A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Background
2.1. Pathology of AD
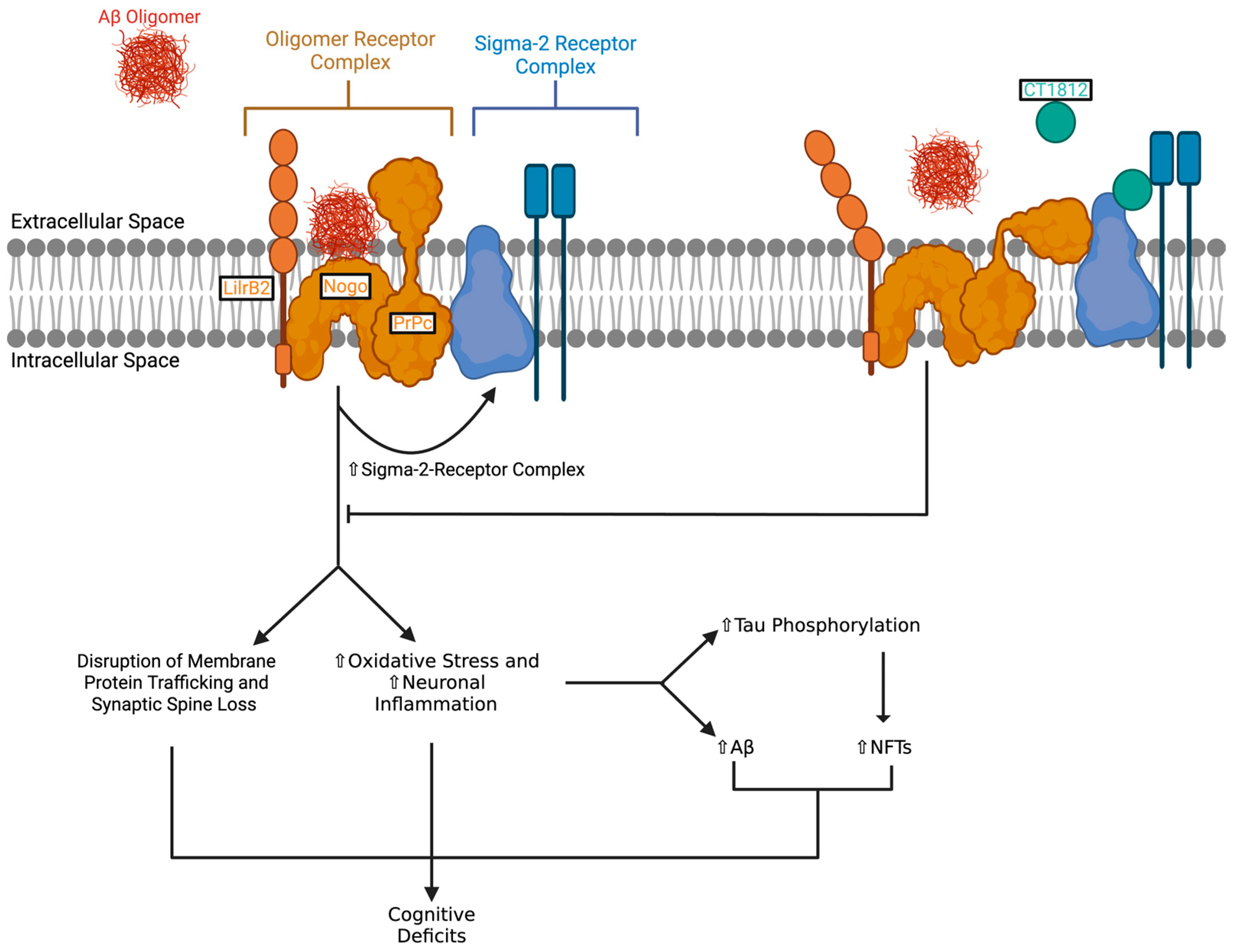
2.2. Mechanism of Action of CT1812
3. Current Research
3.1. Preclinical Studies
3.2. Clinical Trials
3.2.1. Safety and Tolerability of CT1812 Across Clinical Trials
3.2.2. Amyloid Displacement and Tau Modulation
3.2.3. Synaptic Function Modulation
3.2.4. Cognitive Function
3.3. Limitations and Future Directions
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-beta |
| BBB | Blood–brain barrier |
| PrPc | Cellular prion protein |
| NMDA | N-methyl-D-aspartate |
| CSF | Cerebrospinal fluid |
| NFTs | Neurofibrillary tangles |
| p-tau | Hyperphosphorylated tau protein |
| AEs | Adverse Effects |
| PK | Pharmacokinetics |
| PD | Pharmacodynamics |
| CYP | Cytochrome P450 |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s Disease: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Disease-Modifying Drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Paul, D.; Agrawal, R.; Singh, S. Alzheimer’s Disease and Clinical Trials. J. Basic. Clin. Physiol. Pharmacol. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Staniszewski, A.; To, L.; Fa, M.; Teich, A.F.; Saeed, F.; Wostein, H.; Walko, T.; Vaswani, A.; Wardius, M.; et al. Alzheimer’s Therapeutics Targeting Amyloid Beta 1-42 Oligomers I: Abeta 42 Oligomer Binding to Specific Neuronal Receptors Is Displaced by Drug Candidates That Improve Cognitive Deficits. PLoS ONE 2014, 9, e111898. [Google Scholar] [CrossRef]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural Oligomers of the Alzheimer Amyloid-β Protein Induce Reversible Synapse Loss by Modulating an NMDA-Type Glutamate Receptor-Dependent Signaling Pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Yuede, C.M.; LaBarbera, K.M.; Limegrover, C.S.; Rehak, C.; Yurko, R.; Waybright, L.; Look, G.; Rishton, G.; Safferstein, H.; et al. Preclinical and Clinical Biomarker Studies of CT1812: A Novel Approach to Alzheimer’s Disease Modification. Alzheimer’s Dement. 2021, 17, 1365–1382. [Google Scholar] [CrossRef]
- Kaur, G.; Dar, Z.A.; Bajpai, A.; Singh, R.; Bansal, R. Clinical Update on an Anti-Alzheimer Drug Candidate CT1812: A Sigma-2 Receptor Antagonist. Clin. Ther. 2024, 46, e21–e28. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Zaheer, A.B.; Munawwar, A.; Sarfraz, Z.; Sarfraz, A.; Robles-Velasco, K.; Cherrez-Ojeda, I. The Allosteric Antagonist of the Sigma-2 Receptors—Elayta (CT1812) as a Therapeutic Candidate for Mild to Moderate Alzheimer’s Disease: A Scoping Systematic Review. Life 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, P.; Brimson, J.M.; Moreira, P.I.; Avila, J.; Galimberti, D.; Pappolla, M.A.; Plascencia-Villa, G.; Sorensen, A.A.; Zhu, X.; Perry, G. CT1812, a Small Molecule Sigma-2 Receptor Antagonist for Alzheimer’s Disease Treatment: A Systematic Review of Available Clinical Data. J. Alzheimer’s Dis. 2024, 101, S115–S128. [Google Scholar] [CrossRef]
