2′), 145.8 (C-8), 134.8 (C-13), 132.7 (C-5′), 131.0 (C-3′), 126.1 (C-4′), 120.6 (C-1′), 118.7 (C-7′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.6 (C-19), 55.7 (C-12), 53.0 (C-5), 53.0 (C-9), 41.3 (C-4), 39.4 (C-10), 38.3 (C-7), 37.0 (C-1), 29.4 (C-11), 24.8 (C-6), 24.2 (C-2), 22.4 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 587 [M + H]+.
- (12R)-4-(4-methoxyphenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (16): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 4-ethynylanisole (10.2 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 16 (17 mg, 43%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.79 (2H, d, J = 8.8 Hz, H-4′), 7.72 (1H, s, H-1′), 7.36 (2H, d, J = 8.8 Hz, H-5′), 7.36 (1H, q, J = 1.6 Hz, H-14), 5.52 (1H, br d, J = 11.5 Hz, H-12), 5.05 (1H, s, H-17a), 4.99 (1H, s, H-17b), 4.87* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.78* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.47 (1H, dd, J = 11.6, 4.8 Hz, H-3), 4.31 (1H, d, J = 11.7 Hz, H-19a), 4.06 (1H, d, J = 11.7 Hz, H-19b), 3.84 (3H, s, H-7′), 2.01 (3H, s, H-19OCOCH3), 1.99 (3H, s, H-3OCOCH3), 1.24 (1H, m, H-9), 0.92 (3H, s, H-18), 0.74 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.9 (C-19OCOCH3), 170.3 (C-3OCOCH3), 159.8 (C-6′), 147.7 (C-14), 147.7 (C-2′), 145.9 (C-8), 133.6 (C-13), 127.1 (C-4′), 122.9 (C-3′), 119.6 (C-1′), 114.4 (C-5′), 108.6 (C-17), 79.4 (C-3), 70.5 (C-15), 64.7 (C-19), 55.8 (C-12), 55.4 (C-7′), 54.9 (C-5), 51.8 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.4 (C-1), 28.2 (C-11), 24.8 (C-6), 24.1 (C-2), 22.3 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C20) ppm. ESIMS m/z 592 [M + H]+.
- (12S)-4-(4-methoxyphenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (17): Obtained from reaction of compound 4 (30 mg, 0.065 mmol) with 4-ethynylanisole (10.2 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 17 (7 mg, 18%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.95 (1H, s, H-1′), 7.74 (2H, d, J = 8.7 Hz, H-4′), 7.64 (1H, br s, H-14), 6.95 (2H, d, J = 8.7 Hz, H-5′), 5.62 (1H, dd, J = 11.2, 3.6 Hz, H-12), 5.00 (1H, s, H-17a), 4.91 (2H, br s, H-15), 4.66 (1H, s, H-17b), 4.59 (1H, dd, J = 11.5, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.10 (1H, d, J = 11.8 Hz, H-19b), 3.84 (3H, s, H-7′), 2.05 (3H, s, H-3OCOCH3), 2.03 (3H, s, H-19OCOCH3), 1.52 (1H, m, H-9), 1.01 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 159.7 (C-6′), 150.5 (C-14), 147.7 (C-2′), 145.9 (C-8), 131.6 (C-13), 127.1 (C-4′), 123.1 (C-3′), 118.6 (C-1′), 114.2 (C-5′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.7 (C-19), 55.4 (C-12), 55.3 (C-7′), 52.9 (C-5), 51.8 (C-9), 41.3 (C-4), 39.3 (C-10), 38.2 (C-7), 36.5 (C-1), 29.4 (C-11), 23.9 (C-6), 24.2 (C-2), 22.2 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.3 (C-20) ppm. ESIMS m/z 592 [M + H]+.
- (12R)-4-(4-trifluoromethoxy)phenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (18): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 4-(trifluoromethoxy)phenylacetylene (12 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 18 (22 mg, 51%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.89 (2H, dm, J = 8.8 Hz, H-4′), 7.84 (1H, s, H-1′), 7.41 (1H, q, J = 1.6 Hz, H-14), 7.29 (2H, dd, J = 8.8 Hz, H-5′), 5.54 (1H, br d, J = 11.7 Hz, H-12), 5.06 (1H, s, H-17a), 4.96 (1H, s, H-17b), 4.88* (1H, dt, J = 18.5, 1.4 Hz, H-15a), 4.80* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.47 (1H, dd, J = 11.5, 4.9 Hz, H-3), 4.30 (1H, d, J = 11.7 Hz, H-19a), 4.06 (1H, d, J = 11.7 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 2.00 (3H, s, H-19OCOCH3), 1.10 (1H, m, H-9), 0.92 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.8 (C-19OCOCH3), 170.4 (C-3OCOCH3), 149.2 (C-6′), 148.0 (C-14), 146.3 (C-2′), 145.8 (C-8), 133.4 (C-13), 129.0 (C-3′), 127.2 (C-4′), 121.5 (C-5’), 120.6 (C-1’), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 55.9 (C-12), 55.0 (C-5), 51.9 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.5 (C-1), 28.8 (C-11), 24.8 (C-6), 24.1 (C-2), 22.5 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 646 [M + H]+.
