Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness
Abstract
1. Introduction
2. Results
3. Discussion
Limitations
4. Methods
4.1. Study Design and Data Source
4.2. Study Measurements
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, D.I. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J. Support. Oncol. 2007, 5 (Suppl. S4), 23–31. [Google Scholar] [PubMed]
- Russo, G.; Haddad, R.; Posner, M.; Machtay, M. Radiation treatment breaks and UM in head and neck cancer. Oncologist 2008, 13, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Rybkin, A.; Kang, J.J.; Lee, A.; Kitpanit, S.; Fan, M.; Mohamed, N.; Cartano, O.; Zakeri, K.; Gelblum, D.; Sherman, E.; et al. The effect of short radiation treatment breaks on chemo-radiotherapy for oropharyngeal cancers. Head Neck 2021, 43, 3796–3809. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.H.M.; Silva, C.L.A.; Pedra, R.C.; Rosado, L.P.L.; Verner, F.S.; Aquino, S.N. Probability of oral complications of radiotherapy and chemotherapy for head and neck cancer. Gen. Dent. 2021, 69, 70–74. [Google Scholar] [PubMed]
- Bhide, S.A.; Gulliford, S.; Fowler, J.; Rosenfelder, N.; Newbold, K.; Harrington, K.J.; Nutting, C.M. Characteristics of response of oral and pharyngeal mucosa in patients receiving chemo-IMRT for head and neck cancer using hypofractionated accelerated radiotherapy. Radiother. Oncol. 2010, 97, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Krc, R.F.; Singh, S.A.; Fang, W.; Weir, J.S. Photobiomodulation During Chemoradiation for Head and Neck Cancer: Effect on Mucositis, Weight Loss, and Feeding Tube Dependence. Adv. Radiat. Oncol. 2023, 8, 101216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satheeshkumar, P.S.; Mohan, M.P. Association and risk factors of healthcare-associated infection and burden of illness among chemotherapy-induced ulcerative mucositis patients. Clin. Oral Investig. 2022, 26, 1323–1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miranda-Silva, W.; Gomes-Silva, W.; Zadik, Y.; Yarom, N.; Al-Azri, A.R.; Hong, C.H.L.; Ariyawardana, A.; Saunders, D.P.; Correa, M.E.; Arany, P.R.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis: Sub-analysis of current interventions for the management of oral mucositis in pediatric cancer patients. Support. Care Cancer 2021, 29, 3539–3562. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, P.S.; Blijlevens, N.; Sonis, S.T. Application of big data analyses to compare the impact of oral and gastrointestinal mucositis on risks and outcomes of febrile neutropenia and septicemia among patients hospitalized for the treatment of leukemia or multiple myeloma. Support. Care Cancer 2023, 31, 199. [Google Scholar] [CrossRef] [PubMed]
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T.; Riley, P. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2011, 4, CD000978. [Google Scholar]
- Donnelly, J.P.; Bellm, L.A.; Epstein, J.B.; Sonis, S.T.; Symonds, R.P. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect. Dis. 2003, 3, 405–412. [Google Scholar] [PubMed]
- Bjordal, J.M.; Bensadoun, R.J.; Tunèr, J.; Frigo, L.; Gjerde, K.; Lopes-Martins, R.A. A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support. Care Cancer. 2011, 19, 1069–1077. [Google Scholar]
- Fornaini, C.; Arany, P.; Rocca, J.P.; Merigo, E. Photobiomodulation in Pediatric Dentistry: A Current State-of-the-Art. Photobiomodul. Photomed. Laser Surg. 2019, 37, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Cerchietti, L.C.; Navigante, A.H.; Bonomi, M.R.; Zaderajko, M.A.; Menéndez, P.R.; Pogany, C.E.; Roth, B.M. Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 2002, 95, 2230–2236, Erratum in Cancer 2003, 97, 1137. [Google Scholar] [CrossRef] [PubMed]
- Cerchietti, L.C.; Navigante, A.H.; Körte, M.W.; Cohen, A.M.; Quiroga, P.N.; Villaamil, E.C.; Bonomi, M.R.; Roth, B.M. Potential utility of the peripheral analgesic properties of morphine in stomatitis-related pain: A pilot study. Pain 2003, 105, 265–273. [Google Scholar] [CrossRef]
- Cerchietti, L. Morphine mouthwashes for painful mucositis. Support. Care Cancer 2007, 15, 115–116. [Google Scholar]
- Vayne-Bossert, P.; Escher, M.; de Vautibault, C.G.; Dulguerov, P.; Allal, A.; Desmeules, J.; Herrmann, F.R.; Pautex, S. Effect of topical morphine (mouthwash) on oral pain due to chemotherapy- and/or radiotherapy-induced mucositis: A randomized double-blinded study. J. Palliat. Med. 2010, 13, 125–128. [Google Scholar] [CrossRef]
