Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Animals
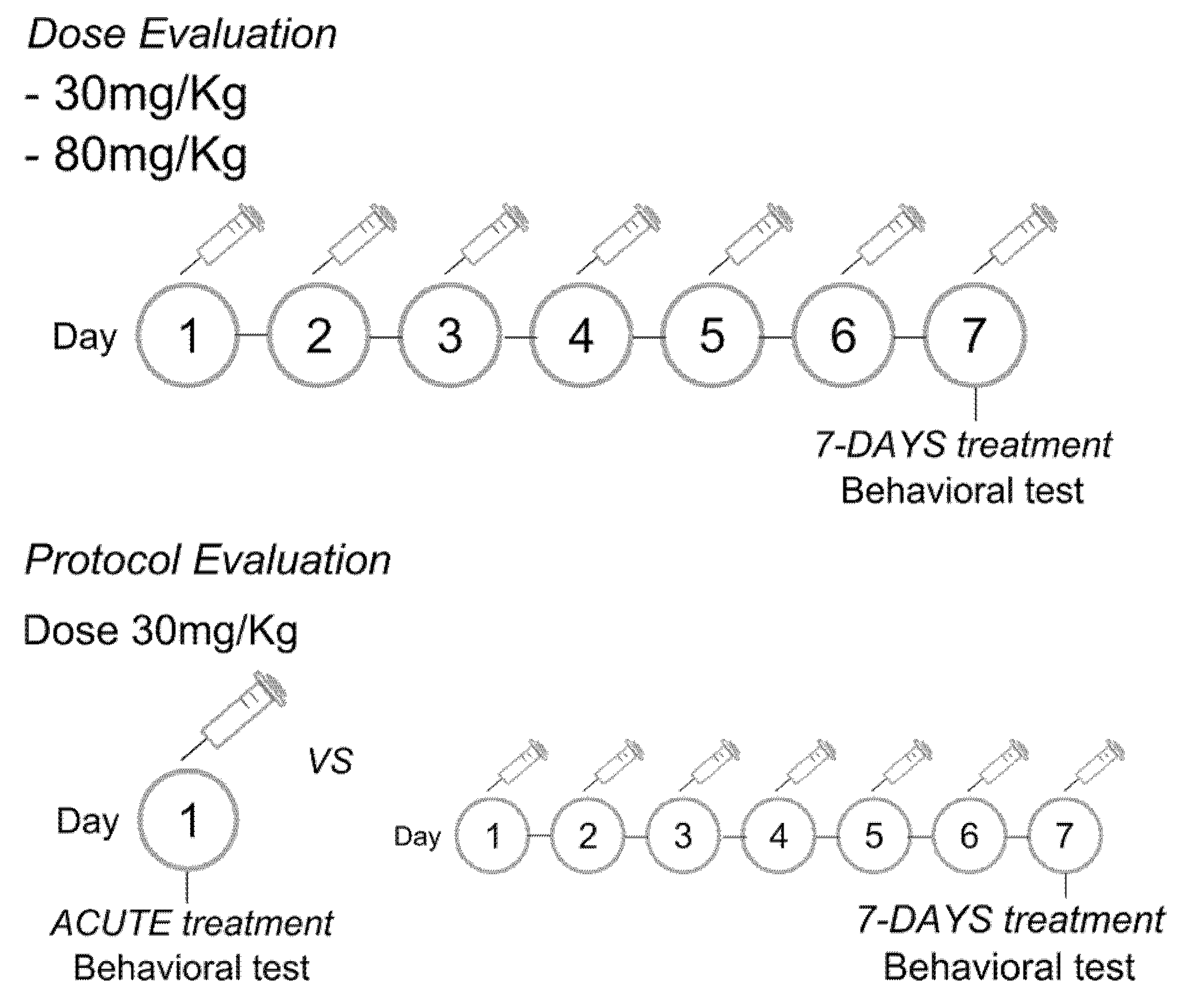
2.3. Experimental Protocol
2.4. Behavioral Test
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of Lavandula burnatii Essential Oil
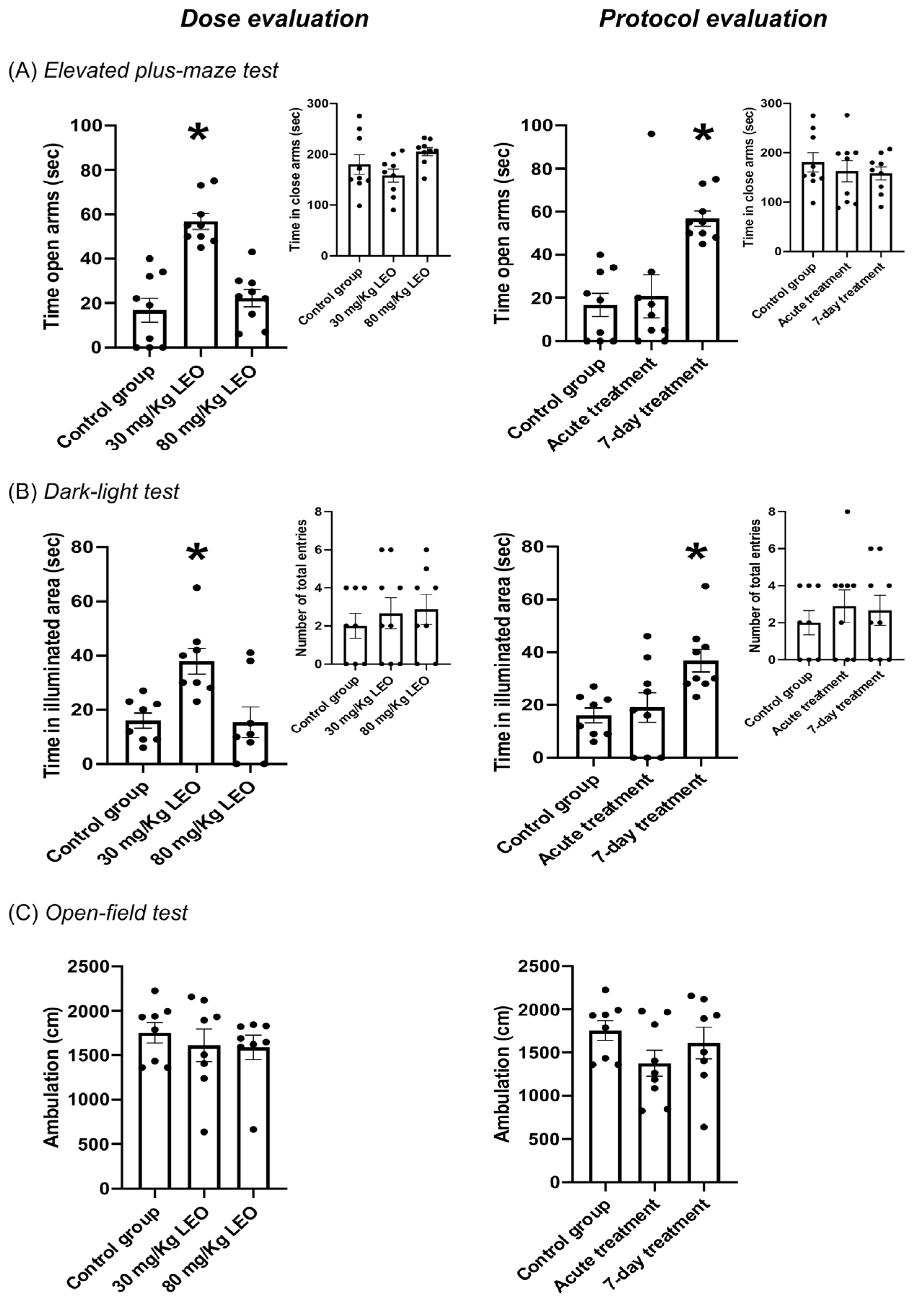
3.2. Effect of Lavandula burnatii Essential Oil on Elevated Plus Maze Test
3.3. Effect of Lavandula burnatii Essential Oil on Dark–Light Test
3.4. Effect of Lavandula burnatii Essential Oil in Open-Field Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Lépine, J.-P.; ESEMeD/MHEDEA 2000 Scientific Committee. Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD). J. Clin. Psychiatry 2007, 68, 3–9. [Google Scholar]
- Kessler, R.C. The global burden of anxiety and mood disorders: Putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. J. Clin. Psychiatry 2007, 68, 10–19. [Google Scholar]
- Scott, K.; Bruffaerts, R.; Tsang, A.; Ormel, J.; Alonso, J.; Angermeyer, M.; Benjet, C.; Bromet, E.; de Girolamo, G.; de Graaf, R.; et al. Depression–anxiety relationships with chronic physical conditions: Results from the World Mental Health surveys. J. Affect. Disord. 2007, 103, 113–120. [Google Scholar] [CrossRef]