- (12S)-4-(4-trifluoromethoxy)phenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (19): Obtained from reaction of compound 4 (30 mg, 0.065 mmol) with 4-(trifluoromethoxy)phenylacetylene (12 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 19 (11 mg, 25%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.06 (1H, s, H-1′), 7.85 (2H, dd, J = 8.8 Hz, H-4′), 7.65 (1H, br s, H-14), 7.26 (2H, dm, J = 8.8 Hz, H-5′), 5.65 (1H, dd, J = 11.1, 3.5 Hz, H-12), 5.00 (1H, s, H-17a), 4.92 (2H, br s, H-15), 4.66 (1H, s, H-17b), 4.59 (1H, dd, J = 11.6, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.10 (1H, d, J = 11.8 Hz, H-19b), 2.05 (3H, s, H-3OCOCH3), 2.03 (3H, s, H-19OCOCH3), 1.53 (1H, m, H-9), 1.01 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.7 (C-14), 149.1 (C-6′), 146.6 (C-2′), 145.9 (C-8), 131.3 (C-13), 129.2 (C-3′), 127.2 (C-4′), 121.3 (C-5′), 119.6 (C-1′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.6 (C-19), 55.5 (C-12), 55.3 (C-5), 52.9 (C-9), 41.3 (C-4), 39.4 (C-10), 38.3 (C-7), 36.9 (C-1), 28.7 (C-11), 24.8 (C-6), 24.2 (C-2), 22.6 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 646 [M + H]+.
- (12R)-4-(3-biphenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (20): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 3-ethynylbiphenyl (14.0 mg, 0.78 mmol). The residue was purified by column chromatography to afford compound 20 (32 mg, 76%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.94 (2H, d, J = 8.4 Hz, H-4′), 7.86 (1H, s, H-1′), 7.68 (2H, d, J = 8.4 Hz, H-5′), 7.63 (2H, br d, J = 7.2 Hz, H-8′), 7.45 (2H, t, J = 7.2 Hz, H-9′), 7.40 (1H, q, J = 1.5 Hz, H-14), 7.38–7.32 (1H, m, H-10′), 5.55 (1H, br d, J = 11.4 Hz, H-12), 5.06 (1H, s, H-17a), 5.00 (1H, s, H-17b), 4.88* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.79* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.48 (1H, dd, J = 11.6, 4.8 Hz, H-3), 4.31 (1H, d, J = 11.8 Hz, H-19a), 4.06 (1H, d, J = 11.8 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 1.99 (3H, s, H-19OCOCH3), 1.25 (1H, m, H-9), 0.92 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.9 (C-19OCOCH3), 170.3 (C-3OCOCH3), 148.0 (C-14), 147.2 (C-2′), 145.9 (C-8), 141.2 (C-6′), 140.5 (C-7′), 133.5 (C-13), 129.1 (C-3′), 128.8 (C-9′), 127.6 (C-10′), 127.5 (C-5′), 127.0 (C-8′), 126.2 (C-4′), 120.5 (C-1′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 55.9 (C-12), 54.9 (C-5), 51.9 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.4 (C-1), 28.9 (C-11), 24.8 (C-6), 24.1 (C-2), 22.5 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 638 [M + H]+.
- (12S)-4-(3-biphenyl)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (21): Obtained from reaction of compound 4 (20 mg, 0.044 mmol) with 3-ethynylbiphenyl (9.3 mg, 0.52 mmol). The residue was purified by column chromatography to afford compound 21 (12 mg, 44%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.08 (1H, s, H-1′), 7.89 (2H, d, J = 8.4, Hz, H-4′), 7.66 (2H, d, J = 8.4 Hz, H-5′), 7.66 (1H, s, H-14), 7.62 (2H, d, J = 7.3 Hz, H-8′), 7.45 (2H, t, J = 7.3 Hz, H-9′), 7.39–7.31 (1H, m, H-10′), 5.65 (1H, dd, J = 11.2, 3.6 Hz, H-12), 5.01 (1H, s, H-17a), 4.92 (2H, br s, H-15), 4.68 (1H, s, H-17b), 4.59 (1H, dd, J = 11.6, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.11 (1H, d, J = 11.8 Hz, H-19b), 2.06 (3H, s, H-3OCOCH3), 2.04 (3H, s, H-19OCOCH3), 1.59 (1H, m, H-9), 1.01 (3H, s, H-18), 0.76 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.6 (C-14), 147.6 (C-2′), 145.9 (C-8), 141.0 (C-6′), 140.6 (C-7′), 131.5 (C-13), 129.3 (C-3′), 128.8 (C-9′), 127.5 (C-10′), 127.4 (C-5′), 127.0 (C-8′), 126.1 (C-4′), 119.4 (C-1′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.7 (C-19), 55.5 (C-12), 55.4 (C-5), 53.0 (C-9), 41.3 (C-4), 39.9 (C-10), 38.2 (C-7), 36.6 (C-1), 29.0 (C-11), 24.8 (C-6), 24.3 (C-2), 22.6 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.6 (C-20) ppm. ESIMS m/z 638 [M + H]+.
