Effects of Anethole on Renal Function of Swiss Mice
Abstract
1. Introduction
2. Results
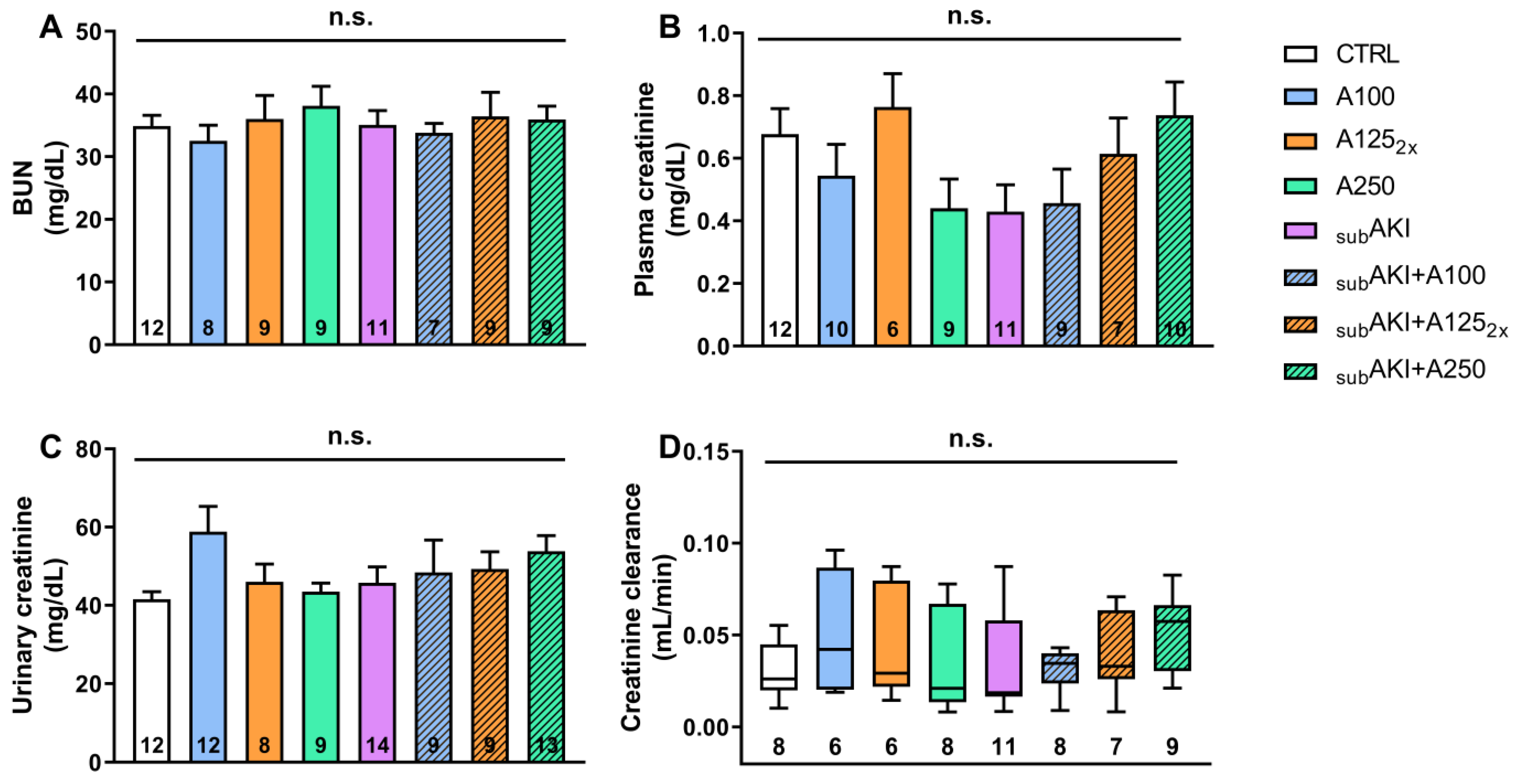
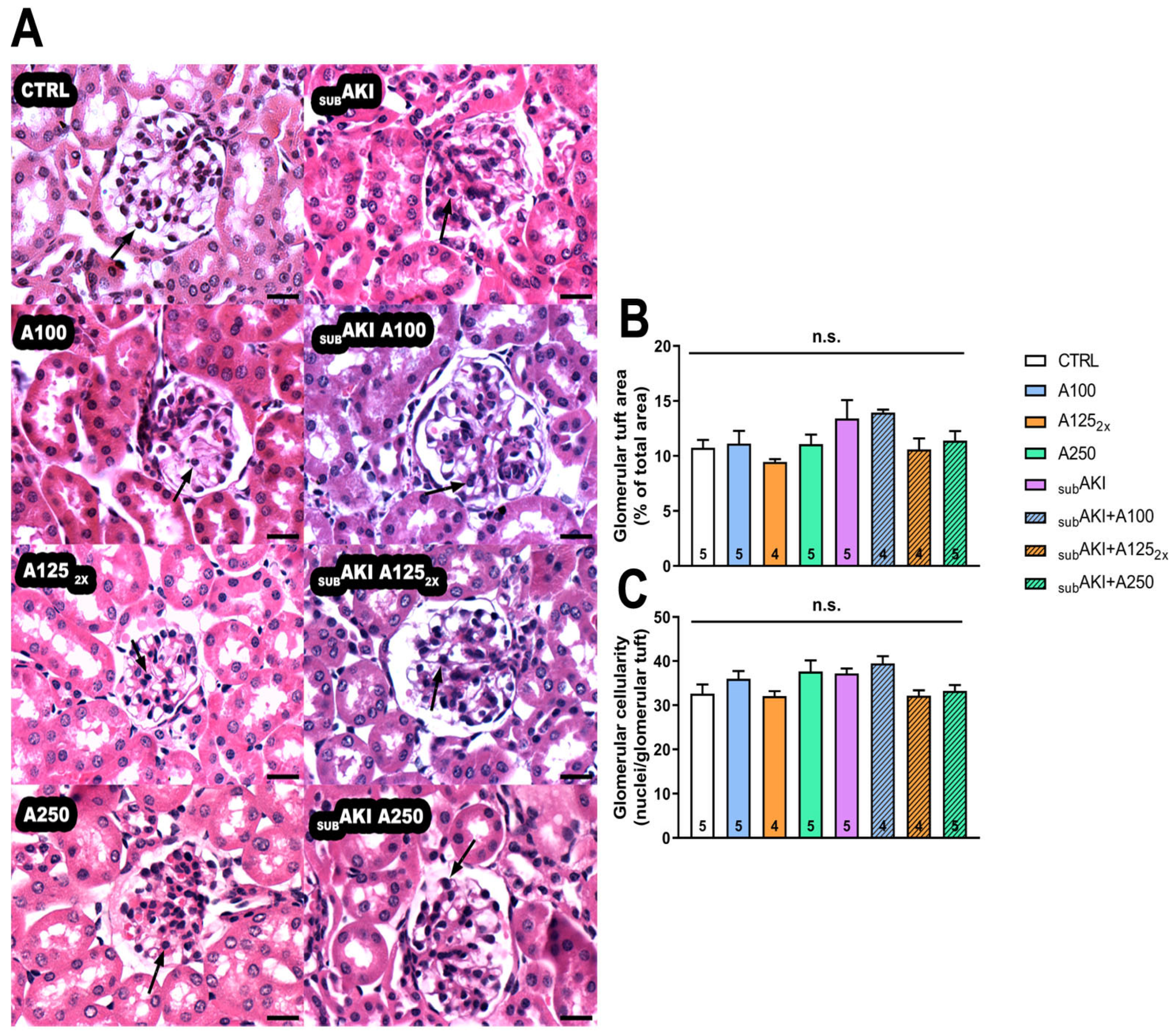
2.1. Effects of Anethole Treatment on Glomerular Renal Function
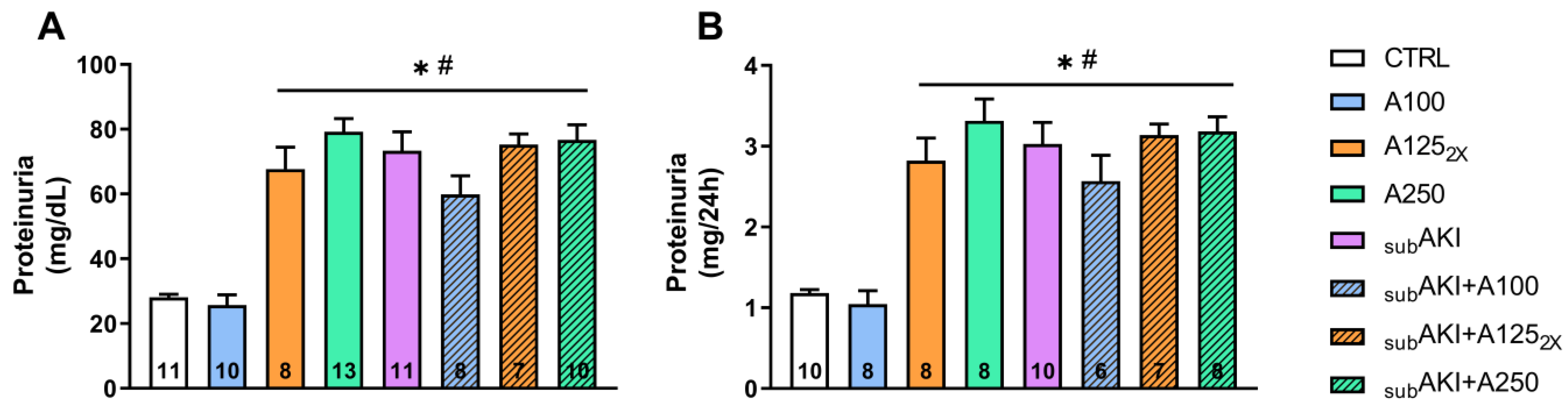
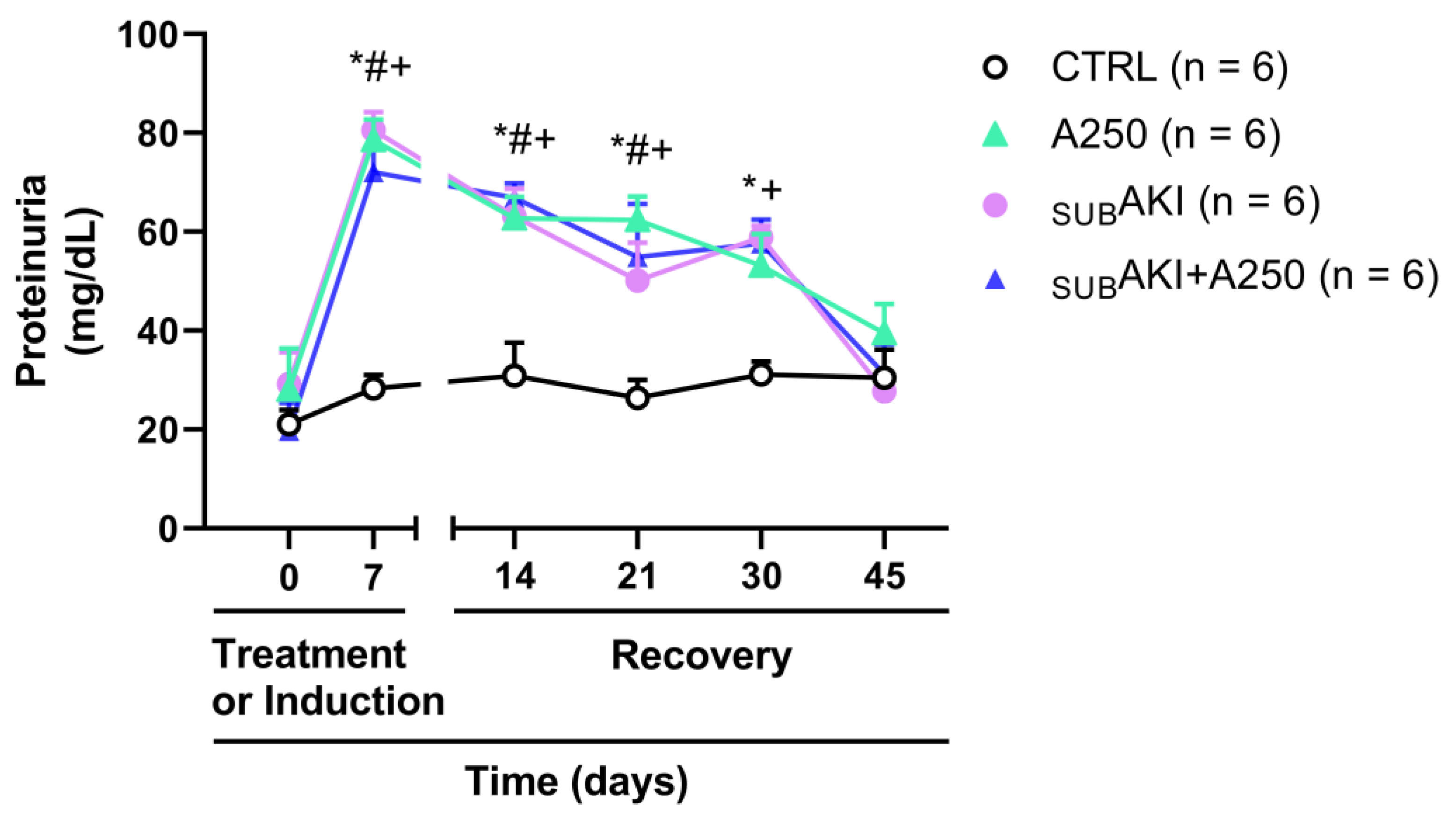
2.2. Effects of Anethole Treatment on Urinary Protein Excretion
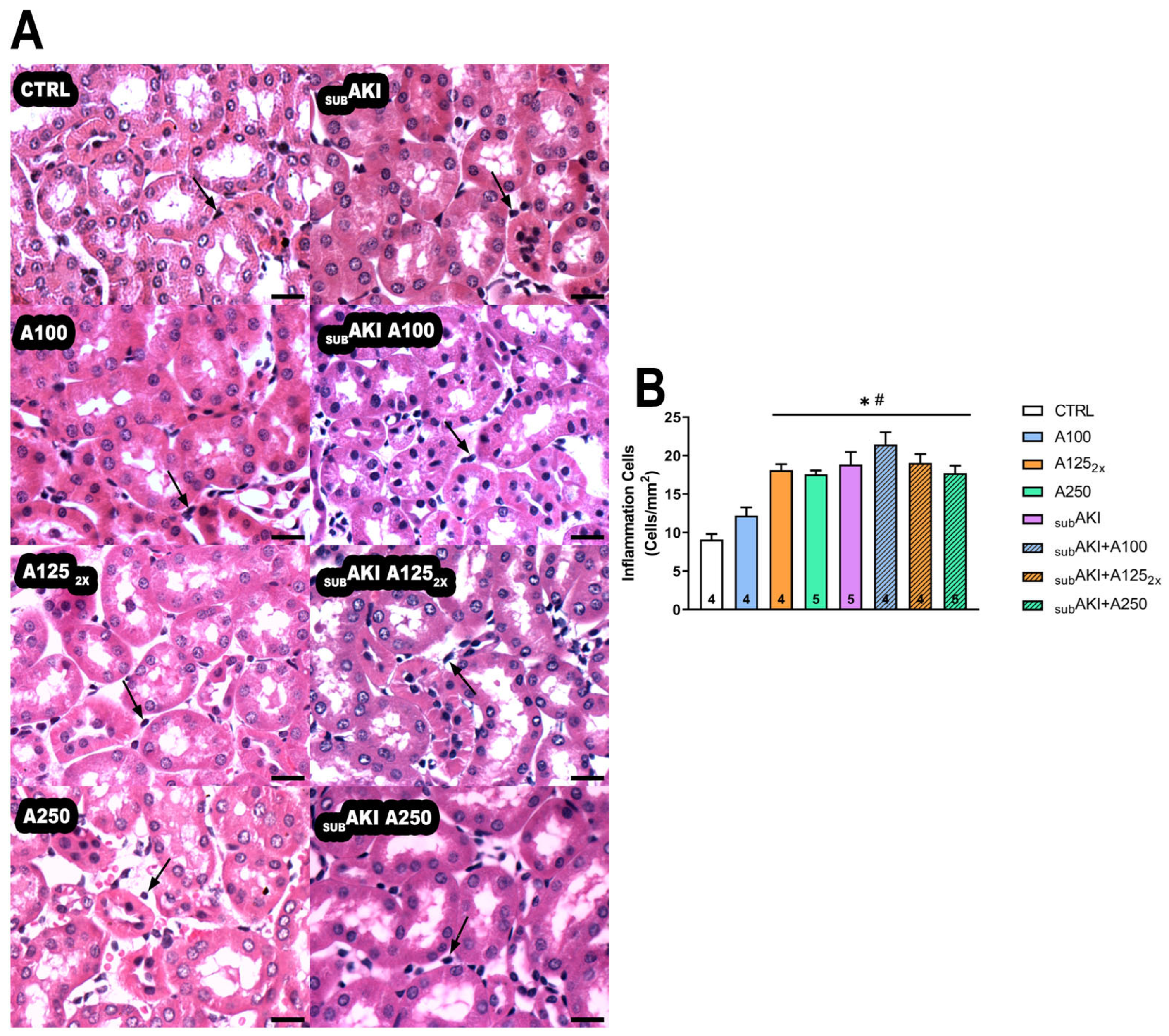
2.3. Effects of Anethole Treatment on Tubular Interstitial Cell Infiltrate
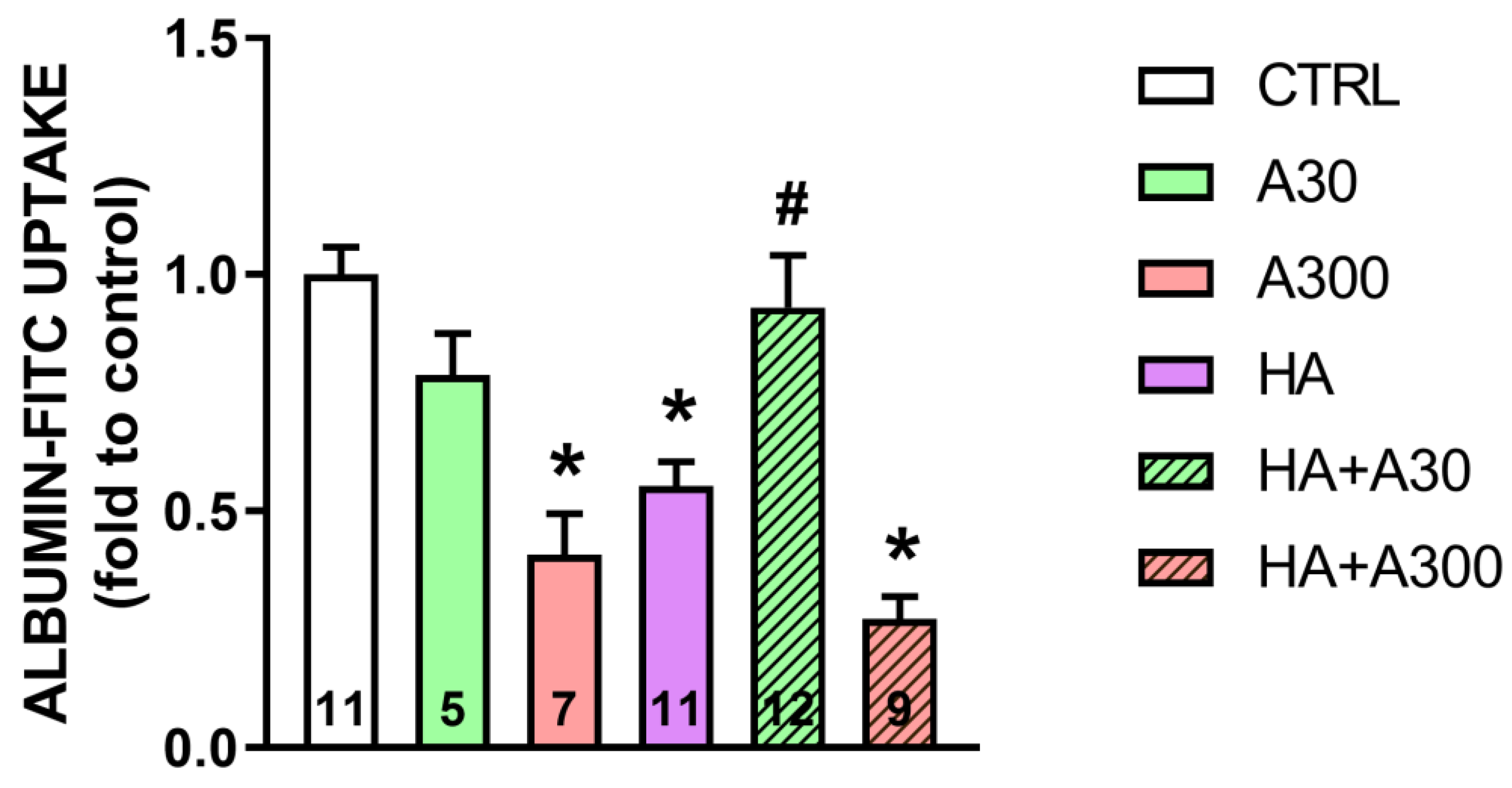
2.4. Effects of Anethole Treatment on in Vitro Albumin Endocytosis
2.5. Spontaneous Reversibility of Effects of Anethole Treatment on Tubulointerstitial Injury
3. Discussion
3.1. Major Discoveries
3.2. Renal Effect of High Doses of Anethole: Lesion Restricted to Tubules and Tubular Inflammation
3.3. Possible Explanation of Mechanistic of Action
3.4. The SUBAKI Model
3.5. Effects of Anethole on Tubular Albumin Uptake in Vitro and Renal Effect of Low Doses of Anethole
3.6. Therapeutic Potential of Anethole: Animal Models and Human Outcomes
4. Materials and Methods
4.1. Animals
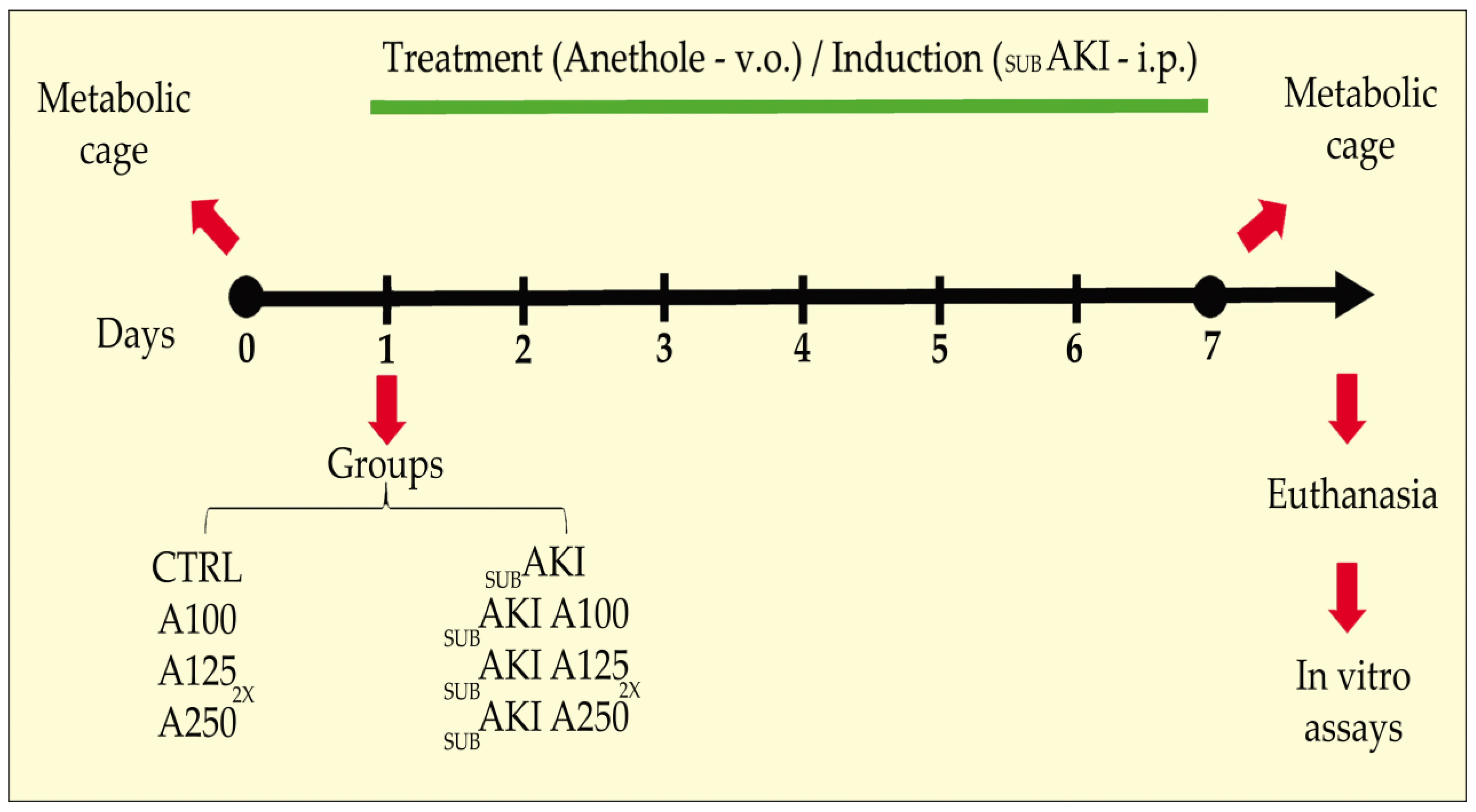
4.2. Subclinical AKI Animal Model and Anethole Treatment
4.3. Renal Function Analysis
4.4. Histological Analyses
4.5. Cell Culture
4.6. Albumin-FITC Endocytosis Assay
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aćimović, M.G.; Tešević, V.; Todosijević, M.; Djisalov, J.; Oljaca, S. Compositional Characteristics of the Essential Oil of Pimpinella Anisum and Foeniculum Vulgare Grown in Serbia. Bot. Serbica 2015, 39, 9–14. [Google Scholar]
- Aprotosoaie, A.C.; Costache, I.-I.; Miron, A. Anethole and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 247–267. ISBN 978-3-319-41342-6. [Google Scholar]
- Cavalcanti, J.M.; Leal-Cardoso, J.H.; Diniz, L.R.L.; Portella, V.G.; Costa, C.O.; Linard, C.F.B.M.; Alves, K.; de Paula Rocha, M.V.A.; Lima, C.C.; Cecatto, V.M.; et al. The Essential Oil of Croton Zehntneri and Trans-Anethole Improves Cutaneous Wound Healing. J. Ethnopharmacol. 2012, 144, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cardoso, J.H.; Fonteles, M.C. Pharmacological Effects of Essential Oils of Plants of the Northeast of Brazil. An. Acad. Bras. Cienc. 1999, 71, 207–213. [Google Scholar]
