Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients
Abstract
1. Introduction
2. Results
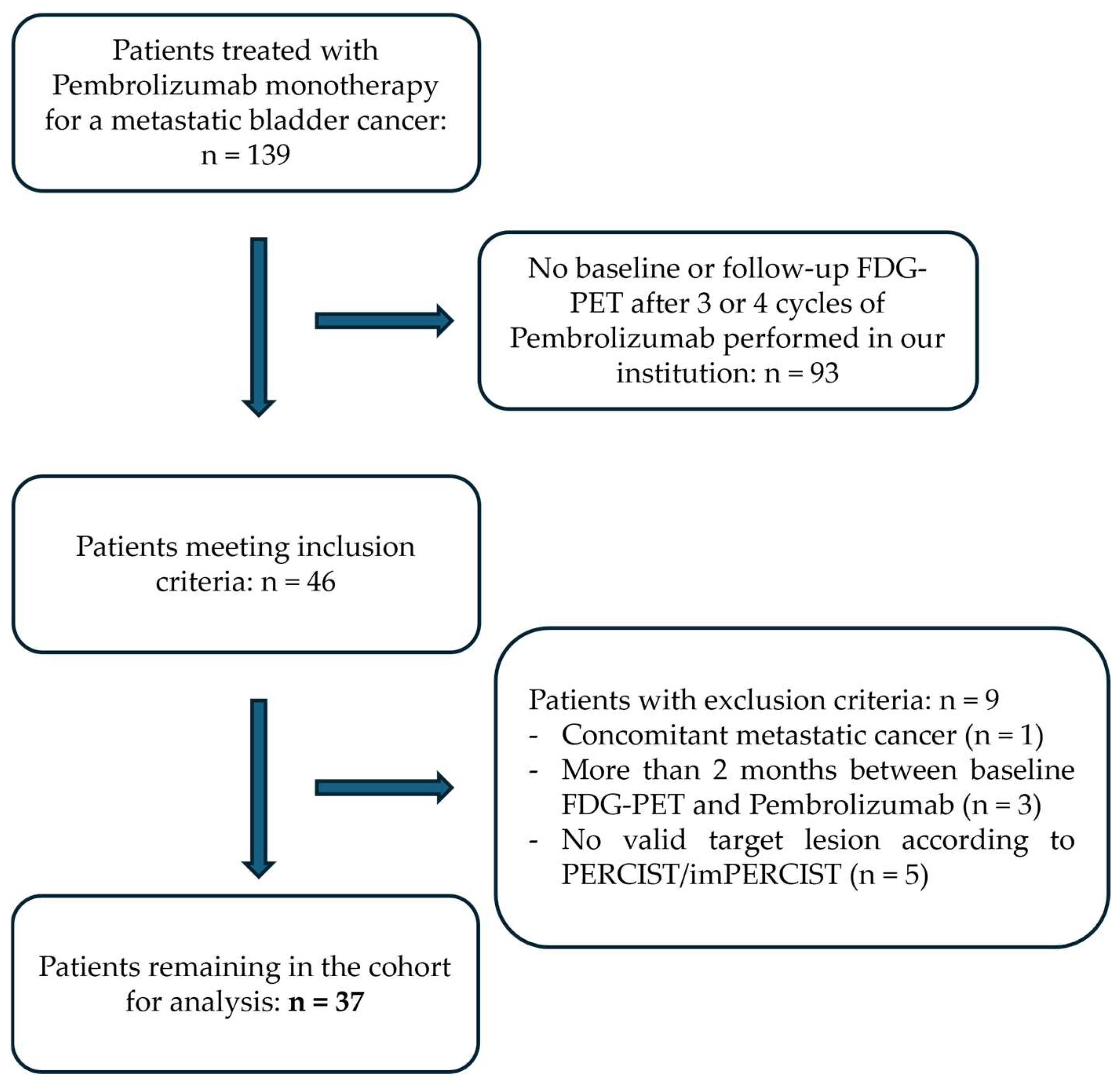
2.1. Patient Characteristics
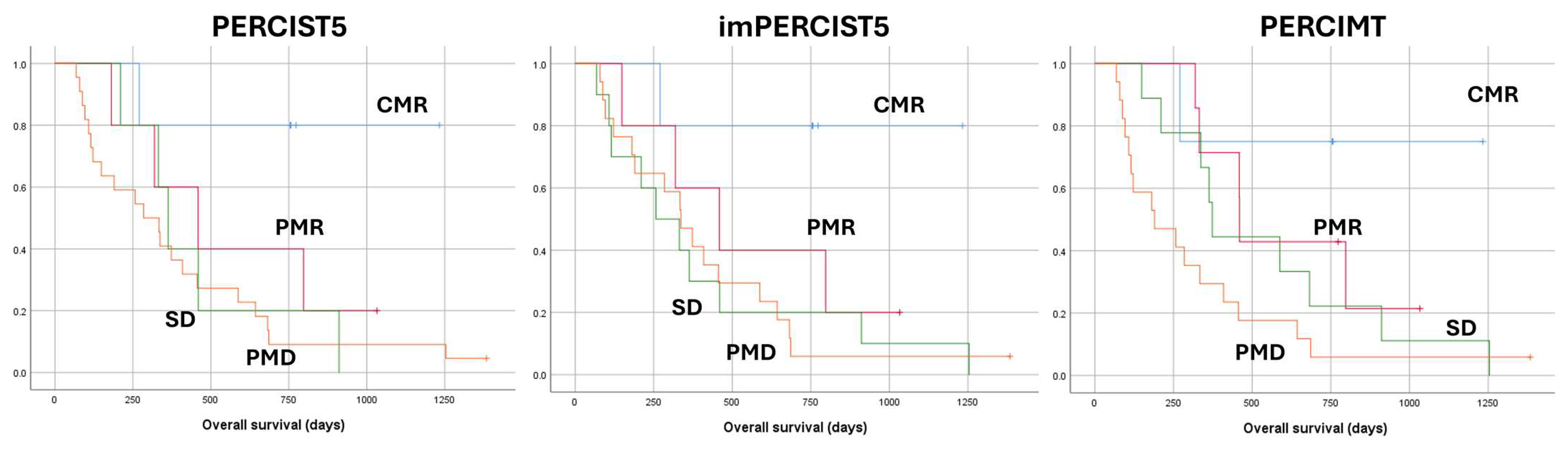
2.2. PERCIST5
2.3. ImPERCIST5
2.4. PERCIMT
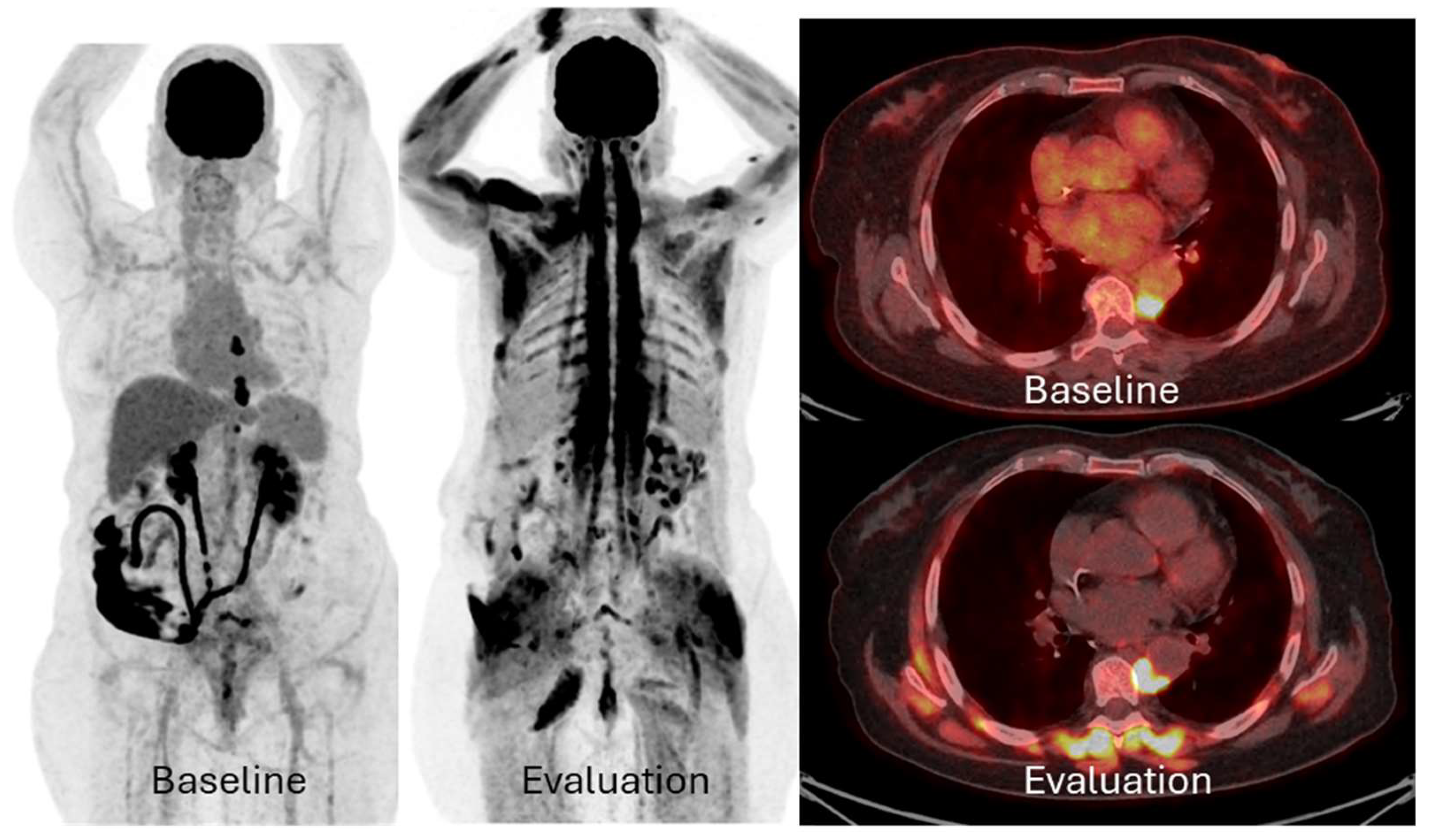
2.5. Pseudoprogression and Immune-Related Adverse Effects
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Follow-Up
4.2. FDG-PET/CT Protocol
4.3. FDG-PET/CT Analysis
4.4. Histological Analysis
4.5. Statistical Analysis
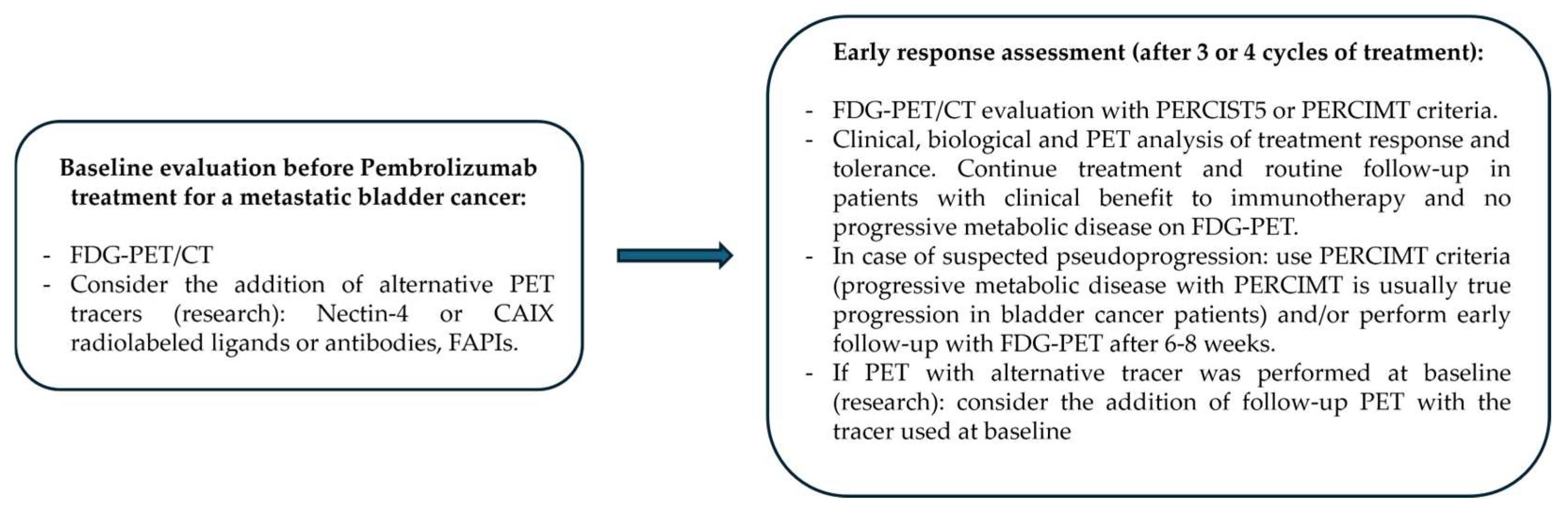
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Ayati, N.; Sadeghi, R.; Kiamanesh, Z.; Lee, S.T.; Zakavi, S.R.; Scott, A.M. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Brezun, J.; Aide, N.; Peroux, E.; Lamboley, J.-L.; Gutman, F.; Lussato, D.; Helissey, C. [18F]FDG PET/CT Integration in Evaluating Immunotherapy for Lung Cancer: A Clinician’s Practical Approach. Diagnostics 2024, 14, 2104. [Google Scholar] [CrossRef] [PubMed]
- Einerhand, S.M.; van Gennep, E.J.; Mertens, L.S.; Hendricksen, K.; Donswijk, M.L.; van der Poel, H.G.; van Rhijn, B.W. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in muscle-invasive bladder cancer. Curr. Opin. Urol. 2020, 30, 654–664. [Google Scholar] [CrossRef]
- Soubra, A.; Gencturk, M.; Froelich, J.; Balaji, P.; Gupta, S.; Jha, G.; Konety, B.R. FDG-PET/CT for Assessing the Response to Neoadjuvant Chemotherapy in Bladder Cancer Patients. Clin. Genitourin. Cancer 2018, 16, 360–364. [Google Scholar] [CrossRef]
- Marandino, L.; Capozza, A.; Bandini, M.; Raggi, D.; Farè, E.; Pederzoli, F.; Gallina, A.; Capitanio, U.; Bianchi, M.; Gandaglia, G.; et al. [18F]Fluoro-Deoxy-Glucose positron emission tomography to evaluate lymph node involvement in patients with muscle-invasive bladder cancer receiving neoadjuvant pembrolizumab. Urol. Oncol. Semin. Orig. Investig. 