- Izzo, N.J.; Xu, J.; Zeng, C.; Kirk, M.J.; Mozzoni, K.; Silky, C.; Rehak, C.; Yurko, R.; Look, G.; Rishton, G.; et al. Alzheimer’s Therapeutics Targeting Amyloid Beta 1–42 Oligomers II: Sigma-2/PGRMC1 Receptors Mediate Abeta 42 Oligomer Binding and Synaptotoxicity. PLoS ONE 2014, 9, e111899. [Google Scholar] [CrossRef]
- Wang, T.; Jia, H. The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 24, 12025. [Google Scholar] [CrossRef]
- Colom-Cadena, M.; Toombs, J.; Simzer, E.; Holt, K.; McGeachan, R.; Tulloch, J.; Jackson, R.J.; Catterson, J.H.; Spires-Jones, M.P.; Rose, J.; et al. Transmembrane Protein 97 Is a Potential Synaptic Amyloid Beta Receptor in Human Alzheimer’s Disease. Acta Neuropathol. 2024, 147, 32. [Google Scholar] [CrossRef]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A Phase 1 Clinical Trial of the Sigma-2 Receptor Complex Allosteric Antagonist CT1812, a Novel Therapeutic Candidate for Alzheimer’s Disease. Alzheimer’s Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef]
- Lizama, B.N.; North, H.A.; Pandey, K.; Williams, C.; Duong, D.; Cho, E.; Di Caro, V.; Ping, L.; Blennow, K.; Zetterberg, H.; et al. An Interim Exploratory Proteomics Biomarker Analysis of a Phase 2 Clinical Trial to Assess the Impact of CT1812 in Alzheimer’s Disease. Neurobiol. Dis. 2024, 199, 106575. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Mecca, A.P.; O’Dell, R.S.; Bartlett, H.H.; Diepenbrock, N.G.; Huang, Y.; Hamby, M.E.; Grundman, M.; Catalano, S.M.; Caggiano, A.O.; et al. A Pilot Study to Evaluate the Effect of CT1812 Treatment on Synaptic Density and Other Biomarkers in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2024, 16, 20. [Google Scholar] [CrossRef]
- Vijverberg, E.; de Haan, W.; Scheijbeler, E.; Hamby, M.E.; Catalano, S.; Scheltens, P.; Grundman, M.; Caggiano, A.O. A Pilot Electroencephalography Study of the Effect of CT1812 Treatment on Synaptic Activity in Patients with Mild to Moderate Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2024, 11, 1809–1817. [Google Scholar] [CrossRef]
- Catalano, S.; Grundman, M.; Schneider, L.S.; DeKosky, S.; Morgan, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; Izzo, N.J.; Safferstein, H.; et al. P4-381: A Two-Part, Double-Blind, Placebo-Controlled, Phase 1 Study of the Safety and Pharmacokinetics of Single and Multiple Ascending Doses of CT1812 in Healthy Volunteers. Alzheimer’s Dement. 2016, 12, P1183–P1184. [Google Scholar] [CrossRef]
