Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials
Abstract
1. Introduction
1.1. Glaucoma
1.1.1. Anatomy and Physiology
1.1.2. Primary Open-Angle Glaucoma
1.1.3. Pathogenesis of POAG
1.1.4. Diagnosis
1.2. The Natural History of POAG
1.2.1. Medical Treatments for POAG
1.2.2. Surgical Treatments for POAG
1.3. Approved ROCK Inhibitors
1.3.1. Mechanism of Action of ROCK Inhibitors
1.3.2. Increased Ocular Perfusion
1.3.3. Neuroprotective Effects of ROCK Inhibitors
1.3.4. Cellular Effects of ROCK Inhibitors
1.4. Review Aims
2. Results
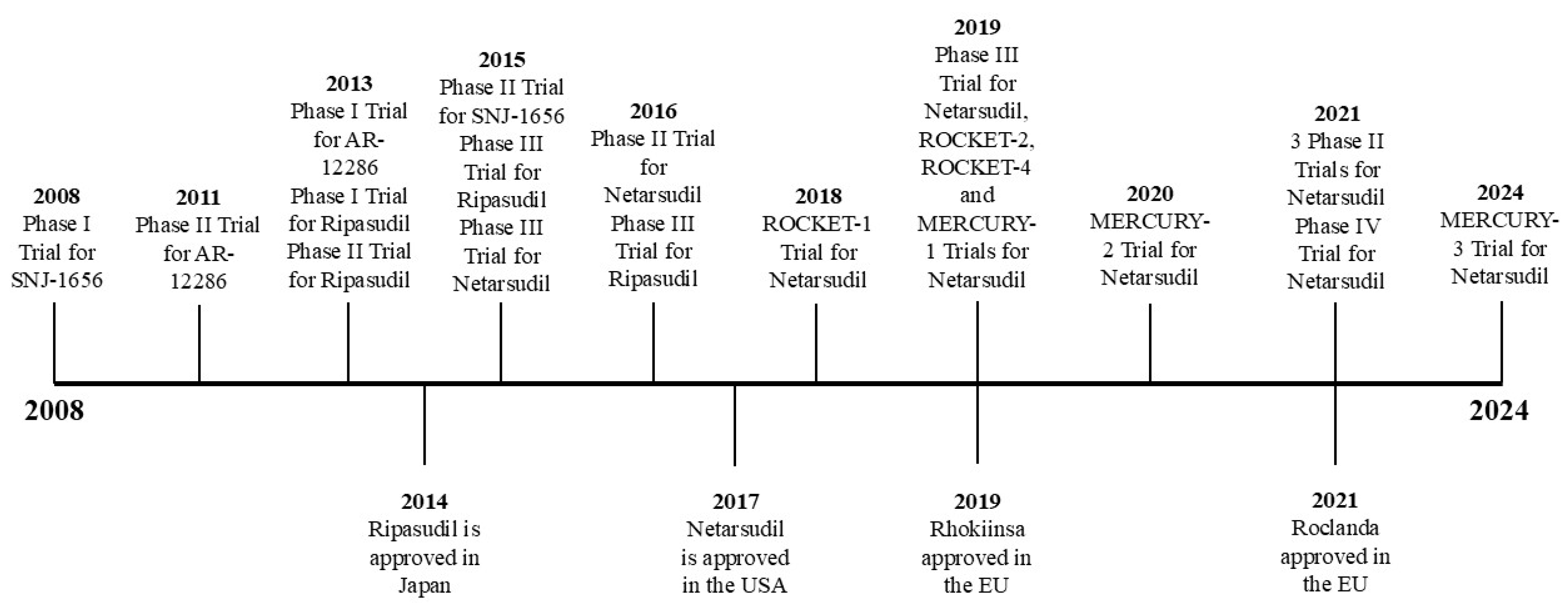
2.1. ROCK Inhibitor Trials
2.2. Patient Characteristics
2.3. Efficacy and Safety of ROCK Inhibitors
2.4. Efficacy of ROCK Inhibitors
2.4.1. ROCK Inhibitors vs. Placebo
2.4.2. ROCK Inhibitor Monotherapy vs. Other
2.4.3. ROCK Inhibitor Combination Therapy vs. Other
2.4.4. Adverse Events
2.5. Anti-Fibrotic Effects of ROCK Inhibitors After Glaucoma Filtration Surgery
2.6. The Economy of ROCK Inhibitors
3. Discussion
General Limitations of the Trials
4. Conclusions
Future Directions
5. Materials and Methods
Inclusion and Exclusion Criteria
- Safety and efficacy outcomes of rho kinase inhibitor trials.
