Real-World Safety Profile of Proton Pump Inhibitors in Infants as Reported in the FDA Adverse Event Reporting System (FAERS): Tiny Tummies, Key Decisions
Abstract
1. Introduction
2. Results
3. Discussion
3.1. PPI Use Trends Worldwide
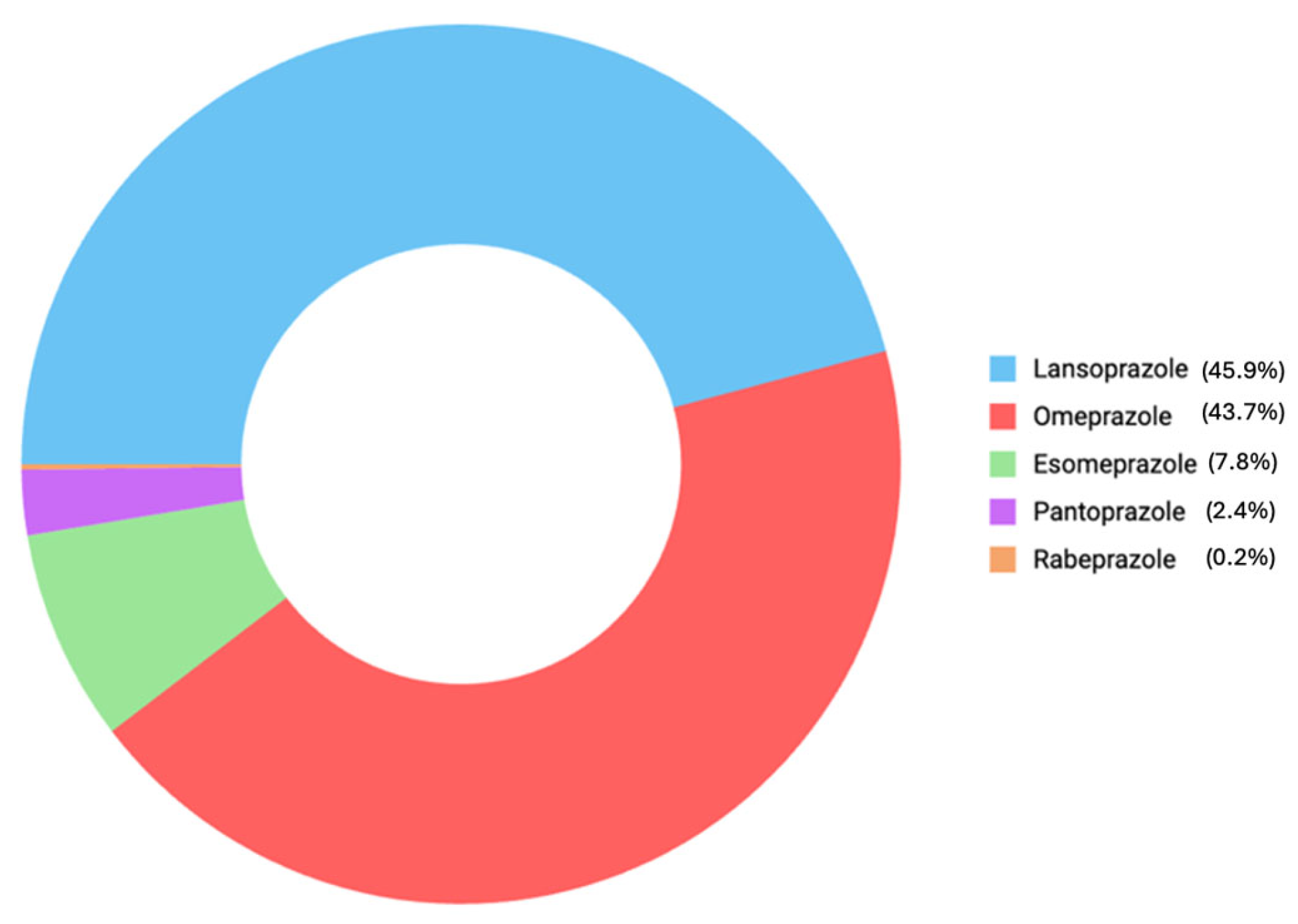
3.2. Comparison of Each PPI
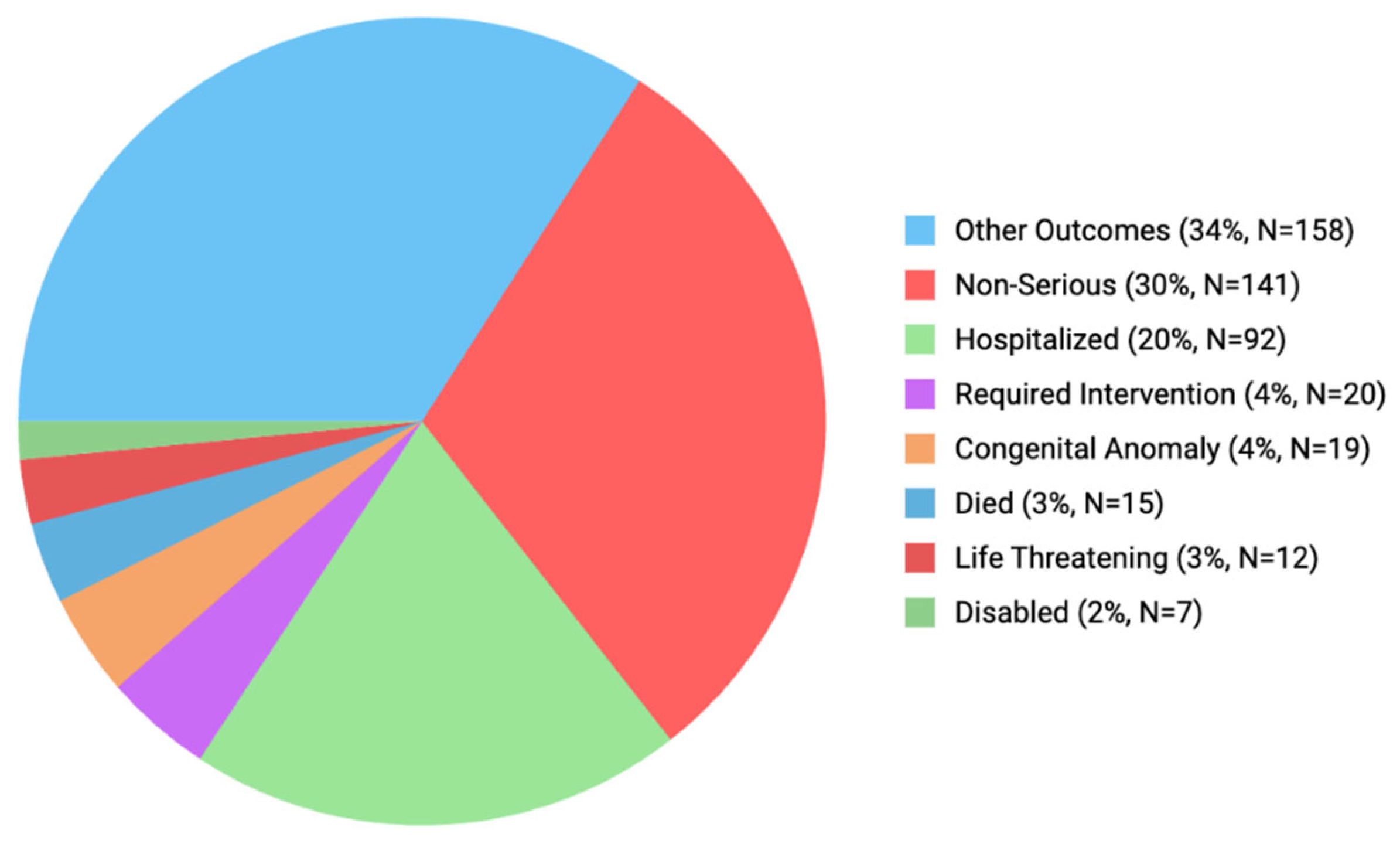
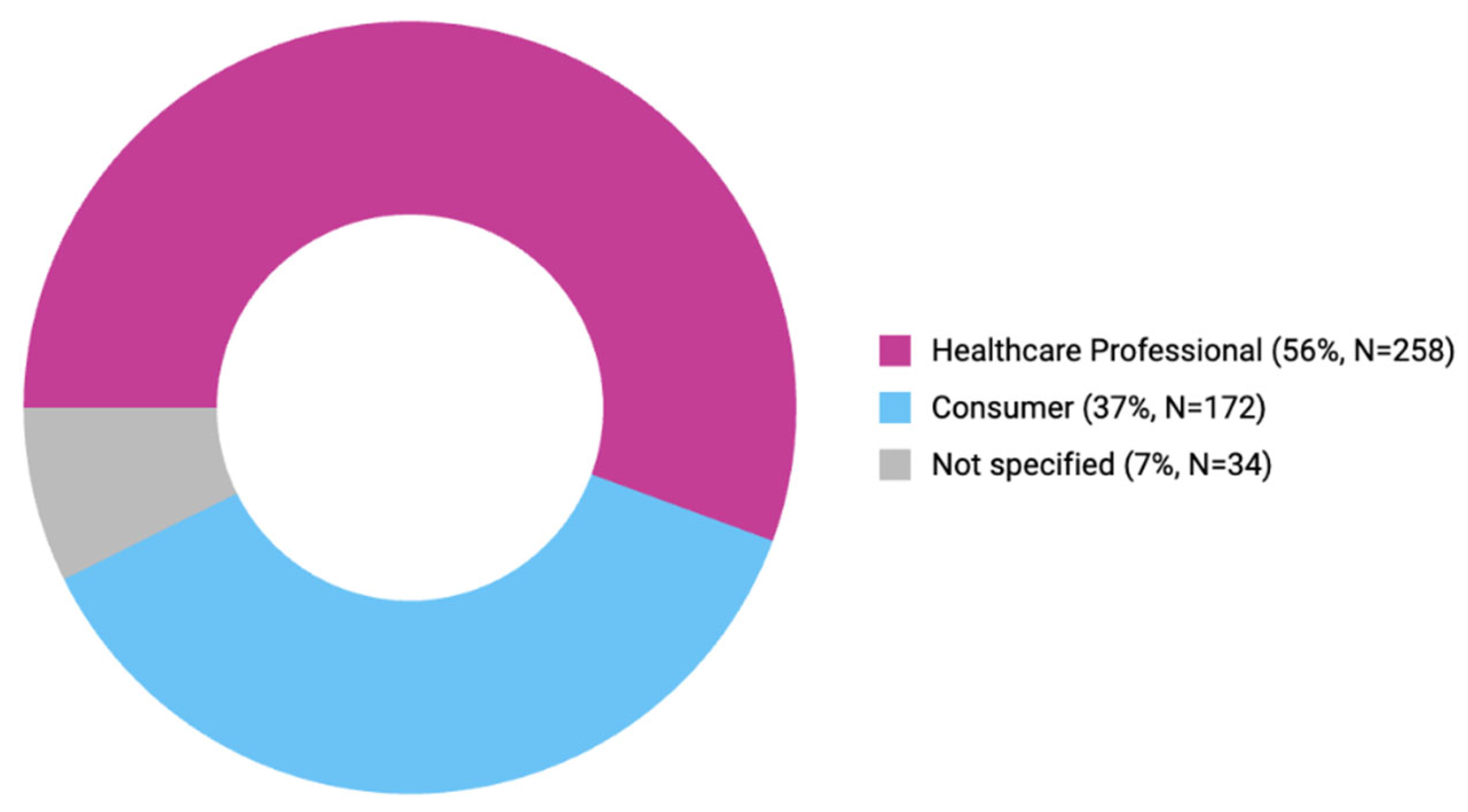
3.3. Comparison of Reporters and Outcomes of Each ADE
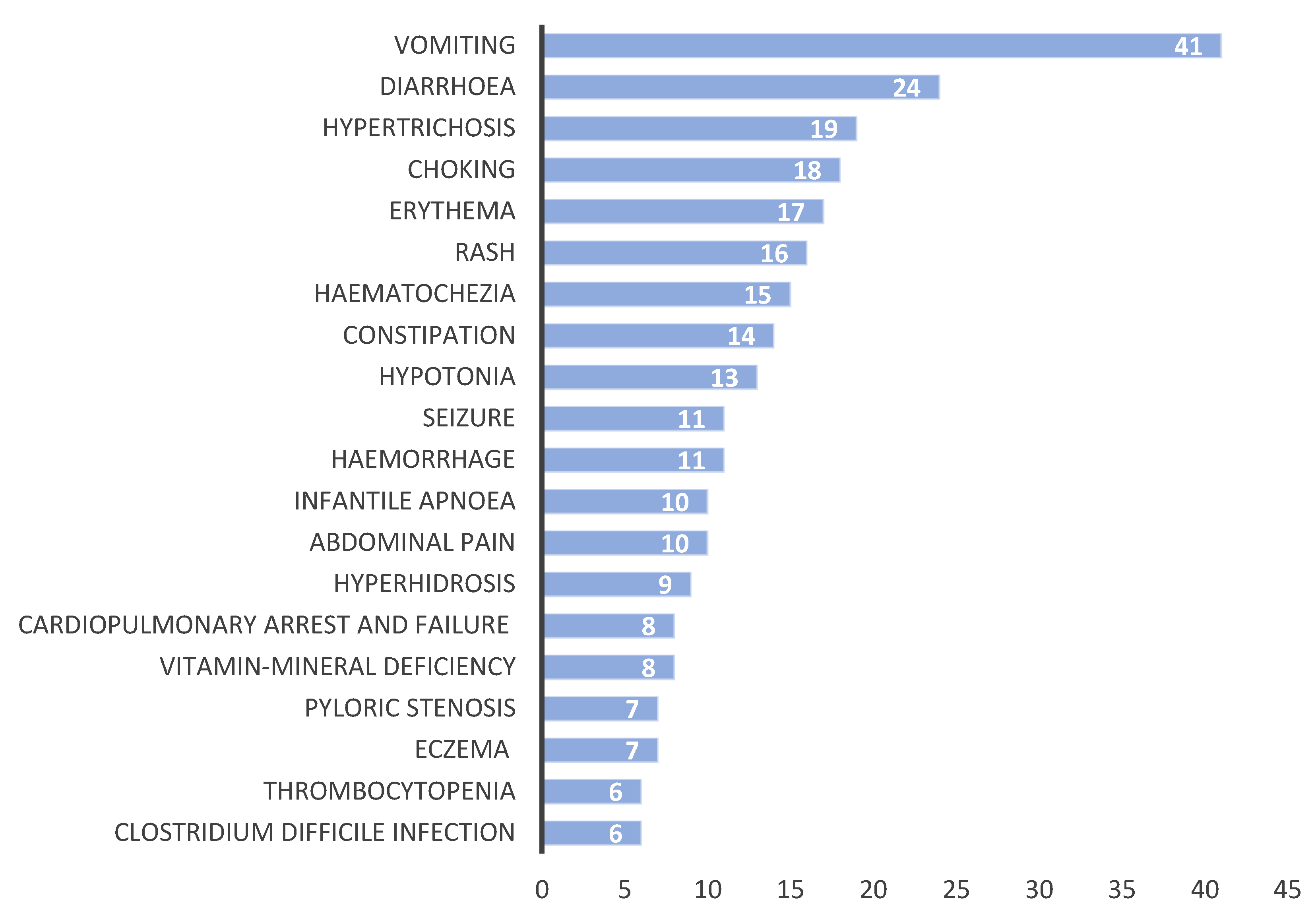
3.4. Comparison of ADEs for Each Organ System
3.4.1. Gastrointestinal Adverse Events
3.4.2. Dermal Adverse Events
3.4.3. Cardiopulmonary Adverse Events
3.4.4. Thrombocytopenia, Hemorrhage, and Hematochezia
3.4.5. Nutrient Deficiencies
3.4.6. Infections
3.4.7. Allergy and Hypersensitivity Reactions
3.4.8. Congenital Anomaly
3.5. Medication Dosing or Administration Errors
3.6. Strengths and Limitations
3.7. Recommendations
4. Materials and Methods
4.1. Study Design
4.2. Data Extraction from the FAERS Database
4.3. Data Handling and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, C.H.; Israel, D.M.; Schreiber, R.; Goldman, R.D. Proton pump inhibitors for irritable infants. Can. Fam. Physician 2013, 59, 153–156. [Google Scholar] [PubMed]
- Dipasquale, V.; Cicala, G.; Spina, E.; Romano, C. A Narrative Review on Efficacy and Safety of Proton Pump Inhibitors in Children. Front. Pharmacol. 2022, 13, 839972. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, S.M.; Moreno-Alvarez, A.; Solar-Boga, A. Proton Pump Inhibitors in Pediatric Gastroesophageal Reflux Disease: A Systematic Review of Randomized Controlled Trials. Children 2024, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.I.; Salvatore, S.; Vandenplas, Y.; de Winter, J.P. Prescription of acid inhibitors in infants: An addiction hard to break. Eur. J. Pediatr. 2020, 179, 1957–1961. [Google Scholar] [CrossRef]
