Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model
Abstract
1. Introduction
2. Results
2.1. Animal Welfare
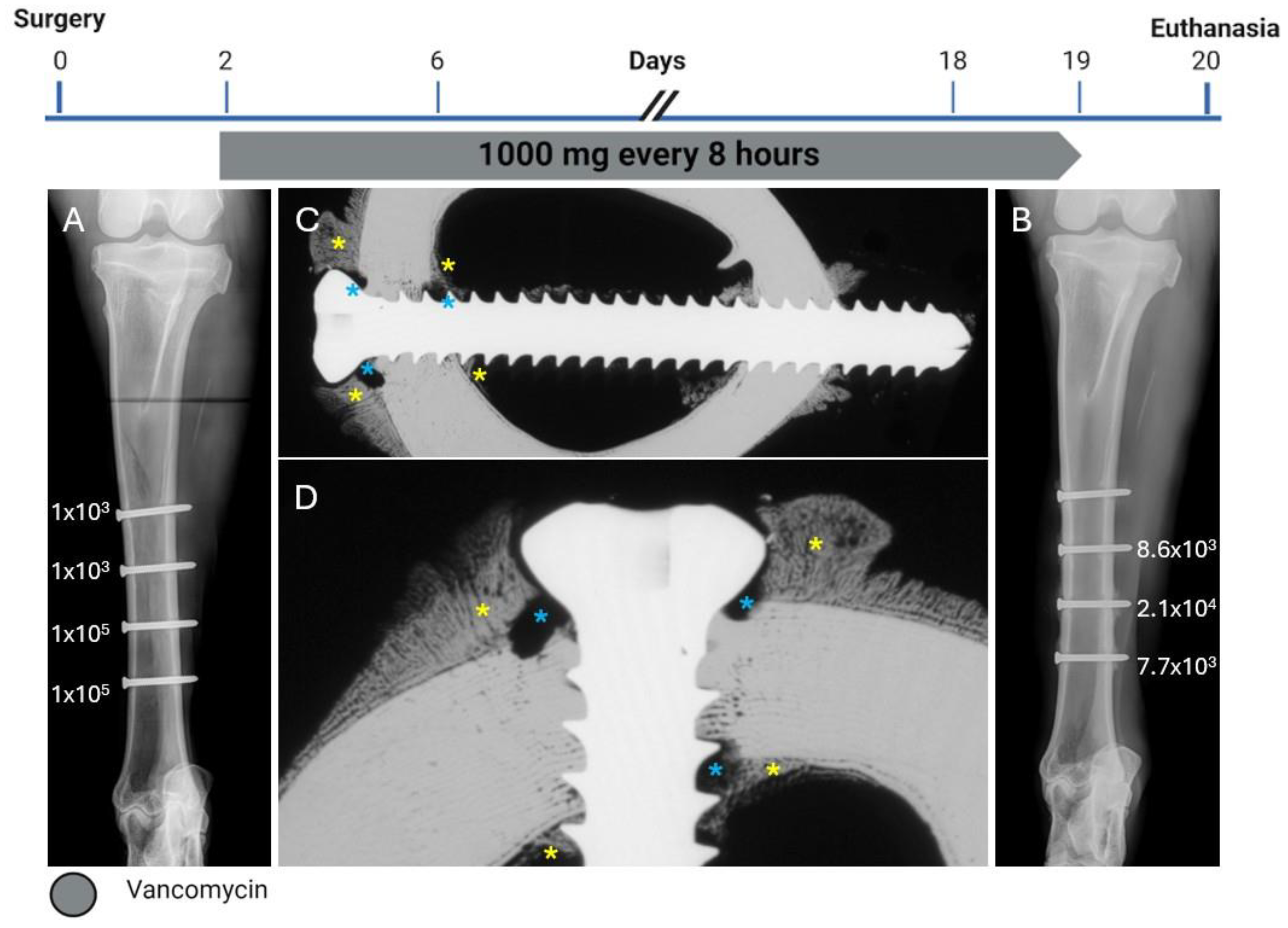
2.2. Sheep One
2.3. Sheep Two
2.4. Sheep Three
2.5. Sheep Four—HBCS 4 mg/kg—9 Administrations
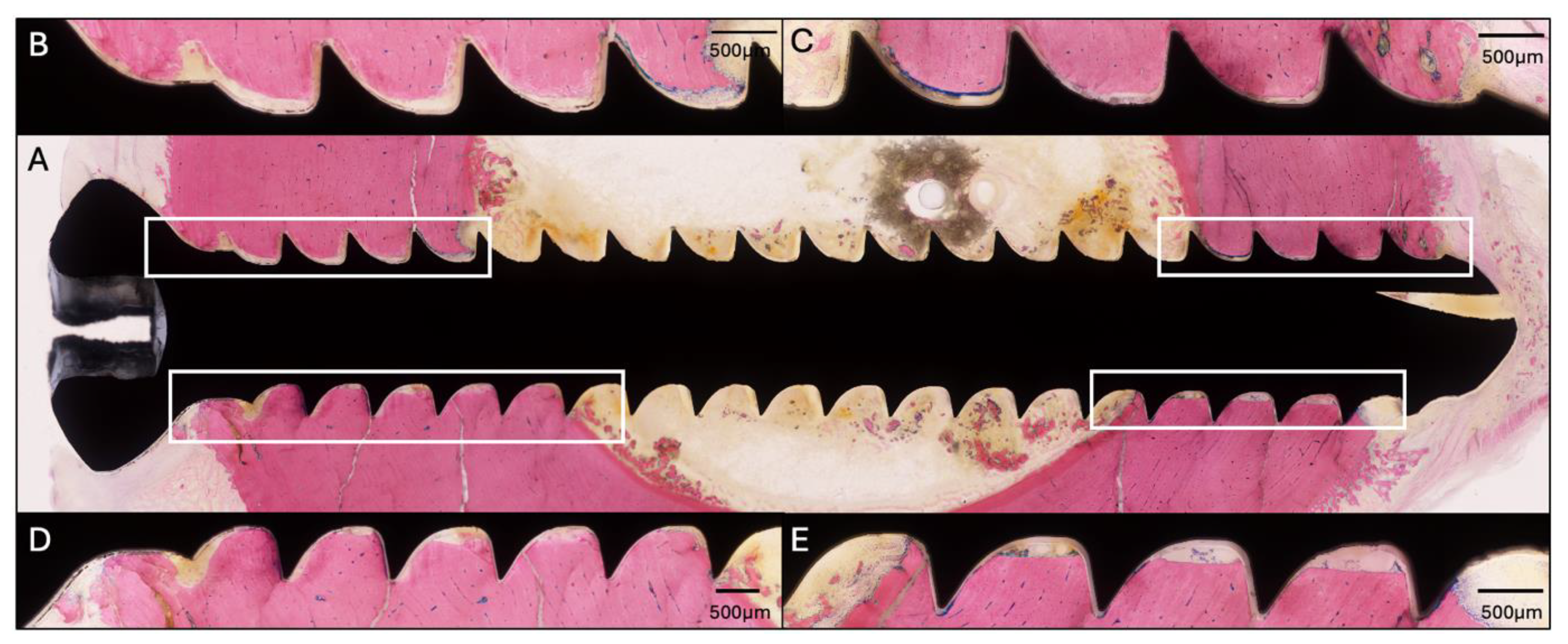
2.6. Transmission Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Study Overview
4.2. Animals, Ethical Approval, Preoperative Animal Care, Anesthesia, and Analgesia
4.3. Screw Inoculation
4.4. Surgical Protocol
4.5. Antibiotic Treatment
4.6. HBCS Administration
4.7. In Vivo Analysis and Euthanasia
4.8. Sample Harvest, Ex Vivo Radiology, Quantitative Bacteriology, and Histology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBCS | Hydroxybisphosphonate conjugated sitafloxacin |
References
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus Osteomyelitis: Bone, Bugs, and Surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Metsemakers, W.J.; Morgenstern, M.; Hofstee, M.I.; Vallejo Diaz, A.; Cassat, J.E.; Wildemann, B.; Depypere, M.; Schwarz, E.M.; Richards, R.G. Fracture-related infection. Nat. Rev. Dis. Primers 2022, 8, 67. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Injury 2017, 48, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; Obremskey, W.; McNally, M.A.; Metsemakers, W.J.; Verhofstad, M.H.J. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61–72. [Google Scholar] [CrossRef]
- de Mesy Bentley, K.L.; Trombetta, R.; Nishitani, K.; Bello-Irizarry, S.N.; Ninomiya, M.; Zhang, L.; Chung, H.L.; McGrath, J.L.; Daiss, J.L.; Awad, H.A.; et al. Evidence of Staphylococcus aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J. Bone Miner. Res. 2017, 32, 985–990. [Google Scholar] [CrossRef]
- de Mesy Bentley, K.L.; MacDonald, A.; Schwarz, E.M.; Oh, I. Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes: A Case Report. JBJS Case Connect. 2018, 8, e8. [Google Scholar] [CrossRef]
