Exploring the Potential of s-Triazine Derivatives as Novel Antifungal Agents: A Review
Abstract
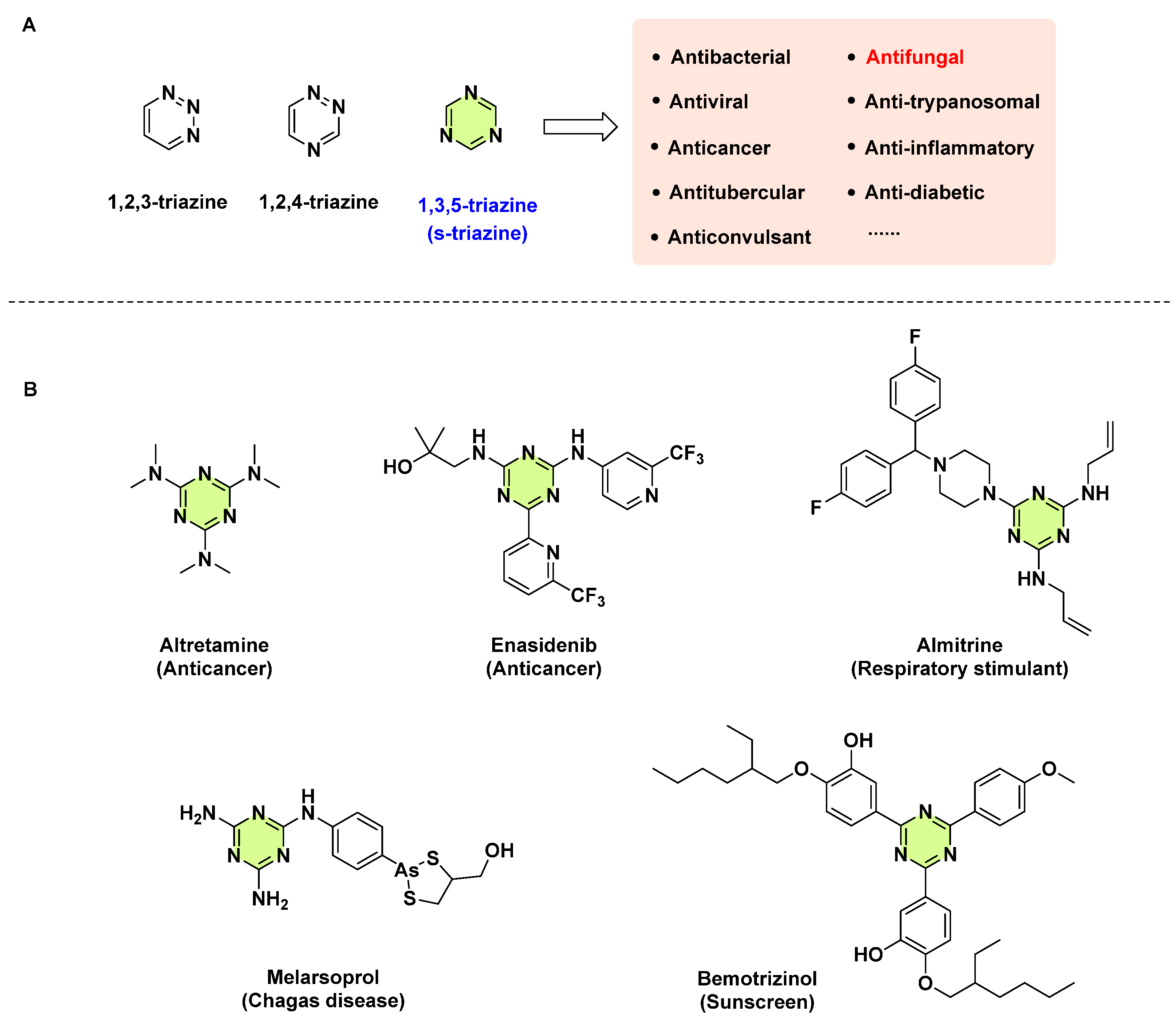
1. Introduction
2. Methodology
- (a)
- Inclusion: the literature (1) published between 2014 and 2024, (2) available in English or with an English abstract if published in another language, and (3) studies on pathogenic fungi.