- (12R)-4-(4-pyridine)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (22): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 3-ethynylthiophene (8.1 mg, 0.78 mmol). The residue was purified by column chromatography to afford compound 22 (16 mg, 44%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.68 (2H, br s, H-5′), 7.99 (1H, s, H-1′), 7.75 (2H, d, J = 5.7 Hz, H-4′), 7.43 (1H, q, J = 1.5 Hz, H-14), 5.56 (1H, br d, J = 11.5 Hz, H-12), 5.06 (1H, s, H-17a), 4.93 (1H, s, H-17b), 4.89* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.80* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.47 (1H, dd, J = 11.5, 4.9 Hz, H-3), 4.30 (1H, d, J = 11.8 Hz, H-19a), 4.06 (1H, d, J = 11.8 Hz, H-19b), 2.01 (3H, s, H-3OCOCH3), 1.98 (3H, s, H-19OCOCH3), 1.14 (1H, m, H-9), 0.91 (3H, s, H-18), 0.75 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.9 (C-16), 170.9 (C-19OCOCH3), 170.4 (C-3OCOCH3), 150.5 (C-5′), 148.1 (C-14), 145.8 (C-8), 145.1 (C-2′), 137.6 (C-3′), 133.1 (C-13), 122.1 (C-4′), 120.0 (C-1′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 56.0 (C-12), 55.0 (C-5), 51.9 (C-9), 42.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.4 (C-1), 28.8 (C-11), 24.8 (C-6), 24.1 (C-2), 22.2 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 563 [M + H]+.
- (12S)-4-(4-pyridine)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (23): Obtained from reaction of compound 4 (20 mg, 0.044 mmol) with 3-ethynylthiophene (5.4 mg, 0.52 mmol). The residue was purified by column chromatography to afford compound 23 (5 mg, 21%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.66 (1H, br s, H-5′), 7.72 (2H, d, J = 5.1 Hz, H-4′), 7.64 (1H, br s, H-14), 5.68 (1H, dd, J = 11.5, 3.4 Hz, H-12), 5.02 (1H, s, H-17a), 4.94 (2H, br s, H-15), 4.66 (1H, s, H-17b), 4.59 (1H, dd, J = 11.6, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.11 (1H, d, J = 11.8 Hz, H-19b), 2.05 (3H, s, H-3OCOCH3), 2.04 (3H, s, H-19OCOCH3), 1.02 (3H, s, H-18), 0.76 (3H, s, H-20) ppm. ESIMS m/z 563 [M + H]+.
- (12R)-4-pyridine-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (24): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 2-ethynylpyridine (7.9 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 24 (32 mg, 88%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.58 (1H, ddd, J = 4.9, 1.8, 0.9 Hz, H-7′), 8.21 (1H, dt, J = 7.8, 1.2 Hz, H-4′), 8.16 (1H, s, H-1′), 7.80 (1H, td, J = 7.8, 1.8 Hz, H-5′), 7.29 (1H, q, J = 1.6 Hz, H-14), 7.24 (1H, ddd, J = 7.5, 4.9, 1.2 Hz, H-6′), 5.56 (1H, br d, J = 11.5 Hz, H-12), 5.04 (1H, s, H-17a), 5.03 (1H, s, H-17b), 4.86* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.76* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.45 (1H, dd, J = 11.4, 5.0 Hz, H-3), 4.30 (1H, d, J = 11.8 Hz, H-19a), 4.04 (1H, d, J = 11.8 Hz, H-19b), 2.00 (3H, s, H-3OCOCH3), 1.98 (3H, s, H-19OCOCH3), 1.17 (1H, m, H-9), 0.90 (3H, s, H-18), 0.73 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.7 (C-16), 170.8 (C-19OCOCH3), 170.4 (C-3OCOCH3), 149.9 (C-3′), 149.5 (C-7′), 148.2 (C-2′), 147.6 (C-14), 145.6 (C-8), 137.0 (C-5′), 133.6 (C-13), 123.1 (C-6′), 122.8 (C-1′), 120.4 (C-4′), 108.7 (C-17), 79.5 (C-3), 70.5 (C-15), 64.7 (C-19), 55.3 (C-12), 54.9 (C-5), 51.9 (C-9), 41.2 (C-4), 38.9 (C-10), 38.1 (C-7), 36.4 (C-1), 28.9 (C-11), 24.8 (C-6), 24.1 (C-2), 22.5 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 564 [M + H]+.
- (12R)-4-(3-thiophene)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (25): Obtained from reaction of compound 3 (30 mg, 0.065 mmol) with 3-ethynylthiophene (7.8 µL, 0.78 mmol). The residue was purified by column chromatography to afford compound 25 (30 mg, 82%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 7.72 (1H, dd, J = 3.0, 1.3 Hz, H-6′), 7.71 (1H, s, H-1′), 7.47 (1H, dd, J = 5.0, 3.0 Hz, H-4′), 7.39 (1H, dd, J = 5.0, 1.3 Hz, H-5′), 7.35 (1H, q, J = 1.6 Hz, H-14), 5.50 (1H, br d, J = 11.5 Hz, H-12), 5.04 (1H, s, H-17a), 4.97 (1H, s, H-17b), 4.86* (1H, dt, J = 18.5, 1.5 Hz, H-15a), 4.77* (1H, dt, J = 18.5, 1.7 Hz, H-15b), 4.46 (1H, dd, J = 11.6, 4.8 Hz, H-3), 4.30 (1H, d, J = 11.7 Hz, H-19a), 4.05 (1H, d, J = 11.7 Hz, H-19b), 2.00 (3H, s, H-3OCOCH3), 1.99 (3H, s, H-19OCOCH3), 1.17 (1H, m, H-9), 0.91 (3H, s, H-18), 0.74 (3H, s, H-20) ppm. *AB part of an ABXY spin system with similar coupling values of A (J ≅ 1.5) and B (J ≅ 1.7) to X and Y. 13C-NMR (75 MHz, CDCl3) δ = 171.4 (C-16), 170.9 (C-19OCOCH3), 170.3 (C-3OCOCH3), 147.9 (C-14), 145.9 (C-8), 143.8 (C-2′), 133.5 (C-13), 131.4 (C-3′), 126.5 (C-5′), 125.7 (C-4′), 121.5 (C-6′), 120.3 (C-1′), 108.5 (C-17), 79.4 (C-3), 70.6 (C-15), 64.7 (C-19), 55.8 (C-12), 54.9 (C-5), 51.8 (C-9), 41.2 (C-4), 39.0 (C-10), 38.2 (C-7), 36.4 (C-1), 28.8 (C-11), 24.8 (C-6), 24.1 (C-2), 22.2 (C-18), 21.1 (C-3OCOCH3), 21.0 (C-19OCOCH3), 14.7 (C-20) ppm. ESIMS m/z 568 [M + H]+.
