Polyphenolic Composition, Mineral Profile, and Biological Activities in Different Organs of Alpine Woundwort—Insights into Antioxidant and Enzyme Inhibitory Potential
Abstract
1. Introduction
2. Results and Discussion
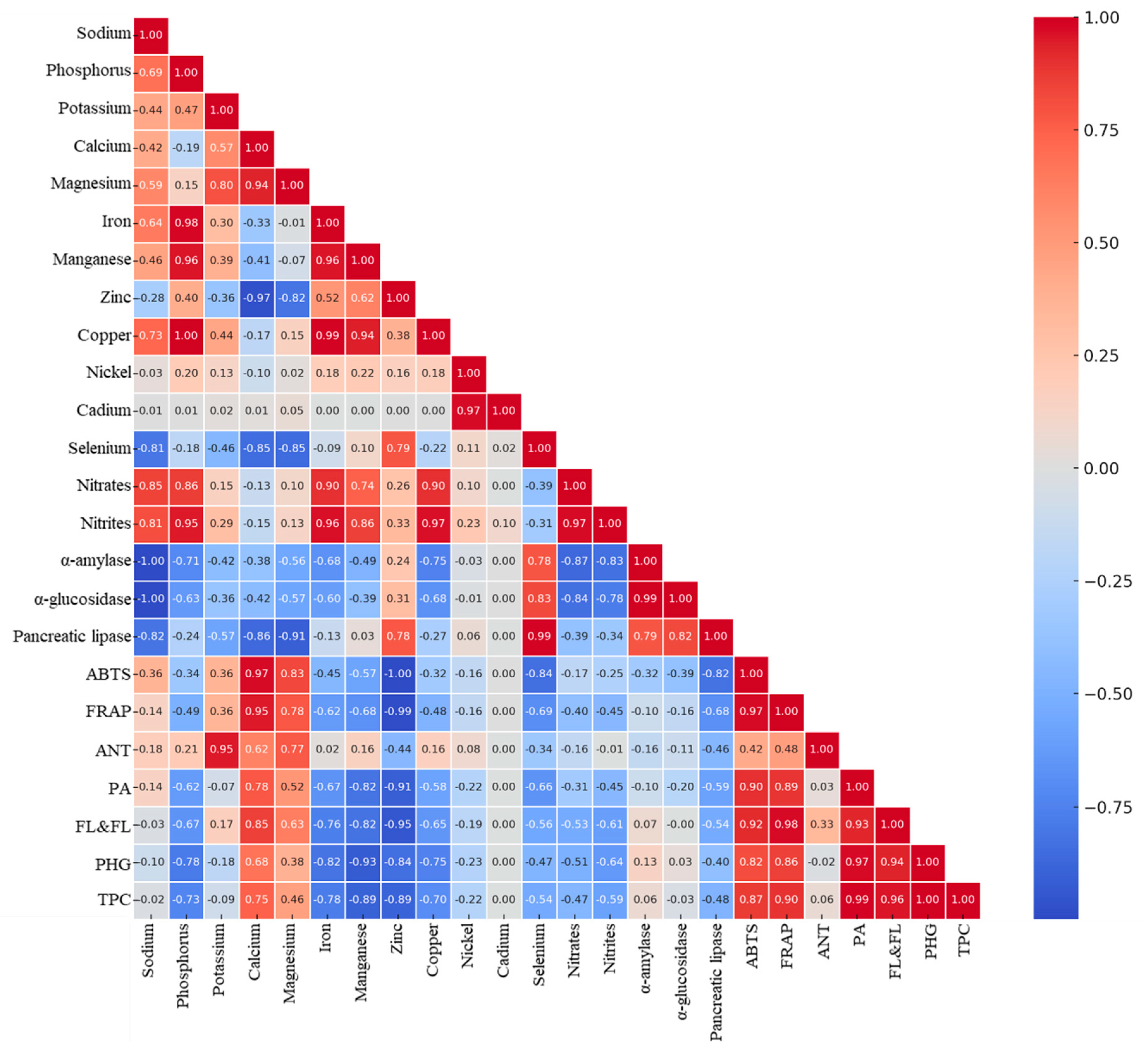
2.1. Mineral Compounds and Nitrates and Nitrites
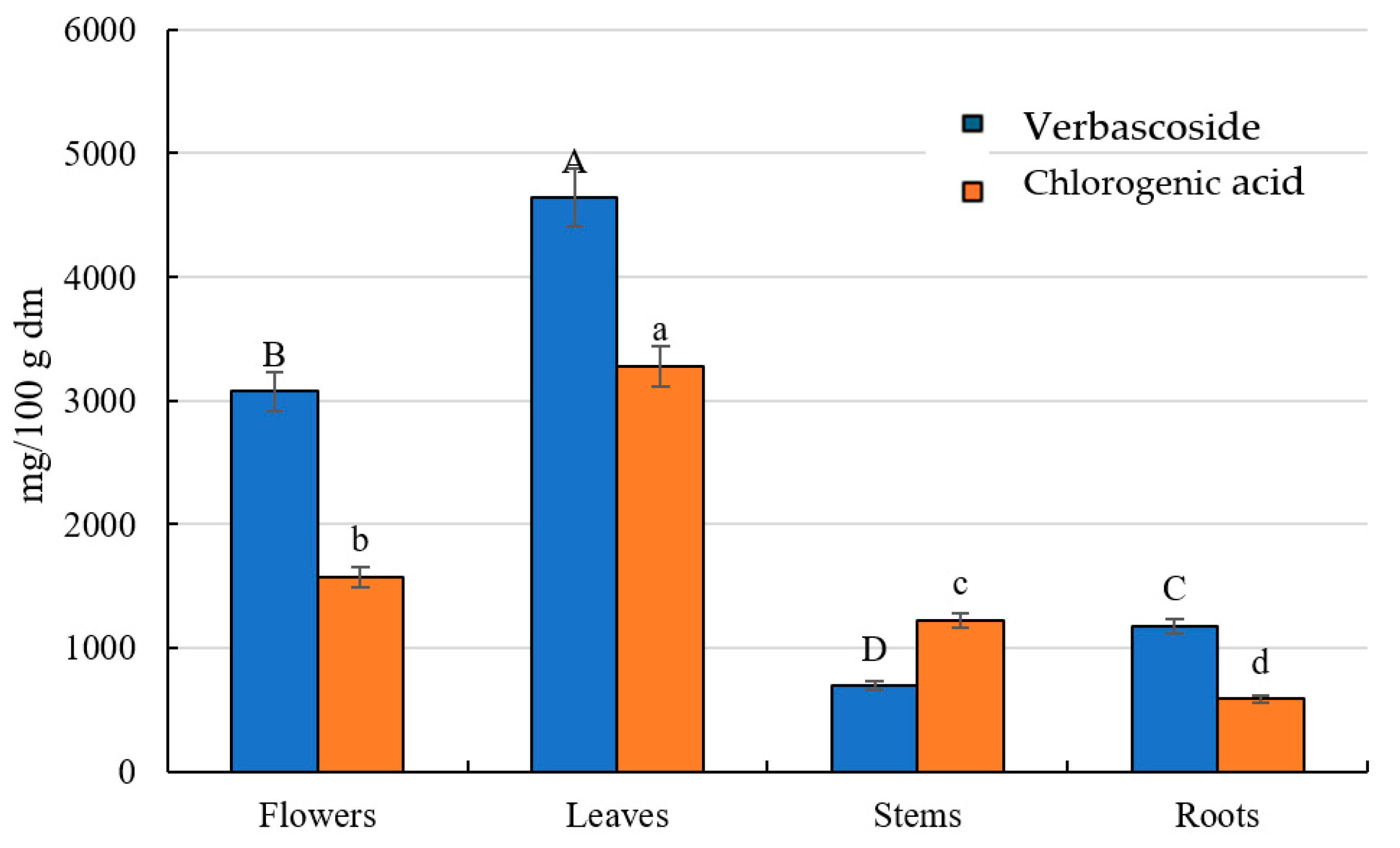
2.2. Identification and Quantification of Polyphenolic Compounds
2.3. Pro-Healthy Properties
Enzyme Inhibition Potency
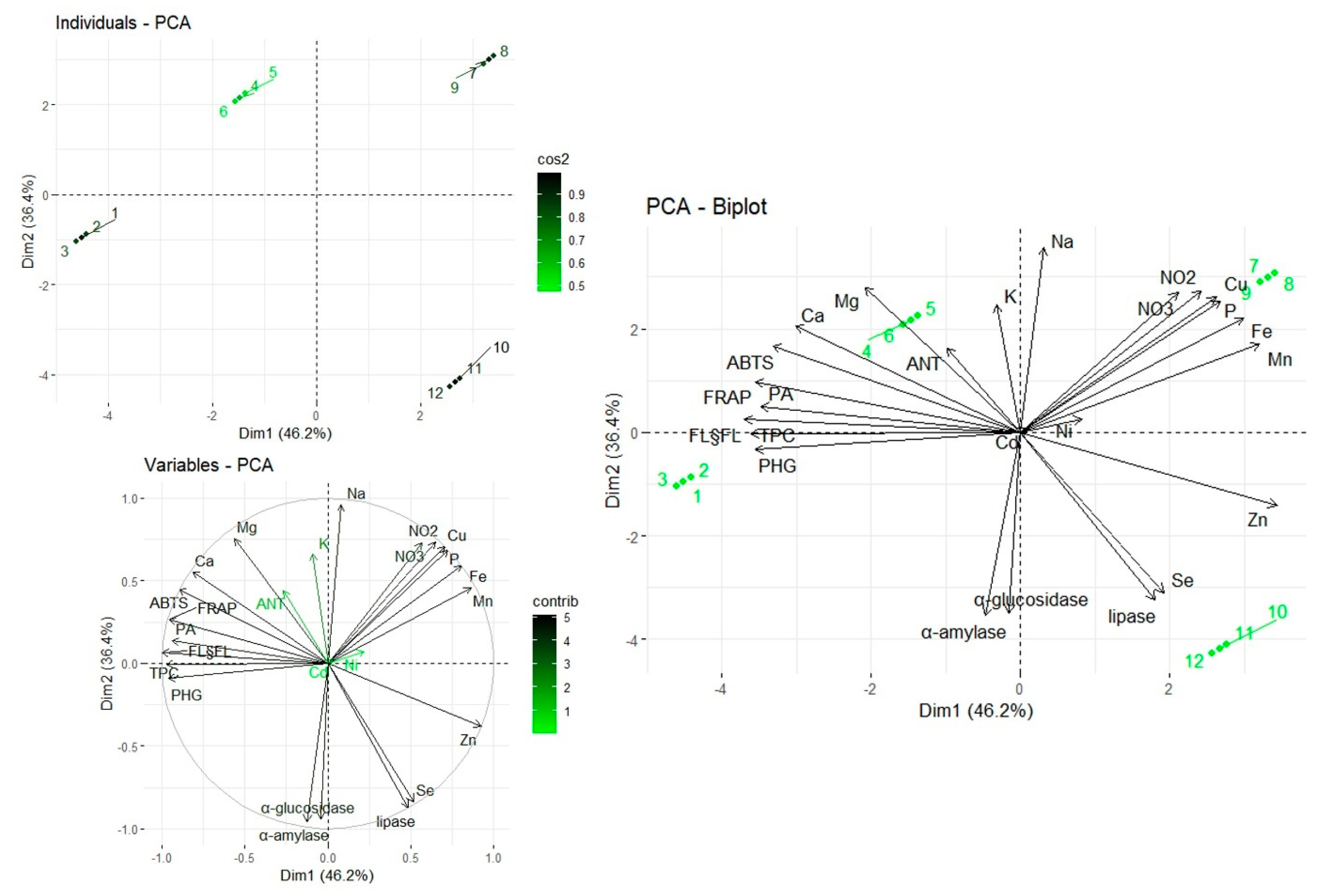
2.4. Multivariate Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Determination of Micro- and Macroelements and Heavy Metals
3.3. Determination of Polyphenolic Compounds
3.4. Determination of Procyanidins
3.5. Determination of Enzyme Inhibition Potency
3.6. Determination of Antioxidant Activity
3.7. Statistical Analysis
4. Limitations and Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, M.; Morteza-Semnani, K.; Mahdavi, M.R.; Rahimi, F. Antimicrobial studies on extracts of four species of Stachys. Indian J. Pharm. Sci. 2008, 70, 403. [Google Scholar]
- Pashova, S.; Karcheva-Bahchevanska, D.; Ivanov, K.; Ivanova, S. Genus Stachys—Phytochemistry, Traditional Medicinal Uses, and Future Perspectives. Molecules 2024, 29, 5345. [Google Scholar] [CrossRef]