- Peter, C.S.; Sprodowski, N.; Bohnhorst, B.; Silny, J.; Poets, C.F. Gastroesophageal reflux and apnea of prematurity: No temporal relationship. Pediatrics 2002, 109, 8–11. [Google Scholar] [CrossRef]
- King, E.; Horn, D.; Gluchowski, N.; O’Reilly, D.; Bruschettini, M.; Cooper, C.; Soll, R.F. Safety and efficacy of proton pump inhibitors in preterm infants with gastroesophageal reflux disease. Cochrane Database Syst. Rev. 2025, 3, CD015127. [Google Scholar] [CrossRef]
- Zou, S.; Ouyang, M.; Cheng, Q.; Shi, X.; Sun, M. Acid-suppressive drugs: A systematic review and network meta-analysis of their nocturnal acid-inhibitory effect. Pharmacotherapy 2024, 44, 171–183. [Google Scholar] [CrossRef]
- Yalcin, N.; Flint, R.B.; van Schaik, R.H.N.; Simons, S.H.P.; Allegaert, K. The Impact of Pharmacogenetics on Pharmacokinetics and Pharmacodynamics in Neonates and Infants: A Systematic Review. Pharmgenom. Pers. Med. 2022, 15, 675–696. [Google Scholar] [CrossRef]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; DiLorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [CrossRef]
- U.S. Centers for Medicare & Medicaid Services (CMS). Proton Pump Inhibitors: Use in Pediatric Patients. Available online: https://www.cms.gov/medicare-medicaid-coordination/fraud-prevention/medicaid-integrity-education/pharmacy-education-materials/downloads/ppi-pediatric-factsheet11-14.pdf (accessed on 11 March 2025).
- Cloostermans, L.; Allegaert, K.; Smits, A.; Van Neste, M. The Deuterium Oxide Dilution Method to Quantify Human Milk Intake Volume of Infants: A Systematic Review-A Contribution from the ConcePTION Project. Nutrients 2024, 16, 4205. [Google Scholar] [CrossRef]