- (12S)-4-(3-thiophene)-1,2,3-triazole-3,19-diacetoxy-14-deoxy-andrographolide (26): Obtained from reaction of compound 4 (20 mg, 0.044 mmol) with 3-ethynylthiophene (5.2 µL, 0.52 mmol). The residue was purified by column chromatography to afford compound 26 (5 mg, 21%) as a white amorphous powder. 1H-NMR (300 MHz, CDCl3) δ = 8.66 (1H, s, H-6′), 8.22 (1H, s, H-1′), 7.75–7.70 (2H, m, H-4′ and H-5′), 7.67 (1H, br s, H-14), 5.68 (1H, dd, J = 11.4, 3.5 Hz, H-12), 5.02 (1H, s, H-17a), 4.94 (2H, br s, H-15), 4.66 (1H, s, H-17b), 4.59 (1H, dd, J = 11.6, 4.9 Hz, H-3), 4.35 (1H, d, J = 11.8 Hz, H-19a), 4.11 (1H, d, J = 11.8 Hz, H-19b), 2.05 (3H, s, H-3OCOCH3), 2.04 (3H, s, H-19OCOCH3), 1.60 (1H, m, H-9), 1.02 (3H, s, H-18), 0.76 (3H, s, H-20) ppm. 13C-NMR (75 MHz, CDCl3) δ = 172.0 (C-16), 170.8 (C-19OCOCH3), 170.6 (C-3OCOCH3), 150.5 (C-14), 146.0 (C-8), 144.0 (C-2′), 131.6 (C-13), 131.5 (C-3′), 126.3 (C-5′), 125.8 (C-4′), 121.3 (C-6′), 119.2 (C-1′), 108.1 (C-17), 79.6 (C-3), 70.5 (C-15), 64.6 (C-19), 55.7 (C-12), 55.4 (C-5), 52.9 (C-9), 41.3 (C-4), 39.3 (C-10), 38.2 (C-7), 36.4 (C-1), 28.5 (C-11), 24.8 (C-6), 24.2 (C-2), 22.3 (C-18), 21.2 (C-3OCOCH3), 21.1 (C-19OCOCH3), 14.7 (C20) ppm. ESIMS m/z 568 [M + H]+.
3.2. Biology
3.2.1. Human Cell Lines and Growth Conditions
3.2.2. Sulforhodamine B (SRB) Assay
3.2.3. Cell Cycle and Apoptosis Analyses
3.2.4. Western Blot
3.2.5. Wound Healing Assay
3.2.6. Combination Therapy with 5-Fluorouracil
3.2.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, V.; Antoine, M.; Dumont, J.E.; Dom, G.; Detours, V.; Maenhaut, C. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L. Biological Properties of Andrographolide, an Active Ingredient of Andrographis Paniculata: A Narrative Review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Tundis, R.; Patra, J.K.; Bonesi, M.; Das, S.; Nath, R.; Das Talukdar, A.; Das, G.; Loizzo, M.R. Anti-Cancer Agent: The Labdane Diterpenoid-Andrographolide. Plants 2023, 12, 1969. [Google Scholar] [CrossRef]
- Wanandi, S.I.; Limanto, A.; Yunita, E.; Syahrani, R.A.; Louisa, M.; Wibowo, A.E.; Arumsari, S. In Silico and in Vitro Studies on the Anti-Cancer Activity of Andrographolide Targeting Survivin in Human Breast Cancer Stem Cells. PLoS ONE 2020, 15, e0240020. [Google Scholar] [CrossRef]
- Wang, X.-R.; Jiang, Z.-B.; Xu, C.; Meng, W.-Y.; Liu, P.; Zhang, Y.-Z.; Xie, C.; Xu, J.-Y.; Xie, Y.-J.; Liang, T.-L.; et al. Andrographolide Suppresses Non-Small-Cell Lung Cancer Progression through Induction of Autophagy and Antitumor Immune Response. Pharmacol. Res. 2022, 179, 16198. [Google Scholar] [CrossRef]
- Forestier-Román, I.S.; López-Rivas, A.; Sánchez-Vázquez, M.M.; Rohena-Rivera, K.; Nieves-Burgos, G.; Ortiz-Zuazaga, H.; Torres-Ramos, C.A.; Martínez-Ferrer, M. Andrographolide Induces DNA Damage in Prostate Cancer Cells. Oncotarget 2019, 10, 1085–1101. [Google Scholar] [CrossRef]
- Yang, T.; Yao, S.; Zhang, X.; Guo, Y. Andrographolide Inhibits Growth of Human T-Cell Acute Lymphoblastic Leukemia Jurkat Cells by Downregulation of PI3K/AKT and Upregulation of P38 MAPK Pathways. Drug Des. Dev. Ther. 2016, 10, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, L.; He, Z.; Chen, M.; Ding, Y.; Yao, Y. Andrographolide Suppresses the Growth and Metastasis of Luminal-Like Breast Cancer by Inhibiting the NF-κB/MiR-21-5p/PDCD4 Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 643525. [Google Scholar] [CrossRef]
- Chen, S.R.; Li, F.; Ding, M.Y.; Wang, D.; Zhao, Q.; Wang, Y.; Zhou, G.C.; Wang, Y. Andrographolide Derivative as STAT3 Inhibitor That Protects Acute Liver Damage in Mice. Bioorg. Med. Chem. 2018, 26, 5053–5061. [Google Scholar] [CrossRef]