- Ponte, E.L.; Sousa, P.L.; Rocha, M.V.A.P.; Soares, P.M.G.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; Assreuy, A.M.S. Comparative Study of the Anti-Edematogenic Effects of Anethole and Estragole. Pharmacol. Rep. 2012, 64, 984–990. [Google Scholar] [CrossRef]
- Ritter, A.M.V.; Domiciano, T.P.; Verri, W.A.; Zarpelon, A.C.; da Silva, L.G.; Barbosa, C.P.; Natali, M.R.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Antihypernociceptive Activity of Anethole in Experimental Inflammatory Pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef]
- Kang, P.; Kim, K.Y.; Lee, H.S.; Min, S.S.; Seol, G.H. Anti-Inflammatory Effects of Anethole in Lipopolysaccharide-Induced Acute Lung Injury in Mice. Life Sci. 2013, 93, 955–961. [Google Scholar] [CrossRef]
- Ritter, A.M.V.; Hernandes, L.; Da Rocha, B.A.; Estevão-Silva, C.F.; Wisniewski-Rebecca, E.S.; Cezar, J.D.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Anethole Reduces Inflammation and Joint Damage in Rats with Adjuvant-Induced Arthritis. Inflamm. Res. 2017, 66, 725–737. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.S.; Seol, G.H. Anti-Inflammatory Effects of Trans-Anethole in a Mouse Model of Chronic Obstructive Pulmonary Disease. Biomed. Pharmacother. 2017, 91, 925–930. [Google Scholar] [CrossRef]
- Serra, D.S.; Gomes, M.D.M.; Cavalcante, F.S.Á.; Leal-Cardoso, J.H. Essential Oil of Croton Zehntneri Attenuates Lung Injury in the OVA-Induced Asthma Model. J. Asthma 2019, 56, 1–10. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants 2022, 11, 535. [Google Scholar] [CrossRef]
- Choo, E.J.; Rhee, Y.-H.; Jeong, S.-J.; Lee, H.-J.; Kim, H.S.; Ko, H.S.; Kim, J.-H.; Kwon, T.-R.; Jung, J.H.; Kim, J.H.; et al. Anethole Exerts Antimetatstaic Activity via Inhibition of Matrix Metalloproteinase 2/9 and AKT/Mitogen-Activated Kinase/Nuclear Factor Kappa B Signaling Pathways. Biol. Pharm. Bull. 2011, 34, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.A.; Pinheiro, D.C.S.N. Synthesis and Antioxidant, Anti-Inflammatory and Gastroprotector Activities of Anethole and Related Compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- de Siqueira, R.J.B.