- (b)
- Exclusion: the literature on (1) purely computational, structural, or in silico studies or (2) without full-text availability.
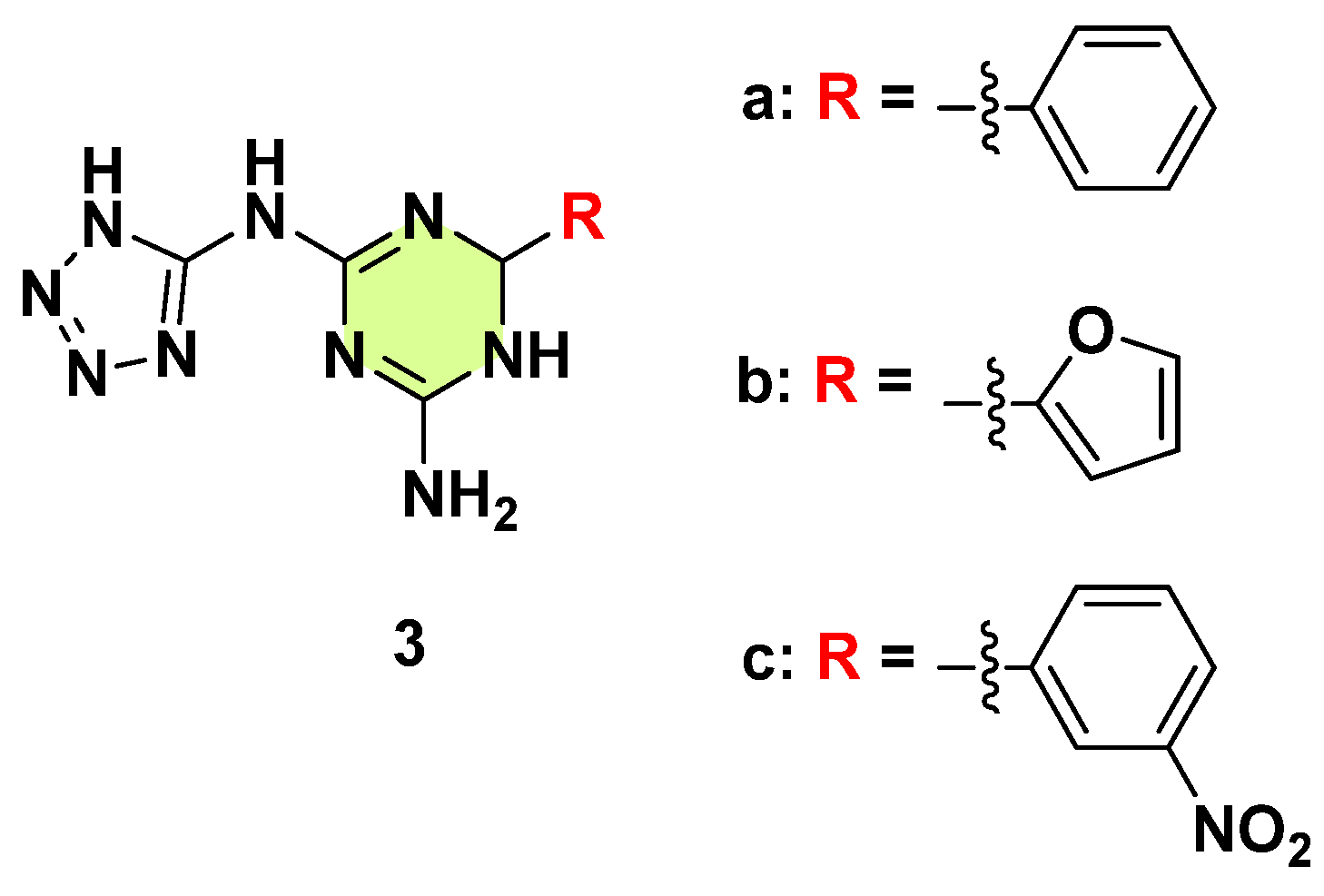
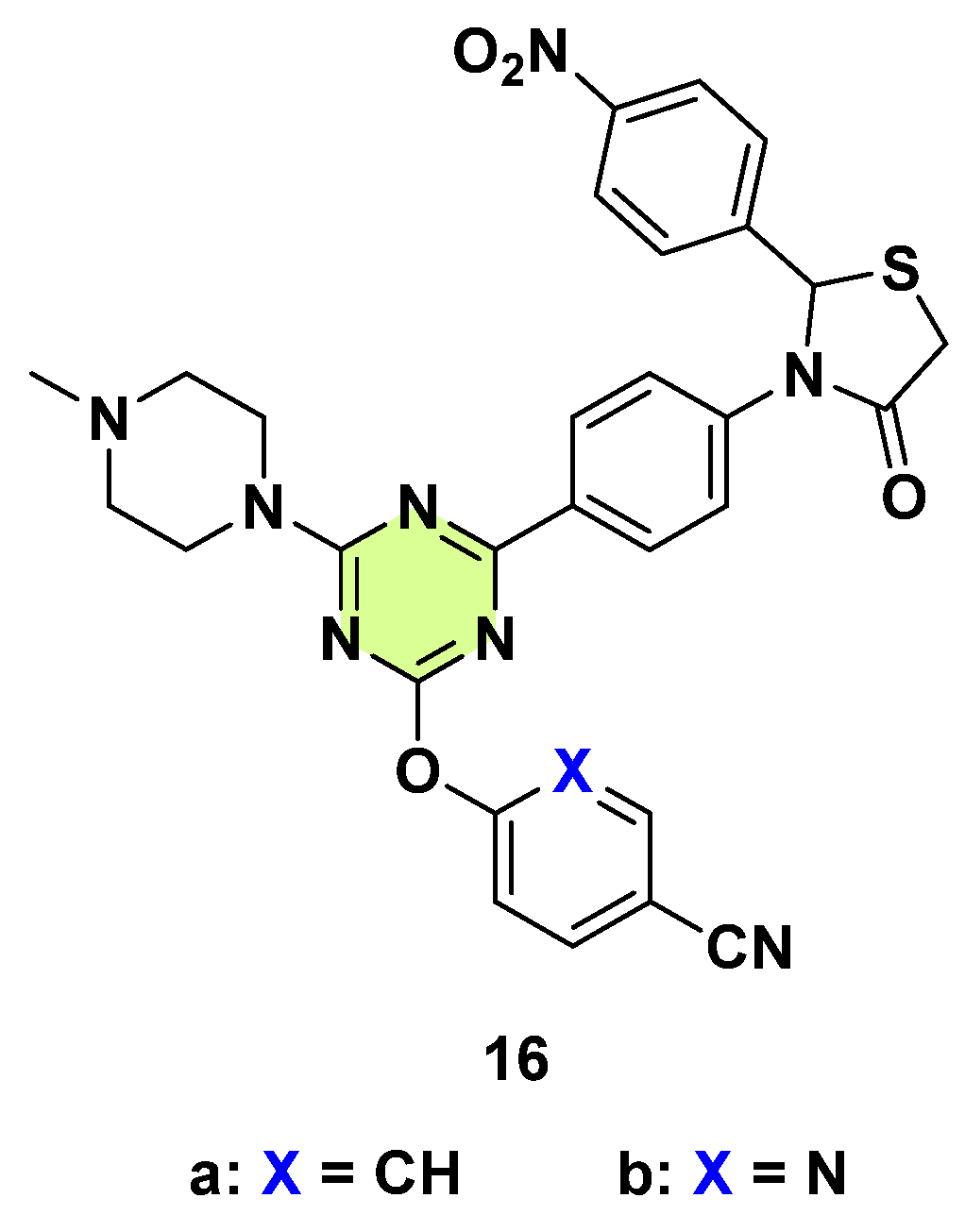
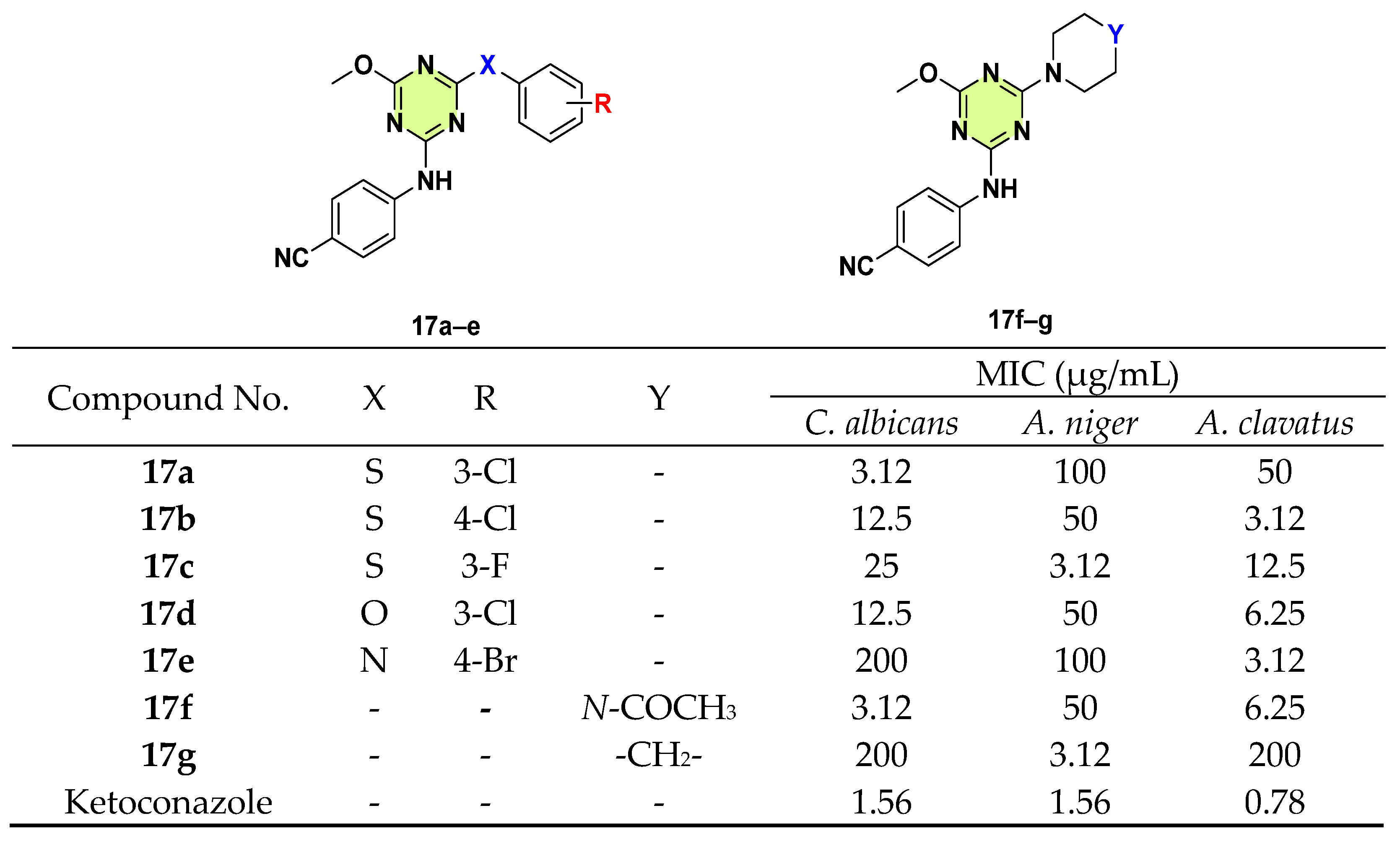
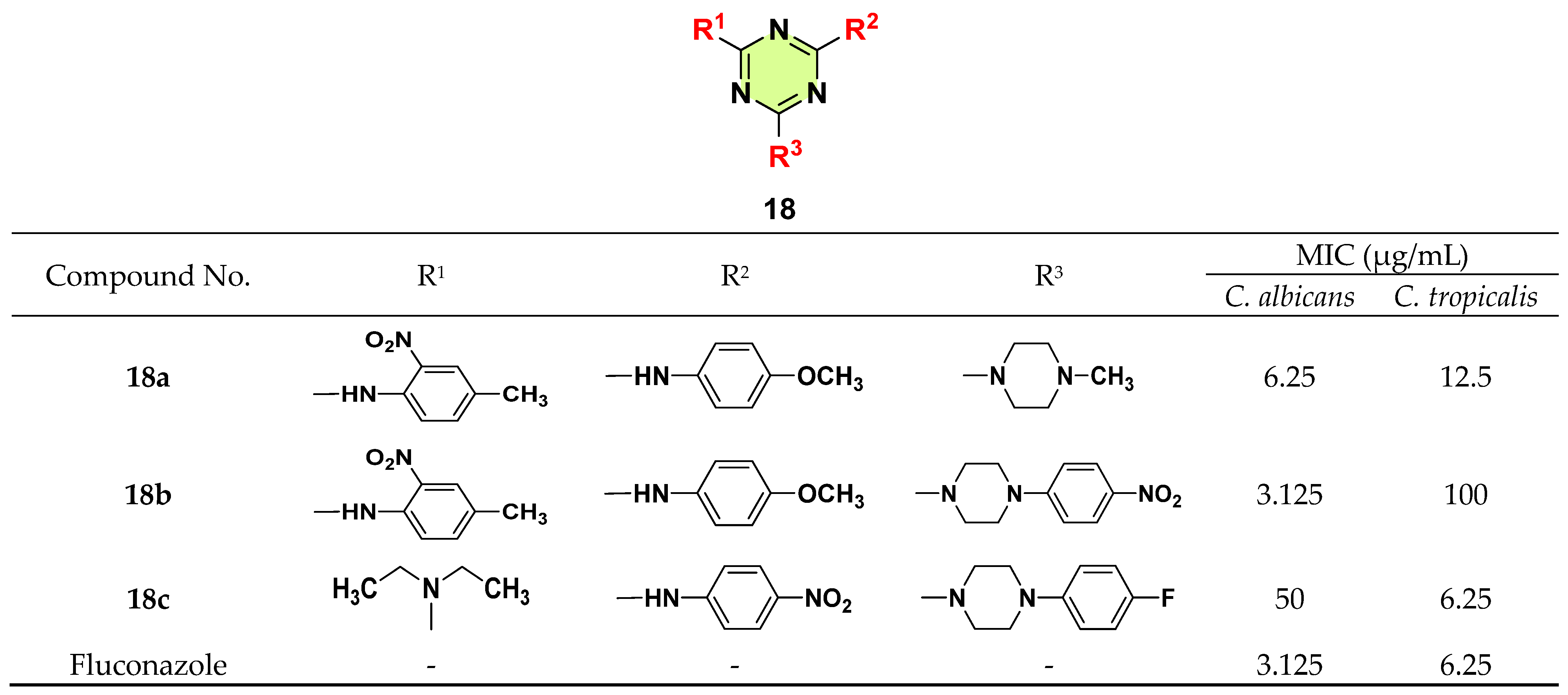
