Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models
Abstract
1. Introduction
2. Results
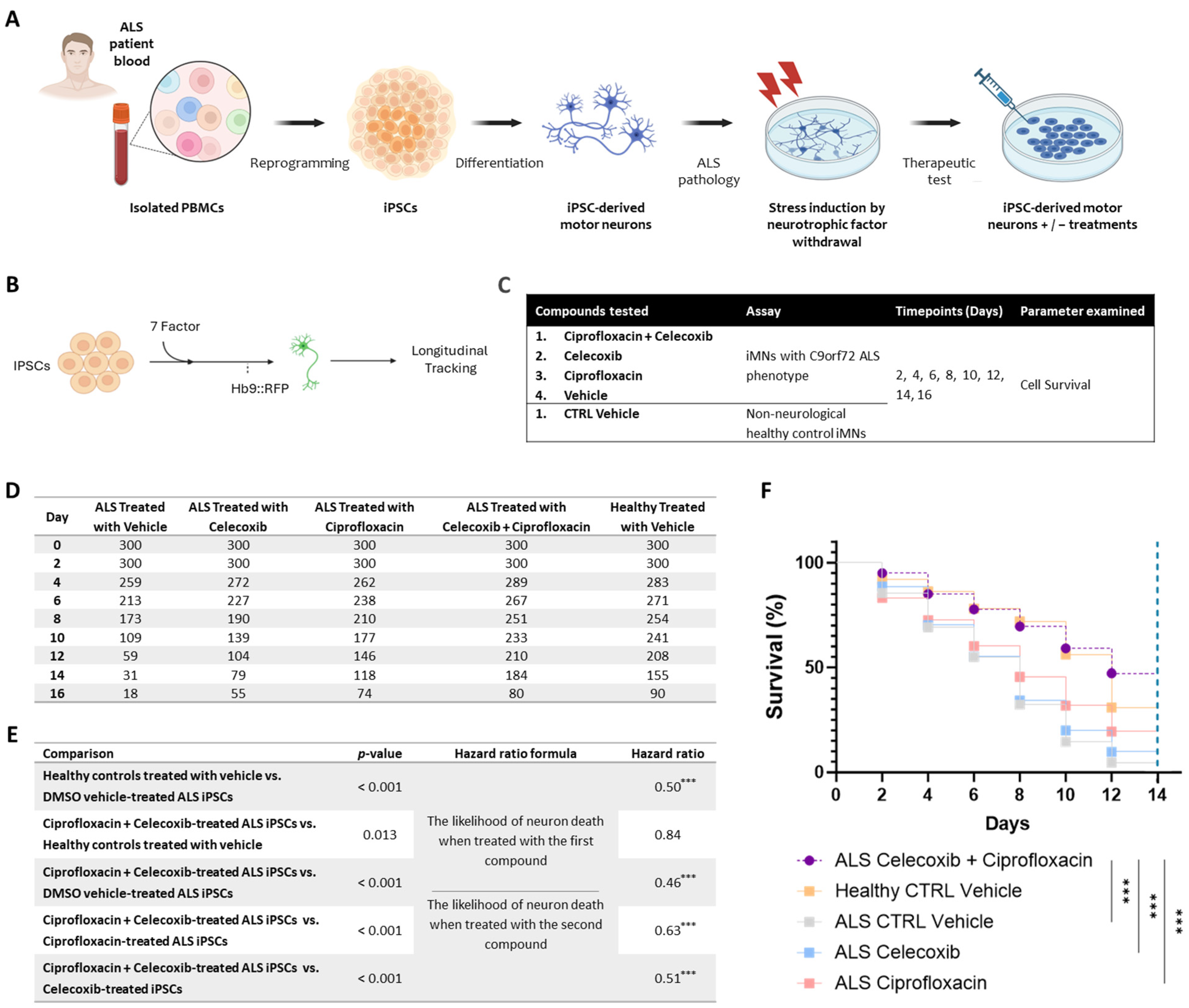
2.1. PrimeC Combination Improves Survival of ALS Patient-Derived iMNs
2.2. Enhanced Brain Exposure Following PrimeC Combination Administration in Mice
3. Discussion
4. Materials and Methods
4.1. Induced Pluripotent Stem Cell (iPSC)-Derived Motor Neuron (MN) Survival
4.1.1. Peripheral Blood Mononuclear Cell (PBMC) Isolation
4.1.2. iPSC Generation
4.1.3. iPSC-Derived iMNs
4.1.4. iMN Assay
4.1.5. Statistical Analysis
4.2. Drug Concentration Profiling of the PrimeC Combination in C57BL Mice
4.2.1. Mice
4.2.2. Formulation Preparation
4.2.3. Ciprofloxacin Liquid Chromatography with Triple Quadrupole Mass Spectrometer (LCMS-MS)
4.2.4. Pharmacokinetic Analysis
- The terminal data points were apparently randomly distributed about a single straight line (on visual inspection).