- Bilušić Vundać, V. Taxonomical and phytochemical characterisation of 10 Stachys taxa recorded in the Balkan Peninsula flora: A review. Plants 2019, 8, 32. [Google Scholar] [CrossRef]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 2018; Volume 3, p. 243. [Google Scholar]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:459286-1 (accessed on 30 May 2024).
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; 370p. [Google Scholar]
- Nelson, J.B.; Rayner, D.A. A new hedge-nettle (Stachys: Lamiaceae) from South Carolina, USA. J. Bot. Res. Inst. Tex. 2014, 8, 431–440. [Google Scholar]
- Tomou, E.-M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef]
- Háznagy-Radnai, E.; Balogh, A.; Czigle, S.; Máthé, I.; Hohmann, J.; Blazsó, G. Antiinflammatory activities of Hungarian Stachys species and their iridoids. Phytother. Res. 2012, 26, 505–509. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F.; Tomás-Lorente, F. Flavonoid p-coumaroylglucosides and 8-hydroxyflavone allosylglucosides in some Labiatae. Phytochemistry 1992, 31, 3097–3102. [Google Scholar] [CrossRef]
- Háznagy-Radnai, E.; Czigle, S.; Janicsák, G.; Máthé, I. Iridoids of Stachys species growing in Hungary. J. Planar Chromatogr. Mod. TLC 2006, 19, 187–190. [Google Scholar] [CrossRef]
- Arceusz, A.; Wesolowski, M.; Radecka, I. Macro-and micro-elements in some herbal drug raw materials and their water extracts consumed in Poland. Cent. Eur. J. Chem. 2011, 9, 917–924. [Google Scholar] [CrossRef]
- Arceusz, A.; Radecka, I.; Wesolowski, M. Identification of diversity in elements content in medicinal plants belonging to different plant families. Food Chem. 2010, 120, 52–58. [Google Scholar] [CrossRef]