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Cardiovascular Effects of the Essential Oil of Croton Zehntneri Leaves and Its Main Constituents, Anethole and Estragole, in Normotensive Conscious Rats. Life Sci. 2006, 78, 2365–2372. [Google Scholar] [CrossRef]
- Seo, E.; Kang, P.; Seol, G.H. Trans-Anethole Prevents Hypertension Induced by Chronic Exposure to Both Restraint Stress and Nicotine in Rats. Biomed. Pharmacother. 2018, 102, 249–253. [Google Scholar] [CrossRef]
- Lu, J.; Hou, W.; Yang, S.; Chen, D.; Wang, F.; Liu, L.; Shen, Z. Trans-Anethole Pretreatment Ameliorates Hepatic Ischemia-Reperfusion Injury via Regulation of Soluble Epoxide Hydrolase. Int. Immunopharmacol. 2023, 124, 110809. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Leal-Cardoso, J.H.; Santos, C.F.; Morais, S.M.; Coelho-de-Souza, A.N. Antinociceptive Effects of the Essential Oil of Croton Zehntneri in Mice. Braz. J. Med. Biol. Res. 2001, 34, 1471–1474. [Google Scholar] [CrossRef]
- Lima, C.C.; de Holanda-Angelin-Alves, C.M.; Pereira-Gonçalves, Á.; Kennedy-Feitosa, E.; Evangelista-Costa, E.; Bezerra, M.A.C.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Antispasmodic Effects of the Essential Oil of Croton Zehnteneri, Anethole, and Estragole, on Tracheal Smooth Muscle. Heliyon 2020, 6, e05445. [Google Scholar] [CrossRef]
- Morais, S.M.; Cavalcanti, E.S.B.; Bertini, L.M.; Oliveira, C.L.L.; Rodrigues, J.R.B.; Cardoso, J.H.L. Larvicidal Activity of Essential Oils from Brazilian Croton Species against Aedes aegypti L. J. Am. Mosq. Control Assoc. 2006, 22, 161–164. [Google Scholar] [CrossRef]
- Ferreira-da-Silva, F.W.; da Silva-Alves, K.S.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Essential Oil of Croton Zehntneri Prevents Electrophysiological Alterations in Dorsal Root Ganglia of Streptozotocin-Induced Diabetes Mellitus in Rats. Phytomedicine Plus 2023, 3, 100443. [Google Scholar] [CrossRef]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Essential Oil of Croton Zehntneri Prevents Conduction Alterations Produced by Diabetes Mellitus on Vagus Nerve. Plants 2021, 10, 893. [Google Scholar] [CrossRef]