- Tohkayomatee, R.; Reabroi, S.; Tungmunnithum, D.; Parichatikanond, W.; Pinthong, D. Andrographolide Exhibits Anticancer Activity against Breast Cancer Cells (MCF-7 and MDA-MB-231 Cells) through Suppressing Cell Proliferation and Inducing Cell Apoptosis via Inactivation of ER-α Receptor and PI3K/AKT/MTOR Signaling. Molecules 2022, 27, 3544. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and Future Perspectives of Multi-Target Drugs in Cancer Treatment: The next Generation Anti-Cancer Agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Sun, Y.; Sheng, W.; Liao, D. Designing Multi-Targeted Agents: An Emerging Anticancer Drug Discovery Paradigm. Eur. J. Med. Chem. 2017, 136, 195–211. [Google Scholar] [CrossRef]
- Othman, N.S.; Mohd Azman, D.K. Andrographolide Induces G2/M Cell Cycle Arrest and Apoptosis in Human Glioblastoma DBTRG-05MG Cell Line via ERK1/2 /c-Myc/P53 Signaling Pathway. Molecules 2022, 27, 6686. [Google Scholar] [CrossRef]
- Khan, I.; Mahfooz, S.; Saeed, M.; Ahmad, I.; Ansari, I.A. Andrographolide Inhibits Proliferation of Colon Cancer SW-480 Cells via Downregulating Notch Signaling Pathway. Anticancer Agents Med. Chem. 2021, 21, 487–497. [Google Scholar] [CrossRef]
- Dai, J.; Lin, Y.; Duan, Y.; Li, Z.; Zhou, D.; Chen, W.; Wang, L.; Zhang, Q.Q. Andrographolide Inhibits Angiogenesis by Inhibiting the Mir-21-5p/TIMP3 Signaling Pathway. Int. J. Biol. Sci. 2017, 13, 660–668. [Google Scholar] [CrossRef]
- Wang, W.; Guo, W.; Li, L.; Fu, Z.; Liu, W.; Gao, J.; Shu, Y.; Xu, Q.; Sun, Y.; Gu, Y. Andrographolide Reversed 5-FU Resistance in Human Colorectal Cancer by Elevating BAX Expression. Biochem. Pharmacol. 2016, 121, 8–17. [Google Scholar] [CrossRef]
- Lakra, D.S.; Bharathiraja, P.; Dhanalakshmi, T.; Prasad, N.R. Andrographolide Reverts Multidrug Resistance in KBChR 8-5 Cells through AKT Signaling Pathway. Cell Biochem. Funct. 2024, 42, e3948. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.R.L.; Szemerédi, N.; Gonçalves, B.M.F.; Spengler, G.; Afonso, C.A.M.; Ferreira, M.J.U. Nitrogen-Containing Andrographolide Derivatives with Multidrug Resistance Reversal Effects in Cancer Cells. RSC Med. Chem. 2024, 15, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, T.; Tang, L.; Liu, W.; Yang, Z.; Zhou, J.; Zheng, Z.; Cai, Z.; Hu, M.; Liu, Z. Poor Oral Bioavailability of a Promising Anticancer Agent Andrographolide Is Due to Extensive Metabolism and Efflux by P-Glycoprotein. J. Pharm. Sci. 2011, 100, 5007–5011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cui, Q. Biodistribution of Andrographolide to Assess the Interior-Exterior Relationship between the Lung and Intestine Using MicroPET. Thorac. Cancer 2020, 11, 3365–3374. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhang, Z.M.; Zhang, Z.Q.; Wang, Y.; Zhou, W. Andrographolide Induced Acute Kidney Injury: Analysis of 26 Cases Reported in Chinese Literature. Nephrology 2014, 19, 21–26. [Google Scholar] [CrossRef]
- Chung, W.-J.; Chan, K.-L.; Lee, C.-Y. Comparing the Pharmacokinetics of 13α,21-Dihydroeurycomanone and Eurycomanone Exclusively Enriched in Eurycoma Longifolia Extracts and Their Spermatogenesis Enhancement in Andrographolide-Induced Oligospermia in Rats. J. Pharm. Pharmacol. 2021, 73, 161–168. [Google Scholar] [CrossRef]
- Cai, W.; Li, J.; Chen, C.; Wu, J.; Li, J.; Xue, X. Design, Synthesis, and Anticancer Evaluation of Novel Andrographolide Derivatives Bearing an α,β-Unsaturated Ketone Moiety. Bioorg. Chem. 2021, 112, 104941. [Google Scholar] [CrossRef]