- Nielsen, B.N.; Friis, S.M.; Schmiegelow, K.; Henneberg, S.; Rømsing, J. Evaluation of topical morphine for treatment of oral mucositis in cancer patients. Br. J. Pain 2021, 15, 411–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 185–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, J.A.; Balneaves, L.G. Cancer patient decision making related to clinical trial participation: An integrative review with implications for patients’ relational autonomy. Support. Care Cancer 2015, 23, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R. The value of observational cohort studies for cancer drugs. Biotechnol. Healthc. 2010, 7, 18–24. [Google Scholar] [PubMed] [PubMed Central]
- Rothwell, P.M. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. Lancet 2005, 365, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Califf, R.M.; Pryor, D.B.; Rosati, R.A. Regression modelling strategies for improved prognostic prediction. Stat. Med. 1984, 3, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Senn, S.; Graf, E.; Caputo, A. Stratification for the propensity score compared with linear regression techniques to assess the effect of treatment or exposure. Stat. Med. 2007, 26, 5529–5544, Erratum in Stat. Med. 2008, 27, 4615; Erratum in Stat. Med. 2019, 38, 4475. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williamson, E.; Morley, R.; Lucas, A.; Carpenter, J. Propensity scores: From naive enthusiasm to intuitive understanding. Stat. Methods Med. Res. 2012, 21, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, P.G.; Ray, W.A. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am. J. Epidemiol. 2011, 174, 613–620. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zylla, D.; Steele, G.; Shapiro, A.; Richter, S.; Gupta, P. Impact of opioid use on health care utilization and survival in patients with newly diagnosed stage IV malignancies. Support. Care Cancer 2018, 26, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Jacobs, J.M.; Cialic, R.; Mor, E.E.; Stessman, J. Opioids, survival, and advanced cancer in the hospice setting. J. Am. Med. Dir. Assoc. 2011, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, H.M.; Woodrell, C.; Rustgi, S.D.; Aronson, A.; Kessel, E.; Amin, S.; Lucas, A.L. Opioid Prescription Is Associated with Increased Survival in Older Adult Patients With Pancreatic Cancer in the United States: A Propensity Score Analysis. JCO Oncol. Pract. 