- Stordal, K.; Ma, A.; Beck, C.E. Reducing the use of proton pump inhibitors in infants with reflux symptoms. BMJ 2024, 385, e074588. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, M.; Zureik, M.; Dray-Spira, R. Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children. JAMA Pediatr. 2023, 177, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Bueno de Mesquita, M.; Mimouni, F.B. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: A 10 years literature review. Br. J. Clin. Pharmacol. 2015, 80, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.I.; Hoang, D.M.; Vandenplas, Y. The effects of proton pump inhibitors on the microbiome in young children. Acta Paediatr. 2020, 109, 1531–1538. [Google Scholar] [CrossRef]
- Lemmens, A.S.; Huysentruyt, K.; Vandenplas, Y. Why think twice before prescribing proton pump inhibitors. Eur. J. Pediatr. 2025, 184, 227. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wintzell, V.; Ludvigsson, J.F.; Svanstrom, H.; Pasternak, B. Proton pump inhibitor use and risk of depression and anxiety in children: Nationwide cohort study. Clin. Transl. Sci. 2022, 15, 1112–1122. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wintzell, V.; Ludvigsson, J.F.; Svanstrom, H.; Pasternak, B. Association Between Proton Pump Inhibitor Use and Risk of Fracture in Children. JAMA Pediatr. 2020, 174, 543–551. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, T.J.; Yang, J.; Bao, D.N. Use of acid-suppressive drugs and risk of fracture in children and young adults: A meta-analysis of observational studies. Eur. J. Clin. Pharmacol. 2022, 78, 365–373. [Google Scholar] [CrossRef]