- Comparison of rho kinase inhibitors with conventional treatments for primary open-angle glaucoma.
- Additional benefits of rho kinase inhibitors in glaucoma management, including neuroprotective and anti-fibrotic effects.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Embase 1974–5 April 2022 | ||
| Search Lines | Search Terms | Search Results |
| 1 | glaucoma * | 100,842 |
| 2 | exp glaucoma/ | 91,390 |
| 3 | 1 or 2 | 105,022 |
| 4 | rho kinase inhibitor | 4177 |
| 5 | rho-kinase inhibitor | 4177 |
| 6 | rock inhibitor | 2146 |
| 7 | ripasudil * | 219 |
| 8 | netarsudil * | 229 |
| 9 | fasudil * | 3030 |
| 10 | 5 or 6 or 7 or 8 or 9 | 7594 |
| 11 | 3 or 10 | 112,087 |
| 12 | 3 and 10 | 529 |
| * Was the symbol used for truncated search terms | ||
Appendix B
| Medline 1946–5 April 2022 | ||
| Search Lines | Search Terms | Search Results |
| 1 | glaucoma * | 77,322 |
| 2 | exp glaucoma/ | 56,949 |
| 3 | 1 or 2 | 77,541 |
| 4 | rho kinase inhibitor | 1589 |
| 5 | rho-kinase inhibitor | 1589 |
| 6 | rock inhibitor | 1366 |
| 7 | ripasudil * | 131 |
| 8 | netarsudil * | 113 |
| 9 | fasudil * | 1374 |
| 10 | 5 or 6 or 7 or 8 or 9 | 3699 |
| 11 | 3 or 10 | 81,018 |
| 12 | 3 and 10 | 222 |
| * Was the symbol used for truncated search terms | ||
References
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Glaucoma. Available online: https://cks.nice.org.uk/topics/glaucoma/background-information/definition/ (accessed on 12 January 2025).
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Keller, K.E.; Acott, T.S. The Juxtacanalicular Region of Ocular Trabecular Meshwork: A Tissue with a Unique Extracellular Matrix and Specialized Function. J. Ocul. Biol. 2013, 1, 3. [Google Scholar]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713, discussion 829–730. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Mt-Isa, S.; Owen, C.G.; Cook, D.G.; Ashby, D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: A Bayesian meta-analysis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4254–4261. [Google Scholar] [CrossRef]
- Abu-Amero, K.; Kondkar, A.A.; Chalam, K.V. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int. J. Mol. Sci. 2015, 16, 28886–28911. [Google Scholar] [CrossRef]
- Craig, J.E.; Han, X.; Qassim, A.; Hassall, M.; Cooke Bailey, J.N.; Kinzy, T.G.; Khawaja, A.P.; An, J.; Marshall, H.; Gharahkhani, P.; et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet. 2020, 52, 160–166. [Google Scholar] [CrossRef]
- Hardy, K.M.; Hoffman, E.A.; Gonzalez, P.; McKay, B.S.; Stamer, W.D. Extracellular trafficking of myocilin in human trabecular meshwork cells. J. Biol. Chem. 2005, 280, 28917–28926. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Cooke Bailey, J.N.; Wareham, N.J.; Scott, R.A.; Simcoe, M.; Igo, R.P., Jr.; Song, Y.E.; Wojciechowski, R.; Cheng, C.Y.; Khaw, P.T.; et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018, 50, 778–782. [Google Scholar] [CrossRef]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Heon, E.; Krupin, T.; Ritch, R.; Kreutzer, D.; et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Ying, X.; Khaw, P.T.; Raisman, G. An energy theory of glaucoma. Glia 2015, 63, 1537–1552. [Google Scholar] [CrossRef]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Hare, W.; WoldeMussie, E.; Lai, R.; Ton, H.; Ruiz, G.; Feldmann, B.; Wijono, M.; Chun, T.; Wheeler, L. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv. Ophthalmol. 2001, 45 (Suppl. S3), S284–S289, discussion S295-286. [Google Scholar] [CrossRef]
