5-Fluorouracil Toxicity: Revisiting the Relevance of Pharmacokinetic Parameters
Abstract
1. Introduction
2. Results
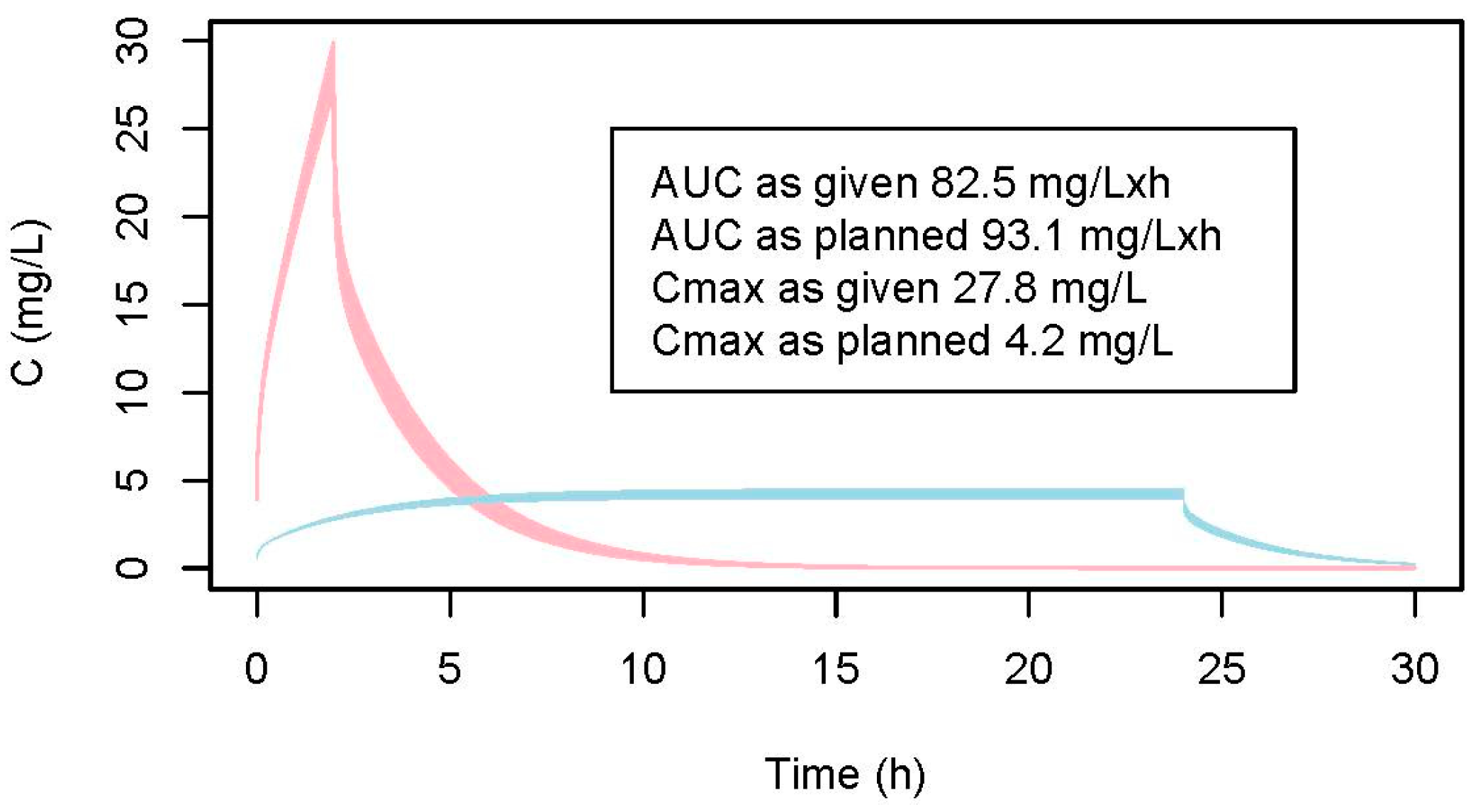
2.1. Model Evaluation
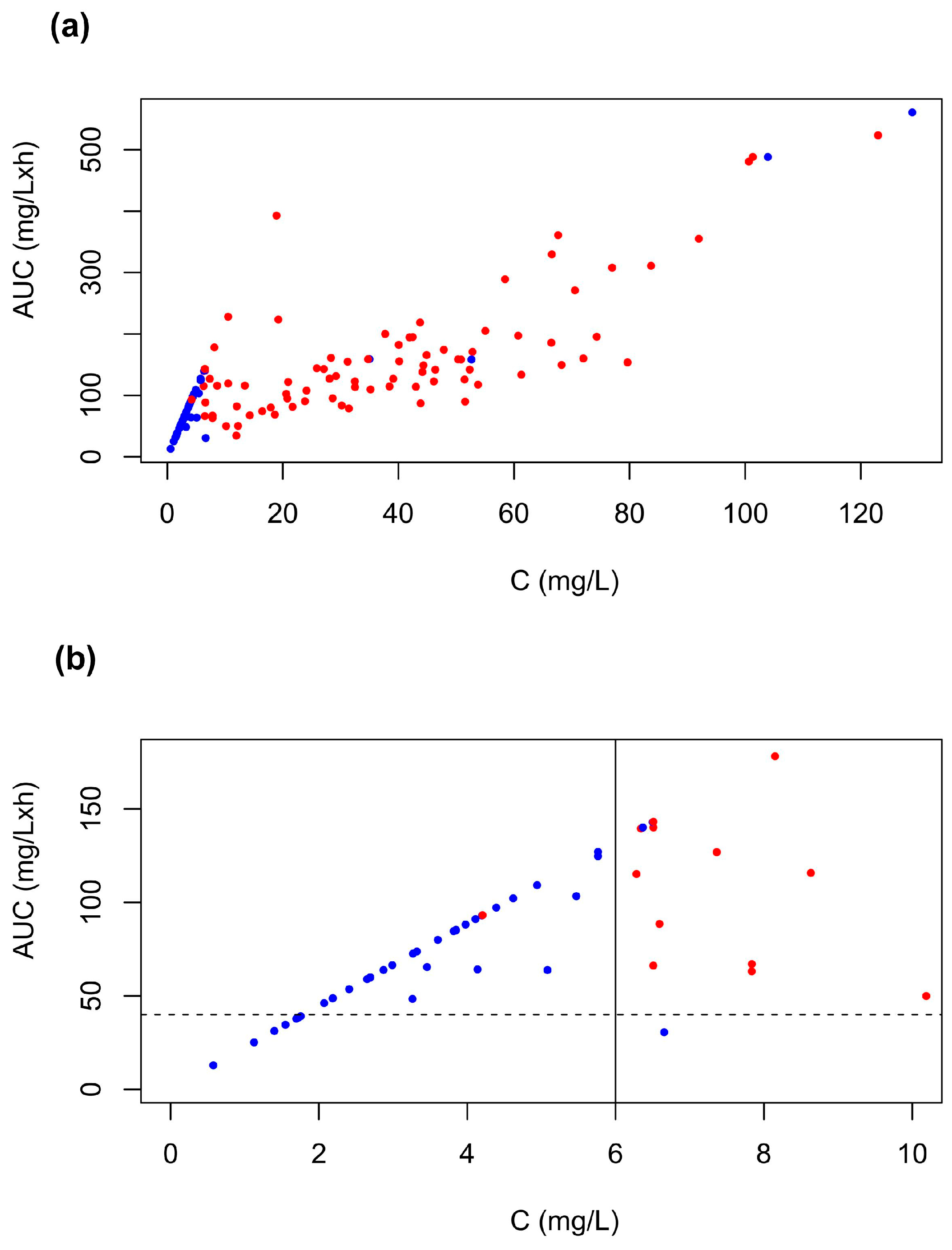
2.2. Modeled Css and AUC
2.2.1. Data of the Patient
2.2.2. Data from the Literature
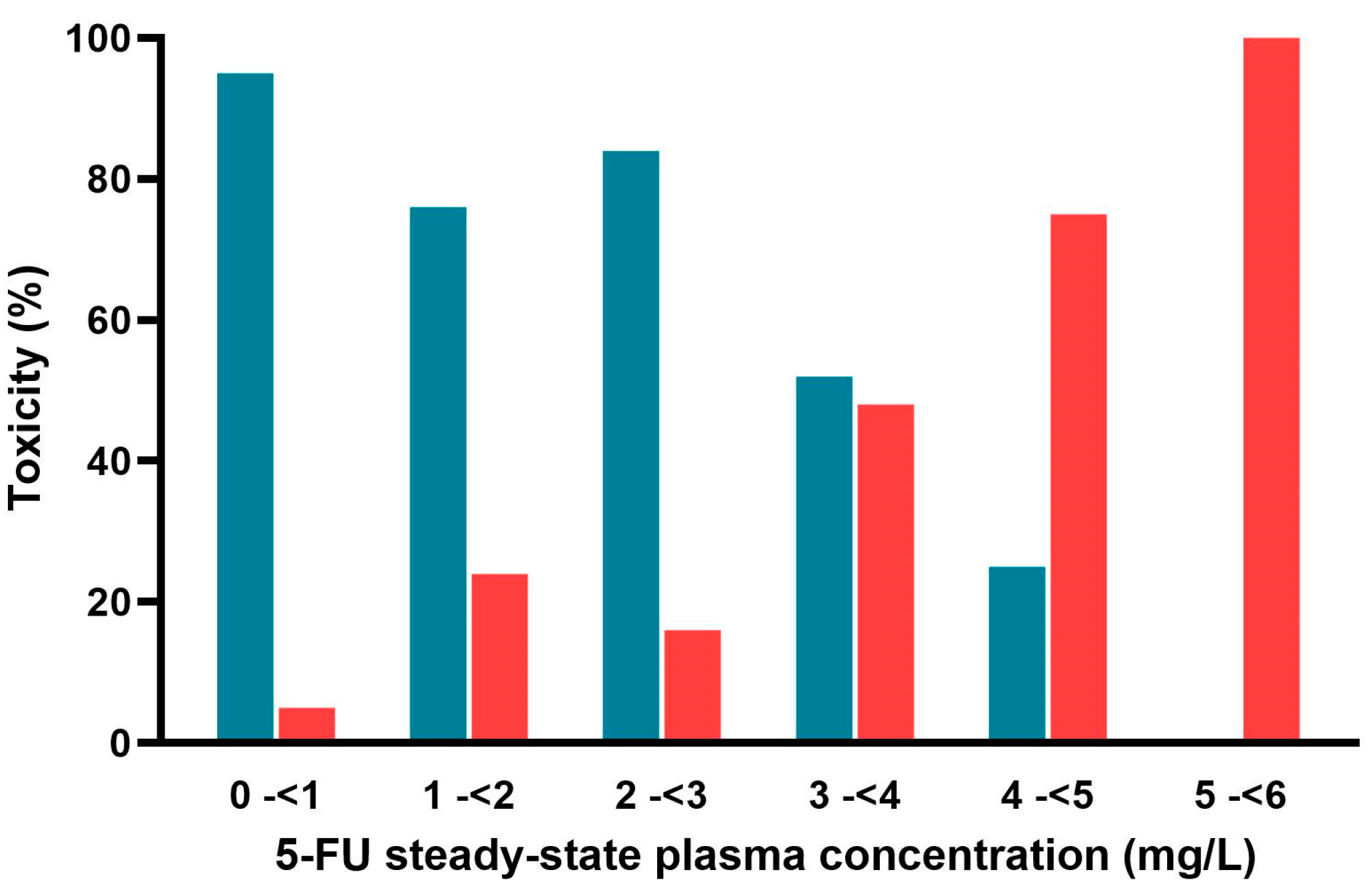
2.3. Reported AUCs and Estimated Css
3. Discussion
4. Materials and Methods
4.1. Data Search
4.2. Model-Independent Estimations of CSS from Data on Dosing, Corresponding Exposure and Toxicity Outcomes
4.3. Model-Dependent Estimations of CSS and Auc from Data on Dosing and Corresponding Toxicity Outcomes
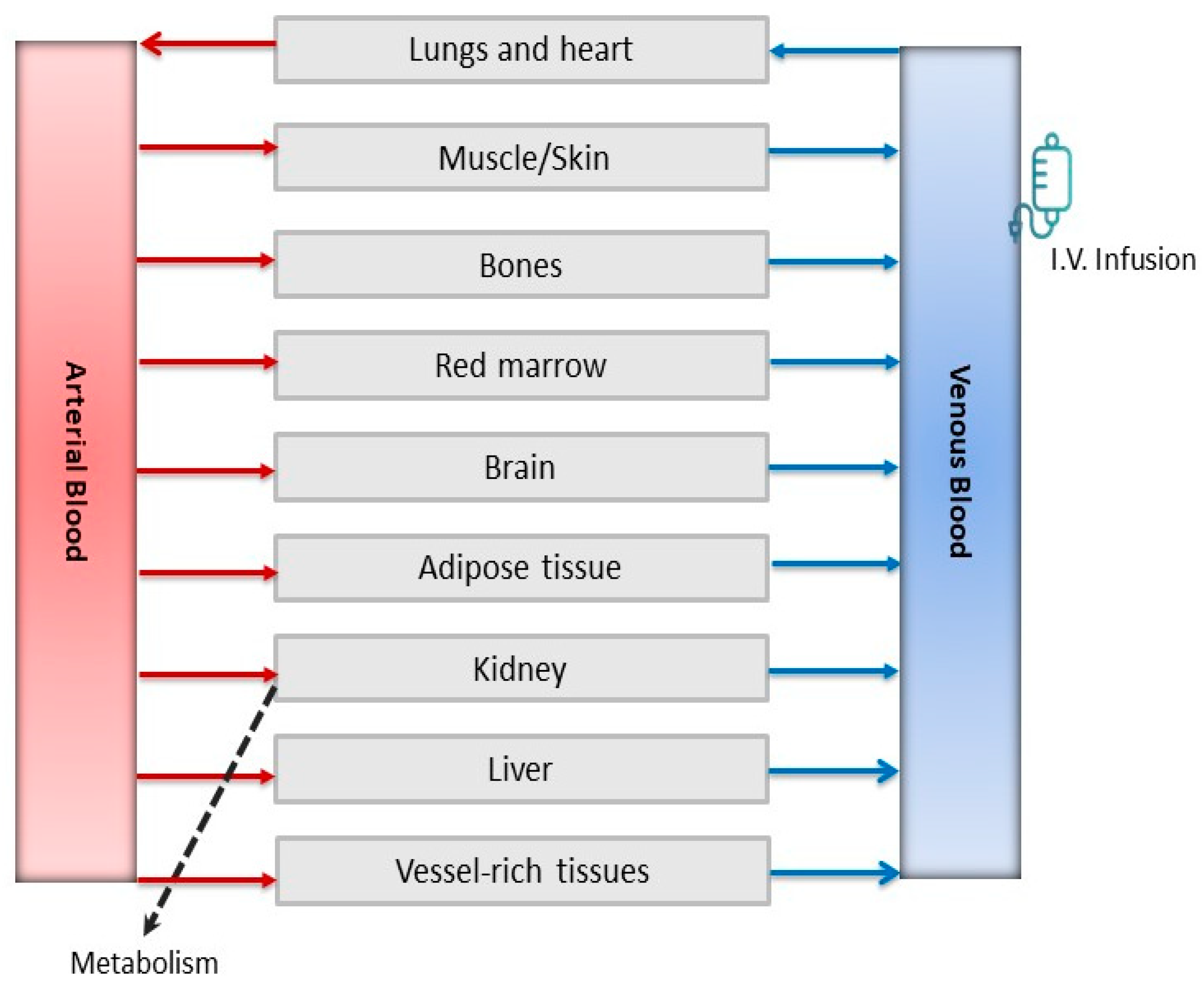
4.3.1. Model Development and Evaluation
4.3.2. Model-Dependent Estimations
4.4. Evaluation of the Relationship Between Toxicity, Css and AUC