- Allana, A.; Bashir, S.; Hand, I. Quality Improvement Project to Improve Adherence to Best Practices to Decrease Incidence of Necrotizing Enterocolitis in Preterm Infants. Children 2025, 12, 176. [Google Scholar] [CrossRef]
- Kalaiselvan, V.; Kumar, P.; Mishra, P.; Singh, G.N. System of adverse drug reactions reporting: What, where, how, and whom to report? Indian J. Crit. Care Med. 2015, 19, 564–566. [Google Scholar] [CrossRef]
- Davies, I.; Burman-Roy, S.; Murphy, M.S.; Guideline Development, G. Gastro-oesophageal reflux disease in children: NICE guidance. BMJ 2015, 350, g7703. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.Y.; Rafferty, G.F.; Hannam, S.; Greenough, A. Acid gastroesophageal reflux in convalescent preterm infants: Effect of posture and relationship to apnea. Pediatr. Res. 2007, 62, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Illueca, M.; Alemayehu, B.; Shoetan, N.; Yang, H. Proton pump inhibitor prescribing patterns in newborns and infants. J. Pediatr. Pharmacol. Ther. 2014, 19, 283–287. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, P.; Christiaens, T.; Vander Stichele, R.; Van Winckel, M. Changes in prescription patterns of acid-suppressant medications by Belgian pediatricians: Analysis of the national database, [1997–2009]. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 220–225. [Google Scholar] [CrossRef]
- O’Reilly, D.; Conway, R.; O’Connor, L.; Fitzpatrick, P. Use of anti-reflux medications in infants under 1 year of age: A retrospective drug utilization study using national prescription reimbursement data. Eur. J. Pediatr. 2020, 179, 1963–1967. [Google Scholar] [CrossRef]