- Yang, T.C.; Chiang, Y.J.; Chiang, P.Y.; Chen, H.Y.; Zhuang, K.R.; Wang, Y.C.; Lin, C.H.; Lo, L.C.; Fu, S.L. Design, Synthesis, and Anti-Cancer Evaluation of C-14 Arylcarbamate Derivatives of Andrographolide. Bioorg. Med. Chem. 2024, 98, 117582. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J.; Sun, Y.; Chan, J.Y.W.; Sheng, D.; Wang, K.; Wei, P.; Ouyang, P.; Wang, D.; Lee, S.M.Y.; et al. SAR Studies of 3,14,19-Derivatives of Andrographolide on Anti-Proliferative Activity to Cancer Cells and Toxicity to Zebrafish: An in Vitro and in Vivo Study. RSC Adv. 2015, 5, 22510–22526. [Google Scholar] [CrossRef]
- Devendar, P.; Nayak, V.L.; Yadav, D.K.; Kumar, A.N.; Kumar, J.K.; Srinivas, K.V.N.S.; Sridhar, B.; Khan, F.; Sastry, K.P.; Ramakrishna, S. Synthesis and Evaluation of Anticancer Activity of Novel Andrographolide Derivatives. MedChemComm 2015, 6, 898–904. [Google Scholar] [CrossRef]
- Messire, G.; Rollin, P.; Gillaizeau, I.; Berteina-Raboin, S. Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview. Molecules 2024, 29, 2884. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, C.; Deevi, D.S.; Rajagopalan, R.; Srinivas, N.; Rajagopal, S. DRF 3188 a Novel Semi-Synthetic Analog of Andrographolide: Cellular Response to MCF 7 Breast Cancer Cells. BMC Cancer 2004, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Nyavanandi, V.K.; Sanjeeva Rao Thunuguntla, S.; Kasu, S.; Pallerla, M.K.; Sai Ram, P.; Rajagopal, S.; Ajaya Kumar, R.; Ramanujam, R.; Moses Babu, J.; et al. Synthesis and Structure-Activity Relationships of Andrographolide Analogues as Novel Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. [Google Scholar] [CrossRef]
- Jada, S.R.; Subur, G.S.; Matthews, C.; Hamzah, A.S.; Lajis, N.H.; Saad, M.S.; Stevens, M.F.G.; Stanslas, J. Semisynthesis and in Vitro Anticancer Activities of Andrographolide Analogues. Phytochemistry 2007, 68, 904–912. [Google Scholar] [CrossRef]
- Kumar, G.; Thapa, S.; Tali, J.A.; Singh, D.; Sharma, B.K.; Panda, K.N.; Singh, S.K.; Shankar, R. Site-Selective Synthesis of C-17 Ester Derivatives of Natural Andrographolide for Evaluation as a Potential Anticancer Agent. ACS Omega 2023, 8, 6099–6123. [Google Scholar] [CrossRef] [PubMed]
- Kandanur, S.G.S.; Golakoti, N.R.; Nanduri, S. Synthesis and in Vitro Cytotoxicity of Novel C-12 Substituted-14-Deoxy-Andrographolide Derivatives as Potent Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2015, 25, 5781–5786. [Google Scholar] [CrossRef]
- Kandanur, S.G.S.; Nanduri, S.; Golakoti, N.R. Synthesis and Biological Evaluation of New C-12(α/β)-(N-) Sulfamoyl-Phenylamino-14-Deoxy-Andrographolide Derivatives as Potent Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2854–2862. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhao, S.; Wang, X.; Liu, B.; Xu, H. Click Chemistry in Natural Product Modification. Front. Chem. 2021, 9, 774977. [Google Scholar] [CrossRef]
- Ma, N.; Wang, Y.; Zhao, B.X.; Ye, W.C.; Jiang, S. The Application of Click Chemistry in the Synthesis of Agents with Anticancer Activity. Drug Des. Dev. Ther. 2015, 9, 1585–1599. [Google Scholar] [CrossRef]
- Meghani, N.M.; Amin, H.H.; Lee, B.J. Mechanistic Applications of Click Chemistry for Pharmaceutical Drug Discovery and Drug Delivery. Drug Discov. Today 2017, 22, 1604–1619. [Google Scholar] [CrossRef]