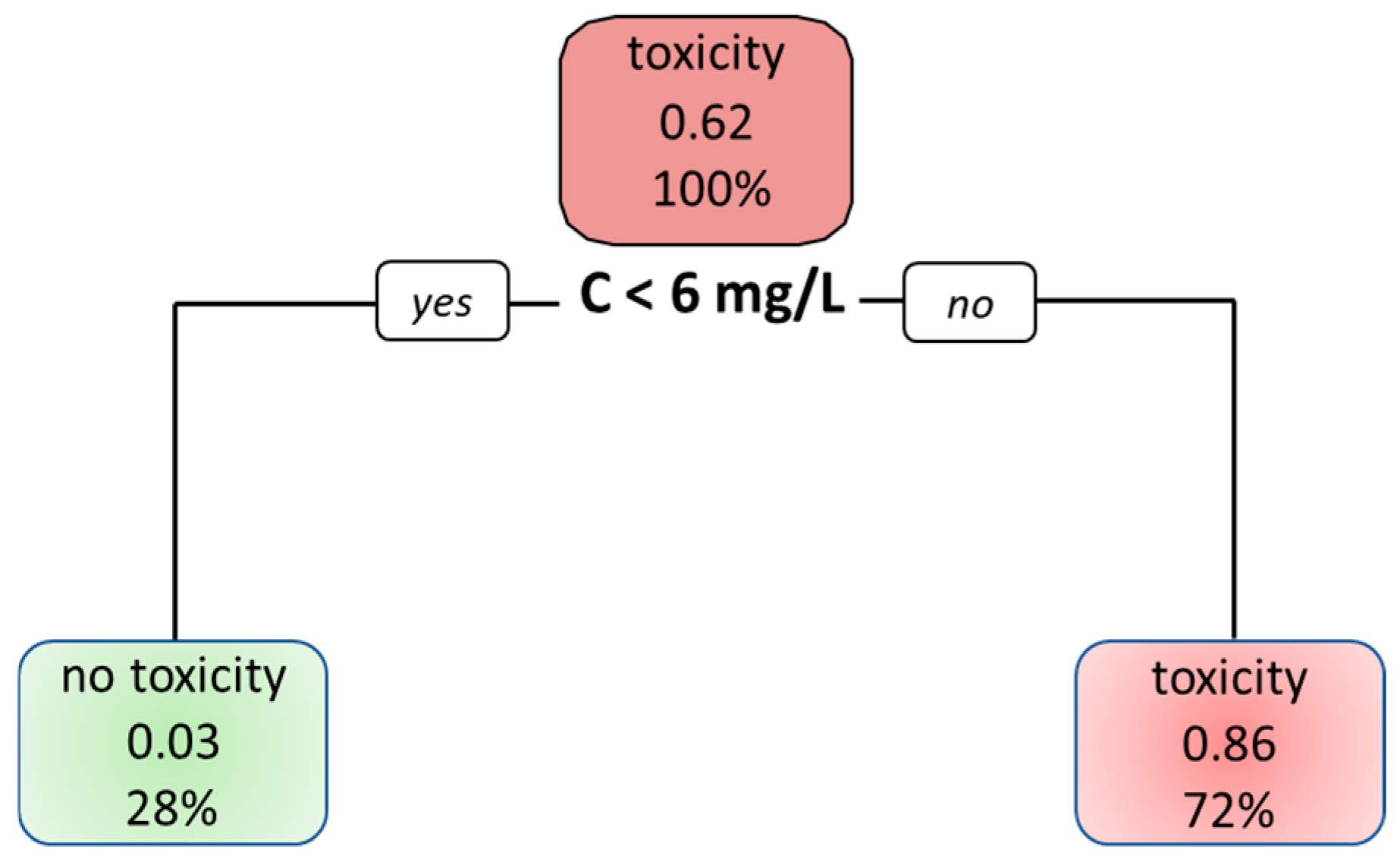
4.5. Decision Tree
| Physiological Data | ||
|---|---|---|
| Cardiac output (Qc) (L/h) [47] | 401.7 | |
| Body weight (bw) (kg) | 73 | |
| Blood flow through the organs (L/h) [47] | Organ volumes (L) [47] | |
| Fat | 19.5 | 18.2 |
| Liver | 99.5 | 1.8 |
| Brain | 46.8 | 1.45 |
| Kidney | 74.1 | 0.31 |
| Muscle | 65.8 | 0.40 × bw |
| Skin | 20 | 0.0371 × bw |
| Vessel-rich tissue (excluding red marrow) | 44.8 | 2.6 |
| Skeleton | 7.8 | 6.9 |
| Red marrow | 11.7 | 1.2 |
| Substance-Specific Data | ||
| Log Ko/w [49] | −0.89 | |
| pKa [49] | 8.02 | |
| Protein binding (%) [49] | 8–12 | |
| Partition coefficient 1 | ||
| Fat/blood | 0.20 | |
| Liver/blood | 0.92 | |
| Brain/blood | 1.35 | |
| Kidney/blood | 0.98 | |
| Muscle | 2.19 | |