- Lyamouri, M.; Marild, K.; Nielsen, R.G.; Stordal, K. Proton pump inhibitors for infants in three Scandinavian countries increased from 2007 to 2020 despite international recommendations. Acta Paediatr. 2022, 111, 2222–2228. [Google Scholar] [CrossRef]
- Yalçın, N. Drug-Induced Problems in Neonatal Patients. Yenidoğan Hastalarda Ilaç Kaynaklı Sorunlar. 2022. Available online: https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp (accessed on 11 May 2025).
- Dipasquale, V.; Cicala, G.; Lagana, F.; Cutroneo, P.; Felicetti, P.; Potenza, S.; Trimarchi, G.; Spina, E.; Romano, C. Adverse reactions related to proton pump inhibitors in pediatric population: An analysis of spontaneous reporting data. Expert. Opin. Drug Saf. 2022, 21, 127–132. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cicala, G.; Lagana, F.; Cutroneo, P.; Trimarchi, G.; Spina, E.; Romano, C. Spontaneous reporting of adverse reactions related to proton pump inhibitors. Dig. Liver Dis. 2023, 55, 595–600. [Google Scholar] [CrossRef]
- Springer, M.; Atkinson, S.; North, J.; Raanan, M. Safety and pharmacodynamics of lansoprazole in patients with gastroesophageal reflux disease aged <1 year. Paediatr. Drugs 2008, 10, 255–263. [Google Scholar] [CrossRef]
- Sandstrom, M.; Davidson, G.; Tolia, V.; Sullivan, J.E.; Langstrom, G.; Lundborg, P.; Brown, K. Phase I, multicenter, randomized, open-label study evaluating the pharmacokinetics and safety profile of repeated once-daily doses of intravenous esomeprazole in children 0 to 17 years of age. Clin. Ther. 2012, 34, 1828–1838. [Google Scholar] [CrossRef]