- Jurado, J.M.; Alcázar, A.; Pablos, F.; Martín, M.J. LC Determination of Anethole in Aniseed Drinks. Chromatographia 2006, 64, 223–226. [Google Scholar] [CrossRef]
- Newberne, P.; Smith, R.L.; Doull, J.; Goodman, J.I.; Munro, I.C.; Portoghese, P.S.; Wagner, B.M.; Weil, C.S.; Woods, L.A.; Adams, T.B.; et al. The FEMA GRAS Assessment of Trans-Anethole Used as a Flavouring Substance. Food Chem. Toxicol. 1999, 37, 789–811. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Rogers, A.E.; et al. Safety Assessment of Allylalkoxybenzene Derivatives Used as Flavouring Substances—Methyl Eugenol and Estragole. Food Chem. Toxicol. 2002, 40, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Yea, S.S.; Jeong, H.-S.; Choi, C.Y.; Park, K.-R.; Oh, S.; Shin, J.-G.; Yun, C.-H. Inhibitory Effect of Anethole on T-Lymphocyte Proliferation and Interleukin-2 Production through down-Regulation of the NF-AT and AP-1. Toxicol. Vitr. 2006, 20, 1098–1105. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Rocha, M.V.A.P.; Oliveira, K.A.; Vasconcelos, Y.A.G.; Santos, E.C.; Silva-Alves, K.S.; Diniz, L.R.L.; Ferreira-da-Silva, F.W.; Oliveira, A.C.; Ponte, E.L.; et al. Volatile Oil of Croton Zehntneri per Oral Sub-Acute Treatment Offers Small Toxicity: Perspective of Therapeutic Use. Rev. Bras. Farmacogn. 2019, 29, 228–233. [Google Scholar] [CrossRef]
- Silva, K.F.; Peruchetti, D.B.; Sirtoli, G.M.; Takiya, C.M.; Pinheiro, A.A.S.; Leal-Cardoso, J.H.; Caruso-Neves, C. High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage. Plants 2021, 10, 1400. [Google Scholar] [CrossRef]
- Strausser, S.A.; Nakano, D.; Souma, T. Acute Kidney Injury to Chronic Kidney Disease Transition: Insufficient Cellular Stress Response. Curr. Opin. Nephrol. Hypertens. 2018, 27, 314–322. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Luft, F.C. Biomarkers and Predicting Acute Kidney Injury. Acta Physiol. 2021, 231, e13479. [Google Scholar] [CrossRef]