2021, 39, e15–e235. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. 18F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Balar, A.; Castellano, D.; Grivas, P.; Vaughn, D.; Powles, T.; Vuky, J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.; et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: Results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann. Oncol. 2023, 34, 289–299. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, K.W.; Pyo, J.; Suh, C.H.; Yoon, S.; Hatabu, H.; Nishino, M. Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis. Radiology 2020, 297, 87–96. [Google Scholar] [CrossRef]
- Ayati, N.; Jamshidi-Araghi, Z.; Hoellwerth, M.; Schweighofer-Zwink, G.; Hitzl, W.; Koelblinger, P.; Pirich, C.; Beheshti, M. Predictive value and accuracy of [18F]FDG PET/CT modified response criteria for checkpoint immunotherapy in patients with advanced melanoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Weru, V.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The prognostic value of [18F]FDG PET/CT based response monitoring in metastatic melanoma patients undergoing immunotherapy: Comparison of different metabolic criteria. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2699–2714. [Google Scholar] [CrossRef]
- Homburg, S.; Christensen, C.B.; Pedersen, M.; Sørensen, S.G.; Donia, M.; Svane, I.M.; Hendel, H.W.; Ellebaek, E. Prospective Assessment of Fluorine-18-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) for Early Identification of Checkpoint-Inhibitor-Induced Pseudoprogression. Cancers 2024, 16, 964. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.; Pruim, J.; Price, P. Measurement of Clinical and Subclinical Tumour Response Using [18F]-fluorodeoxyglucose and Positron Emission Tomography: Review and 1999 EORTC Recommendations. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.-C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; McKenna, C.; et al. Immune-Modified Response Evaluation Criteria in Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Comparison of Metabolic and Morphological Response Criteria for Early Prediction of Response and Survival in NSCLC Patients Treated with Anti-PD-1/PD-L1. Front. Oncol. 2020, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zou, Y.; Wang, W.; Li, Q.; Tian, R. The role of baseline 18F-FDG PET/CT for survival prognosis in NSCLC patients undergoing immunotherapy: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2024, 16, 17588359241293364. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- McDonald, S.; Keane, K.G.; Gauci, R.; Hayne, D. Nuclear Medicine and Molecular Imaging in Urothelial Cancer: Current Status and Future Directions. Cancers 2025, 17, 232. [Google Scholar] [CrossRef]
- Kubota, Y.; Sato, T.; Hozumi, C.; Han, Q.; Aoki, Y.; Masaki, N.; Obara, K.; Tsunoda, T.; Hoffman, R.M. Superiority of [11C]methionine over [18F]deoxyglucose for PET Imaging of Multiple Cancer Types Due to the Methionine Addiction of Cancer. Int. J. Mol. Sci. 2023, 24, 1935. [Google Scholar] [CrossRef]
- Golan, S.; Sopov, V.; Baniel, J.; Groshar, D. Comparison of 11C-Choline With 18F-FDG in Positron Emission Tomography/Computerized Tomography for Staging Urothelial Carcinoma: A Prospective Study. J. Urol. 2011, 186, 436–441. [Google Scholar] [CrossRef]
- Kim, S.J.; Koo, P.J.; Pak, K.; Kim, I.J.; Kim, K. Diagnostic accuracy of C-11 choline and C-11 acetate for lymph node staging in patients with bladder cancer: A systematic review and meta-analysis. World J. Urol. 2018, 36, 331–340. [Google Scholar] [CrossRef]