2022, 18, e659–e668. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431, Erratum in Cancer 2021, 127, 3700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herzig, S.J.; Rothberg, M.B.; Cheung, M.; Ngo, L.H.; Marcantonio, E.R. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J. Hosp. Med. 2014, 9, 73–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bigliardi, P.L.; Bigliardi-Qi, M.; Buechner, S.; Rufli, T. Expression of mu-opiate receptor in human epidermis and keratinocytes. J. Investig. Dermatol. 1998, 111, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Imai, Y.; Uhl, G.R.; Stein, C. Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not mu-opioid receptor transcription in dorsal root ganglia. Eur. J. Pharmacol. 1995, 279, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Küchler, S.; Wolf, N.B.; Heilmann, S.; Weindl, G.; Helfmann, J.; Yahya, M.M.; Stein, C.; Schäfer-Korting, M. 3D-wound healing model: Influence of morphine and solid lipid nanoparticles. J. Biotechnol. 2010, 148, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.B.; Küchler, S.; Radowski, M.R.; Blaschke, T.; Kramer, K.D.; Weindl, G.; Kleuser, B.; Haag, R.; Schäfer-Korting, M. Influences of opioids and nanoparticles on in vitro wound healing models. Eur. J. Pharm. Biopharm. 2009, 73, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gysler, A.; Lange, K.; Korting, H.C.; Schäfer-Korting, M. Prednicarbate biotransformation in human foreskin keratinocytes and fibroblasts. Pharm. Res. 1997, 14, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.M.; Bruce, A.J.; Wolf, R.C.; Litzow, M.R.; Hogan, W.J.; Patnaik, M.S.; Kremers, W.K.; Phillips, G.L.; Hashmi, S.K. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol. Blood Marrow Transplant. 2016, 22, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.Z.; Zhang, Y. Efficacy and safety of transdermal fentanyl for the treatment of oral mucositis pain caused by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Support. Care Cancer 2015, 23, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Gündogdu, F.; Sayar, S. Oncology nursing practices in the management of chemotherapy-related oral mucositis in accordance with evidence-based guidelines: A descriptive and cross-sectional study. Support. Care Cancer 2022, 30, 9549–9557. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Ferrell, B. The management of cancer pain. CA Cancer J. Clin. 2011, 61, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.A.; Shea, T.; Olajida, O.; Serody, J.S.; Comeau, T. The effect of oral mucositis on morbidity and mortality in bone marrow transplant. Semin. Oncol. 2003, 30 (Suppl. S18), 76–83. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Hong, S.Y.; Jeong, Y.M.; Choi, K.S.; Lee, E.; Lee, E.; Kim, Y.J.; Bang, S.M. Drug use evaluation of opioid analgesics in pain management among patients with hematopoietic stem cell transplantation. Blood Res. 2020, 55, 151–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fanning, S.R.; Rybicki, L.; Kalaycio, M.; Andresen, S.; Kuczkowski, E.; Pohlman, B.; Sobecks, R.; Sweetenham, J.; Bolwell, B. Severe mucositis is associated with reduced survival after autologous stem cell transplantation for lymphoid malignancies. Br. J. Haematol. 2006, 135, 374–381. [Google Scholar] [CrossRef] [PubMed]
- HCUP-US. NIS Overview. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 25 February 2020).
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Lumley, T. Survey: Analysis of Complex Survey Samples. 2019. Available online: https://CRAN.R-project.org/package=survey (accessed on 4 September 2020).
- Bland, J.M.; Altman, D.G. Transformations, means, and confidence intervals. BMJ 1996, 312, 1079. [Google Scholar] [CrossRef]
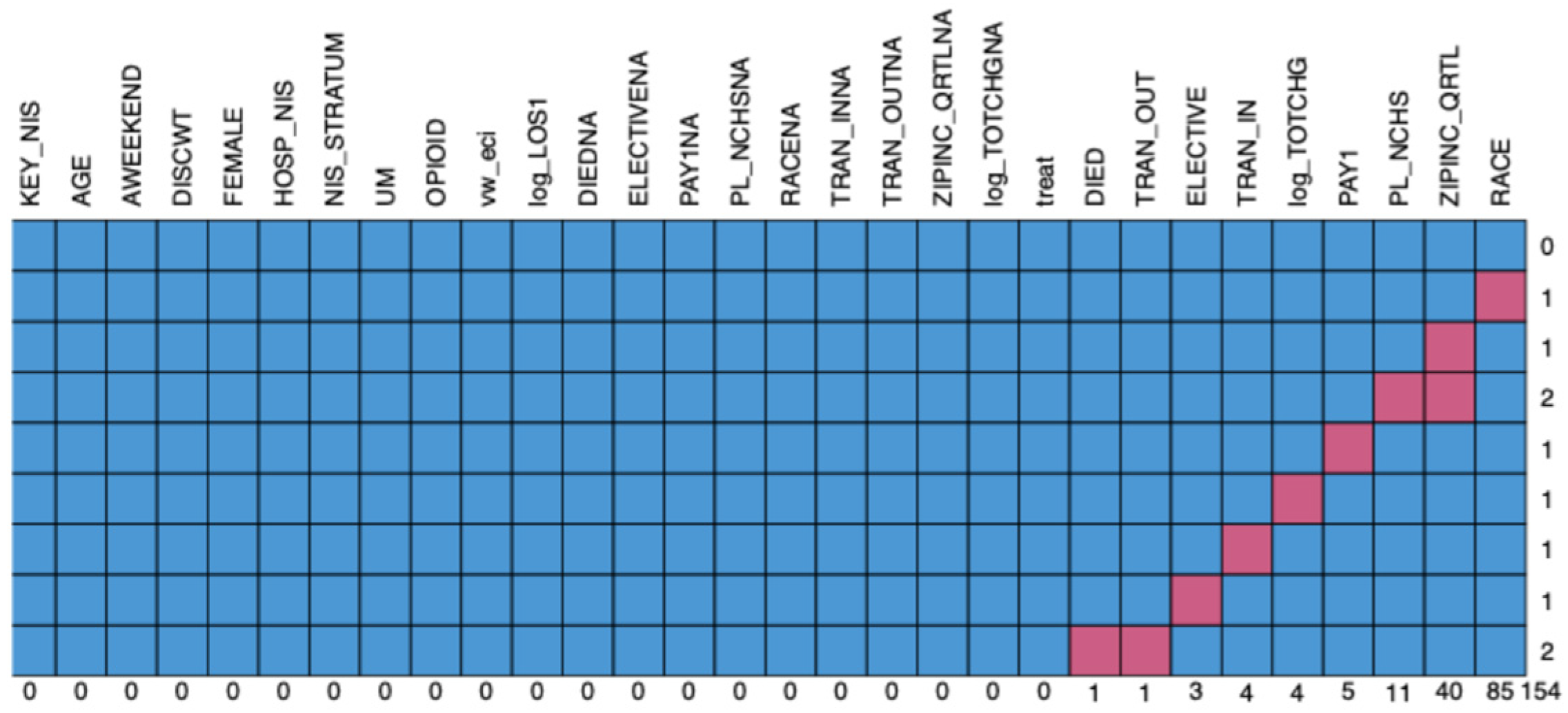
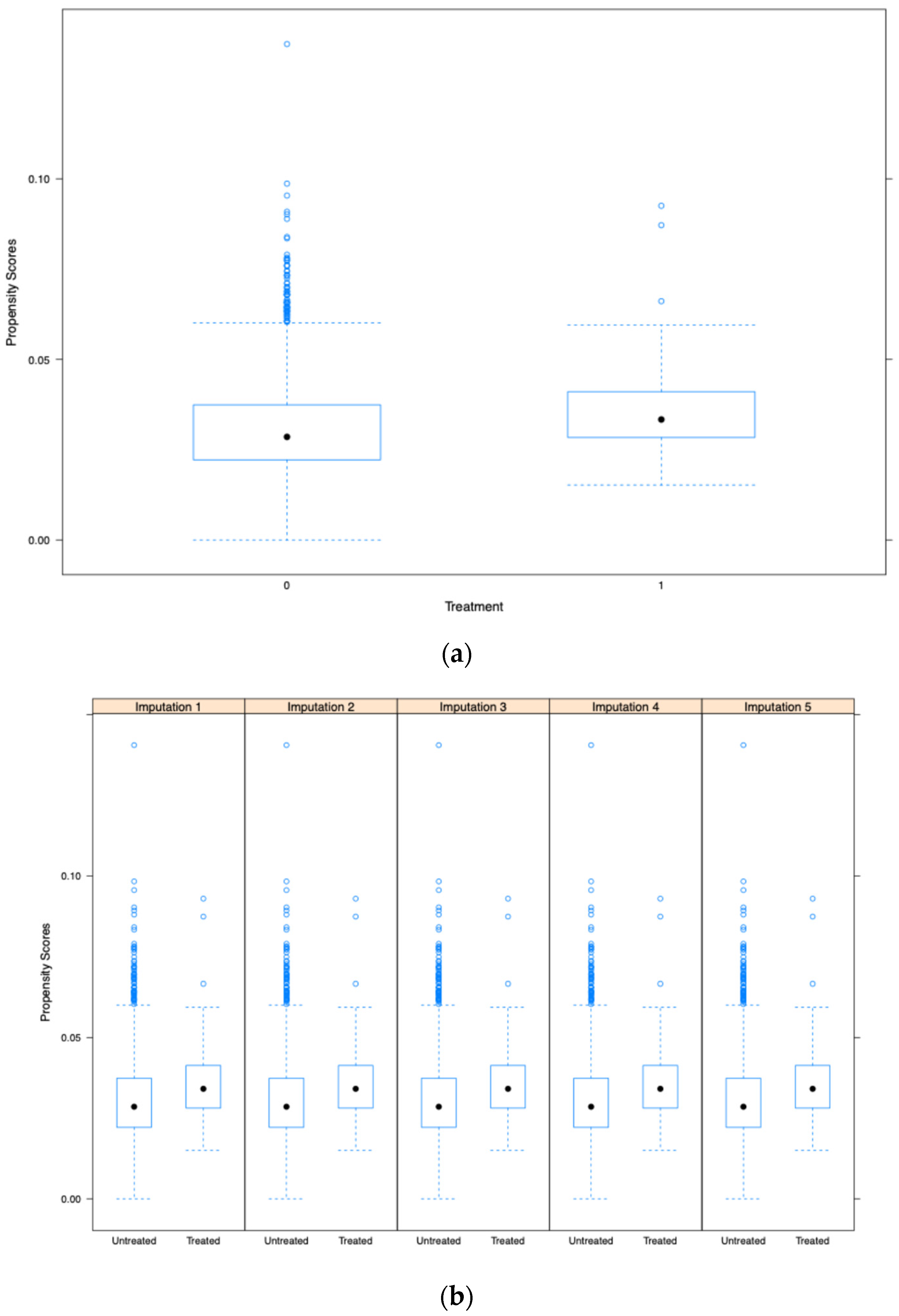
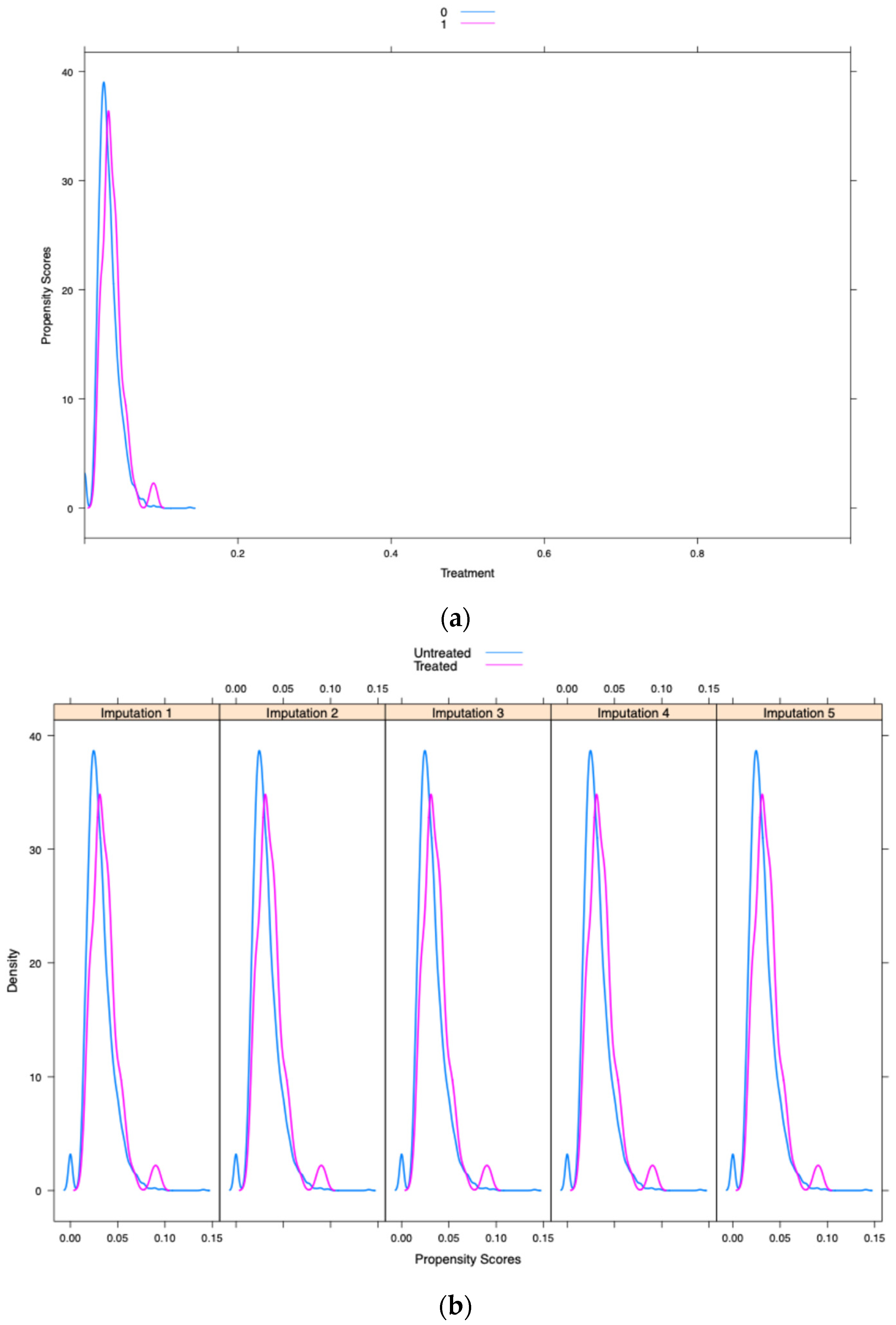


| Ulcerative Mucositis Without Opioid Use (Survey Weighted) | Ulcerative Mucositis with Opioid Use (Survey Weighted) | p-Value | |
|---|---|---|---|
| n | 11,400 (96.9%) | 365 (3.1%) | |
| Age (mean (SD)) | 56.88 (15.9) | 56.14 (14.4) | 0.64 |
| Sex (%) | 0.79 | ||
| Female | 5900.0 (51.8) | 195.0 (53.4) | |
| Race (%) | 0.08 | ||
| White | 7665.0 (69.7) | 220.0 (62.9) | |
| Black | 1170.0 (10.6) | 70.0 (20.0) | |
| Hispanic | 1200.0 (10.9) | 25.0 (7.1) | |
| Asian and others | 955.0 (8.7) | 35.0 (10.0) | |
| Median household income (based on current year) | 0.94 | ||
| 0–25th percentile | 2445.0 (21.8) | 70.0 (19.2) | |
| 26th to 50th percentile | 2840.0 (25.4) | 95.0 (26.0) | |