- Serafini, M.; Pirali, T.; Tron, G.C. Chapter Three—Click 1,2,3-Triazoles in Drug Discovery and Development: From the Flask to the Clinic? In Advances in Heterocyclic Chemistry; Meanwell, N.A., Lolli, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 101–148. ISBN 9780128201817. [Google Scholar]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-Triazole Ring as a Bioisostere in Medicinal Chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Paterna, A.; Kincses, A.; Spengler, G.; Mulhovo, S.; Molnár, J.; Ferreira, M.J.U. Dregamine and Tabernaemontanine Derivatives as ABCB1 Modulators on Resistant Cancer Cells. Eur. J. Med. Chem. 2017, 128, 247–257. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Kincses, A.; Nové, M.; Spengler, G.; Mulhovo, S.; Aires-de-Sousa, J.; dos Santos, D.J.V.A.; Ferreira, M.J.U. Alkylated Monoterpene Indole Alkaloid Derivatives as Potent P-Glycoprotein Inhibitors in Resistant Cancer Cells. Eur. J. Med. Chem. 2021, 210, 112985. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Szemerédi, N.; Spengler, G.; Mulhovo, S.; Dos Santos, D.J.V.A.; Ferreira, M.J.U. Exploring the Monoterpene Indole Alkaloid Scaffold for Reversing P-Glycoprotein-Mediated Multidrug Resistance in Cancer. Pharmaceuticals 2021, 14, 862. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, L.; Paterna, A.; Calheiros, J.; Ribeiro, J.; Cardoso, D.S.P.; Piga, I.; Neto, S.J.; Hegan, D.; Glazer, P.M.; Indraccolo, S.; et al. BBIT20 Inhibits Homologous DNA Repair with Disruption of the BRCA1–BARD1 Interaction in Breast and Ovarian Cancer. Br. J. Pharmacol. 2021, 178, 3627–3647. [Google Scholar] [CrossRef]
- Reis, M.A.; Matos, A.M.; Duarte, N.; Ahmed, O.B.; Ferreira, R.J.; Lage, H.; Ferreira, M.J.U. Epoxylathyrane Derivatives as MDR-Selective Compounds for Disabling Multidrug Resistance in Cancer. Front. Pharmacol. 2020, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, E.F.P.; Gonåalves, L.M.; Carvalho, L.A.R.; Guedes, R.C.; Hofbauer, S.; Brito, J.A.; Archer, M.; Moreira, R.; Lucas, S.D. Clickable 4-Oxo-b-Lactam-Based Selective Probing for Human Neutrophil Elastase Related Proteomes. ChemMedChem 2016, 11, 2037–2042. [Google Scholar] [CrossRef]
- Kasemsuk, S.; Sirion, U.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. 12-Amino-Andrographolide Analogues: Synthesis and Cytotoxic Activity. Arch. Pharm. Res. 2013, 36, 1454–1464. [Google Scholar] [CrossRef]
- Collins, K.; Jacks, T.; Pavletich, N.P. The Cell Cycle and Cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 2776–2778. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A Novel Assay for Apoptosis. Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Honda, H. Wound Healing Mechanisms in Epithelial Tissues Cell Adhesion to Bsal Lamina. In Proceedings of the 2006 WSEAS International Conference on Cellular & Molecular Biology, Biophyhsics & Bioengineering, Athens, Greece, 14–16 July 2006; pp. 111–116. [Google Scholar]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. Cell Adhesion & Migration An Introduction to the Wound Healing Assay Using Livecell Microscopy An Introduction to the Wound Healing Assay Using Livecell Microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Owen, A.E.; Louis, H.; Ejiofor, E.U.; Emori, W.; Gber, T.E.; Benjamin, I.; Cheng, C.R.; Orosun, M.M.; Ling, L.; Adeyinka, A.S. Natural Andrographolide Isolated from Andrographis Paniculata as Potent Epileptic Agent: Spectroscopy, Molecular Structure, and Molecular Docking Investigation. Chem. Afr. 2023, 6, 2445–2461. [Google Scholar] [CrossRef]
- Villedieu-Percheron, E.; Ferreira, V.; Campos, J.F.; Destandau, E.; Pichon, C.; Berteina-Raboin, S. Quantitative Determination of Andrographolide and Related Compounds in Andrographis Paniculata Extracts and Biological Evaluation of Their Anti-Inflammatory Activity. Foods 2019, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Raimundo, L.; Pereira, N.A.L.; Dos Santos, D.J.V.A.; Pérez, M.; Queiroz, G.; Leão, M.; Santos, M.M.M.; Saraiva, L. A Tryptophanol-Derived Oxazolopiperidone Lactam Is Cytotoxic against Tumors via Inhibition of P53 Interaction with Murine Double Minute Proteins. Pharmacol. Res. 2015, 95–96, 42–52. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Raimundo, L.; Espadinha, M.; Soares, J.; Loureiro, J.B.; Alves, M.G.; Santos, M.M.M.; Saraiva, L. Improving Anticancer Activity towards Colon Cancer Cells with a New P53-Activating Agent. Br. J. Pharmacol. 2018, 175, 3947–3962. [Google Scholar] [CrossRef]