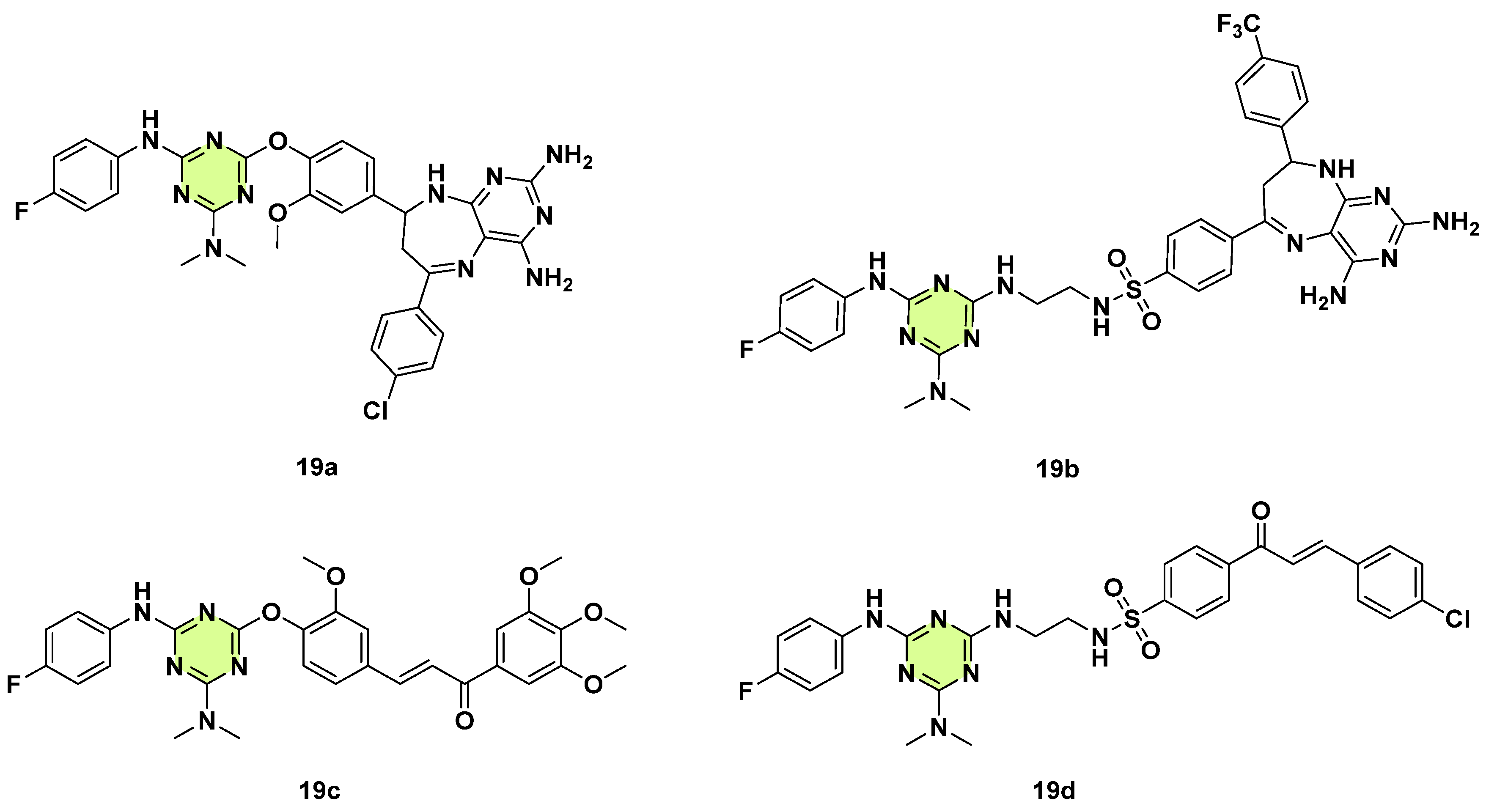
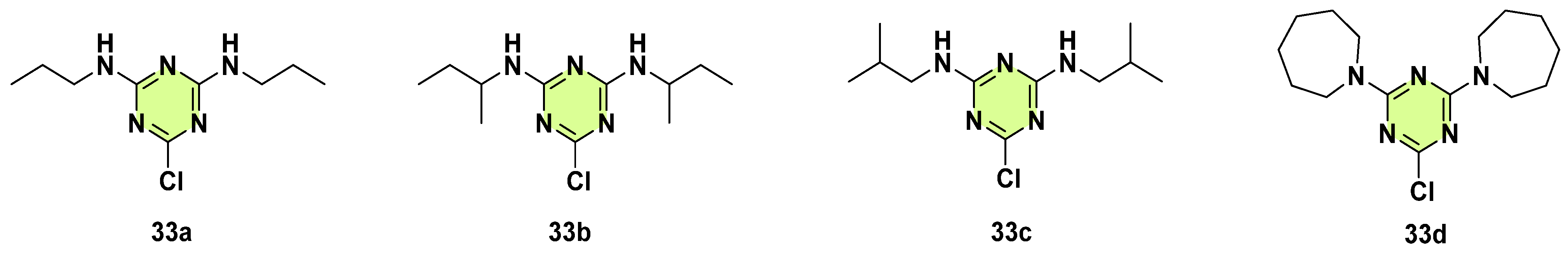
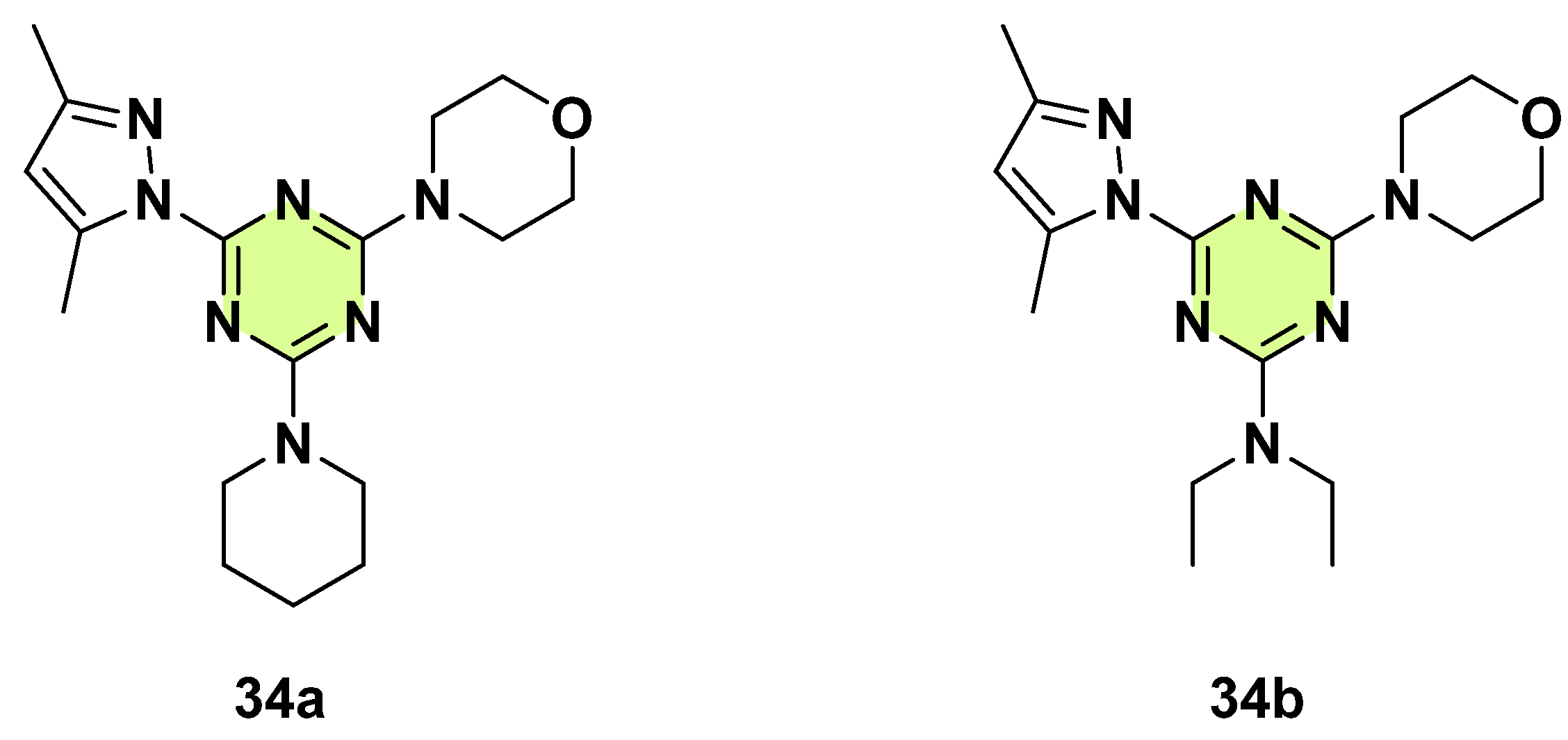
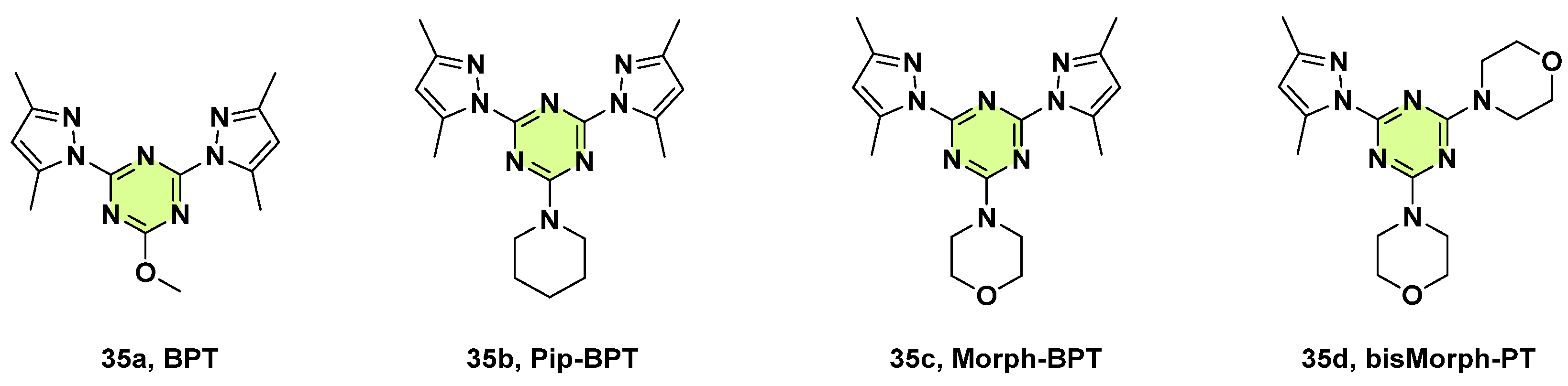
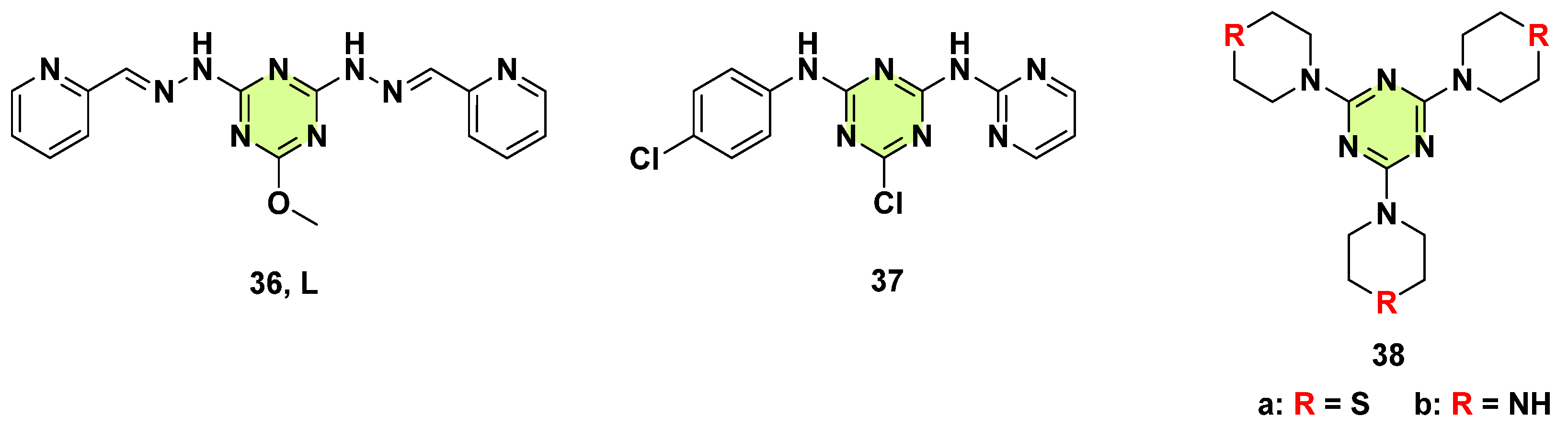
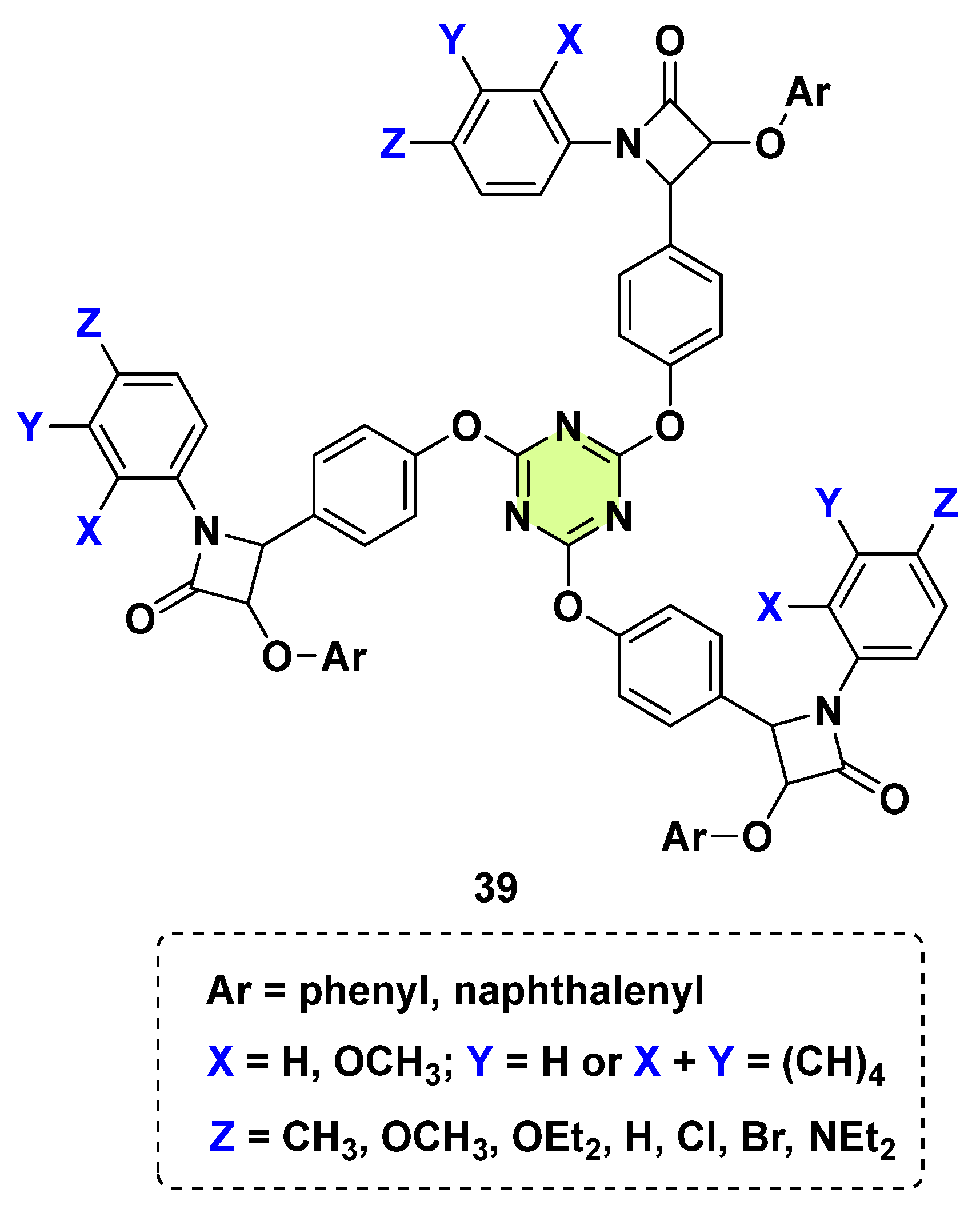
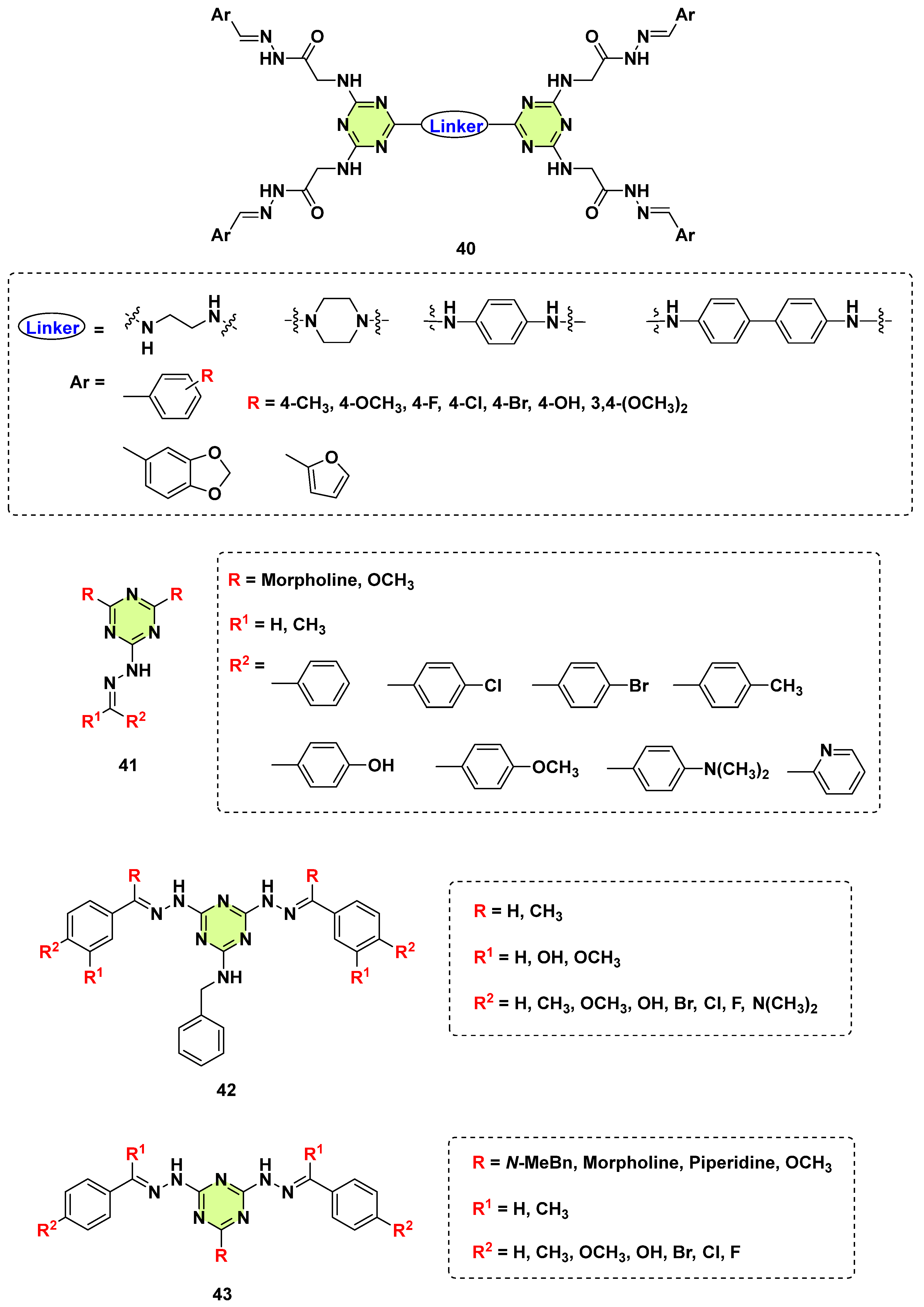
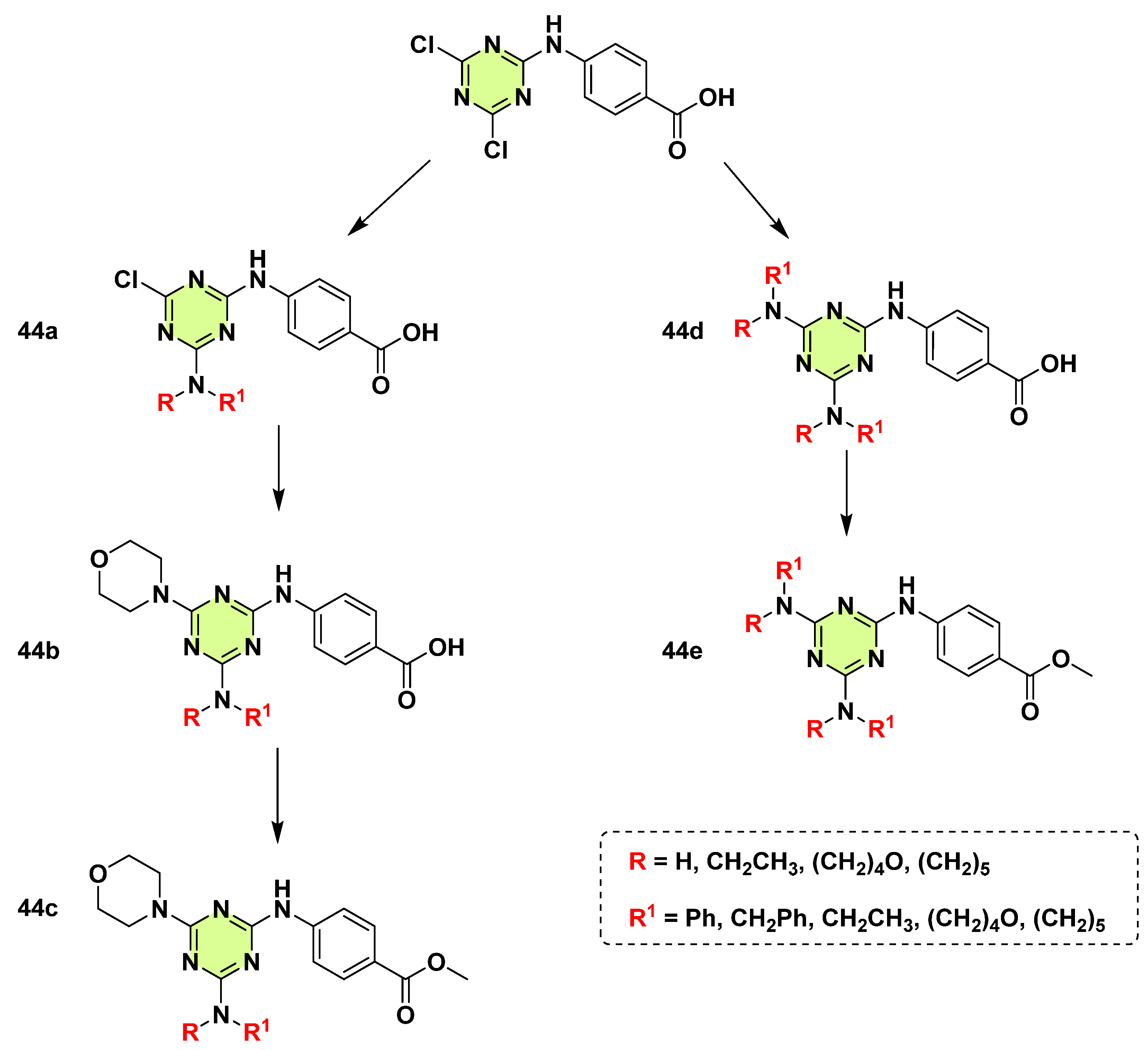
3. Antifungal Activities of s-Triazine Based Derivatives
4. Discussion
- Substituents on the s-triazine core with tetrazole, pyridine, pyrimidine, morpholine, piperazine, piperidine, coumarin, chalcone, and quinoline are favored for improving their antifungal activity against Candida, Cryptococcus, and Aspergillus species.