- Masters, E.A.; de Mesy Bentley, K.L.; Gill, A.L.; Hao, S.P.; Galloway, C.A.; Salminen, A.T.; Guy, D.R.; McGrath, J.L.; Awad, H.A.; Gill, S.R.; et al. Identification of Penicillin Binding Protein 4 (PBP4) as a critical factor for Staphylococcus aureus bone invasion during osteomyelitis in mice. PLoS Pathog. 2020, 16, e1008988. [Google Scholar] [CrossRef]
- Adjei-Sowah, E.; Peng, Y.; Weeks, J.; Jonason, J.H.; de Mesy Bentley, K.L.; Masters, E.; Morita, Y.; Muthukrishnan, G.; Cherian, P.; Hu, X.E.; et al. Development of Bisphosphonate-Conjugated Antibiotics to Overcome Pharmacodynamic Limitations of Local Therapy: Initial Results with Carbamate Linked Sitafloxacin and Tedizolid. Antibiotics 2021, 10, 732. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Sun, S.; Junka, A.F.; Richard, E.; Sadrerafi, K.; Mahabady, S.; Bakhshalian, N.; Tjokro, N.; Bartoszewicz, M.; Oleksy, M.; et al. Design, Synthesis, and Antimicrobial Evaluation of a Novel Bone-Targeting Bisphosphonate-Ciprofloxacin Conjugate for the Treatment of Osteomyelitis Biofilms. J. Med. Chem. 2017, 60, 2326–2343. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Cherian, P.; Roshandel, S.; Tjokro, N.; Chen, C.; Junka, A.F.; Hu, E.; Neighbors, J.; Pawlak, J.; Russell, R.G.G.; et al. Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates. Int. J. Mol. Sci. 2023, 24, 1985. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J. From molds and macrophages to mevalonate: A decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif. Tissue Int. 2004, 75, 451–461. [Google Scholar] [CrossRef]