- Elosua-Gonzalez, M.; Campos-Dominguez, M.; Bancalari, D.; Noguera-Morel, L.; Hernandez-Martin, A.; Huerta-Aragones, J.; Torrelo, A. Omeprazole-induced hypertrichosis in two children. Pediatr. Dermatol. 2018, 35, e212–e214. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.D.; Stark, J.E.; Vesta, K.S. Pantoprazole-induced thrombocytopenia. Ann. Pharmacother. 2006, 40, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Binnetoglu, E.; Akbal, E.; Sen, H.; Gunes, F.; Erbag, G.; Asik, M.; Bozkurt, N.; Uludag, A.; Tekin, M.; Tekin, S.Z. Pantoprazole-induced thrombocytopenia in patients with upper gastrointestinal bleeding. Platelets 2015, 26, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.B.; Nagaraja, V.; Kapur, A.; Eslick, G.D. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: A systematic review and meta-analysis. Intern. Med. J. 2015, 45, 409–416. [Google Scholar] [CrossRef]
- Stark, C.M.; Nylund, C.M. Side Effects and Complications of Proton Pump Inhibitors: A Pediatric Perspective. J. Pediatr. 2016, 168, 16–22. [Google Scholar] [CrossRef]
- Tavares, M.; Amil-Dias, J. Proton-Pump Inhibitors: Do Children Break a Leg by Using Them? J. Pediatr. Gastroenterol. Nutr. 2021, 73, 665–669. [Google Scholar] [CrossRef]
- Yadlapati, R.; Kahrilas, P.J. The “dangers” of chronic proton pump inhibitor use. J. Allergy Clin. Immunol. 2018, 141, 79–81. [Google Scholar] [CrossRef]
- De Bruyne, P.; Ito, S. Toxicity of long-term use of proton pump inhibitors in children. Arch. Dis. Child. 2018, 103, 78–82. [Google Scholar] [CrossRef]
- Anjewierden, S.; Han, Z.; Foster, C.B.; Pant, C.; Deshpande, A. Risk factors for Clostridium difficile infection in pediatric inpatients: A meta-analysis and systematic review. Infect. Control Hosp. Epidemiol. 2019, 40, 420–426. [Google Scholar] [CrossRef]