- A minimum of three data points was available for the regression.
- The regression coefficients ≥ 0.85.
- The interval including the data points chosen for the regression was at least two-fold greater than the half-life itself.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| AIDS | Acquired immunodeficiency syndrome |
| iPSCs | Induced pluripotent stem cells |
| PK | Pharmacokinetic |
| RNA | Ribonucleic acid |
| miRNA | MicroRNA |
| TRBP | Transactivation response element RNA-binding protein |
| RISC | RNA-induced Silencing Complex |
| NSAID | Non-steroidal anti-inflammatory drug |
| COX-2 | Cyclooxygenase-2 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| PALS | People living with ALS |
| TDP-43 | TAR DNA-binding protein 43 |
| PgJ2 | 15-deoxy-Δ12,14-prostaglandin J2 |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| ALSFRS-R | Revised Amyotrophic Lateral Sclerosis Functional Rating Scale |
| SVC | Slow vital capacity |
| FDA | Food and Drug Administration |
| MNs | Motor neurons |
| iMNs | Induced motor neurons |
| C9orf72 | Chromosome 9 open reading frame 72 |
| PBMCs | Peripheral blood mononuclear cells |
| DMSO | Dimethyl sulfoxide |
| NINDS | National Institute of Neurological Disorders and Stroke |
| C9 | C9orf72 samples |
| CTRL | Controls |
| shRNA | Short hairpin RNA |
| Oct4 | Octamer-binding transcription factor 4 |
| Sox2 | Sex-determining region Y-box 2 |
| KLF4 | Kruppel-like factor 4 |
| L-Myc | L-myc-1 proto-oncogene protein |
| Lin28 | Lin-28 homolog A protein gene |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| MEM | Minimum essential medium |
| GDNF | Glial cell line-derived neurotrophic factor |
| BDNF | Brain-derived neurotrophic factor |
| CTNF | Ciliary neurotrophic factor |
| RFP | Red fluorescent protein |
| FGF-2 | Fibroblast Growth Factor 2 |
| ANOVA | Analysis of variance |
| PO | Per os |
| NIH | National Institute of Health |
| AAALAC | Association for Assessment and Accreditation of Laboratory Animal Care |
| SOP | Standard operating procedure |
| BW | Body weight |
| API | Active pharmaceutical ingredients |
| LCMS-MS | Liquid Chromatography with Triple Quadrupole Mass Spectrometer |
| mg | Milligram |
| kg | Kilogram |
| ng | Nanogram |
| mL | Milliliter |
| h | Hour |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| CI | Confidence interval |
| AUC | Area under the curve |
| t1/2 | Terminal half life |
| tmax | Transport maximum |
| Cmax | Maximum concentration |
| BBB | Blood–brain barrier |
| MDR1 | Multi-drug resistance 1 |
References
- Brown, R.H., Jr.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 1602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Ndayisaba, A.; Kaindlstorfer, C.; Wenning, G.K. Iron in Neurodegeneration—Cause or Consequence? Front. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef]
- Campos-Melo, D.; Droppelmann, C.A.; He, Z.; Volkening, K.; Strong, M.J. Altered microRNA expression profile in Amyotrophic Lateral Sclerosis: A role in the regulation of NFL mRNA levels. Mol. Brain 2013, 6, 26. [Google Scholar] [CrossRef]
- Shan, G.; Li, Y.; Zhang, J.; Li, W.; Szulwach, K.E.; Duan, R.; Faghihi, M.A.; Khalil, A.M.; Lu, L.; Paroo, Z.; et al. A small molecule enhances RNA interference and promotes microRNA processing. Nat. Biotechnol. 2008, 26, 933–940. [Google Scholar] [CrossRef]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef]