- Trombetta, R.P.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. A High-Throughput Screening Approach To Repurpose FDA-Approved Drugs for Bactericidal Applications against Staphylococcus aureus Small-Colony Variants. mSphere 2018, 3, e00422-18. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xue, T.; Rainbolt, J.; Bentley, K.L.M.; Galloway, C.A.; Liu, Y.; Cherian, P.; Neighbors, J.; Hofstee, M.I.; Ebetino, F.H.; et al. Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of “Target & Release” Kinetics and Killing of Bacteria Within Canaliculi. Front. Cell. Infect. Microbiol. 2022, 12, 910970. [Google Scholar] [CrossRef]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; de Mesy Bentley, K.L.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef]
- Kuhn, E.M.A.; Sominsky, L.A.; Chitto, M.; Schwarz, E.M.; Moriarty, T.F. Antibacterial Mechanisms and Clinical Impact of Sitafloxacin. Pharmaceuticals 2024, 17, 1537. [Google Scholar] [CrossRef]
- Ren, Y.; Weeks, J.; Xue, T.; Rainbolt, J.; de Mesy Bentley, K.L.; Shu, Y.; Liu, Y.; Masters, E.; Cherian, P.; McKenna, C.E.; et al. Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis. Bone Res. 2023, 11, 51. [Google Scholar] [CrossRef]
- Salamaga, B.; Kong, L.; Pasquina-Lemonche, L.; Lafage, L.; von Und Zur Muhlen, M.; Gibson, J.F.; Grybchuk, D.; Tooke, A.K.; Panchal, V.; Culp, E.J.; et al. Demonstration of the role of cell wall homeostasis in Staphylococcus aureus growth and the action of bactericidal antibiotics. Proc. Natl. Acad. Sci. USA 2021, 118, e2106022118. [Google Scholar] [CrossRef]
- Sutton, J.A.F.; Carnell, O.T.; Lafage, L.; Gray, J.; Biboy, J.; Gibson, J.F.; Pollitt, E.J.G.; Tazoll, S.C.; Turnbull, W.; Hajdamowicz, N.H.; et al. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 2021, 17, e1009468. [Google Scholar] [CrossRef]