- Mathew, S.J.; Price, R.B.; Charney, D.S. Recent advances in the neurobiology of anxiety disorders: Implications for novel therapeutics. Am. J. Med Genet. Part C Semin. Med. Genet. 2008, 148C, 89–98. [Google Scholar] [CrossRef]
- McEvoy, P.M.; Grove, R.; Slade, T. Epidemiology of Anxiety Disorders in the Australian General Population: Findings of the 2007 Australian National Survey of Mental Health and Wellbeing. Aust. N. Z. J. Psychiatry 2011, 45, 957–967. [Google Scholar] [CrossRef]
- Kessler, R.C.; Ruscio, A.M.; Shear, K.; Wittchen, H.-U. Epidemiology of anxiety disorders. Curr. Top. Behav. Neurosci. 2010, 2, 21–35. [Google Scholar]
- Stein, D.J.; Scott, K.M.; de Jonge, P.; Kessler, R.C. Epidemiology of anxiety disorders: From surveys to nosology and back. Dialog. Clin. Neurosci. 2017, 19, 127–136. [Google Scholar] [CrossRef]
- Toft, T.; Fink, P.; Oernboel, E.; Christensen, K.; Frostholm, L.; Olesen, F. Mental disorders in primary care: Prevalence and co-morbidity among disorders. Results from the Functional Illness in Primary care (FIP) study. Psychol. Med. 2005, 35, 1175–1184. [Google Scholar] [CrossRef]
- Nash, J.R.; Nutt, D.J. Pharmacotherapy of Anxiety. In Anxiety and Anxiolytic Drugs; Springer: Berlin/Heidelberg, Germany, 2005; pp. 469–501. [Google Scholar] [CrossRef]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N. Essential Oils and Anxiolytic Aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, D. Medicinal plants for insomnia: A review of their pharmacology, efficacy and tolerability. J. Psychopharmacol. 2005, 19, 414–421. [Google Scholar] [CrossRef]
- Brennan, S.E.; McDonald, S.; Murano, M.; McKenzie, J.E. Effectiveness of aromatherapy for prevention or treatment of disease, medical or preclinical conditions, and injury: Protocol for a systematic review and meta-analysis. Syst. Rev. 2022, 11, 148. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Community Herbal Monograph on Lavandula angustifolia Mill., Aetheroleum; Committee on Herbal Medicinal Products. EMA/HMPC/143181/2010; European Medicines Agency: Amsterdam, The Netherlands, 2012.