- Baloch, S. Essential and non-essential elements in medicinal plants: A review. Biomed. J. Sci. Tech. Res. 2021, 33, 26098–26100. [Google Scholar] [CrossRef]
- Szentmihalyi, K.; Then, M. Examination of microelements in medicinal plants of the Carpathian Basin. Acta Aliment. 2007, 36, 231–236. [Google Scholar] [CrossRef]
- Pourimani, R.; Kashian, S.; Fathivand, A.A. Measurement of trace elements in five popular medicinal plants using Instrumental Neutron Activation Analysis method (INAA) in Arak, Iran. arXiv 2019, arXiv:1903.10859. [Google Scholar]
- Bouasla, I.; Hamel, T.; Barour, C.; Bouasla, A.; Hachouf, M.; Bouguerra, O.M.; Messarah, M. Evaluation of solvent influence on phytochemical content and antioxidant activities of two Algerian endemic taxa: Stachys marrubiifolia Viv. and Lamium flexuosum Ten. (Lamiaceae). Eur. J. Integr. Med. 2021, 42, 101267. [Google Scholar] [CrossRef]
- Geng, Y.J. The Nitrate–Nitrite–Nitric Oxide Pathway in Traditional Herbal Medicine and Dietary Supplements with Potential Benefits for Cardiovascular Diseases. In Nitrite and Nitrate in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2017; pp. 279–291. [Google Scholar]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS) Guidance for submission for food additive evaluations. EFSA J. 2012, 10, 2760. Available online: www.efsa.europa.eu/efsajournal (accessed on 11 January 2025).