- Ronco, C.; Kellum, J.A.; Haase, M. Subclinical AKI Is Still AKI. Crit. Care 2012, 16, 313. [Google Scholar] [CrossRef]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI—An Emerging Syndrome with Important Consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Vanmassenhove, J.; Van Biesen, W.; Vanholder, R.; Lameire, N. Subclinical AKI: Ready for Primetime in Clinical Practice? J. Nephrol. 2019, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ishola, D.A.; Van Der Giezen, D.M.; Hahnel, B.; Goldschmeding, R.; Kriz, W.; Koomans, H.A.; Joles, J.A. In Mice, Proteinuria and Renal Inflammatory Responses to Albumin Overload Are Strain-Dependent. Nephrol. Dial. Transplant. 2006, 21, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M. Renal tubule albumin transport. Annu. Rev. Physiol. 2005, 67, 573–594. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Martinez-Castelao, A. Proteinuria: Detection and Role in Native Renal Disease Progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef]
- Chevalier, R.L. The Proximal Tubule Is the Primary Target of Injury and Progression of Kidney Disease: Role of the Glomerulotubular Junction. Am. J. Physiol. Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Eddy, A.A.; Neilson, E.G. Chronic Kidney Disease Progression. J. Am. Soc. Nephrol. 2006, 17, 2964–2966. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole Induces Anti-Oral Cancer Activity by Triggering Apoptosis, Autophagy and Oxidative Stress and by Modulation of Multiple Signaling Pathways. Sci. Rep. 2021, 11, 13087. [Google Scholar] [CrossRef]
- De, S.; Kuwahara, S.; Saito, A. The Endocytic Receptor Megalin and Its Associated Proteins in Proximal Tubule Epithelial Cells. Membranes 2014, 4, 333–355. [Google Scholar] [CrossRef]
- López-Hernández, T.; Haucke, V.; Maritzen, T. Endocytosis in the Adaptation to Cellular Stress. Cell Stress 2020, 4, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Proteinuria and Interstitial Injury. Nephrol. Dial. Transplant. 2004, 19, 277–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eddy, A.A. Interstitial Nephritis Induced by Protein-Overload Proteinuria. Am. J. Pathol. 1989, 135, 719–733. [Google Scholar] [PubMed]
- Murugan, R.; Kellum, J.A. Acute Kidney Injury: What’s the Prognosis? Nat. Rev. Nephrol. 2011, 7, 209–217. [Google Scholar] [CrossRef]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The Proximal Tubule and Albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef]