- Bagheri-Nesami, M.; Espahbodi, F.; Nikkhah, A.; Shorofi, S.A.; Charati, J.Y. The effects of lavender aromatherapy on pain following needle insertion into a fistula in hemodialysis patients. Complement. Ther. Clin. Pract. 2014, 20, 1–4. [Google Scholar] [CrossRef]
- Antar, A.; Abdel-Rehiem, E.S.; Al-Khalaf, A.A.; Abuelsaad, A.S.A.; Abdel-Gabbar, M.; Shehab, G.M.G.; Abdel-Aziz, A.M. Therapeutic Efficacy of Lavandula dentata’s Oil and Ethanol Extract in Regulation of the Neuroinflammation, Histopathological Alterations, Oxidative Stress, and Restoring Balance Treg Cells Expressing FoxP3+ in a Rat Model of Epilepsy. Pharmaceuticals 2024, 18, 35. [Google Scholar] [CrossRef]
- Kumar, V. Characterization of anxiolytic and neuropharmacological activities of Silexan. Wien. Med. Wochenschr. 2013, 163, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Li, Y.; Tang, P.; Zhang, Y.; Cao, M.; Ni, J.; Xing, M. Effectiveness of Aromatherapy Massage and Inhalation on Symptoms of Depression in Chinese Community-Dwelling Older Adults. J. Altern. Complement. Med. 2018, 24, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Vidal-García, E.; Vallhonrat-Bueno, M.; Pla-Consuegra, F.; Orta-Ramírez, A. Efficacy of Lavender Essential Oil in Reducing Stress, Insomnia, and Anxiety in Pregnant Women: A Systematic Review. Healthcare 2024, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Prasad, N.; Beautily, V.; Thenmozhi, P.; Madaswamy, R.; Deepika, D. Effect of Lavender Oil on Social Anxiety Among First-Year College Students. J. Pharm. Bioallied Sci. 2024, 16, S2907–S2909. [Google Scholar] [CrossRef]
- Marchevsky, S. Real-world outcomes of long-term use of silexan in patients with anxiety disorders: A single-centre experience. Int. J. Psychiatry Clin. Pract. 2024, 28, 138–141. [Google Scholar] [CrossRef]
- Faustino, T.T.; de Almeida, R.B.; Andreatini, R. Plantas medicinais no tratamento do transtorno de ansiedade generalizada: Uma revisão dos estudos clínicos controlados. Rev. Bras. Psiquiatr. 2010, 32, 429–436. [Google Scholar] [CrossRef]
- Wagner, J.K.; Gambell, E.; Gibbons, T.; Martin, T.J.; Kaplan, J.S. Sex Differences in the Anxiolytic Properties of Common Cannabis Terpenes, Linalool and β-Myrcene, in Mice. NeuroSci 2024, 5, 635–649. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Toroudi, H.P. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef]
- File, S.E.; Lippa, A.S.; Beer, B.; Lippa, M.T. Animal Tests of Anxiety. Curr. Protoc. Neurosci. 2004, 26, 8.3.1–8.3.22. [Google Scholar] [CrossRef]
- SMART VIDEO TRACKING Software, SMART v3.0.03; Panlab Harvard Apparatus: Barcelona, Spain. Available online: https://www.panlab.com/en/products/smart-video-tracking-software-panlab (accessed on 24 January 2025).
- Ennaceur, A.; Chazot, P.L. Preclinical animal anxiety research—Flaws and prejudices. Pharmacol. Res. Perspect. 2016, 4, e00223. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, A.; Steckler, T. Pharmacology of Anxiety or Pharmacology of Elevated Plus Maze? Biol. Psychiatry 2021, 89, e73. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Wirz, R.; Loretan, A.V.; Sutter, N.A.; da Cunha, C.T.P.; Jaric, I.; Würbel, H.; Voelkl, B. Reliability of common mouse behavioural tests of anxiety: A systematic review and meta-analysis on the effects of anxiolytics. Neurosci. Biobehav. Rev. 2022, 143, 104928. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. What’s Wrong With My Mouse? Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Kumar, V.; Bhat, Z.A.; Kumar, D. Animal models of anxiety: A comprehensive review. J. Pharmacol. Toxicol. Methods 2013, 68, 175–183. [Google Scholar] [CrossRef]