- Halogen atoms, especially fluorine, chlorine, and bromine, are present in the s-triazine derivatives’ chemical structures, which suggests the significant importance of halogen bonding in interactions with corresponding targets.
- Tri-substituted s-triazine derivatives are generally more active than di-substituted and mono-substituted, possibly due to the introduction of more pharmacophore fragments.
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infection. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69–81. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Moghimi, S.; Shafiei, M.; Foroumadi, A. Drug design strategies for the treatment azole-resistant candidiasis. Expert Opin. Drug Discov. 2022, 17, 879–895. [Google Scholar] [CrossRef]
- Ullah, A.; Iftikhar, F.; Arfan, M.; Kazmi, S.T.B.; Anjum, M.N.; Haq, I.U.; Ayaz, M.; Farooq, S.; Rashid, U. Amino acid conjugated antimicrobial drugs: Synthesis, lipophilicity-activity relationship, antibacterial and urease inhibition activity. Eur. J. Med. Chem. 2018, 145, 140–153. [Google Scholar] [CrossRef]
- Cesarini, S.; Vicenti, I.; Poggialini, F.; Secchi, M.; Giammarino, F.; Varasi, I.; Lodola, C.; Zazzi, M.; Dreassi, E.; Maga, G.; et al. Privileged scaffold decoration for the identification of the first trisubstituted triazine with anti-SARS-CoV-2 activity. Molecules 2022, 27, 8829. [Google Scholar] [CrossRef]
- Sun, Q.; Chu, Y.; Zhang, N.; Chen, R.; Wang, L.; Wu, J.; Dong, Y.; Li, H.; Wang, L.; Tang, L.; et al. Design, synthesis, formulation, and bioevaluation of trisubstituted triazines as highly selective mTOR inhibitors for the treatment of human breast cancer. J. Med. Chem. 2024, 67, 7330–7358. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Inoyama, D.; Russo, R.; Li, S.G.; Jadhav, R.; Stratton, T.P.; Mittal, N.; Bilotta, J.A.; Singleton, E.; Kim, T.; et al. Antitubercular triazines: Optimization and intrabacterial metabolism. Cell Chem. Biol. 2020, 27, 172–185.e111. [Google Scholar] [CrossRef] [PubMed]
- Alhamzani, A.G.; Yousef, T.A.; Abou-Krisha, M.M.; Raghu, M.S.; Kumar, K.Y.; Prashanth, M.K.; Jeon, B.H. Design, synthesis, molecular docking and pharmacological evaluation of novel triazine-based triazole derivatives as potential anticonvulsant agents. Bioorg. Med. Chem. Lett. 2022, 77, 129042. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhou, S.; Cheng, D.; Li, Y.; Liu, J.; Xie, Y.; Li, Y.; Li, Z. Design, synthesis and herbicidal activity study of aryl 2,6-disubstituted sulfonylureas as potent acetohydroxyacid synthase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3365–3369. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A review on recent treatment technology for herbicide atrazine in contaminated environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef]
- Kubo, T.; Figg, C.A.; Swartz, J.L.; Brooks, W.L.A.; Sumerlin, B.S. Multifunctional homopolymers: Postpolymerization modification via sequential nucleophilic aromatic substitution. Macromolecules 2016, 49, 2077–2084. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Nik, W.B.W.; Srivastava, V. Triazines as a potential class of corrosion inhibitors: Present scenario, challenges and future perspectives. J. Mol. Liq. 2021, 321, 114747. [Google Scholar] [CrossRef]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef]
- Sharma, A.; Sheyi, R.; de la Torre, B.G.; El-Faham, A.; Albericio, F. s-Triazine: A privileged structure for drug discovery and bioconjugation. Molecules 2021, 26, 864. [Google Scholar] [CrossRef]
- Shahari, M.S.B.; Dolzhenko, A.V. A closer look at N2,6-substituted 1,3,5-triazine-2,4-diamines: Advances in synthesis and biological activities. Eur. J. Med. Chem. 2022, 241, 114645. [Google Scholar] [CrossRef]
- Ali, M.I.; Naseer, M.M. Recent biological applications of heterocyclic hybrids containing s-triazine scaffold. RSC Adv. 2023, 13, 30462–30490. [Google Scholar] [CrossRef] [PubMed]
- Bareth, D.; Jain, S.; Kumawat, J.; Kishore, D.; Dwivedi, J.; Hashmi, S.Z. Synthetic and pharmacological developments in the hybrid s-triazine moiety: A review. Bioorg. Chem. 2024, 143, 106971. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Sonika, J.; Namita, M.; Jaya, D.; Dharma, K. 1,3,5-Triazine: Recent development in synthesis of its analogs and biological profile. Mini-Rev. Med. Chem. 2024, 24, 2019–2071. [Google Scholar]
- Patil, V.; Noonikara-Poyil, A.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Lewis, A.M.; Bugarin, A. Synthesis, molecular docking studies, and in vitro evaluation of 1,3,5-triazine derivatives as promising antimicrobial agents. J. Mol. Struct. 2020, 1220, 128687. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Abuo-Rahma, G.E.-D.A.; Abd-Elmonem, M.; Yahia, R.; Hisham, M.; Hayallah, A.M.; Mostafa, S.M.; Abo-Elsoud, F.A.; Sadek, K.U. New s-Triazine/Tetrazole conjugates as potent antifungal and antibacterial agents: Design, molecular docking and mechanistic study. J. Mol. Struct. 2022, 1267, 133615. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, Y.F.; Xu, Z. Tetrazole hybrids and their antifungal activities. Eur. J. Med. Chem. 2019, 170, 225–234. [Google Scholar] [CrossRef]