| 51st to 75th percentile | 2960.0 (26.4) | 105.0 (28.8) | |
| 76th to 100th percentile | 2955.0 (26.4) | 95.0 (26.0) | |
| Expected primary payer (%) | 0.89 | ||
| Medicare | 4205.0 (37.0) | 145.0 (39.7) | |
| Medicaid | 1570.0 (13.8) | 45.0 (12.3) | |
| Private insurance | 4980.0 (43.8) | 150.0 (41.1) | |
| Self-pay, no charge, and other | 620.0 (5.5) | 25.0 (6.8) | |
| Patient location: NCHS urban–rural code (%) | 0.36 | ||
| “Central” counties of metro areas of >=1 million population | 3615.0 (31.9) | 90.0 (24.7) | |
| “Fringe” counties of metro areas of >=1 million population | 2930.0 (25.8) | 105.0 (28.8) | |
| Counties in metro areas of 250,000–999,999 population | 2335.0 (20.6) | 65.0 (17.8) | |
| Counties in metro areas of 50,000–249,999 population and micropolitan counties and not metropolitan or micropolitan counties | 2465.0 (21.7) | 105.0 (28.8) | |
| Admission type (%) | 0.40 | ||
| Elective | 3830.0 (33.6) | 105.0 (28.8) | |
| Indicator of a transfer out of the hospital | 0.95 | ||
| Transferred out | 960.0 (8.4) | 30.0 (8.2) | |
| Weighted Elixir score mean (SD) | 14.13 (9.48) | 16.79 (9.29) | 0.02 |
| Length of stay (geometric mean) | 9.1 days | 5.9 days | <0.001 |
| Total charge (geometric mean) | USD 89,524 | USD 46,340 | <0.001 |
| Mortality (%) | 395.0 (3.5) | 15.0 (4.1) | 0.76 |
| Lymphoma (%) | 3135.0 (27.5) | 110.0 (30.1) | 0.63 |
| Solid tumors with metastatic cancers (%) | 2460.0 (21.6) | 110.0 (30.1) | 0.12 |
| Solid tumors without metastatic cancers (%) | 4450.0 (39.0) | 210.0 (57.5) | 0.002 |
| Ulcerative Mucositis Without Opioid Use (Weighted) | Ulcerative Mucositis with Opioid Use (Weighted) | p-Value | |
|---|---|---|---|
| n | 73 | 73 | |
| Age (mean (SD)) | 55.3 (16.7) | 56.14 (14.4) | 0.75 |
| Sex (%) | 0.74 | ||
| Female | 42.0 (57.8) | 39.0 (53.4) | |
| Race (%) | 0.17 | ||
| White | 47.0 (64.4) | 46.0 (63.0) | |
| Black | 26.0 (35.6) | 15.0 (36.9) | |
| Median household income (based on current year) | 0.79 | ||
| 0–25th percentile | 14.0 (19.2) | 14.0 (19.2) | |
| 26th to 50th percentile | 24.0 (32.9) | 19.0 (26.0) | |
| 51st to 75th percentile | 17.0 (23.3) | 21.0 (28.8) | |
| 76th to 100th percentile | 18.0 (24.7) | 19.0 (26.0) | |
| Expected primary payer (%) | 0.77 | ||
| Medicare and Medicaid | 50.0 (56.1) | 47.0 (42.0) | |