- LaBarbera, K.M.; Sheline, Y.I.; Izzo, N.J.; Yuede, C.M.; Waybright, L.; Yurko, R.; Edwards, H.M.; Gardiner, W.D.; Blennow, K.; Zetterberg, H.; et al. A Phase 1b Randomized Clinical Trial of CT1812 to Measure Aβ Oligomer Displacement in Alzheimer’s Disease Using an Indwelling CSF Catheter. Transl. Neurodegener. 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug Treatments in Alzheimer’s Disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Burke, A.D.; Goldfarb, D.; Bollam, P.; Khokher, S. Diagnosing and Treating Depression in Patients with Alzheimer’s Disease. Neurol. Ther. 2019, 8, 325–350. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Wilkinson, C.W.; Trittschuh, E.H.; Chapmana, D.; Watson, G.S.; Cholerton, B.; Plymate, S.R.; Arbuckle, M.; Craft, S. Sex and ApoE Genotype Differences in Treatment Response to Two Doses of Intranasal Insulin in Adults with Mild Cognitive Impairment or Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 35, 789–797. [Google Scholar] [CrossRef]
- Lizama, B.N.; Williams, C.; North, H.A.; Pandey, K.; Duong, D.; Di Caro, V.; Mecca, A.P.; Blennow, K.; Zetterberg, H.; Levey, A.I.; et al. CT1812 Biomarker Signature from a Meta-Analysis of CSF Proteomic Findings from Two Phase 2 Clinical Trials in Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 6860–6880. [Google Scholar] [CrossRef]
| Trial Name (ID) | Study Design | Primary Outcome(s) | Results |
|---|---|---|---|
| NCT02570997 (COG0101) | A two-part, phase 1, randomized, double-blind, placebo-controlled, parallel-assignment study in healthy adults evaluating safety, tolerability, and pharmacokinetics (PK) and determining the maximum tolerated dose (MTD) of CT1812. Part A (54 participants, aged 18–64) was a single ascending dose (SAD) study with 6 cohorts (10–1120 mg; 6 active–2 placebo) and one food-effect cohort (90 mg; n = 6). Part B (39 participants, aged 18–75) was a 14-day multiple ascending dose (MAD) study with 3 young adult cohorts (280–840 mg; 8 active–2 placebo) and 1 elderly cohort aged ≥65 (560 mg; 7 active:2 placebo). CSF sampling and cognitive testing were conducted in Part B. | Safety and Tolerability | Safety and Tolerability Safe and well tolerated Most AEs were mild or moderate AE rates comparable between CT1812 and placebo groups Pharmacokinetics (PK) Elderly subjects (≥65 yrs) showed ~1.5× higher exposure vs. younger adults CSF concentrations increased dose-dependently, confirming BBB penetration, with levels corresponding to >80% receptor occupancy Cognitive Testing (Elderly Cohort) No treatment-related cognitive decline was observed in elderly subjects receiving CT1812 |
| NCT02907567 (COG0102) | A randomized, double-blind, placebo-controlled, parallel-group phase 1b/2a study evaluating the safety, tolerability, and PK of three once-daily doses of CT1812 (90 mg, 280 mg, and 560 mg) vs. placebo in adults with mild-to-moderate AD. A total of 19 participants (mean age 70.2) were randomized to receive either CT1812 or placebo for 28 days. | Safety and Tolerability | Target Engagement Dose-dependent increase in CSF CT1812 levels Significant rise in CSF Aβ oligomers vs. placebo, consistent