- Dinari, M.; Gharahi, F.; Asadi, P. Synthesis, spectroscopic characterization, antimicrobial evaluation and molecular docking study of novel triazine-quinazolinone based hybrids. J. Mol. Struct. 2018, 1156, 43–50. [Google Scholar] [CrossRef]
- Zala, A.R.; Kumar, D.; Razakhan, U.; Rajani, D.P.; Ahmad, I.; Patel, H.; Kumari, P. Molecular modeling and biological investigation of novel s-triazine linked benzothiazole and coumarin hybrids as antimicrobial and antimycobacterial agents. J. Biomol. Struct. Dyn. 2023, 42, 3814–3825. [Google Scholar] [CrossRef]
- Sweta, D.D.; Arvind, G.M. Design, synthesis, characterization and biological evaluation of various N-substituted piperazine annulated s-triazine derivatives. Res. J. Chem. Sci. 2014, 4, 14–19. [Google Scholar]
- Bhat, H.R.; Masih, A.; Shakya, A.; Ghosh, S.K.; Singh, U.P. Design, synthesis, anticancer, antibacterial, and antifungal evaluation of 4-aminoquinoline-1,3,5-triazine derivatives. J. Heterocycl. Chem. 2019, 57, 390–399. [Google Scholar] [CrossRef]
- Masih, A.; Shrivastava, J.K.; Bhat, H.R.; Singh, U.P. Potent antibacterial activity of dihydydropyrimidine-1,3,5-triazines via inhibition of DNA gyrase and antifungal activity with favourable metabolic profile. Chem. Biol. Drug Des. 2020, 96, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Makwana, A.H.; Rajpara, K.M. Synthesis and study of 1,3,5-triazine based thiazole derivatives as antimicrobial agents. J. Saudi Chem. Soc. 2016, 20, S334–S341. [Google Scholar] [CrossRef]
- Desai, N.C.; Makwana, A.H.; Senta, R.D. Synthesis, characterization and antimicrobial activity of some novel 4-(4-(arylamino)-6-(piperidin-1-yl)-1,3,5-triazine-2-ylamino)-N-(pyrimidin-2-yl)benzenesulfonamides. J. Saudi Chem. Soc. 2016, 20, 686–694. [Google Scholar] [CrossRef]
- Noureen, S.; Ali, S.; Iqbal, J.; Zia, M.A.; Hussain, T. Synthesis, comparative theoretical and experimental characterization of some new 1,3,5 triazine based heterocyclic compounds and in vitro evaluation as promising biologically active agents. J. Mol. Struct. 2022, 1268, 133622. [Google Scholar] [CrossRef]
- Mohamed-Ezzat, R.A.; Elgemeie, G.H. Novel synthesis of new triazine sulfonamides with antitumor, anti-microbial and anti-SARS-CoV-2 activities. BMC Chem. 2024, 18, 58. [Google Scholar] [CrossRef]
- Kumawat, J.; Jain, S.; Patel, S.; Misra, N.; Jain, P.; Hashmi, S.Z.; Dwivedi, J.; Kishore, D. Synthesis, biological evaluation, and DFT analysis of s-triazine analogues with medicinal potential integrated with bioactive heterocyclic scaffolds. J. Mol. Struct. 2024, 1313, 138668. [Google Scholar] [CrossRef]
- Shinde, R.S.; Dake, S.A.; Pawar, R.P. Design, synthesis and antimicrobial activity of some triazine chalcone derivatives. Anti-Infect. Agents 2021, 18, 332–338. [Google Scholar] [CrossRef]
- Patel, A.B.; Chikhalia, K.H.; Kumari, P. An efficient synthesis of new thiazolidin-4-one fused s-triazines as potential antimicrobial and anticancer agents. J. Saudi Chem. Soc. 2014, 18, 646–656. [Google Scholar] [CrossRef]
- Mewada, N.S.; Shah, D.R.; Lakum, H.P.; Chikhalia, K.H. Synthesis and biological evaluation of novel s-triazine based aryl/heteroaryl entities: Design, rationale and comparative study. J. Assoc. Arab Univ. Basic Appl. Sci. 2018, 20, 8–18. [Google Scholar] [CrossRef]
- Singh, R.B.; Das, N.; Zaman, M.K. Facile synthesis, characterization, and in vitro antimicrobial screening of a new series of 2,4,6-trisubstituted-s-triazine based compounds. Int. J. Med. Chem. 2015, 2015, 571836. [Google Scholar] [CrossRef]
- Moreno, L.M.; Quiroga, J.; Abonia, R.; Crespo, M.D.P.; Aranaga, C.; Martínez-Martínez, L.; Sortino, M.; Barreto, M.; Burbano, M.E.; Insuasty, B. Synthesis of novel triazine-based chalcones and 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as potential leads in the search of anticancer, antibacterial and antifungal agents. Int. J. Mol. Sci. 2024, 25, 3623. [Google Scholar] [CrossRef] [PubMed]
- Maliszewski, D.; Demirel, R.; Wróbel, A.; Baradyn, M.; Ratkiewicz, A.; Drozdowska, D. s-Triazine derivatives functionalized with alkylating 2-chloroethylamine fragments as promising antimicrobial agents: Inhibition of bacterial DNA gyrases, molecular docking studies, and antibacterial and antifungal activity. Pharmaceuticals 2023, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.A.; Kim, H.; Qasim, M.; Djehal, A.; Hernday, A.D.; Désaubry, L.; Rauceo, J.M. Triazine-based small molecules: A potential new class of compounds in the antifungal toolbox. Pathogens 2023, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Mena, L.; Billamboz, M.; Charlet, R.; Desprès, B.; Sendid, B.; Ghinet, A.; Jawhara, S. Two new compounds containing pyridinone or triazine heterocycles have antifungal properties against Candida albicans. Antibiotics 2022, 11, 72. [Google Scholar] [CrossRef]