- Xie, C.; Ren, Y.; Weeks, J.; Rainbolt, J.; Kenney, H.M.; Xue, T.; Allen, F.; Shu, Y.; Tay, A.J.H.; Lekkala, S.; et al. Longitudinal intravital imaging of the bone marrow for analysis of the race for the surface in a murine osteomyelitis model. J. Orthop. Res. 2024, 42, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, K.; Sutipornpalangkul, W.; de Mesy Bentley, K.L.; Varrone, J.J.; Bello-Irizarry, S.N.; Ito, H.; Matsuda, S.; Kates, S.L.; Daiss, J.L.; Schwarz, E.M. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J. Orthop. Res. 2015, 33, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- de Mesy Bentley, K.L.; Galloway, C.A.; Muthukrishnan, G.; Echternacht, S.R.; Masters, E.A.; Zeiter, S.; Schwarz, E.M.; Leckenby, J.I. Emerging electron microscopy and 3D methodologies to interrogate Staphylococcus aureus osteomyelitis in murine models. J. Orthop. Res. 2021, 39, 376–388. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanvelk, N.; Tapia-Dean, J.; Zeiter, S.; de Mesy Bentley, K.; Xie, C.; Ebetino, F.H.; Sun, S.; Neighbors, J.; Schwarz, E.M.; Moriarty, T.F. Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model. Pharmaceuticals 2025, 18, 675. https://doi.org/10.3390/ph18050675
Vanvelk N, Tapia-Dean J, Zeiter S, de Mesy Bentley K, Xie C, Ebetino FH, Sun S, Neighbors J, Schwarz EM, Moriarty TF. Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model. Pharmaceuticals. 2025; 18(5):675. https://doi.org/10.3390/ph18050675
Chicago/Turabian StyleVanvelk, Niels, James Tapia-Dean, Stephan Zeiter, Karen de Mesy Bentley, Chao Xie, Frank Hal Ebetino, Shuting Sun, Jeffrey Neighbors, Edward M. Schwarz, and Thomas Fintan Moriarty. 2025. "Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model" Pharmaceuticals 18, no. 5: 675. https://doi.org/10.3390/ph18050675
APA StyleVanvelk, N., Tapia-Dean, J., Zeiter, S., de Mesy Bentley, K., Xie, C., Ebetino, F. H., Sun, S., Neighbors, J., Schwarz, E. M., & Moriarty, T. F. (2025). Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model. Pharmaceuticals, 18(5), 675. https://doi.org/10.3390/ph18050675