- Leheste, J.-R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.Ø.; Otto, A.; Christensen, E.I.; et al. Megalin Knockout Mice as an Animal Model of Low Molecular Weight Proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef]
- Raychowdhury, R.; Niles, J.L.; McCluskey, R.T.; Smith, J.A. Autoimmune Target in Heymann Nephritis Is a Glycoprotein with Homology to the LDL Receptor. Science 1989, 244, 1163–1165. [Google Scholar] [CrossRef]
- Marzolo, M.-P.; Farfán, P. New Insights into the Roles of Megalin/LRP2 and the Regulation of Its Functional Expression. Biol. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic Kidney Disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global Epidemiology and Outcomes of Acute Kidney Injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L.; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ferreira, B.D.S.; da Silva, F.E.R.; Gomes-Vasconcelos, Y.D.A.; Joca, H.C.; Coelho-de-Souza, A.N.; Ferreira-da-Silva, F.W.; Leal-Cardoso, J.H.; da Silva-Alves, K.S. Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats. Int. J. Mol. Sci. 2024, 25, 8133. [Google Scholar] [CrossRef]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a Medicinal Plant Compound, Decreases the Production of Pro-Inflammatory TNF-α and IL-1β in a Rat Model of LPS-Induced Periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar]
- Raposo, A.; Raheem, D.; Zandonadi, R.P.; Suri, N.; Olukosi, A.; de Lima, B.R.; Carrascosa, C.; Sharifi-Rad, J.; Ryu, H.B.; Han, H.; et al. Anethole in Cancer Therapy: Mechanisms, Synergistic Potential, and Clinical Challenges. Biomed. Pharmacother. 2024, 180, 117449. [Google Scholar] [CrossRef]
- Chen, C.H.; deGraffenried, L.A. Anethole Suppressed Cell Survival and Induced Apoptosis in Human Breast Cancer Cells Independent of Estrogen Receptor Status. Phytomedicine 2012, 19, 763–767. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, É.B.V.; de Sousa, D.P. Antitumor Phenylpropanoids Found in Essential Oils. BioMed Res. Int. 2015, 2015, 392674. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Chung, P.-S.; Kim, S.-H.; Ahn, J.C. CXCR4 and PTEN Are Involved in the Anti-Metastatic Regulation of Anethole in DU145 Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2014, 447, 557–562. [Google Scholar] [CrossRef]