- Galley, H.F.; Dhillon, J.K.; Paterson, R.L.; Webster, N.R. Effect of ciprofloxacin on the activation of the transcription factors nuclear factor kappaB, activator protein-1 and nuclear factor-interleukin-6, and interleukin-6 and interleukin-8 mRNA expression in a human endothelial cell line. Clin. Sci. 2000, 99, 405–410. [Google Scholar]
- Badal, S.; Her, Y.F.; Maher, L.J., 3rd. Nonantibiotic Effects of Fluoroquinolones in Mammalian Cells. J. Biol. Chem. 2015, 290, 22287–22297. [Google Scholar] [CrossRef]
- Nunez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Knopfova, L.; Smarda, J. The use of Cox-2 and PPARgamma signaling in anti-cancer therapies. Exp. Ther. Med. 2010, 1, 257–264. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Tsai, G.; Kuncl, R.W.; Clawson, L.; Cornblath, D.R.; Drachman, D.B.; Pestronk, A.; Stauch, B.L.; Coyle, J.T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1990, 28, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Consilvio, C.; Vincent, A.M.; Feldman, E.L. Neuroinflammation, COX-2, and ALS—A dual role? Exp. Neurol. 2004, 187, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Li, K.; Yuan, D.; Yang, S.; Zhou, L.; Zhao, Y.; Miao, S.; Lv, C.; Zhao, J. COX-2/PGE2 Pathway Inhibits the Ferroptosis Induced by Cerebral Ischemia Reperfusion. Mol. Neurobiol. 2022, 59, 1619–1631. [Google Scholar] [CrossRef]
- Yoshihara, T.; Ishigaki, S.; Yamamoto, M.; Liang, Y.; Niwa, J.; Takeuchi, H.; Doyu, M.; Sobue, G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 2002, 80, 158–167. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Shefner, J.M.; Schoenfeld, D.A.; Zhang, H.; Andreasson, K.I.; Rothstein, J.D.; Drachman, D.B. Trial of celecoxib in amyotrophic lateral sclerosis. Ann. Neurol. 2006, 60, 22–31. [Google Scholar] [CrossRef]
- Schachtel, B.P.; McCabe, D.; Berger, M.; Zhang, R.; Sanner, K.M.; Savino, L.; Rizouk, J.; Schachtel, E.P. Efficacy of low-dose celecoxib in patients with acute pain. J. Pain 2011, 12, 756–763. [Google Scholar] [CrossRef]
- Dey, R.; Sultana, S.; Bishayi, B. Combination treatment of celecoxib and ciprofloxacin attenuates live S. aureus induced oxidative damage and inflammation in murine microglia via regulation of cytokine balance. J. Neuroimmunol. 2018, 316, 23–39. [Google Scholar] [CrossRef]
- Goldshtein, H.; Muhire, A.; Petel Legare, V.; Pushett, A.; Rotkopf, R.; Shefner, J.M.; Peterson, R.T.; Armstrong, G.A.B.; Russek-Blum, N. Efficacy of Ciprofloxacin/Celecoxib combination in zebrafish models of amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1883–1897. [Google Scholar] [CrossRef]
- Salomon-Zimri, S.; Pushett, A.; Russek-Blum, N.; Van Eijk, R.P.A.; Birman, N.; Abramovich, B.; Eitan, E.; Elgrart, K.; Beaulieu, D.; Ennist, D.L.; et al. Combination of ciprofloxacin/celecoxib as a novel therapeutic strategy for ALS. Amyotroph. Lateral. Scler. Front. Degener. 2023, 24, 263–271. [Google Scholar] [CrossRef]
- El Hokayem, J.; Cukier, H.N.; Dykxhoorn, D.M. Blood Derived Induced Pluripotent Stem Cells (iPSCs): Benefits, Challenges and the Road Ahead. J. Alzheimers Dis. Park. 2016, 6, 275. [Google Scholar] [CrossRef]
- Koh, S.; Piedrahita, J.A. Generation of Induced Pluripotent Stem Cells (iPSCs) from Adult Canine Fibroblasts. Methods Mol. Biol. 2015, 1330, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Liang, P.; Wu, J.C. Induced pluripotent stem cells as a disease modeling and drug screening platform. J. Cardiovasc. Pharmacol. 2012, 60, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Ishikawa, M.; Otomo, A.; Atsuta, N.; Nakamura, R.; Akiyama, T.; Hadano, S.; Aoki, M.; Saya, H.; Sobue, G.; et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018, 24, 1579–1589. [Google Scholar] [CrossRef]