- Oshima, T.; Wu, L.; Li, M.; Fukui, H.; Watari, J.; Miwa, H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: A systematic review and meta-analysis. J. Gastroenterol. 2018, 53, 84–94. [Google Scholar] [CrossRef]
- Bavbek, S.; Kepil Ozdemir, S.; Bonadonna, P.; Atanaskovic-Markovic, M.; Barbaud, A.; Brockow, K.; Laguna Martinez, J.; Nakonechna, A.; Pagani, M.; Arcolaci, A.; et al. Hypersensitivity reactions to proton pump inhibitors. An EAACI position paper. Allergy 2024, 79, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Hviid, A. Use of proton-pump inhibitors in early pregnancy and the risk of birth defects. N. Engl. J. Med. 2010, 363, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Peron, A.; Ripoche, E.; Picot, C.; Ajiji, P.; Cucherat, M.; Cottin, J. Use of proton pump inhibitors during pregnancy: A systematic review and meta-analysis of congenital malformations. Reprod. Toxicol. 2023, 119, 108419. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Noh, Y.; Jeong, H.E.; Choi, E.Y.; Man, K.K.C.; Han, J.Y.; Kim, H.S.; Yon, D.K.; Shin, J.Y. Association Between Proton Pump Inhibitor Use During Early Pregnancy and Risk of Congenital Malformations. JAMA Netw. Open 2023, 6, e2250366. [Google Scholar] [CrossRef]
- Matalova, P.; Buchta, M.; Drietomska, V.; Spicakova, A.; Wawruch, M.; Ondra, P.; Urbanek, K. Acute drug intoxication in childhood: A 10-year retrospective observational single-centre study and case reports. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2023, 167, 294–302. [Google Scholar] [CrossRef]
- NHS GGC Proton Pump Inhibitor (PPI) Guideline for Neonates and Paediatrics. Available online: https://clinicalguidelines.scot.nhs.uk/media/2849/proton-pump-inhibitor-guideline-for-neonates-and-paediatrics.pdf (accessed on 18 March 2025).