| Compound | Cell Line/IC50 (µM) ± SEM | ||||
|---|---|---|---|---|---|
| PANC-1 | HCT116 | A375 | MCF-7 | CCD-18Co | |
| 1 | 3.1 ± 0.1 | 5.1 ± 1.5 | 4.0 ± 0.1 | 13.5 ± 0.5 | 6.2 ± 1.1 |
| 2 | 1.9 ± 0.2 | 1.4 ± 0.1 | 1.2 ± 0.2 | 4.9 ± 0.1 | 5.6 ± 1.3 |
| 3 | 1.9 ± 0.1 | 7.9 ± 2.1 | 1.3 ± 0.1 | 6.6 ± 0.1 | 3.8 ± 0.7 |
| 4 | 3.0 ± 0.2 | 1.5 ± 0.2 | 1.2 ± 0.2 | 6.9 ± 0.8 | 2.2 ± 0.8 |
| 5 | 8.5 ± 1.0 | 8.4 ± 1.1 | 7.7 ± 1.3 | 25.0 ± 3.0 | 7.1 ± 2.6 |
| 6 | 3.4 ± 0.9 | 1.2 ± 0.1 | 1.3 ± 0.3 | 3.8 ± 0.2 | 4.4 ± 0.2 |
| 7 | 4.1 ± 0.3 | 1.6 ± 0.4 | 3.6 ± 0.9 | 18.0 ± 0.1 | 12.9 ± 2.2 |
| 8 | 3.1 ± 0.4 | 2.1 ± 0.6 | 23.0 ± 2.0 | 15.5 ± 0.5 | 8.7 ± 1.9 |
| 9 | 2.0 ± 0.1 | 1.9 ± 0.6 | 2.1 ± 0.3 | 8.1 ± 0.1 | 3.8 ± 0.9 |
| 10 | 3.8 ± 0.7 | 2.2 ± 0.1 | 7.7 ± 1.0 | 7.8 ± 0.8 | 7.2 ± 0.6 |
| 11 | 3.4 ± 0.6 | 1.9 ± 0.3 | 8.2 ± 0.1 | 4.2 ± 0.8 | 3.3 ± 0.4 |
| 12 | 3.4 ± 0.9 | 1.7 ± 0.2 | 1.7 ± 0.0 | 6.8 ± 0.3 | 9.6 ± 2.3 |
| 13 | 6.5 ± 0.4 | 4.8 ± 1.3 | 6.8 ± 0.9 | 26.0 ± 3.0 | 30.0 ± 2.0 |
| 14 | 2.5 ± 0.3 | 2.0 ± 0.6 | 1.7 ± 0.2 | 7.9 ± 0.6 | 3.7 ± 0.9 |
| 15 | 3.3 ± 0.9 | 1.3 ± 0.1 | 1.2 ± 0.2 | 5.6 ± 0.2 | 6.2 ± 1.2 |
| 16 | 2.3 ± 0.2 | 2.3 ± 0.9 | 1.5 ± 0.2 | 9.3 ± 0.1 | 6.8 ± 1.0 |
| 17 | 2.1 ± 0.1 | 1.7 ± 0.5 | 0.9 ± 0.1 | 5.6 ± 0.2 | 4.4 ± 0.9 |
| 18 | 3.5 ± 1.6 | 3.7 ± 0.5 | 6.6 ± 0.8 | 9.5 ± 0.5 | 5.1 ± 0.1 |
| 19 | 3.1 ± 0.8 | 4.1 ± 0.2 | 7.1 ± 0.1 | 7.9 ± 1.5 | 6.6 ± 0.9 |
| 20 | 3.0 ± 1.1 | 1.4 ± 0.3 | 3.3 ± 1.3 | 28.0 ± 2.0 | 3.5 ± 0.3 |
| 21 | 3.5 ± 0.3 | 1.2 ± 0.2 | 1.3 ± 0.3 | 10.4 ± 0.7 | 4.4 ± 0.8 |
| 22 | 5.0 ± 0.4 | 1.6 ± 0.3 | 1.3 ± 0.2 | 5.9 ± 0.1 | 3.2 ± 0.9 |
| 23 | 6.3 ± 1.9 | 2.8 ± 0.8 | 3.3 ± 1.0 | 11.0 ± 0.2 | 4.1 ± 0.9 |
| 24 | 1.8 ± 0.1 | 6.7 ± 3.2 | 1.3 ± 0.3 | 7.0 ± 0.2 | 4.6 ± 1.4 |
| 25 | 4.5 ± 2.0 | 1.7 ± 0.3 | 1.8 ± 0.3 | 6.9 ± 0.1 | 8.9 ± 0.2 |
| 26 | 4.3 ± 1.4 | 1.3 ± 0.1 | 1.4 ± 0.1 | 6.1 ± 0.2 | 6.2 ± 1.4 |
| Etoposide | 2.8 ± 0.05 | 0.65 ± 0.09 | 0.82 ± 0.07 | 1.8 ± 0.03 | 0.76 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.R.L.; Calheiros, J.; Silva, R.A.M.; Gonçalves, B.M.F.; Afonso, C.A.M.; Saraiva, L.; Ferreira, M.-J.U. Exploring the Anticancer Properties of 1,2,3-Triazole-Substituted Andrographolide Derivatives. Pharmaceuticals 2025, 18, 750. https://doi.org/10.3390/ph18050750
Ribeiro JRL, Calheiros J, Silva RAM, Gonçalves BMF, Afonso CAM, Saraiva L, Ferreira M-JU. Exploring the Anticancer Properties of 1,2,3-Triazole-Substituted Andrographolide Derivatives. Pharmaceuticals. 2025; 18(5):750. https://doi.org/10.3390/ph18050750
Chicago/Turabian StyleRibeiro, Joana R. L., Juliana Calheiros, Rita A. M. Silva, Bruno M. F. Gonçalves, Carlos A. M. Afonso, Lucília Saraiva, and Maria-José U. Ferreira. 2025. "Exploring the Anticancer Properties of 1,2,3-Triazole-Substituted Andrographolide Derivatives" Pharmaceuticals 18, no. 5: 750. https://doi.org/10.3390/ph18050750
APA StyleRibeiro, J. R. L., Calheiros, J., Silva, R. A. M., Gonçalves, B. M. F., Afonso, C. A. M., Saraiva, L., & Ferreira, M.-J. U. (2025). Exploring the Anticancer Properties of 1,2,3-Triazole-Substituted Andrographolide Derivatives. Pharmaceuticals, 18(5), 750. https://doi.org/10.3390/ph18050750