- Peruchetti, D.B.; Silva-Filho, J.L.; Silva-Aguiar, R.P.; Teixeira, D.E.; Takiya, C.M.; Souza, M.C.; Henriques, M.D.G.; Pinheiro, A.A.S.; Caruso-Neves, C. IL-4 Receptor α Chain Protects the Kidney Against Tubule-Interstitial Injury Induced by Albumin Overload. Front. Physiol. 2020, 11, 172. [Google Scholar] [CrossRef]
- Silva, C.M.; Ornellas, D.S.; Ornellas, F.M.; Santos, R.S.; Martini, S.V.; Ferreira, D.; Muiler, C.; Cruz, F.F.; Takiya, C.M.; Rocco, P.R.M.; et al. Early Effects of Bone Marrow-Derived Mononuclear Cells on Lung and Kidney in Experimental Sepsis. Respir. Physiol. Neurobiol. 2023, 309, 103999. [Google Scholar] [CrossRef]
- Afonso, L.G.; Silva-Aguiar, R.P.; Teixeira, D.E.; Alves, S.A.S.; Schmaier, A.H.; Pinheiro, A.A.S.; Peruchetti, D.B.; Caruso-Neves, C. The Angiotensin II/Type 1 Angiotensin II Receptor Pathway Is Implicated in the Dysfunction of Albumin Endocytosis in Renal Proximal Tubule Epithelial Cells Induced by High Glucose Levels. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130684. [Google Scholar] [CrossRef]
- Alves, S.A.S.; Florentino, L.S.; Teixeira, D.E.; Silva-Aguiar, R.P.; Peruchetti, D.B.; Oliveira, A.C.; Scharfstein, J.; Marzolo, M.-P.; Pinheiro, A.A.S.; Caruso-Neves, C. Surface Megalin Expression Is a Target to the Inhibitory Effect of Bradykinin on the Renal Albumin Endocytosis. Peptides 2021, 146, 170646. [Google Scholar] [CrossRef]
- Huber, S.M.; Misovic, M.; Mayer, C.; Rodemann, H.-P.; Dittmann, K. EGFR-Mediated Stimulation of Sodium/Glucose Cotransport Promotes Survival of Irradiated Human A549 Lung Adenocarcinoma Cells. Radiother. Oncol. 2012, 103, 373–379. [Google Scholar] [CrossRef]
| Groups | Body Mass (g) | Absolute Kidney Weight (g) | Relative Kidney Weight (%) | Food Intake (g) | Water Intake (mL) | Urinary Volume (µL) |
|---|---|---|---|---|---|---|
| CTRL | 30.50 ± 0.77 | 0.322 ± 0.01 | 1.059 ± 0.03 | 4.863 ± 0.28 | 7.21 ± 0.69 | 700.0 ± 89.61 |