| Skin | 10.04 | |
| Vessel-rich tissue (excluding red marrow) | 0.98 | |
| Skeleton | 0.63 | |
| Red marrow | 0.92 | |
| Metabolic constants 2 | ||
| Vmax (mg/h) | 1221.7 | |
| Km (mg/L) | 11.7 | |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| AUC | Area under the plasma concentration–time curve |
| PBPK | Physiologically-based pharmacokinetic modeling |
| TS | Thymidylate synthase |
| DPD | Dihydropyrimidine dehydrogenase |
| TDM | Therapeutic drug monitoring |
| IATDMCT | International Association of Therapeutic Drug Monitoring and Clinical Toxicology |
| PK | Pharmacokinetic |
| Css | Steady-state concentration |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
References
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Sobrero, A.; Guglielmi, A.; Grossi, F.; Puglisi, F.; Aschele, C. Mechanism of action of fluoropyrimidines: Relevance to the new developments in colorectal cancer chemotherapy. Semin. Oncol. 2000, 27 (Suppl. 10), 72–77. [Google Scholar] [PubMed]
- White, C.; Kendall, G.; Millington, T.; Corcoran, B.; Paul, C.; Scott, R.J.; Ackland, S. Evaluation of early fluoropyrimidine toxicity in solid organ cancer patients: A retrospective observational study in Australia. Intern. Med. J. 2024, 54, 1506–1514. [Google Scholar] [CrossRef]
- Mueller, F.; Büchel, B.; Köberle, D.; Schürch, S.; Pfister, B.; Krähenbühl, S.; Froehlich, T.K.; Largiader, C.R.; Joerger, M. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: Results from a prospective population pharmacokinetic study. Cancer Chemother. Pharmacol. 2013, 71, 361–370. [Google Scholar] [CrossRef]
- Hodroj, K.; Barthelemy, D.; Lega, J.-C.; Grenet, G.; Gagnieu, M.-C.; Walter, T.; Guitton, J.; Payen-Gay, L. Issues and limitations of available biomarkers for fluoropyrimidine-based chemotherapy toxicity, a narrative review of the literature. ESMO Open 2021, 6, 100125. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Chu, E.; Allegra, C.; Tanigawara, Y.; Milano, G.; Diasio, R.; Kim, T.W.; Mathijssen, R.H.; Zhang, L.; Arnold, D.; et al. Therapeutic Drug Monitoring in Oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology Recommendations for 5-Fluorouracil Therapy. Clin. Pharmacol. Ther. 2019, 105, 598–613. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency (EMA). Available online: https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-and-flucytosine (accessed on 23 October 2024).