- Duan, X.; Xia, L.; Zhang, Z.; Ren, Y.; Pomper, M.G.; Rowe, S.P.; Li, X.; Li, N.; Zhang, N.; Zhu, H.; et al. First-in-Human Study of the Radioligand 68Ga-N188 Targeting Nectin-4 for PET/CT Imaging of Advanced Urothelial Carcinoma. Clin. Cancer Res. 2023, 29, 3395–3407. [Google Scholar] [CrossRef]
- Campbell, D.O.; Noda, A.; Verlinsky, A.; Snyder, J.; Fujita, Y.; Murakami, Y.; Fushiki, H.; Miyoshi, S.; Lacayo, S.; Cabral, E.; et al. Preclinical Evaluation of an Anti-Nectin-4 ImmunoPET Reagent in Tumor-Bearing Mice and Biodistribution Studies in Cynomolgus Monkeys. Mol. Imaging Biol. 2016, 18, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidi, M.; Viswambaram, P.; McCombie, S.; Liow, E.; Lenzo, N.; Ferguson, T.; Redfern, A.D.; Gauci, R.; Hayne, D. 89Zirconium-labelled girentuximab (89Zr-TLX250) PET in Urothelial Cancer Patients (ZiPUP): Protocol for a phase I trial of a novel staging modality for urothelial carcinoma. BMJ Open 2022, 12, e060478. [Google Scholar] [CrossRef]
- Hofman, M.S.; Tran, B.; Feldman, D.R.; Pokorska-Bocci, A.; Pichereau, S.; Wessen, J.; Haskali, M.B.; Sparks, R.B.; Vlasyuk, O.; Galetic, I. First-in-Human Safety, Imaging, and Dosimetry of a Carbonic Anhydrase IX–Targeting Peptide, [68Ga]Ga-DPI-4452, in Patients with Clear Cell Renal Cell Carcinoma. J. Nucl. Med. 2024, 65, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Skovgaard, D.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Nielsen, C.H.; Thurison, T.; Klausen, T.L.; Holm, S.; Loft, A.; et al. First-in-human uPAR PET: Imaging of Cancer Aggressiveness. Theranostics 2015, 5, 1303–1316. [Google Scholar] [CrossRef]