- Dong, G.; Liu, Y.; Wu, Y.; Tu, J.; Chen, S.; Liu, N.; Sheng, C. Novel non-peptidic small molecule inhibitors of secreted aspartic protease 2 (SAP2) for the treatment of resistant fungal infections. Chem. Commun. 2018, 54, 13535–13538. [Google Scholar] [CrossRef]
- Alhameed, R.A.; Almarhoon, Z.; Sholkamy, E.N.; Khan, S.A.; Ul-Haq, Z.; Sharma, A.; de la Torre, B.G.; Albericio, F.; El-Faham, A. Novel 4,6-disubstituted s-triazin-2-yl amino acid derivatives as promising antifungal agents. J. Fungi 2020, 6, 237. [Google Scholar] [CrossRef]
- Dongre, R.P.; Rathod, S.D. Synthesis of novel isoxazoline derivatives containing s-triazine via chalcones and their anti-microbial studies. Der Pharma Chem. 2017, 9, 68–71. [Google Scholar]
- Li, L.; Zhang, T.; Xu, J.; Wu, J.; Wang, Y.; Qiu, X.; Zhang, Y.; Hou, W.; Yan, L.; An, M.; et al. The synergism of the small molecule ENOblock and fluconazole against fluconazole-resistant Candida albicans. Front. Microbiol. 2019, 10, 2071. [Google Scholar] [CrossRef]
- Jung, D.W.; Kim, W.H.; Park, S.H.; Lee, J.; Kim, J.; Su, D.; Ha, H.H.; Chang, Y.T.; Williams, D.R. A unique small molecule inhibitor of enolase clarifies its role in fundamental biological processes. ACS Chem. Biol. 2013, 8, 1271–1282. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chen, H.F.; Kuo, T.J.; Lin, C.Y. Mutations on CaENO1 in Candida albicans inhibit cell growth in the presence of glucose. J. Biomed. Sci. 2006, 13, 313–321. [Google Scholar] [CrossRef]
- Xie, F.; Hao, Y.; Liu, J.; Bao, J.; Ni, T.; Liu, Y.; Chi, X.; Wang, T.; Yu, S.; Jin, Y.; et al. Discovery of novel thiosemicarbazides containing 1,3,5-triazines derivatives as potential synergists against fluconazole-resistant Candida albicans. Pharmaceutics 2022, 14, 2334. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Hao, Y.; Liu, Y.; Bao, J.; Wang, R.; Chi, X.; Wang, T.; Yu, S.; Jin, Y.; Li, L.; et al. From synergy to monotherapy: Discovery of novel 2,4,6-trisubstituted triazine hydrazone derivatives with potent antifungal potency in vitro and in vivo. J. Med. Chem. 2024, 67, 4007–4025. [Google Scholar] [CrossRef] [PubMed]
- Haiba, N.S.; Khalil, H.H.; Moniem, M.A.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinical isolates. Bioorg. Chem. 2019, 89, 103013. [Google Scholar] [CrossRef]
- Salaković, B.; Kovačević, S.; Banjac, M.K.; Podunavac-Kuzmanović, S.; Jevrić, L.; Pajčin, I.; Grahovac, J. New perspective on comparative chemometric and molecular modeling of antifungal activity and herbicidal potential of alkyl and cycloalkyl s-triazine derivatives. Processes 2023, 11, 358. [Google Scholar] [CrossRef]
- Sharma, A.; Ghabbour, H.; Khan, S.T.; de la Torre, B.G.; Albericio, F.; El-Faham, A. Novel pyrazolyl-s-triazine derivatives, molecular structure and antimicrobial activity. J. Mol. Struct. 2017, 1145, 244–253. [Google Scholar] [CrossRef]
- Mondal, J.; Sivaramakrishna, A. Functionalized triazines and tetrazines: Synthesis and applications. Top. Curr. Chem. 2022, 380, 34. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Synthesis, structure and biological activity of zinc(II) pincer complexes with 2,4-bis(3,5-dimethyl-1H-pyrazol-1-yl)-6-methoxy-1,3,5-triazine. Inorg. Chim. Acta 2020, 508, 119627. [Google Scholar] [CrossRef]
- Refaat, H.M.; Alotaibi, A.A.M.; Dege, N.; El-Faham, A.; Soliman, S.M. Synthesis, structure and biological evaluations of Zn(II) pincer complexes based on s-triazine type chelator. Molecules 2022, 27, 3625. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Syntheses, structure, Hirshfeld analysis and antimicrobial activity of four new Co(II) complexes with s-triazine-based pincer ligand. Inorg. Chim. Acta 2020, 510, 119753. [Google Scholar] [CrossRef]
- Soliman, S.M.; Al-Rasheed, H.H.; Elsilk, S.E.; El-Faham, A. A novel centrosymmetric Fe(III) complex with anionic bis-pyrazolyl-s-triazine ligand; Synthesis, structural investigations and antimicrobial evaluations. Symmetry 2021, 13, 1247. [Google Scholar] [CrossRef]
- Soliman, S.M.; Almarhoon, Z.; Sholkamy, E.N.; El-Faham, A. Bis-pyrazolyl-s-triazine Ni(II) pincer complexes as selective gram positive antibacterial agents; synthesis, structural and antimicrobial studies. J. Mol. Struct. 2019, 1195, 315–322. [Google Scholar] [CrossRef]
- Soliman, S.M.; Al-Rasheed, H.H.; Albering, J.H.; El-Faham, A. Fe(III) complexes based on mono- and bis-pyrazolyl-s-triazine ligands: Synthesis, molecular structure, Hirshfeld, and antimicrobial evaluations. Molecules 2020, 25, 5750. [Google Scholar] [CrossRef] [PubMed]