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Agric. Food Chem. 2006, 54, 2727–2735. [Google Scholar] [CrossRef]
- Seidler-Łożykowska, K.; Kaźmierczak, K.; Kucharski, W.A.; Mordalski, R.; Buchwald, W. Plonowanie i jakość surowca bazylii pospolitej i majeranku ogrodowego z upraw ekologicznych. J. Res. Appl. Agric. Eng. 2006, 51, 157–160. [Google Scholar]
- Vundać, V.B.; Brantner, A.H.; Plazibat, M. Content of polyphenolic constituents and antioxidant activity of some Stachys taxa. Food Chem. 2007, 104, 1277–1281. [Google Scholar] [CrossRef]
- Karioti, A.; Bolognesi, L.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC–DAD and HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2010, 53, 15–23. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Ursu, T.M.; Zanfirescu, A.; Negres, S.; Chirita, C.; Radu, G.L. Anti-inflammatory and antioxidant activities of the Impatiens noli-tangere and Stachys officinalis polyphenolic-rich extracts. Rev. Bras. Farmacogn. 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Lachowicz-Wiśniewska, S.; Pratap-Singh, A.; Kapusta, I.; Kruszyńska, A.; Rapak, A.; Ochmian, I.; Rubiński, P. Flowers and leaves extracts of Stachys palustris L. exhibit stronger anti-proliferative, antioxidant, anti-diabetic, and anti-obesity potencies than stems and roots due to more phenolic compounds as revealed by UPLC-PDA-ESI-TQD-MS/MS. Pharmaceuticals 2022, 15, 785. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Lorenzetti, L.M.; Maggi, F.; Serafini, M.; Bianco, A. Reassessment of the polar fraction of Stachys alopecuros (L.) Benth. subsp. divulsa (Ten.) Grande (Lamiaceae) from the Monti Sibillini National Park: A potential source of bioactive compounds. J. Intercult. Ethnopharmacol. 2017, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Zinchenko, T.V. Phenolic compounds of Stachys palustris. Chem. Nat. Compd. 1970, 6, 261–262. [Google Scholar] [CrossRef]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira Island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical profile and biological activity of endemic Sideritis sipylea Boiss. in North Aegean Greek islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- Properties, A.I. Phenolic characterization of Leonurus cardiaca L. Characterization of phenolic constituents of medicinal plants and evaluation of pharmacological activities: Focus in antioxidant and anti-inflammatory properties. 2013, 3, 57. Available online: https://gredos.usal.es/bitstream/handle/10366/123041/DFIFA_Rodriguezpereira_phenolicconstituensofmedicalplants.pdf?sequence=1 (accessed on 7 April 2025).
- Ergun, M.; Süslüoğlu, Z. Evaluating carrot as a functional food. Middle East J. Sci. 2018, 4, 113–119. [Google Scholar] [CrossRef]
- Meriç, Z.; Özdemir Nath, E.; Doğan, A.; Bitiş, L. Antioxidant, anti-tyrosinase activities and characterization of phenolic compounds for some plants from the Marmara Region, Türkiye. J. Res. Pharm. 2024, 28, 2. [Google Scholar]
- Badmos, S.; Lee, S.H.; Kuhnert, N. Comparison and quantification of chlorogenic acids for differentiation of green Robusta and Arabica coffee beans. Food Res. Int. 2019, 126, 108544. [Google Scholar] [CrossRef]
- Yamaguchi, M.A.; Kawanobu, S.; Maki, T.; Ino, I. Cyanidin 3-malonylglucoside and malonyl-coenzyme A: Anthocyanidin malonyltransferase in Lactuca sativa leaves. Phytochemistry 1996, 42, 661–663. [Google Scholar] [CrossRef]
- Donner, H.; Gao, L.; Mazza, G. Separation and characterization of simple and malonylated anthocyanins in red onions, Allium cepa L. Food Res. Int. 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Jaiswal, R.; Kuhnert, N. Profiling and characterisation by liquid chromatography/multi-stage mass spectrometry of the chlorogenic acids in Gardeniae Fructus. Rapid Commun. Mass Spectrom. 2010, 24, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic compounds of mountain tea from the Balkans: LC/DAD/ESI/MSn profile and content. Nat. Prod. Commun. 2011, 6, 1934578X1100600107. [Google Scholar] [CrossRef]
- Kharazian, N.; Dehkordi, F.J.; Xiang, C.L. Metabolomics-based profiling of five Salvia L. (Lamiaceae) species using untargeted data analysis workflow. Phytochem. Anal. 2025, 36, 113–143. [Google Scholar] [CrossRef]