- Bestebreurtje, P.; de Koning, B.A.E.; Roeleveld, N.; Knibbe, C.A.J.; Tibboel, D.; van Groen, B.; van de Ven, C.P.; Plotz, F.B.; de Wildt, S.N. Rectal Omeprazole in Infants With Gastroesophageal Reflux Disease: A Randomized Pilot Trial. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 635–643. [Google Scholar] [CrossRef]
- Leopoldino, R.W.; Rocha, L.C.A.; Fernandes, F.E.M.; de Lima Costa, H.T.M.; Vale, L.M.P.; Oliveira, A.G.; Martins, R.R. Assessment of severity and avoidability of adverse drug reactions in neonates: A reproducibility study of the Hartwig tool and LAAT. Eur. J. Clin. Pharmacol. 2025, 81, 123–127. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The Reporting of a Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Saf. 2024, 47, 575–584. [Google Scholar] [CrossRef]
- Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Yalcin, N.; Kasikci, M.; Celik, H.T.; Allegaert, K.; Demirkan, K.; Yigit, S.; Yurdakok, M. An Artificial Intelligence Approach to Support Detection of Neonatal Adverse Drug Reactions Based on Severity and Probability Scores: A New Risk Score as Web-Tool. Children 2022, 9, 1826. [Google Scholar] [CrossRef]
- Anderson, P.O.; Manoguerra, A.S.; Valdes, V. A Review of Adverse Reactions in Infants From Medications in Breastmilk. Clin. Pediatr. 2016, 55, 236–244. [Google Scholar] [CrossRef]
- Du, W.; Lehr, V.T.; Lieh-Lai, M.; Koo, W.; Ward, R.M.; Rieder, M.J.; Van Den Anker, J.N.; Reeves, J.H.; Mathew, M.; Lulic-Botica, M.; et al. An algorithm to detect adverse drug reactions in the neonatal intensive care unit. J. Clin. Pharmacol. 2013, 53, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Leopoldino, R.W.D.; de Oliveira, L.V.S.; Fernandes, F.E.M.; de Lima Costa, H.T.M.; Vale, L.M.P.; Oliveira, A.G.; Martins, R.R. Causality assessment of adverse drug reactions in neonates: A comparative study between Naranjo’s algorithm and Du’s tool. Int. J. Clin. Pharm. 2023, 45, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, N.; van den Anker, J.; Samiee-Zafarghandy, S.; Allegaert, K. Drug related adverse event assessment in neonates in clinical trials and clinical care. Expert. Rev. Clin. Pharmacol. 2024, 17, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024, 47, 585–599. [Google Scholar] [CrossRef]
- Loke, Y.K. Not Just Another Reporting Guideline? Here’s Why READUS-PV is a Major Step Forward. Drug Saf. 2024, 47, 571–573. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Khouri, C.; Raschi, E. The reporting of disproportionality analysis in pharmacovigilance: Spotlight on the READUS-PV guideline. Front. Pharmacol. 2024, 15, 1488725. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef]
- Ojo, A.; Patel, P.; Boehning, A.P. Conversion Weights. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557608/ (accessed on 29 September 2024).


| Additional Information | Number of Reports | % |
|---|---|---|
| No Adverse Event | 53 | 11.4 |
| Medication Error | 50 | 10.8 |
| Product Administered to Patient of Inappropriate Age | 43 | 9.3 |
| Wrong Technique in Product Usage Process | 39 | 8.4 |
| Off-Label Use | 36 | 7.8 |
| Incorrect Dose Administered | 31 | 6.7 |
| Product Prescribing Error | 30 | 6.5 |
| Accidental Exposure | 24 | 5.2 |
| Product Quality-Substitution Issue | 10 | 2.2 |
| Oral Administration Complication | 7 | 1.5 |
| Adverse Event of Interest | All Other Adverse Events | Total | |
|---|---|---|---|
| Drug of interest | a | b | a + b |
| All other drugs | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tezel Yalçın, H.; Yalçın, N.; Allegaert, K. Real-World Safety Profile of Proton Pump Inhibitors in Infants as Reported in the FDA Adverse Event Reporting System (FAERS): Tiny Tummies, Key Decisions. Pharmaceuticals 2025, 18, 730. https://doi.org/10.3390/ph18050730
Tezel Yalçın H, Yalçın N, Allegaert K. Real-World Safety Profile of Proton Pump Inhibitors in Infants as Reported in the FDA Adverse Event Reporting System (FAERS): Tiny Tummies, Key Decisions. Pharmaceuticals. 2025; 18(5):730. https://doi.org/10.3390/ph18050730
Chicago/Turabian StyleTezel Yalçın, Hülya, Nadir Yalçın, and Karel Allegaert. 2025. "Real-World Safety Profile of Proton Pump Inhibitors in Infants as Reported in the FDA Adverse Event Reporting System (FAERS): Tiny Tummies, Key Decisions" Pharmaceuticals 18, no. 5: 730. https://doi.org/10.3390/ph18050730
APA StyleTezel Yalçın, H., Yalçın, N., & Allegaert, K. (2025). Real-World Safety Profile of Proton Pump Inhibitors in Infants as Reported in the FDA Adverse Event Reporting System (FAERS): Tiny Tummies, Key Decisions. Pharmaceuticals, 18(5), 730. https://doi.org/10.3390/ph18050730