with oligomer displacement No change in Aβ40 or Aβ42 monomers between groups or over time Synaptic Effects (CT1812 vs. Placebo) Reduced CSF Nrgn and Syt1 Proteomics revealed significantly altered synaptic proteins, including GSK3β and Wnt signalling components Tau Phosphorylation (CT1812 vs. Placebo) Reduced phosphorylation at multiple sites No change in unphosphorylated or total tau GSK3β levels trended lower, consistent with potential upstream modulation of tau kinases Disease Modification (CT1812 vs. Placebo) Partial normalization of AD-altered protein (involved in key AD-disrupted pathways) |
| NCT03716427 (COG0103) | A phase 1, single-centre, open-label, single-sequence drug–drug interaction study was conducted in 16 healthy adult volunteers (aged 18–55 years) to assess the effects of CT1812 on the PK of four CYP probe drugs (tolbutamide, midazolam, dextromethorphan, and omeprazole) administered before and after 6 consecutive daily oral doses of 560 mg CT1812 (steady state). | Area under the plasma-concentration time curve of CT1812 compared to the baseline values | Weak CYP2D6 (dextromethorphan) inhibition and CYP3A4 (midazolam) induction; no clinically relevant effects on CYP2C19 (omeprazole) or CYP2C9 (tolbutamide) |
| NCT03522129 (COG0104) | A multicenter, phase 1b, randomized, double-blind, placebo-controlled, parallel-group trial evaluating single-dose CT1812 (560 mg) vs. placebo in adults (50–80 years) with mild-to-moderate AD. Only 3 participants were enrolled due to recruitment challenges associated with invasive procedures. | Measuring the displacement of Aβ oligomers into CSF | Safety CT1812 was safe and well tolerated Target Engagement Significant exposure-dependent increases in CSF Aβ oligomer levels in CT1812-treated patients (vs. placebo), indicating oligomer displacement No significant change in Aβ40/Aβ42 monomers PK/PD Plasma and CSF CT1812 levels were measured and positively correlated with Aβ oligomer increases |
| NCT03493282 (SPARC; COG0105) | A phase 1/2 randomized, double-blind, placebo-controlled trial evaluating safety, PK, and PD of oral CT1812 (100/300 mg daily) in 23 mild-to-moderate AD patients (aged 50–85) over 24 weeks (+optional 24-week extension). Assessments included clinical measures, CSF biomarkers, and neuroimaging (SV2A PET, FDG-PET, volumetric MRI). | Safety and Tolerability | Safety and Tolerability CT1812 was safe and well tolerated Most AEs were mild, with headache being the most common Neuroimaging (CT1812 vs. Placebo) SV2A PET: No significant differences FDG-PET: No significant differences Volumetric MRI: Trend toward tissue preservation overall; nominally significant preservation observed in pericentral, prefrontal, and hippocampal cortices Cognitive and Clinical Outcomes (CT1812 vs. Placebo) No significant differences in ADAS-Cog11, MMSE, and CDR-SB ADCS-ADL: 300 mg group showed decline; pooled CT1812 groups (100 mg + 300 mg) showed a trend toward decline CSF Biomarkers (CT1812 vs. Placebo) No significant dose- or treatment-related changes in Aβ40, Aβ42, total tau, p-tau, Nrgn, or Syt |