- Karioti, A.; Kukić-Marković, J.; Bilia, A.R.; Niketić, M.; Petrović, S. Chemical profiling of six Stachys taxa from the Balkan Peninsula. Biochem. Syst. Ecol. 2022, 104, 104482. [Google Scholar] [CrossRef]
- Mantovska, D.I.; Zhiponova, M.K.; Petrova, D.; Alipieva, K.; Bonchev, G.; Boycheva, I.; Yordanova, Z.P. Exploring the phytochemical composition and biological potential of Balkan endemic species Stachys scardica Griseb. Plants 2023, 13, 30. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C.; Ceylan, O. Metabolite profiling and health benefits of Stachys cretica subsp. mersinaea as a medicinal food. Ind. Crops Prod. 2019, 131, 85–89. [Google Scholar]
- Benabderrahim, M.A.; Sarikurkcu, C.; Elfalleh, W.; Ozer, M.S.; Ceylan, O. Phenolic composition and biological activities of Turkish endemic plant: Stachys cretica subsp. kutahyensis. S. Afr. J. Bot. 2021, 138, 124–128. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 115–124. [Google Scholar]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crops Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Khan, S.; Nazir, M.; Saleem, H.; Raiz, N.; Saleem, M.; Anjum, S.M.M.; Zengin, G.; Mukhtar, M.; Tousif, M.I.; Mahomoodally, F.M.; et al. Valorization of the antioxidant, enzyme inhibition and phytochemical propensities of Berberis calliobotrys Bien. ex Koehne: A multifunctional approach to probe for bioactive natural products. Ind. Crops Prod. 2019, 141, 111693. [Google Scholar] [CrossRef]
- Aldughaylibi, F.S.; Raza, M.A.; Naeem, S.; Rafi, H.; Alam, M.W.; Souayeh, B.; Mir, T.A. Extraction of bioactive compounds for antioxidant, antimicrobial, and antidiabetic applications. Molecules 2022, 27, 5935. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, Y.; Zhang, C.; Zhan, L.; Khan, W.; Siddiqua, S.; Xiao, J. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit. Rev. Food Sci. Nutr. 2022, 62, 3873–3897. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Kalisz, S. Effects of various polysaccharide clarification agents and reaction time on content of polyphenolic compound, antioxidant activity, turbidity, and colour of chokeberry juice. LWT 2018, 92, 347–360. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.H. New insights into the latest advancement in α-amylase inhibitors of plant origin with anti-diabetic effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef]
- Fuchsberger, C.; Flannick, J.; Teslovich, T.M.; Mahajan, A.; Agarwala, V.; Gaulton, K.J.; Koistinen, H.A. The genetic architecture of type 2 diabetes. Nature 2016, 536, 41–47. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kocak, M.S.; Uren, M.C.; Calapoglu, M.; Tepe, A.S. Potential sources for the management of global health problems and oxidative stress: Stachys byzantina and S. iberica subsp. iberica var. densipilosa. Eur. J. Integr. Med. 2016, 8, 631–637. [Google Scholar] [CrossRef]
- Carev, I.; Sarikurkcu, C. LC-MS/MS profiles and in vitro biological activities of extracts of an endemic species from Turkey: Stachys cretica ssp. anatolica. Plants 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.G.; Nazzaro, M.; Di Stasio, M.; Siano, F.; Coppola, R.; De Marco, A. Content of micronutrients, mineral and trace elements in some Mediterranean spontaneous edible herbs. Chem. Cent. J. 2015, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Du, C.; Zheng, S.; Du, Y. In situ monitoring of nitrate content in leafy vegetables using attenuated total reflectance−Fourier-transform mid-infrared spectroscopy coupled with machine learning algorithm. Food Anal. Methods 2021, 14, 2237–2248. [Google Scholar] [CrossRef]
- Kukić, J.; Petrović, S.; Niketić, M. Antioxidant activity of four endemic Stachys taxa. Biol. Pharm. Bull. 2006, 29, 725–729. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, M.; Kumar, S.; Sharma, D.; Yadav, J.P. In vitro antioxidant activities and GC-MS analysis of different solvent extracts of Acacia nilotica leaves. Indian J. Pharm. Sci. 2018, 80, 892–902. [Google Scholar] [CrossRef]