- European Medicine Agency (EMA). Available online: https://www.ema.europa.eu/en/documents/scientific-conclusion/teysuno-h-c-1242-a31-0040-epar-scientific-conclusions_en.pdf (accessed on 1 December 2024).
- Fluorouracil Injection 25 mg/mL. Summary of Product Characteristics. Available online: https://www.medac.eu/fileadmin/user_upload/medac-eu/SPCs/United_Kingdom/Fluorouracil_injection_25_mgml-spc-gb.pdf (accessed on 1 January 2025).
- Lemaitre, F.; Goirand, F.; Launay, M.; Chatelut, E.; Boyer, J.C.; Evrard, A.; Paludetto, M.N.; Guilhaumou, R.; Ciccolini, J.; Schmitt, A. 5-fluorouracil therapeutic drug monitoring: Update and recommendations of the STP-PT group of the SFPT and the GPCO-Unicancer. Bull. Cancer 2018, 105, 790–803. [Google Scholar] [CrossRef]
- Denda, T.; Kanda, M.; Morita, Y.; Kim, H.M.; Kashiwada, T.; Matsuda, C.; Fujieda, S.; Nakata, K.; Murotani, K.; Oba, K.; et al. Pharmacokinetic dose adjustment of 5-FU in modified FOLFOX7 plus bevacizumab for metastatic colorectal cancer in Japanese patients: A-JUST phase II clinical trial. Cancer Chemother. Pharmacol. 2016, 78, 1253–1261. [Google Scholar] [CrossRef]
- Gamelin, E.C.; Danquechin-Dorval, E.M.; Dumesnil, Y.F.; Maillart, P.J.; Goudier, M.J.; Burtin, P.C.; Delva, R.G.; Lortholary, A.H.; Gesta, P.H.; Larra, F.G. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 1996, 77, 441–451. [Google Scholar] [CrossRef]
- Kaldate, R.R.; Haregewoin, A.; Grier, C.E.; Hamilton, S.A.; McLeod, H.L. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist 2012, 17, 296–302. [Google Scholar] [CrossRef]
- Wilhelm, M.; Mueller, L.; Miller, M.C.; Link, K.; Holdenrieder, S.; Bertsch, T.; Kunzmann, V.; Stoetzer, O.J.; Suttmann, I.; Braess, J.; et al. Prospective, Multicenter Study of 5-Fluorouracil Therapeutic Drug Monitoring in Metastatic Colorectal Cancer Treated in Routine Clinical Practice. Clin. Color. Cancer 2016, 15, 381–388. [Google Scholar] [CrossRef]
- Schmulenson, E.; Zimmermann, N.; Mikus, G.; Joerger, M.; Jaehde, U. Current status and future outlooks on therapeutic drug monitoring of fluorouracil. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1407–1422. [Google Scholar] [CrossRef]
- Au, J.L.; Rustum, Y.M.; Ledesma, E.J.; Mittelman, A.; Creaven, P.J. Clinical pharmacological studies of concurrent infusion of 5-fluorouracil and thymidine in treatment of colorectal carcinomas. Cancer Res. 1982, 42, 2930–2937. [Google Scholar]
- Thyss, A.; Milano, G.; Renée, N.; Vallicioni, J.; Schneider, M.; Demard, F. Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother. Pharmacol. 1986, 16, 64–66. [Google Scholar] [CrossRef]
- Trump, D.L.; Egorin, M.J.; Forrest, A.; Willson, J.K.; Remick, S.; Tutsch, K.D. Pharmacokinetic and pharmacodynamic analysis of fluorouracil during 72-hour continuous infusion with and without dipyridamole. J. Clin. Oncol. 1991, 9, 2027–2035. [Google Scholar] [CrossRef]
- van Groeningen, C.J.; Pinedo, H.M.; Heddes, J.; Kok, R.M.; de Jong, A.P.; Wattel, E.; Peters, G.J.; Lankelma, J. Pharmacokinetics of 5-fluorouracil assessed with a sensitive mass spectrometric method in patients on a dose escalation schedule. Cancer Res. 1988, 48, 6956–6961. [Google Scholar]
- Ychou, M.; Duffour, J.; Kramar, A.; Debrigode, C.; Gourgou, S.; Bressolle, F.; Pinguet, F. Individual 5-FU dose adaptation in metastatic colorectal cancer: Results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother. Pharmacol. 2003, 52, 282–290. [Google Scholar] [CrossRef]
- Yoshida, T.; Araki, E.; Iigo, M.; Fujii, T.; Yoshino, M.; Shimada, Y.; Saito, D.; Tajiri, H.; Yamaguchi, H.; Yoshida, S.; et al. Clinical significance of monitoring serum levels of 5-fluorouracil by continuous infusion in patients with advanced colonic cancer. Cancer Chemother. Pharmacol. 1990, 26, 352–354. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yoshida, Y.; Yamada, T.; Aisu, N.; Yoshimatsu, G.; Yoshimura, F.; Hasegawa, S. Current Status of Therapeutic Drug Monitoring of 5-Fluorouracil Prodrugs. Anticancer Res. 2020, 40, 4655–4661. [Google Scholar] [CrossRef]