- Yousri, A.; Gad, S.I.; Abu-Youssef, M.A.M.; El-Faham, A.; Barakat, A.; Tatikonda, R.; Haukka, M.; Soliman, S.M. Synthesis of Co(II), Mn(II), and Ni(II) complexes with 4-(4,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,3,5-triazin-2-yl)morpholine; X-ray structure, Hirshfeld, AIM, and biological studies. Inorg. Chim. Acta 2024, 573, 122320. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A.; Elsilk, S.E.; Farooq, M. Two heptacoordinated manganese(II) complexes of giant pentadentate s-triazine bis-Schiff base ligand: Synthesis, crystal structure, biological and DFT studies. Inorg. Chim. Acta 2018, 479, 275–285. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Badr, A.M.A.; Soliman, S.M.; Slawin, A.M.Z.; Woollins, J.D. Synthesis, characterizations, antitumor and antimicrobial evaluations of novel Mn(II) and Cu(II) complexes with NNN-tridentate s-Triazine-Schiff base ligand. Inorg. Chim. Acta 2023, 555, 121586. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Synthesis, X-ray structure of two hexa-coordinated Ni(II) complexes with s-Triazine hydrazine schiff base ligand. Inorganics 2023, 11, 222. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand. Inorganics 2023, 11, 253. [Google Scholar] [CrossRef]
- Al-Khodir, F.A.I.; Al-Warhi, T.; Abumelha, H.M.A.; Al-Issa, S.A. Synthesis, chemical and biological investigations of new Ru(III) and Se(IV) complexes containing 1,3,5-triazine chelating derivatives. J. Mol. Struct. 2019, 1179, 795–808. [Google Scholar] [CrossRef]
- Martins, E.P.S.; Lima, E.D.O.; Martins, F.T.; de Almeida Vasconcellos, M.L.A.; Rocha, G.B. Synthesis, spectroscopic characterization, DFT studies, and preliminary antimicrobial evaluation of new antimony(III) and bismuth(III) complexes derived from 1,3,5-triazine. J. Mol. Struct. 2019, 1183, 373–383. [Google Scholar] [CrossRef]
- Bashiri, M.; Jarrahpour, A.; Rastegari, B.; Iraji, A.; Irajie, C.; Amirghofran, Z.; Malek-Hosseini, S.; Motamedifar, M.; Haddadi, M.; Zomorodian, K.; et al. Synthesis and evaluation of biological activities of tripodal imines and β-lactams attached to the 1,3,5-triazine nucleus. Monatsh. Chem. 2020, 151, 821–835. [Google Scholar] [CrossRef]
- Vembu, S.; Pazhamalai, S.; Gopalakrishnan, M. Synthesis, spectral characterization, and effective antifungal evaluation of 1H-tetrazole containing 1,3,5-triazine dendrimers. Med. Chem. Res. 2016, 25, 1916–1924. [Google Scholar] [CrossRef]
- Ramadan, D.R.; Elbardan, A.A.; Bekhit, A.A.; El-Faham, A.; Khattab, S.N. Synthesis and characterization of novel dimerics-triazine derivatives as potential anti-bacterial agents against MDR clinical isolates. New J. Chem. 2018, 42, 10676–10688. [Google Scholar] [CrossRef]
- Al-Rasheed, H.H.; Sholkamy, E.N.; Al Alshaikh, M.; Siddiqui, M.R.H.; Al-Obaidi, A.S.; El-Faham, A. Synthesis, characterization, and antimicrobial studies of novel series of 2,4-bis(hydrazino)-6-substituted-1,3,5-triazine and their Schiff base derivatives. J. Chem. 2018, 2018, 8507567. [Google Scholar] [CrossRef]
- Al-Rasheed, H.H.; Al Alshaikh, M.; Khaled, J.M.; Alharbi, N.S.; El-Faham, A. Ultrasonic irradiation: Synthesis, characterization, and preliminary antimicrobial activity of novel series of 4,6-disubstituted-1,3,5-triazine containing hydrazone derivatives. J. Chem. 2016, 2016, 3464758. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Khalil, H.H.; El-Faham, A.; Khattab, S.N. Synthesis, characterization and evaluation of 1,3,5-triazine aminobenzoic acid derivatives for their antimicrobial activity. Chem. Cent. J. 2017, 11, 39. [Google Scholar] [CrossRef]
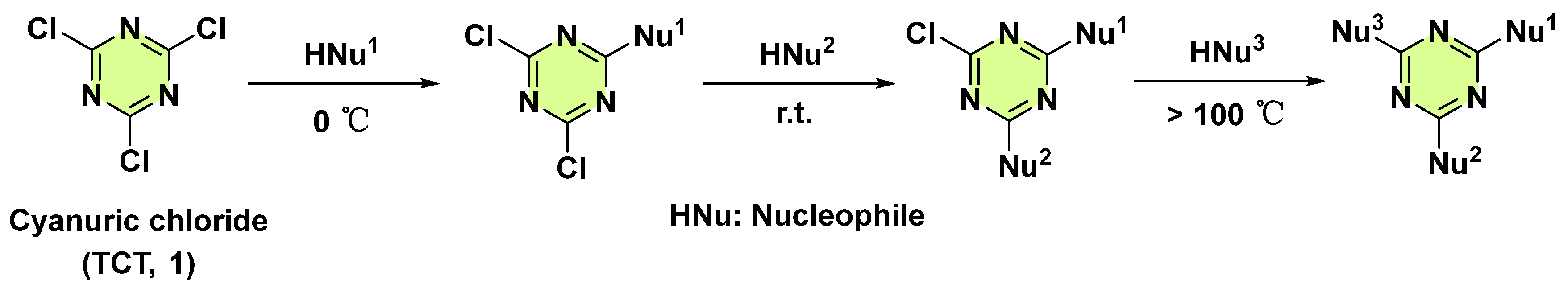
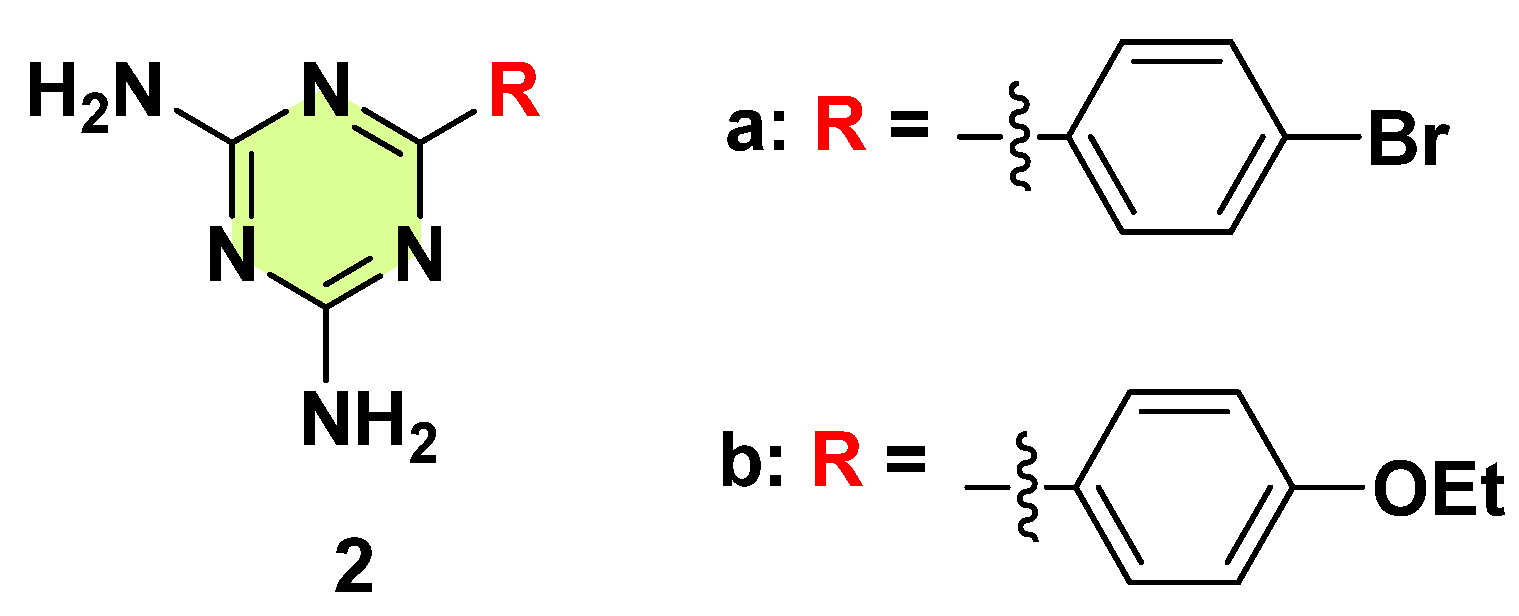



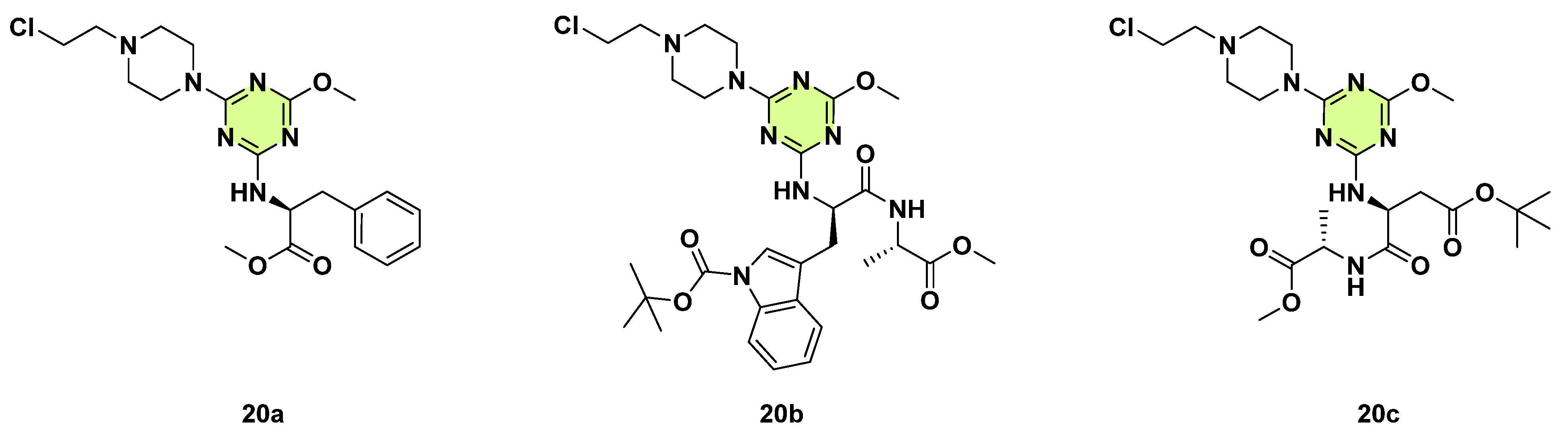
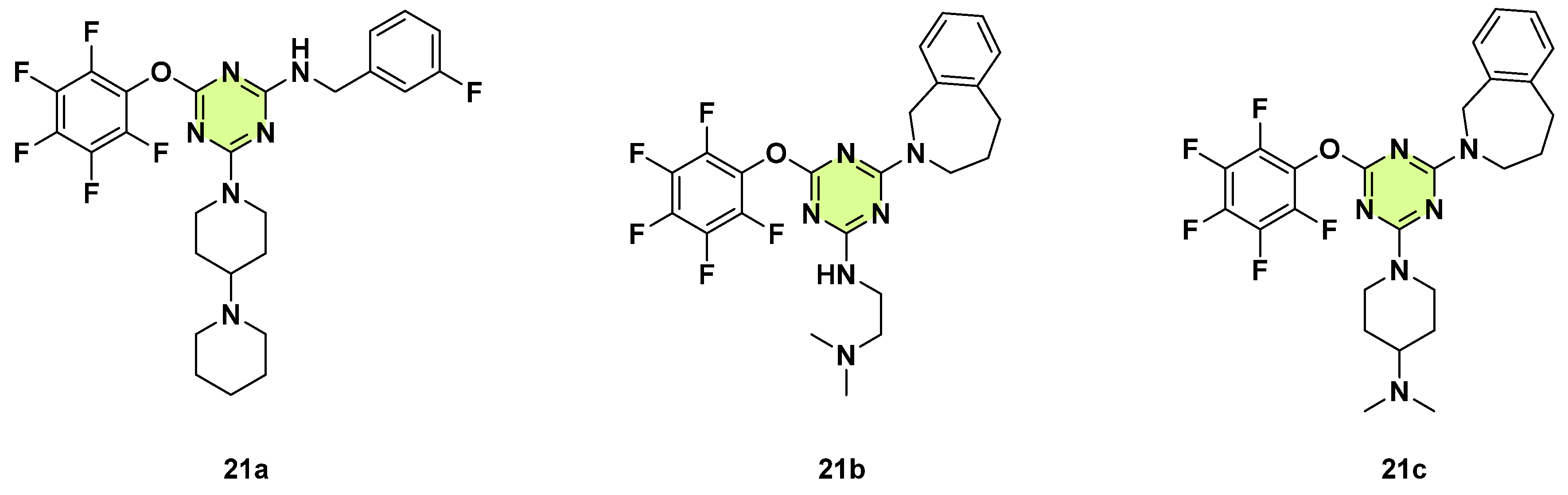
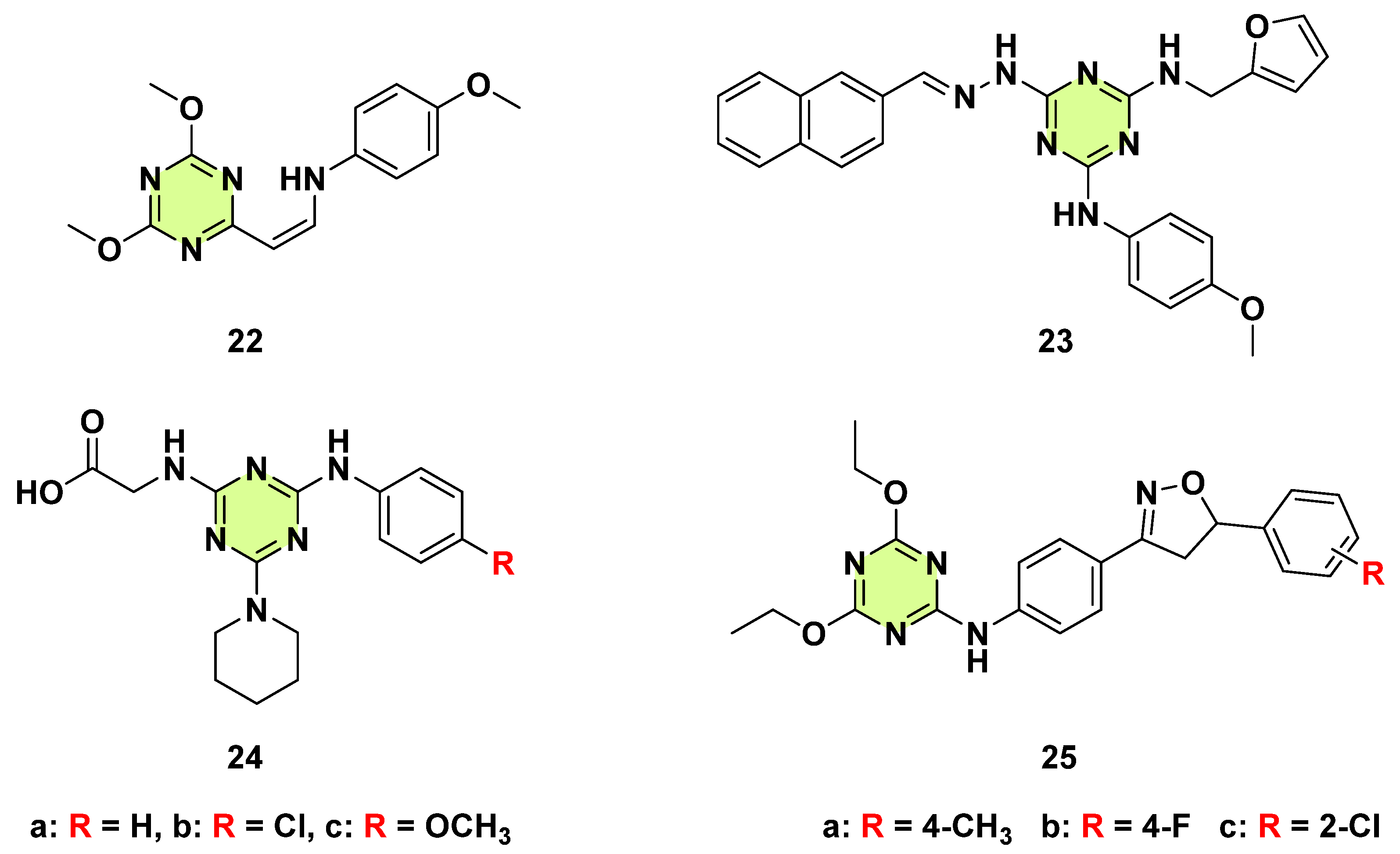
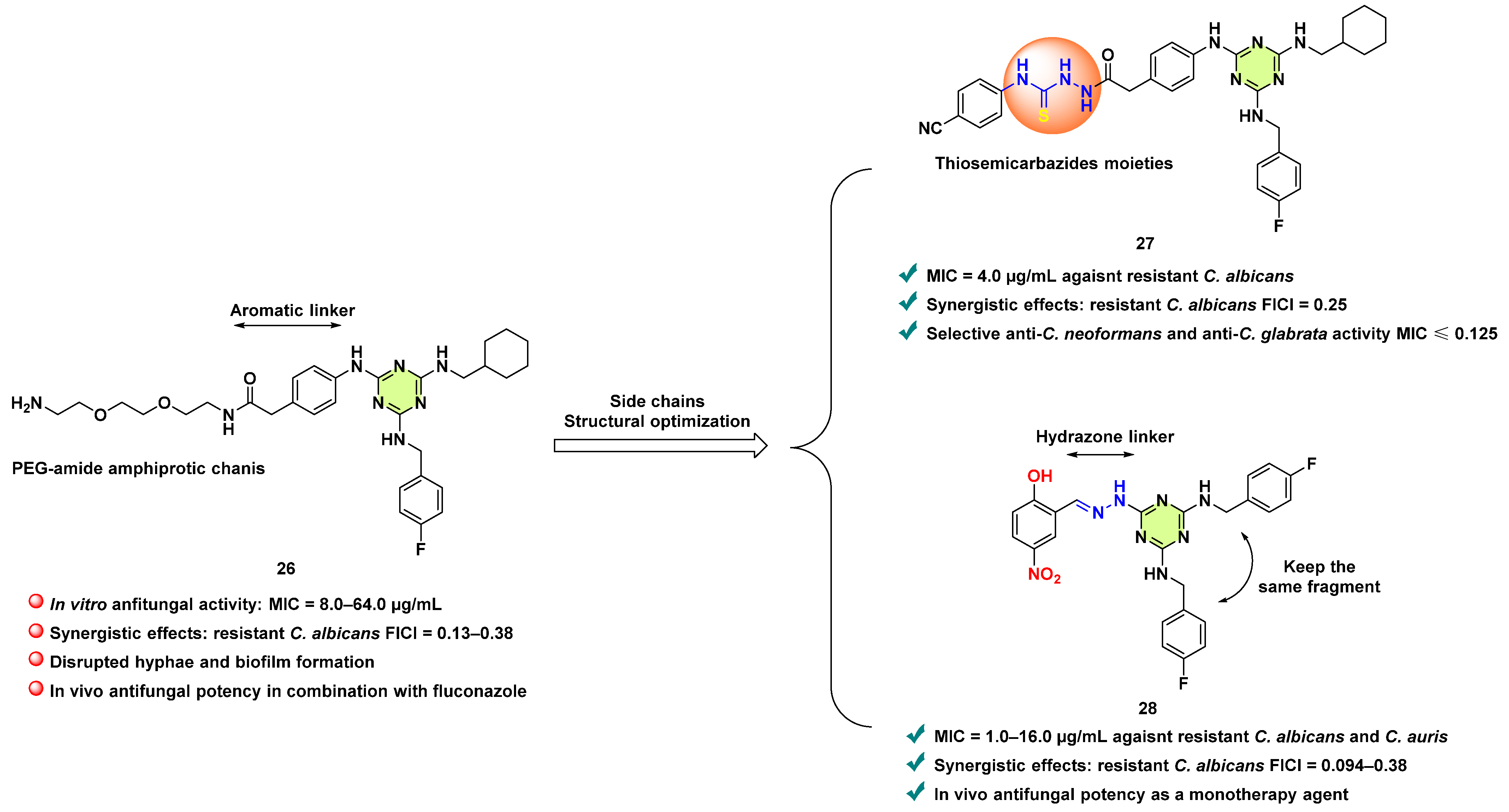

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.; Liu, M.; Wang, M.; Zhang, D.; Hao, Y.; Xie, F. Exploring the Potential of s-Triazine Derivatives as Novel Antifungal Agents: A Review. Pharmaceuticals 2025, 18, 690. https://doi.org/10.3390/ph18050690
Liao H, Liu M, Wang M, Zhang D, Hao Y, Xie F. Exploring the Potential of s-Triazine Derivatives as Novel Antifungal Agents: A Review. Pharmaceuticals. 2025; 18(5):690. https://doi.org/10.3390/ph18050690
Chicago/Turabian StyleLiao, Haoyan, Menglu Liu, Mengyuan Wang, Dazhi Zhang, Yumeng Hao, and Fei Xie. 2025. "Exploring the Potential of s-Triazine Derivatives as Novel Antifungal Agents: A Review" Pharmaceuticals 18, no. 5: 690. https://doi.org/10.3390/ph18050690
APA StyleLiao, H., Liu, M., Wang, M., Zhang, D., Hao, Y., & Xie, F. (2025). Exploring the Potential of s-Triazine Derivatives as Novel Antifungal Agents: A Review. Pharmaceuticals, 18(5), 690. https://doi.org/10.3390/ph18050690