- Harro, J. Animals, anxiety, and anxiety disorders: How to measure anxiety in rodents and why. Behav. Brain Res. 2018, 352, 81–93. [Google Scholar] [CrossRef]
- Haller, J.; Alicki, M. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr. Opin. Psychiatry 2012, 25, 59–64. [Google Scholar] [CrossRef]
- Montgomery, K.C.; Montgomery, K.C. The relation between fear induced by novel stimulation and exploratory drive. J. Comp. Physiol. Psychol. 1955, 48, 254–260. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Takahashi, M.; Yoshino, A.; Yamanaka, A.; Asanuma, C.; Satou, T.; Hayashi, S.; Masuo, Y.; Sadamoto, K.; Koike, K. Effects of Inhaled Lavender Essential Oil on Stress-Loaded Animals: Changes in Anxiety-Related Behavior and Expression Levels of Selected mRNAs and Proteins. Nat. Prod. Commun. 2012, 7, 1539–1544. [Google Scholar] [CrossRef]
- Shustorovich, A.; Corroon, J.; Wallace, M.S.; Sexton, M. Biphasic effects of cannabis and cannabinoid therapy on pain severity, anxiety, and sleep disturbance: A scoping review. Pain Med. 2024, 25, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, G.; Ito, M. Inhalation Administration of the Bicyclic Ethers 1,8- and 1,4-cineole Prevent Anxiety and Depressive-Like Behaviours in Mice. Molecules 2020, 25, 1884. [Google Scholar] [CrossRef] [PubMed]
- Hartley, N.; McLachlan, C.S. Aromas Influencing the GABAergic System. Molecules 2022, 27, 2414. [Google Scholar] [CrossRef]
- Ferreira, L.O.; de Souza, R.D.; Silva, F.d.A.; Costa, F.F.M.; Farias, R.A.F.; Hamoy, A.O.; de Mello, V.J.; Lopes, D.C.F.; Hamoy, M. Electrocorticographic patterns dominated by low-frequency waves in camphor-induced seizures. Sci. Rep. 2020, 10, 18222. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Elisabetsky, E.; Marschner, J.; Souza, D.O. Effects of linalool on glutamatergic system in the rat cerebral cortex. Neurochem. Res. 1995, 20, 461–465. [Google Scholar] [CrossRef]
- Elisabetsky, E.; Brum, L.S.; Souza, D. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 1999, 6, 107–113. [Google Scholar] [CrossRef]
- Brum, L.F.S.; Emanuelli, T.; Souza, D.O.; Elisabetsky, E. Effects of Linalool on Glutamate Release and Uptake in Mouse Cortical Synaptosomes. Neurochem. Res. 2001, 26, 191–194. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kashani, L.; Fotouhi, A.; Jarvandi, S.; Mobaseri, M.; Moin, M.; Khani, M.; Jamshidi, A.H.; Baghalian, K.; Taghizadeh, M. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Baldinger, P.; Höflich, A.S.; Mitterhauser, M.; Hahn, A.; Rami-Mark, C.; Spies, M.; Wadsak, W.; Lanzenberger, R.; Kasper, S. Effects of Silexan on the Serotonin-1A Receptor and Microstructure of the Human Brain: A Randomized, Placebo-Controlled, Double-Blind, Cross-Over Study with Molecular and Structural Neuroimaging. Int. J. Neuropsychopharmacol. 2014, 18, pyu063. [Google Scholar] [CrossRef] [PubMed]
- Schuwald, A.M.; Nöldner, M.; Wilmes, T.; Klugbauer, N.; Leuner, K.; Müller, W.E. Lavender Oil-Potent Anxiolytic Properties via Modulating Voltage Dependent Calcium Channels. PLoS ONE 2013, 8, e59998. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, J.; Bazleh, S.; Janahmadi, M. The effects of linalool on the excitability of central neurons of snail Caucasotachea atrolabiata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 192, 33–39. [Google Scholar] [CrossRef]
- Milanos, S.; Elsharif, S.A.; Janzen, D.; Buettner, A.; Villmann, C. Metabolic Products of Linalool and Modulation of GABAA Receptors. Front. Chem. 2017, 5, 46. [Google Scholar] [CrossRef]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef]
| LEO Compound | Percentage of the Sample (%) | Total in 100 µL (µL) |