| A100 | 29.73 ± 0.83 | 0.310 ± 0.01 | 1.034 ± 0.05 | 4.512 ± 0.32 | 6.60 ± 0.47 | 787.5 ± 47.95 |
| A1252x | 30.77 ± 0.61 | 0.326 ± 0.01 | 1.062 ± 0.04 | 4.382 ± 0.53 | 6.87 ± 0.89 | 766.7 ± 126.9 |
| A250 | 31.56 ± 0.81 | 0.367 ± 0.01 | 1.169 ± 0.06 | 4.542 ± 0.23 | 7.83 ± 0.51 | 677.8 ± 99.69 |
| SUBAKI | 28.37 ± 0.57 | 0.312 ± 0.01 | 1.103 ± 0.05 | 4.567 ± 0.51 | 6.32 ± 0.26 | 636.4 ± 85.57 |
| SUBAKI+A100 | 30.03 ± 0.94 | 0.334 ± 0.01 | 1.126 ± 0.04 | 4.620 ± 0.33 | 7.21 ± 0.53 | 875.0 ± 133.3 |
| SUBAKI+A1252x | 28.89 ± 1.08 | 0.336 ± 0.01 | 1.170 ± 0.03 | 4.572 ± 0.17 | 7.07 ± 0.80 | 766.7 ± 88.19 |
| SUBAKI+A250 | 30.12 ± 1.29 | 0.366 ± 0.01 | 1.223 ± 0.04 | 3.891 ± 0.27 | 5.90 ± 0.64 | 700.0 ± 91.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro-Lustosa, R.; Gondim-Pereira, N.M.S.; Alves, S.A.d.S.; Takiya, C.M.; Silva-Alves, K.S.d.; Pinheiro, A.A.S.; Coelho-de-Souza, A.N.; Moreira-Gomes, M.D.; Caruso-Neves, C.; Leal-Cardoso, J.H. Effects of Anethole on Renal Function of Swiss Mice. Pharmaceuticals 2025, 18, 541. https://doi.org/10.3390/ph18040541
Pinheiro-Lustosa R, Gondim-Pereira NMS, Alves SAdS, Takiya CM, Silva-Alves KSd, Pinheiro AAS, Coelho-de-Souza AN, Moreira-Gomes MD, Caruso-Neves C, Leal-Cardoso JH. Effects of Anethole on Renal Function of Swiss Mice. Pharmaceuticals. 2025; 18(4):541. https://doi.org/10.3390/ph18040541
Chicago/Turabian StylePinheiro-Lustosa, Romário, Neide Maria Silva Gondim-Pereira, Sarah Aparecida dos Santos Alves, Christina Maeda Takiya, Kerly Shamyra da Silva-Alves, Ana Acacia Sá Pinheiro, Andrelina Noronha Coelho-de-Souza, Maria Diana Moreira-Gomes, Celso Caruso-Neves, and José Henrique Leal-Cardoso. 2025. "Effects of Anethole on Renal Function of Swiss Mice" Pharmaceuticals 18, no. 4: 541. https://doi.org/10.3390/ph18040541
APA StylePinheiro-Lustosa, R., Gondim-Pereira, N. M. S., Alves, S. A. d. S., Takiya, C. M., Silva-Alves, K. S. d., Pinheiro, A. A. S., Coelho-de-Souza, A. N., Moreira-Gomes, M. D., Caruso-Neves, C., & Leal-Cardoso, J. H. (2025). Effects of Anethole on Renal Function of Swiss Mice. Pharmaceuticals, 18(4), 541. https://doi.org/10.3390/ph18040541