- Savić, M.M.; Kukić, J.M.; Grayer, R.J.; Milinković, M.M.; Marin, P.D.; Divljaković, J.; Petrović, S.D. Behavioural characterization of four endemic Stachys taxa. Phytother. Res. 2010, 24, 1309–1316. [Google Scholar] [CrossRef]
- Vicente, A.R.; Manganaris, G.A.; Sozzi, G.O.; Crisosto, C.H. Nutritional Quality of Fruits and Vegetables. In Postharvest Handling: A Systems Approach, 2nd ed.; Wiley: Berlin, Germany, 2009; ISBN 978-0-12-374112-7. [Google Scholar]
- Kruczek, A.; Krupa-Małkiewicz, M.; Lachowicz, S.; Oszmiański, J.; Ochmian, I. Health-promoting capacities of in vitro and cultivated goji (Lycium chinense Mill.) fruit and leaves; polyphenols, antimicrobial activity, macro- and microelements and heavy metals. Molecules 2020, 25, 5314. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Oszmiański, J.; Lachowicz, S.; Krupa-Małkiewicz, M. Rootstock effect on physico-chemical properties and content of bioactive compounds of four cultivars Cornelian cherry fruits. Sci. Hortic. 2019, 256, 108588. [Google Scholar] [CrossRef]
- Lachowicz, S.; Świeca, M.; Pejcz, E. Improvement of health-promoting functionality of rye bread by fortification with free and microencapsulated powders from Amelanchier alnifolia Nutt. Antioxidants 2020, 9, 614. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Yousefian, N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbraucherschutz Leb. 2011, 6, 191–195. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lachowicz, S.; Wiśniewski, R.; Ochmian, I.; Drzymała, K.; Pluta, S. Anti-microbiological, anti-hyperglycemic and anti-obesity potency of natural antioxidants in fruit fractions of Saskatoon berry. Antioxidants 2019, 8, 397. [Google Scholar] [CrossRef]

| Group | Element | Leaves | Flowers | Stalk | Root |
|---|---|---|---|---|---|
| Macroelements [mg/kg] | N | 25,300.00 ± 50.60 d ** | 7400.00 ± 14.80 a | 18,700.00 ± 37.40 d | 15,400.00 ± 30.80 b |
| P | 21,000.00 ± 42.00 d | 12,200.00 ± 24.40 b | 15,900.00 ± 31.80 d | 7200.00 ± 14.40 a | |
| K | 10,100.00 ± 20.20 b | 8600.00 ± 17.20 a | 13,600.00 ± 27.20 b | 8600.00 ± 17.20 a | |
| Ca | 5400.00 ± 10.80 b | 2200.00 ± 4.40 a | 10,100.00 ± 20.20 b | 9200.00 ± 18.40 c | |
| Mg | 2900.00 ± 5.80 a | 1800.00 ± 3.60 a | 3900.00 ± 7.80 c | 3100.00 ± 6.20 b | |
| Microelements [mg/kg] | Fe | 89.43 ± 0.18 c | 56.17 ± 0.11 b | 61.10 ± 0.12 b | 34.67 ± 0.07 b |
| Mn | 155.30 ± 0.31 c | 107.67 ± 0.22 b | 114.33 ± 0.23 b | 32.30 ± 0.06 a | |
| Zn | 16.37 ± 0.03 c | 21.00 ± 0.04 d | 7.73 ± 0.02 b | 5.37 ± 0.01 a | |
| Cu | 12.60 ± 0.03 d | 6.23 ± 0.01 b | 8.67 ± 0.02 c | 3.37 ± 0.01 a | |
| Se | <0.01 | <0.01 | <0.01 | <0.01 | |
| Ni | <0.01 | <0.01 | <0.01 | <0.01 | |
| Cd | 0.03 ± 0.001 b | 0.42 ± 0.001 c | 0.02 ± 0.001 ab | 0.01 ± 0.001 a | |
| NO3⁻ [mg/kg] | 109.00 a | 187.50 b | 552.30 c | 102.00 a | |
| NO2⁻ [mg/kg] | 0.70 a | 1.20 b | 1.80 c | 0.90 a | |
| No | λ [nm] | MS-MS [M-H]−/[M-H]+ | Compounds | Rt * [min] | S. alpina L. | |||
|---|---|---|---|---|---|---|---|---|
| Flowers | Leaves | Stems | Roots | |||||
| 1 | 320 | 461/315 | Decaffeoyl-acetoside | 3.67 | 53.13 ± 1.06 b ** | 92.65 ± 1.85 a | 28.07 ± 0.56 c | 15.94 ± 0.32 d |
| 2 | 325 | 353/191/179/135 | 1-Caffeoylquinic acid | 3.73 | 19.83 ± 0.40 b | 29.04 ± 0.58 a | 18.63 ± 0.37 c | 8.26 ± 0.17 d |
| 3 | 325 | 353/179 | 3-Caffeoylquinic acid | 3.87 | 6.70 ± 0.13 c | 13.03 ± 0.26 a | 11.17 ± 0.22 b | 3.42 ± 0.07 d |
| 4 | 320 | 461/315 | Decaffeoyl-acetoside | 4.01 | 9.57 ± 0.19 c | 13.42 ± 0.27 b | 14.41 ± 0.29 a | 9.37 ± 0.19 cd |
| 5 | 329 | 353/294/274/179 | Caffeoylquinic acid | 4.19 | 12.87 ± 0.26 b | 54.83 ± 1.10 a | 4.58 ± 0.09 c | 3.60 ± 0.07 d |
| 6 | 335 | 339/179 | Methoxycinnamic acid hexoside | 4.31 | 6.34 ± 0.13 b | 22.75 ± 0.46 a | 3.74 ± 0.07 c | nd |
| 7 | 325 | 341/179 | Caffeoylglucose | 4.43 | 4.45 ± 0.09 b | 24.96 ± 0.50 a | 2.11 ± 0.04 c | nd |
| 8 | 329 | 339/179 | Methoxycinnamic acid hexoside | 4.63 | 8.42 ± 0.17 b | 36.77 ± 0.74 a | 3.9 ± 0.08 c | nd |
| 9 | 324 | 353/191/179 | 5-Caffeoylquinic acid | 4.87 | 1573.15 ± 31.46 b | 3277.83 ± 65.56 a | 1221.95 ± 24.44 c | 590.35 ± 11.81 d |
| 10 | 517 | 449/287 | Cyanidin 3-glucoside | 5.05 | 31.33 ± 0.63 a | nd | nd | nd |