- Findl, O.; Strenn, K.; Wolzt, M.; Menapace, R.; Vass, C.; Eichler, H.G.; Schmetterer, L. Effects of changes in intraocular pressure on human ocular haemodynamics. Curr. Eye Res. 1997, 16, 1024–1029. [Google Scholar] [CrossRef]
- Grus, F.H.; Joachim, S.C.; Wuenschig, D.; Rieck, J.; Pfeiffer, N. Autoimmunity and glaucoma. J. Glaucoma 2008, 17, 79–84. [Google Scholar] [CrossRef]
- Mursch-Edlmayr, A.S.; Bolz, M.; Strohmaier, C. Vascular Aspects in Glaucoma: From Pathogenesis to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4662. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X. T Cell-Mediated Autoimmunity in Glaucoma Neurodegeneration. Front. Immunol. 2021, 12, 803485. [Google Scholar] [CrossRef]
- Chauhan, B.C.; O’Leary, N.; AlMobarak, F.A.; Reis, A.S.C.; Yang, H.; Sharpe, G.P.; Hutchison, D.M.; Nicolela, M.T.; Burgoyne, C.F. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology 2013, 120, 535–543. [Google Scholar] [CrossRef]
- Yu, M.; Lin, C.; Weinreb, R.N.; Lai, G.; Chiu, V.; Leung, C.K. Risk of Visual Field Progression in Glaucoma Patients with Progressive Retinal Nerve Fiber Layer Thinning: A 5-Year Prospective Study. Ophthalmology 2016, 123, 1201–1210. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B. Early Manifest Glaucoma Trial: Design and baseline data. Ophthalmology 1999, 106, 2144–2153. [Google Scholar] [CrossRef]
- Heijl, A.; Bengtsson, B.; Hyman, L.; Leske, M.C.; Early Manifest Glaucoma Trial, G. Natural history of open-angle glaucoma. Ophthalmology 2009, 116, 2271–2276. [Google Scholar] [CrossRef]
- Wilson, M.R.; Kosoko, O.; Cowan, C.L., Jr.; Sample, P.A.; Johnson, C.A.; Haynatzki, G.; Enger, C.; Crandall, D. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am. J. Ophthalmol. 2002, 134, 399–405. [Google Scholar] [CrossRef]
- Lee, A.J.; Goldberg, I. Emerging drugs for ocular hypertension. Expert. Opin. Emerg. Drugs 2011, 16, 137–161. [Google Scholar] [CrossRef]
- Fogagnolo, P.; Rossetti, L. Medical treatment of glaucoma: Present and future. Expert. Opin. Investig. Drugs 2011, 20, 947–959. [Google Scholar] [CrossRef]
- Cvenkel, B.; Kolko, M. Current Medical Therapy and Future Trends in the Management of Glaucoma Treatment. J. Ophthalmol. 2020, 2020, 6138132. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Skuta, G.L.; Parrish, R.K., 2nd. Wound healing in glaucoma filtering surgery. Surv. Ophthalmol. 1987, 32, 149–170. [Google Scholar] [CrossRef]
- Lee, S.; Park, D.Y.; Huh, M.G.; Cha, S.C. Influence of preoperative glaucoma medication on long-term outcomes of trabeculectomy. Sci. Rep. 2024, 14, 28341. [Google Scholar] [CrossRef]
- Glaukos. iDose® TR Prescribing Information. Available online: https://www.glaukos.com/prescribing-information/idosetr/ (accessed on 15 January 2025).
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef]
- Moshirfar, M.; Parker, L.; Birdsong, O.C.; Ronquillo, Y.C.; Hofstedt, D.; Shah, T.J.; Gomez, A.T.; Hoopes, P.C., Sr. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 101–111. [Google Scholar]
- Agency, E.M. Rhokiinsa. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rhokiinsa (accessed on 25 March 2024).
- Agency, E.M. Roclanda. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/roclanda (accessed on 11 December 2024).