- Avellini, C.; Licini, C.; Lazzarini, R.; Gesuita, R.; Guerra, E.; Tossetta, G.; Castellucci, C.; Giannubilo, S.R.; Procopio, A.; Alberti, S.; et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017, 8, 58642–58653. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-H.; Chen, S.-M.; Qiu, Q.-R.; Gao, R.-C.; Wei, Y.; Zheng, Q.-S.; Miao, W.-B.; Xu, N. Head-to-head comparisons of 68Ga-PSMA-11 and 18F-FDG PET/CT in evaluating patients with upper tract urothelial carcinoma: A prospective pilot study. Int. Urol. Nephrol. 2023, 55, 2753–2764. [Google Scholar] [CrossRef]
- Novruzov, E.; Dendl, K.; Ndlovu, H.; Choyke, P.L.; Dabir, M.; Beu, M.; Novruzov, F.; Mehdi, E.; Guliyev, F.; Koerber, S.A.; et al. Head-to-head Intra-individual Comparison of [68Ga]-FAPI and [18F]-FDG PET/CT in Patients with Bladder Cancer. Mol. Imaging Biol. 2022, 24, 651–658. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Feng, Y.; Lin, Z.; Tao, K.; Zhang, T.; Lan, X. Predicting Pathologic Complete Response in Locally Advanced Rectal Cancer with [68Ga]Ga-FAPI-04 PET, [18F]FDG PET, and Contrast-Enhanced MRI: Lesion-to-Lesion Comparison with Pathology. J. Nucl. Med. 2024, 65, 1548–1556. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Pang, Y.; Fu, K.; Luo, Q.; Sun, L.; Wu, H.; Lin, Q.; Su, G.; Chen, X.; et al. FAP-targeted radioligand therapy with68Ga/177Lu-DOTA-2P(FAPI)2 enhance immunogenicity and synergize with PD-L1 inhibitors for improved antitumor efficacy. J. Immunother. Cancer 2025, 13, e010212. [Google Scholar] [CrossRef]
| Median or N | Mean (±Standard Deviation) [Range] or % | |
|---|---|---|
| Sex | Male: 26 Female: 11 | 70% 30% |
| Age (years) | 72 | 69.7 (±10.1) [33–82] |
| Weight (kg) | 72.5 | 73 (±15.0) [49–118] |
| BMI | 25.1 | 25.5 (±4.8) [17.9–39.0] |
| ECOG PS | 9: 11 22: 25 6: 6 3 or more: 0 | 24% 60% 16% 0% |
| Previous lines of treatment | 1: 25 2: 10 3: 2 | 68% 27% 5% |
| Histology | Classic UC: 26 Variant UC: 6 Non-UC histology: 1 | 79% 18% 3% |
| PDL-1 TPS | 5% <1%: 11 | 18.3 (±29)% [0–90%] 44% |