| NCT03507790 (SHINE; COG0201) | An international, multicenter, randomized, double-blind, placebo-controlled phase 2 study in adults aged 50–85 years with mild-to-moderate AD. Approximately 144 participants were randomized 1:1:1 to receive oral CT1812 (100 mg or 300 mg) or placebo once daily for 6 months. | Safety and Tolerability | Results from an exploratory interim analysis (n = 24) Safety Safe and well tolerated; no serious AEs (SAEs) attributed to CT1812 Cognitive Function (CT1812 vs. Placebo) ADAS-Cog11: 3-point improvement in CT1812 group vs. placebo (clinically meaningful but not statistically significant) CSF Biomarkers (CT1812 vs. Placebo) CT1812 significantly reduced Aβ42 and Aβ40 Partial normalization of AD-associated protein dysregulation Mechanistic Insights Identified pharmacodynamic biomarkers altered by CT1812, confirming target engagement in synaptic, amyloid, and inflammatory pathways |
| NCT04735536 (SEQUEL; COG0202) | A phase 2, single-site, randomized, double-blind, placebo-controlled crossover trial evaluating CT1812 (300 mg) vs. placebo in 16 adults aged 50–85 with mild-to-moderate AD. The 29-day study comprised two 4-week treatment periods separated by a 2-week washout, with participants crossing over between CT1812 and placebo. | Safety and Tolerability Change in brain activity measured by global relative theta power via EEG assessments | EEG Biomarkers (CT1812 vs. Placebo) Global relative theta power: non-significant reduction; consistent trend in frontal, temporal, posterior, and central regions; significant decrease in the central region Safety/Tolerability Safe and well tolerated; only mild-to-moderate treatment-emergent AEs Cognitive and Functional Assessments (CT1812 vs. Placebo) No significant differences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinfield, S.R.; Stenn, D.F.; Chen, H.; Kalisch, B.E. A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease. Pharmaceuticals 2025, 18, 659. https://doi.org/10.3390/ph18050659
Steinfield SR, Stenn DF, Chen H, Kalisch BE. A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease. Pharmaceuticals. 2025; 18(5):659. https://doi.org/10.3390/ph18050659
Chicago/Turabian StyleSteinfield, Sara R., Daniel F. Stenn, Helen Chen, and Bettina E. Kalisch. 2025. "A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease" Pharmaceuticals 18, no. 5: 659. https://doi.org/10.3390/ph18050659
APA StyleSteinfield, S. R., Stenn, D. F., Chen, H., & Kalisch, B. E. (2025). A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease. Pharmaceuticals, 18(5), 659. https://doi.org/10.3390/ph18050659