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharmacol. 2016, 78, 447–464. [Google Scholar] [CrossRef]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, E.; Boisdron-Celle, M.; Delva, R.; Regimbeau, C.; Cailleux, P.E.; Alleaume, C.; Maillet, M.L.; Goudier, M.J.; Sire, M.; Person-Joly, M.C.; et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: Results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J. Clin. Oncol. 1998, 16, 1470–1478. [Google Scholar] [CrossRef]
- Grem, J.L.; McAtee, N.; Balis, F.; Murphy, R.; Venzon, D.; Kramer, B.; Goldspiel, B.; Begley, M.; Allegra, C.J. A phase II study of continuous infusion 5-fluorouracil and leucovorin with weekly cisplatin in metastatic colorectal carcinoma. Cancer 1993, 72, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Arshad, U.; Ploylearmsaeng, S.-A.; Karlsson, M.O.; Doroshyenko, O.; Langer, D.; Schömig, E.; Kunze, S.; Güner, S.A.; Skripnichenko, R.; Ullah, S.; et al. Prediction of exposure-driven myelotoxicity of continuous infusion 5-fluorouracil by a semi-physiological pharmacokinetic-pharmacodynamic model in gastrointestinal cancer patients. Cancer Chemother. Pharmacol. 2020, 85, 711–722. [Google Scholar] [CrossRef]
- Finch, R.E.; Bending, M.R.; Lant, A.F. Plasma levels of 5-fluorouracil after oral and intravenous administration in cancer patients. Br. J. Clin. Pharmacol. 1979, 7, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Etienne, M.C.; Lagrange, J.L.; Dassonville, O.; Fleming, R.; Thyss, A.; Renée, N.; Schneider, M.; Demard, F.; Milano, G. Population study of dihydropyrimidine dehydrogenase in cancer patients. J. Clin. Oncol. 1994, 12, 2248–2253. [Google Scholar] [CrossRef]
- Santini, J.; Milano, G.; Thyss, A.; Renee, N.; Viens, P.; Ayela, P.; Schneider, M.; Demard, F. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br. J. Cancer 1989, 59, 287–290. [Google Scholar] [CrossRef]
- Ploylearmsaeng, S.-A.; Fuhr, U.; Jetter, A. How may anticancer chemotherapy with fluorouracil be individualised? Clin. Pharmacokinet. 2006, 45, 567–592. [Google Scholar] [CrossRef]
- Yang, Q.; Bi, Y.; Li, X.; Liu, Q.; Ma, J.; Zhang, C.; Zhang, J.; He, G. A retrospective analysis of plasma concentration monitoring of fluorouracil in patients with advanced colorectal cancer. Eur. J. Hosp. Pharm. 2020, 27, e36–e40. [Google Scholar] [CrossRef]
- Chao, C.-J.; Gardner, I.; Lin, C.-J.; Yeh, K.-H.; Lu, W.-C.; Abduljalil, K.; Ho, Y.-F. Administration mode matters for 5-fluorouracil therapy: Physiologically based pharmacokinetic evidence for avoidance of myelotoxicity by continuous infusion but not intravenous bolus. Br. J. Clin. Pharmacol. 2025, 91, 1031–1040. [Google Scholar] [CrossRef]
- Marok, F.Z.; Wojtyniak, J.-G.; Selzer, D.; Dallmann, R.; Swen, J.J.; Guchelaar, H.-J.; Schwab, M.; Lehr, T. Personalized Chronomodulated 5-Fluorouracil Treatment: A Physiologically-Based Pharmacokinetic Precision Dosing Approach for Optimizing Cancer Therapy. Clin. Pharmacol. Ther. 2024, 115, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, H.; Yu, L.; Zeng, S. Physiologically Based Pharmacokinetic Modeling for Prediction of 5-FU Pharmacokinetics in Cancer Patients with Hepatic Impairment After 5-FU and Capecitabine Administration. Pharm. Res. 2023, 40, 2177–2194. [Google Scholar] [CrossRef] [PubMed]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar]
- Li, M.; Mindt, S.; Lück, A.; Hutzschenreuter, U.; Kollendt, M.; Lathan, B.; Zöller, T.; Frank-Gleich, S.; Lorentz, C.; Lamberti, C.; et al. Drug monitoring detects under- and overdosing in patients receiving 5-fluorouracil-containing chemotherapy-results of a prospective, multicenter German observational study. ESMO Open 2023, 8, 101201. [Google Scholar] [CrossRef]