|---|---|---|
| Linalool | 25.18 | 25.18 |
| Linalyl acetate | 26.15 | 26.15 |
| Camphor | 10.03 | 10.03 |
| Cineole | 8.45 | 8.45 |
| Borneol | 3.81 | 3.81 |
| Lavandulyl acetate | 3.42 | 3.42 |
| Caryophyllene | 2.60 | 2.60 |
| Limonene | 1.63 | 1.63 |
| β-Farnecene | 1.35 | 1.35 |
| β-Ocimene | 1.26 | 1.26 |
| 4-Terpineol | 0.85 | 0.85 |
| α-Terpineol | 0.42 | 0.42 |
| Eugenol | 0.20 | 0.20 |
| (A) Elevated plus maze test—Dose | ||
| Time in open arms | ||
| One-Way ANOVA | F = 24.67 | p < 0.0001 |
| Tukey’s multiple comparisons test | p | |
| “Control group” vs. “30 mg/kg of LEO” | <0.0001 | |
| “Control group” vs. “80 mg/kg of LEO” | 0.6569 | |
| “30 mg/kg of LEO” vs. “80 mg/kg of LEO” | <0.0001 | |
| Cohen’s analysis | d | |
| “Control group” vs. “30 mg/kg of LEO” | 2.91 | |
| Inferior = 1.53 | Superior = 4.25 | |
| Time in closed arms | ||
| One-Way ANOVA | F = 5.249 | p = 0.0129 |
| Tukey’s multiple comparisons test | p | |
| “Control group” vs. “30 mg/kg of LEO” | 5.868 | |
| “Control group” vs. “80 mg/kg of LEO” | 0.0966 | |
| “30 mg/kg of LEO” vs. “80 mg/kg of LEO” | 0.0112 | |
| (B) Elevated plus maze test—Protocol duration | ||
| Time in open arms | ||
| One-Way ANOVA | F = 10.21 | p = 0.0006 |
| Tukey’s multiple comparisons test | p | |
| “Control group” vs. “Acute” | 9.117 | |
| “Control group” vs. “7-day” | 0.0011 | |
| “Acute” vs. “7-day” | 0.0032 | |
| Cohen’s analysis | d | |
| “Control group” vs. “7-day” | 2.91 | |
| Inferior = 1.53 | Superior = 4.25 | |
| Time in closed arms | ||
| One-Way ANOVA | F = 0.42 | p = 0.6618 |
| (C) Dark–light test—Dose | ||
| Time in the illuminated area | ||
| One-Way ANOVA | F = 8.04 | p = 0.0026 |
| Tukey’s multiple comparisons test | p | |
| “Control group” vs. “30 mg/kg of LEO” | 0.0069 | |
| “Control group” vs. “80 mg/kg of LEO” | 0.9947 | |
| “30 mg/kg of LEO” vs. “80 mg/kg of LEO” | 0.0055 | |
| Cohen’s analysis | d | |
| “Control group” vs. “30 mg/kg of LEO” | 1.41 | |
| Inferior = 0.316 | Superior = 2.47 | |
| Total entries | ||
| One-Way ANOVA | F = 0.3463 | p = 0.7111 |
| (D) Dark–light test—Protocol duration | ||
| Time in the illuminated area | ||
| One-Way ANOVA | F = 6.225 | p = 0.0069 |
| Tukey’s multiple comparisons test | p | |
| “Control group” vs. “Acute” | 0.8887 | |
| “Control group” vs. “7-day” | 0.0104 | |
| “Acute” vs. “7-day” | 0.0246 | |
| Cohen’s analysis | d | |
| “Control group” vs. “30 mg/kg of LEO” | 1.41 | |
| Inferior = 0.316 | Superior = 2.47 | |
| Total entries | ||
| One-Way ANOVA | F = 0.3188 | p = 0.7302 |
| (E) Open-field test—Dose | ||
| Ambulation | ||
| One-Way ANOVA | F = 0.3647 | p = 0.6987 |
| (F) Open-field test—Protocol duration | ||
| Ambulation | ||
| One-Way ANOVA | F = 1.603 | p = 0.2239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo, S.M.; Occhieppo, V.B.; Moya, C.; Crespo, R.; Bregonzio, C. Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation. Pharmaceuticals 2025, 18, 624. https://doi.org/10.3390/ph18050624
Angulo SM, Occhieppo VB, Moya C, Crespo R, Bregonzio C. Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation. Pharmaceuticals. 2025; 18(5):624. https://doi.org/10.3390/ph18050624
Chicago/Turabian StyleAngulo, Sol Micaela, Victoria Belén Occhieppo, Cristian Moya, Rosana Crespo, and Claudia Bregonzio. 2025. "Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation" Pharmaceuticals 18, no. 5: 624. https://doi.org/10.3390/ph18050624
APA StyleAngulo, S. M., Occhieppo, V. B., Moya, C., Crespo, R., & Bregonzio, C. (2025). Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation. Pharmaceuticals, 18(5), 624. https://doi.org/10.3390/ph18050624