- Okano, H.; Yasuda, D.; Fujimori, K.; Morimoto, S.; Takahashi, S. Ropinirole, a New ALS Drug Candidate Developed Using iPSCs. Trends Pharmacol. Sci. 2020, 41, 99–109. [Google Scholar] [CrossRef]
- Morimoto, S.; Takahashi, S.; Ito, D.; Date, Y.; Okada, K.; Kato, C.; Nakamura, S.; Ozawa, F.; Chyi, C.M.; Nishiyama, A.; et al. Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery. Cell Stem Cell 2023, 30, 766–780.E9. [Google Scholar] [CrossRef]
- Amoros, M.A.; Choi, E.S.; Cofre, A.R.; Dokholyan, N.V.; Duzzioni, M. Motor neuron-derived induced pluripotent stem cells as a drug screening platform for amyotrophic lateral sclerosis. Front. Cell Dev. Biol. 2022, 10, 962881. [Google Scholar] [CrossRef]
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards Advanced iPSC-based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021, 27, 263–279. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Marcelin-Jimenez, G.; Angeles, A.P.; Martinez-Rossier, L.; Fernandez, S.A. Ciprofloxacin bioavailability is enhanced by oral co-administration with phenazopyridine: A pharmacokinetic study in a Mexican population. Clin. Drug Investig. 2006, 26, 323–328. [Google Scholar] [CrossRef]
- Gorski, J.C.; Renbarger, J.L.; Vuppalanchi, R.; Miller, M.; Galinsky, R.E.; Hall, S.D. Effect of MDR1 Genotype (G2677T) on the Disposition of Ciprofloxacin in Adults. Clin. Pharmacol. Ther. 2005, 77, 31. [Google Scholar]
- Kalle, A.M.; Rizvi, A. Inhibition of bacterial multidrug resistance by celecoxib, a cyclooxygenase-2 inhibitor. Antimicrob. Agents Chemother. 2011, 55, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, M.R.; Jacob, D.A.; Campos, C.; Miller, D.S.; Maragakis, N.J.; Pasinelli, P.; Trotti, D. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol. Dis. 2012, 47, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Naora, K.; Katagiri, Y.; Ichikawa, N.; Hayashibara, M.; Iwamoto, K. Enhanced entry of ciprofloxacin into the rat central nervous system induced by fenbufen. J. Pharmacol Exp. Ther. 1991, 258, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lin, S.; Staats, K.A.; Li, Y.; Chang, W.H.; Hung, S.T.; Hendricks, E.; Linares, G.R.; Wang, Y.; Son, E.Y.; et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 2018, 24, 313–325. [Google Scholar] [CrossRef]
- Mirian, A.; Moszczynski, A.; Soleimani, S.; Aubert, I.; Zinman, L.; Abrahao, A. Breached Barriers: A Scoping Review of Blood-Central Nervous System Barrier Pathology in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2022, 16, 851563. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomon-Zimri, S.; Kerem, N.; Linares, G.R.; Russek-Blum, N.; Ichida, J.K.; Tracik, F. Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models. Pharmaceuticals 2025, 18, 524. https://doi.org/10.3390/ph18040524
Salomon-Zimri S, Kerem N, Linares GR, Russek-Blum N, Ichida JK, Tracik F. Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models. Pharmaceuticals. 2025; 18(4):524. https://doi.org/10.3390/ph18040524
Chicago/Turabian StyleSalomon-Zimri, Shiran, Nitai Kerem, Gabriel R. Linares, Niva Russek-Blum, Justin K. Ichida, and Ferenc Tracik. 2025. "Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models" Pharmaceuticals 18, no. 4: 524. https://doi.org/10.3390/ph18040524
APA StyleSalomon-Zimri, S., Kerem, N., Linares, G. R., Russek-Blum, N., Ichida, J. K., & Tracik, F. (2025). Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models. Pharmaceuticals, 18(4), 524. https://doi.org/10.3390/ph18040524