| 11 | 277 | 459/283 | Palustrinoside | 5.10 | 2.59 ± 0.05 d | 11.46 ± 0.23 a | 9.37 ± 0.19 b | 8.92 ± 0.18 c |
| 12 | 323 | 397/179 | 5-Sinapoylquinic acid | 5.34 | 85.19 ± 1.70 a | 64.73 ± 1.29 b | 4.30 ± 0.09 d | 6.50 ± 0.13 c |
| 13 | 326 | 353/191/179 | 4-Caffeoylquinic acid | 5.50 | 52.3 ± 1.05 b | 157.20 ± 3.14 a | 27.32 ± 0.55 c | nd |
| 14 | 517 | 535/463/301 | Peonidin-3-O-glucoside | 5.80 | 9.59 ± 0.19 a | nd | nd | nd |
| 15 | 311 | 337/191 | 3-O-p-Coumaroylquinic acid | 5.91 | 33.71 ± 0.67 b | 96.70 ± 1.93 a | 5.46 ± 0.11 c | 2.00 ± 0.04 d |
| 16 | 330 | 623/461/443/315 | Verbascoside | 6.02 | 2.74 ± 0.05 d | 22.87 ± 0.46 a | 11.63 ± 0.23 b | 8.53 ± 0.17 c |
| 17 | 324 | 785/161 | Betonyoside E | 6.16 | 16.29 ± 0.33 b | 21.08 ± 0.42 a | 11.65 ± 0.23 c | nd |
| 18 | 517 | 535/287 | Cyanidin 3-malonylglucoside | 6.27 | 163.27 ± 3.27 a | nd | nd | nd |
| 19 | 331 | 785/161 | Betonyoside E | 6.28 | 11.71 ± 0.23 c | 41.60 ± 0.83 a | 8.04 ± 0.16 d | 15.83 ± 0.32 b |
| 20 | 330 | 785/161 | Betonyoside E isomer | 6.33 | 23.38 ± 0.47 b | 31.09 ± 0.62 a | 4.22 ± 0.08 c | 4.06 ± 0.08 d |
| 21 | 329 | 785/161 | Betonyoside E isomer | 6.40 | 22.67 ± 0.45 a | 20.17 ± 0.40 b | 2.71 ± 0.05 d | 4.35 ± 0.09 c |
| 22 | 307 | 337/191 | 3-O-p-Coumaroylquinic acid | 6.43 | 21.29 ± 0.43 b | 38.60 ± 0.77 a | 1.67 ± 0.03 c | nd |
| 23 | 328 | 785/161 | Betonyoside E isomer | 6.75 | 12.55 ± 0.25 a | 6.97 ± 0.14 b | 4.61 ± 0.09 c | nd |
| 24 | 325 | 623/461/161 | Acteoside | 6.81 | 6.94 ± 0.14 a | 2.60 ± 0.05 b | 2.41 ± 0.05 b | nd |
| 25 | 516 | 549/301 | Peonidin- 3-malonylglucoside | 6.94 | 10.86 ± 0.22 a | nd | nd | nd |
| 26 | 334 | 665/623/503/461/443/299/284 | Chryseriol 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 7.02 | 34.43 ± 0.69 a | 29.27 ± 0.59 b | nd | nd |
| 27 | 334 | 785/623/161 | Echinacoside-glucoronoid | 7.14 | 8.98 ± 0.18 b | 21.99 ± 0.44 a | 2.97 ± 0.06 c | nd |
| 28 | 330 | 785/623/161 | Echinacoside isomer | 7.21 | 9.42 ± 0.19 c | 42.83 ± 0.86 a | 10.09 ± 0.20 b | nd |
| 29 | 331 | 785/623/161 | B-OH-Forsythoside B methylether | 7.29 | 16.95 ± 0.34 d | 58.97 ± 1.18 a | 24.62 ± 0.49 c | 33.73 ± 0.67 b |
| 30 | 328 | 755/623/593/161 | Stachysoside A | 7.40 | 886.73 ± 17.73 c | 3022.8 ± 60.46 a | 892.96 ± 17.86 b | 690.72 ± 13.81 d |
| 31 | 328 | 623/461/315 | Verbascoside | 7.59 | 3072.07 ± 61.44 b | 4618.88 ± 92.38 a | 681.49 ± 13.63 d | 1170.71 ± 23.41 c |
| 32 | 274/305/326 | 609/285 | Isoscutellarein-7-O-allosyl(1→2)]-glucoside | 7.70 | nd | 120.84 ± 2.42 a | nd | nd |
| 33 | 347 | 623/447/285 | Kaempferol hexose glucuronide | 7.72 | 126.13 ± 2.52 a | 98.6 ± 1.97 b | nd | nd |
| 34 | 329 | 755/623/461/161 | Forsythoside B | 7.79 | 15.11 ± 0.30 c | 77.89 ± 1.56 a | 11.48 ± 0.23 d | 19.84 ± 0.4 b |
| 35 | 330 | 623/461/161 | Isoverbascoside | 7.91 | 219.21 ± 4.38 d | 1025.28 ± 20.51 a | 250.56 ± 5.01 c | 297.27 ± 5.95 b |
| 36 | 350 | 609, 447, 285 | Luteolin 7-O-dihexoside | 8.01 | 29.62 ± 0.59 b | 88.40 ± 1.77 a | 13.55 ± 0.27 c | 8.87 ± 0.18 d |
| 37 | 326 | 623/461/161 | Acteoside | 8.06 | 102.33 ± 2.05 b | 316.88 ± 6.34 a | 39.79 ± 0.8 d | 81.61 ± 1.63 c |
| 38 | 326 | 693/651/609/489/471/429/285 | Luteolin 7-O-[6‴-O-acetyl]-allosyl-(1→2)-[6′′-O-acetyl]-glucoside | 8.14 | 25.86 ± 0.52 b | 58.95 ± 1.18 a | 13.67 ± 0.27 c | nd |
| 39 | 329 | 769/593/161 | Allysonoside | 8.34 | 195.22 ± 3.9 b | 260.35 ± 5.21 a | 122.08 ± 2.44 d | 164.81 ± 3.30 c |
| 40 | 317 | 769/593/315/161 | Allysonoside | 8.39 | 55.06 ± 1.10 c | 100.55 ± 2.01 b | 121.23 ± 2.42 a | nd |
| 41 | 329 | 675/593/447/285 | Isoscutellarein-3-glucoside-rhamnoside | 8.59 | 109.33 ± 2.19 a | 61.21 ± 1.22 b | 33.14 ± 0.66 d | 59.44 ± 1.19 c |
| 42 | 344 | 755/623/593/461 | Samioside | 8.78 | nd | 7.98 ± 0.16 a | 1.86 ± 0.04 b | nd |
| 43 | 329 | 461/299 | Chrysoeriol-glucoside | 8.86 | 51.79 ± 1.04 b | 65.23 ± 1.30 a | 34.26 ± 0.69 d | 37.86 ± 0.76 c |
| 44 | 324 | 635/593/461/431/269 | apigenin 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 8.91 | 148.31 ± 2.97 a | 93.2 ± 1.86 b | 10.20 ± 0.20 d | 18.08 ± 0.36 c |
| 45 | 324 | 515/353/191 | 3,4-Dicaffeoylquinic acid | 8.92 | 147.51 ± 2.95 a | 89.12 ± 1.78 b | 7.92 ± 0.16 d | 16.96 ± 0.34 c |