- Heggie, G.D.; Sommadossi, J.P.; Cross, D.S.; Huster, W.J.; Diasio, R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987, 47, 2203–2206. [Google Scholar] [PubMed]
- Breda, M.; Barattè, S. A review of analytical methods for the determination of 5-fluorouracil in biological matrices. Anal. Bioanal. Chem. 2010, 397, 1191–1201. [Google Scholar] [CrossRef]
- Büchel, B.; Sistonen, J.; Joerger, M.; Aebi, Y.; Schürch, S.; Largiadèr, C.R. Comparative evaluation of the My5-FU™ immunoassay and LC-MS/MS in monitoring the 5-fluorouracil plasma levels in cancer patients. Clin. Chem. Lab. Med. 2013, 51, 1681–1688. [Google Scholar] [CrossRef][Green Version]
- Schmitt, W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol. Vitr. 2008, 22, 457–467. [Google Scholar] [CrossRef]
- Bessems, J.G.; Loizou, G.; Krishnan, K.; Clewell, H.J., 3rd; Bernasconi, C.; Bois, F.; Coecke, S.; Collnot, E.M.; Diembeck, W.; Farcal, L.R.; et al. PBTK modelling platforms and parameter estimation tools to enable animal-free risk assessment: Recommendations from a joint EPAA--EURL ECVAM ADME workshop. Regul. Toxicol. Pharmacol. 2014, 68, 119–139. [Google Scholar] [CrossRef]
- Deyme, L.; Barbolosi, D.; Gattacceca, F. Population pharmacokinetics of FOLFIRINOX: A review of studies and parameters. Cancer Chemother. Pharmacol. 2019, 83, 27–42. [Google Scholar] [CrossRef]
- Terret, C.; Erdociain, E.; Guimbaud, R.; Boisdron-Celle, M.; McLeod, H.L.; Féty-Deporte, R.; Lafont, T.; Gamelin, E.; Bugat, R.; Canal, P.; et al. Dose and time dependencies of 5-fluorouracil pharmacokinetics. Clin. Pharmacol. Ther. 2000, 68, 270–279. [Google Scholar] [CrossRef] [PubMed]
- van Kuilenburg, A.B.; Häusler, P.; Schalhorn, A.; Tanck, M.W.; Proost, J.H.; Terborg, C.; Behnke, D.; Schwabe, W.; Jabschinsky, K.; Maring, J.G. Evaluation of 5-fluorouracil pharmacokinetics in cancer patients with a c.1905+1G>A mutation in DPYD by means of a Bayesian limited sampling strategy. Clin. Pharmacokinet. 2012, 51, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Woloch, C.; Di Paolo, A.; Marouani, H.; Bocci, G.; Ciccolini, J.; Lacarelle, B.; Danesi, R.; Iliadis, A. Population pharmacokinetic analysis of 5-FU and 5-FDHU in colorectal cancer patients: Search for biomarkers associated with gastro-intestinal toxicity. Curr. Top. Med. Chem. 2012, 12, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Basic anatomical and physiological data for use in radiological protection: Reference values: ICRP Publication 89. Ann. ICRP 2002, 32, 1–277. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1.23. Available online: https://CRAN.R-project.org/package=rpart (accessed on 1 January 2023).
- Fluorouracil. PubChem, National Centre for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluorouracil#section=Computed-Properties (accessed on 1 November 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielke, H.; Algharably, E.A.E.; Gundert-Remy, U. 5-Fluorouracil Toxicity: Revisiting the Relevance of Pharmacokinetic Parameters. Pharmaceuticals 2025, 18, 653. https://doi.org/10.3390/ph18050653
Mielke H, Algharably EAE, Gundert-Remy U. 5-Fluorouracil Toxicity: Revisiting the Relevance of Pharmacokinetic Parameters. Pharmaceuticals. 2025; 18(5):653. https://doi.org/10.3390/ph18050653
Chicago/Turabian StyleMielke, Hans, Engi Abd Elhady Algharably, and Ursula Gundert-Remy. 2025. "5-Fluorouracil Toxicity: Revisiting the Relevance of Pharmacokinetic Parameters" Pharmaceuticals 18, no. 5: 653. https://doi.org/10.3390/ph18050653
APA StyleMielke, H., Algharably, E. A. E., & Gundert-Remy, U. (2025). 5-Fluorouracil Toxicity: Revisiting the Relevance of Pharmacokinetic Parameters. Pharmaceuticals, 18(5), 653. https://doi.org/10.3390/ph18050653