| Others | 29.0 (44.8) | 30.0 (47.9) | |
| Patient location: NCHS urban–rural code (%) | 0.75 | ||
| “Central” counties of metro areas of >=1 million population | 22.0 (30.1) | 18.0 (24.7) | |
| “Fringe” counties of metro areas of >=1 million population | 23.0 (31.5) | 21.0 (28.8) | |
| Counties in metro areas of 250,000–999,999 population | 12.0 (16.4) | 13.0 (17.8) | |
| Counties in metro areas of 50,000–249,999 population and micropolitan counties and not metropolitan or micropolitan counties | 16.0 (21.9) | 21.0 (28.8) | |
| Admission type (%) | 0.85 | ||
| Elective | 23.0 (31.5) | 21.0 (28.8) | |
| Weighted Elixir score mean (SD) | 17.52 (8.38) | 16.79 (9.29) | 0.62 |
| Length of stay (geometric mean) | 9.1 days | 5.9 days | 0.02 |
| Total charge (geometric mean) | USD 76,863 | USD 46,341 | 0.004 |
| Effect Estimates | Propensity Score-Adjusted Model 1 | Propensity Score with a Deciles-Adjusted Model 2 | Fully Adjusted Model (Non-Propensity Score Estimation) 3 |
|---|---|---|---|
| Total charge | 0.51 (0.42–0.76), p < 0.001 | 0.57 (0.42–0.76), p < 0.001 | 0.56 (0.44–0.71), p < 0.001 |
| Length of stay | 0.67 (0.51–0.87), p = 0.002 | 0.67 (0.51–0.87), p = 0.003 | 0.67 (0.54–0.82), p = 0.0002 |
| In-hospital mortality (aOR) | 0.87 (0.21–2.41), p = 0.81 | 0.85 (0.20–2.39), p = 0.79 | 0.93 (0.31–2.82), p = 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, M.P.; Epstein, J.B.; Meleveedu, K.S.; Padhi, P.; Pili, R.; Satheeshkumar, P.S. Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness. Pharmaceuticals 2025, 18, 536. https://doi.org/10.3390/ph18040536
Mohan MP, Epstein JB, Meleveedu KS, Padhi P, Pili R, Satheeshkumar PS. Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness. Pharmaceuticals. 2025; 18(4):536. https://doi.org/10.3390/ph18040536
Chicago/Turabian StyleMohan, Minu Ponnamma, Joel B. Epstein, Kapil S. Meleveedu, Parikshit Padhi, Roberto Pili, and Poolakkad S. Satheeshkumar. 2025. "Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness" Pharmaceuticals 18, no. 4: 536. https://doi.org/10.3390/ph18040536
APA StyleMohan, M. P., Epstein, J. B., Meleveedu, K. S., Padhi, P., Pili, R., & Satheeshkumar, P. S. (2025). Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness. Pharmaceuticals, 18(4), 536. https://doi.org/10.3390/ph18040536