| 46 | 335 | 635/593/461/431/269 | apigenin 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 9.01 | 48.21 ± 0.96 b | 223.23 ± 4.46 a | 7.18 ± 0.14 c | 7.07 ± 0.14 c |
| 47 | 324 | 515/353/191/179 | 3,5-Dicaffeoylquinic acid | 9.04 | 99.47 ± 1.99 a | 64.21 ± 1.28 b | 1.31 ± 0.03 d | 2.81 ± 0.06 c |
| 48 | 347 | 665/623/299/284 | Chryseriol 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 9.06 | 138.21 ± 2.76 a | 71.16 ± 1.42 b | 3.96 ± 0.08 d | 6.45 ± 0.13 c |
| 49 | 347 | 665/299 | Chrysoeriol-7-O-[6‴-acetylallopyranosyl-(1 → 2)]-glucopyranoside (=stachyspinoside) | 9.18 | 421.86 ± 8.44 b | 454.35 ± 9.09 a | 106.94 ± 2.14 d | 132.26 ± 2.65 c |
| 50 | 330 | 665/623/461/299 | Chryseriol 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 9.36 | 10.33 ± 0.21 a | 5.84 ± 0.12 b | 3.82 ± 0.08 c | 1.79 ± 0.04 d |
| 51 | 329 | 783/607/475/329 | Leonoside B | 9.49 | 30.41 ± 0.61 d | 125.83 ± 2.52 a | 53.91 ± 1.08 b | 36.12 ± 0.72 c |
| 52 | 329 | 623/461/299/284 | 4-O-Methylisoscutellarein-7-O-[allosyl-(1→2)]-glucopyranoside | 9.59 | 10.72 ± 0.21 b | 16.16 ± 0.32 a | nd | 9.50 ± 0.19 c |
| 53 | 349 | 783/607/475/329 | Leonoside B | 9.69 | 38.02 ± 0.76 a | 18.16 ± 0.36 b | 2.27 ± 0.05 c | 2.36 ± 0.05 c |
| 54 | 329 | 651/429/285 | Isoscutellarein-7-O-[6‴-acetyl-allopyranosyl-(1 →2)]-glucopyranoside | 9.85 | 40.87 ± 0.82 b | 143.98 ± 2.88 a | 15.99 ± 0.32 d | 20.84 ± 0.42 c |
| 55 | 323 | 665/623/299/284 | 4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 10.30 | 11.3 ± 0.23 b | 71.66 ± 1.43 a | 5.40 ± 0.11 d | 5.92 ± 0.12 c |
| 56 | 324 | 515/353/191 | 4,5-Dicaffeoylquinic acid | 11.07 | 2.24 ± 0.04 b | 10.9 ± 0.22 a | 1.09 ± 0.02 c | 0.21 ± 0.001 d |
| 57 | 347 | 707/665/647/503/299 | 4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl-(1→2)-[6′′-O-acetyl]-glucoside | 11.26 | 0.99 ± 0.02 c | 52.34 ± 1.05 a | 3.33 ± 0.07 b | 0.48 ± 0.01 cd |
| 58 | 347 | 473/269 | Apigenin hexoside acetyl derivative | 11.80 | 0.19 ± 0.001 c | 10.43 ± 0.21 a | 0.34 ± 0.01 b | 0.13 ± 0.001 c |
| Degree of polymerization | 1.64 ± 0.03 b | 1.94 ± 0.04 a | 1.16 ± 0.02 c | 1.14 ± 0.02 d | ||||
| Parts of S. aplina | α-Amylase IC50 [mg/mL] | α-Glucosidase IC50 [mg/mL] | Pancreatic Lipase IC50 [mg/mL] | ABTS mmol TE/g dm | FRAP mmol TE/g dm |
|---|---|---|---|---|---|
| Leaves | 27.77 ± 0.56 **c | 32.73 ± 0.65 c | 51.28 ± 1.03 c | 19.30 ± 0.39 a | 7.62 ± 0.15 a |
| Flowers | 25.31 ± 0.51 b | 31.33 ± 0.63 b | 45.31 ± 0.91 a | 16.85 ± 0.34 b | 6.65 ± 0.13 b |
| Stalks | 20.07 ± 0.40 a | 24.88 ± 0.50 a | 50.78 ± 1.02 b | 9.70 ± 0.19 c | 1.44 ± 0.03 c |
| Roots | 33.16 ± 0.66 d | 40.60 ± 0.81 d | 88.75 ± 1.77 d | 3.74 ± 0.07 d | 0.46 ± 0.01 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz-Wiśniewska, S.; Ochmian, I.; Oszmiański, J.; Wiśniewski, R.; Bernatek, M.; Rubiński, P.; De Vita, D. Polyphenolic Composition, Mineral Profile, and Biological Activities in Different Organs of Alpine Woundwort—Insights into Antioxidant and Enzyme Inhibitory Potential. Pharmaceuticals 2025, 18, 674. https://doi.org/10.3390/ph18050674
Lachowicz-Wiśniewska S, Ochmian I, Oszmiański J, Wiśniewski R, Bernatek M, Rubiński P, De Vita D. Polyphenolic Composition, Mineral Profile, and Biological Activities in Different Organs of Alpine Woundwort—Insights into Antioxidant and Enzyme Inhibitory Potential. Pharmaceuticals. 2025; 18(5):674. https://doi.org/10.3390/ph18050674
Chicago/Turabian StyleLachowicz-Wiśniewska, Sabina, Ireneusz Ochmian, Jan Oszmiański, Rafał Wiśniewski, Małgorzata Bernatek, Paweł Rubiński, and Daniela De Vita. 2025. "Polyphenolic Composition, Mineral Profile, and Biological Activities in Different Organs of Alpine Woundwort—Insights into Antioxidant and Enzyme Inhibitory Potential" Pharmaceuticals 18, no. 5: 674. https://doi.org/10.3390/ph18050674
APA StyleLachowicz-Wiśniewska, S., Ochmian, I., Oszmiański, J., Wiśniewski, R., Bernatek, M., Rubiński, P., & De Vita, D. (2025). Polyphenolic Composition, Mineral Profile, and Biological Activities in Different Organs of Alpine Woundwort—Insights into Antioxidant and Enzyme Inhibitory Potential. Pharmaceuticals, 18(5), 674. https://doi.org/10.3390/ph18050674