| PDL-1 CPS | 10% <1%: 9 | 21.2 (±29)% [0–90%] 36% |
| Cycles received | 8 | 11.6 (± 11.7) [3–47] |
| PFS (days) | 152 (±29) | 361 (±70) [52–1233] |
| OS (days) | 363 (±47) | 517 (±70) [68–1384] |
| Responders Versus Non-Responders | HR for PFS (95%CI) | HR for OS (95%CI) |
|---|---|---|
| PERCIST5 | 5.6 (1.9–16.5) | 3.1 (1.2–8.2) |
| ImPERCIST5 | 5.4 (1.8–15.9) | 3.1 (1.2–8.1) |
| PERCIMT | 4.9 (1.8–13.3) | 3.0 (1.2–7.3) |
| Non-progressors versus progressors | ||
| PERCIST5 | 5.5 (2.3–13.3) | 2.2 (1.0–4.8) |
| ImPERCIST5 | 3.7 (1.7–8.3) | 1.6 (0.8–3.3) |
| PERCIMT | 5.5 (2.3–13.2) | 2.6 (1.2–5.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertaux, M.; Luo, C.; Radulescu, C.; Beuzeboc, P.; Landais, C.; Touche, P.; Abraham, C.; Seban, M.H.; Camps, E.; Faucheron, A.; et al. Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients. Pharmaceuticals 2025, 18, 701. https://doi.org/10.3390/ph18050701
Bertaux M, Luo C, Radulescu C, Beuzeboc P, Landais C, Touche P, Abraham C, Seban MH, Camps E, Faucheron A, et al. Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients. Pharmaceuticals. 2025; 18(5):701. https://doi.org/10.3390/ph18050701
Chicago/Turabian StyleBertaux, Marc, Caroline Luo, Camelia Radulescu, Philippe Beuzeboc, Cecile Landais, Pauline Touche, Christine Abraham, Marie Homo Seban, Eve Camps, Antoine Faucheron, and et al. 2025. "Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients" Pharmaceuticals 18, no. 5: 701. https://doi.org/10.3390/ph18050701
APA StyleBertaux, M., Luo, C., Radulescu, C., Beuzeboc, P., Landais, C., Touche, P., Abraham, C., Seban, M. H., Camps, E., Faucheron, A., Tourne, M., Fricot, L., Turpin, L., Seban, R.-D., & Khedairia, S. (2025). Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients. Pharmaceuticals, 18(5), 701. https://doi.org/10.3390/ph18050701