- Gieser, D.K.; Tracy Williams, R.; O’Connell, W.; Pasquale, L.R.; Rosenthal, B.P.; Walt, J.G.; Katz, L.M.; Siegartel, L.R.; Wang, L.; Rosenblatt, L.C.; et al. Costs and utilization of end-stage glaucoma patients receiving visual rehabilitation care: A US multisite retrospective study. J. Glaucoma 2006, 15, 419–425. [Google Scholar] [CrossRef]
- Rosenthal, R.; Choritz, L.; Schlott, S.; Bechrakis, N.E.; Jaroszewski, J.; Wiederholt, M.; Thieme, H. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp. Eye Res. 2005, 80, 837–845. [Google Scholar] [CrossRef]
- Waki, M.; Yoshida, Y.; Oka, T.; Azuma, M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr. Eye Res. 2001, 22, 470–474. [Google Scholar] [CrossRef]
- Al-Humimat, G.; Marashdeh, I.; Daradkeh, D.; Kooner, K. Investigational Rho Kinase Inhibitors for the Treatment of Glaucoma. J. Exp. Pharmacol. 2021, 13, 197–212. [Google Scholar] [CrossRef]
- Nourinia, R.; Nakao, S.; Zandi, S.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. ROCK inhibitors for the treatment of ocular diseases. Br. J. Ophthalmol. 2018, 102. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Novel therapies for glaucoma: A patent review 2007–2011. Expert Opin. Ther. Pat. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Loge, C.; Wallez, V.; Scalbert, E.; Cario-Tourmaniantz, C.; Loirand, G.; Pacaud, P.; Lesieur, D. Rho-kinase inhibitors: Pharmacomodulations on the lead compound Y-32885. J. Enzym. Inhib. Med. Chem. 2002, 17, 381–390. [Google Scholar] [CrossRef]
- Moura-Coelho, N.; Tavares Ferreira, J.; Bruxelas, C.P.; Dutra-Medeiros, M.; Cunha, J.P.; Pinto Proenca, R. Rho kinase inhibitors-a review on the physiology and clinical use in Ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1101–1117. [Google Scholar] [CrossRef]
- Stefansson, E.; Pedersen, D.B.; Jensen, P.K.; la Cour, M.; Kiilgaard, J.F.; Bang, K.; Eysteinsson, T. Optic nerve oxygenation. Prog. Retin. Eye Res. 2005, 24, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Weinreb, R.N. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv. Ophthalmol. 1994, 39, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Budzyn, K.; Marley, P.D.; Sobey, C.G. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol. Sci. 2006, 27, 97–104. [Google Scholar] [CrossRef]
- Tokushige, H.; Waki, M.; Takayama, Y.; Tanihara, H. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr. Eye Res. 2011, 36, 964–970. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Goldhagen, B.; Proia, A.D.; Epstein, D.L.; Rao, P.V. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. J. Glaucoma 2012, 21, 530–538. [Google Scholar] [CrossRef]
- Mimura, F.; Yamagishi, S.; Arimura, N.; Fujitani, M.; Kubo, T.; Kaibuchi, K.; Yamashita, T. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J. Biol. Chem. 2006, 281, 15970–15979. [Google Scholar] [CrossRef]
- Van de Velde, S.; De Groef, L.; Stalmans, I.; Moons, L.; Van Hove, I. Towards axonal regeneration and neuroprotection in glaucoma: Rho kinase inhibitors as promising therapeutics. Prog. Neurobiol. 2015, 131, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Di Polo, A.; McKerracher, L. Enhanced survival and regeneration of axotomized retinal neurons by repeated delivery of cell-permeable C3-like Rho antagonists. Neurobiol. Dis. 2007, 25, 65–72. [Google Scholar] [CrossRef]
- Dergham, P.; Ellezam, B.; Essagian, C.; Avedissian, H.; Lubell, W.D.; McKerracher, L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002, 22, 6570–6577. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Inatani, M.; Inomata, Y.; Yonemura, N.; Kawaji, T.; Honjo, M.; Tanihara, H. Y-27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Sang, A.; Wang, Y.; Ho, D.; Douglas, C.; Dia, L.; Goldberg, J.L. Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp. Eye Res. 2017, 158, 33–42. [Google Scholar] [CrossRef]
- Tura, A.; Schuettauf, F.; Monnier, P.P.; Bartz-Schmidt, K.U.; Henke-Fahle, S. Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 452–461. [Google Scholar] [CrossRef]
- Yamamoto, K.; Maruyama, K.; Himori, N.; Omodaka, K.; Yokoyama, Y.; Shiga, Y.; Morin, R.; Nakazawa, T. The novel Rho kinase (ROCK) inhibitor K-115: A new candidate drug for neuroprotective treatment in glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7126–7136. [Google Scholar] [CrossRef]
- Thomas, N.M.; Nagrale, P. Rho Kinase Inhibitors as a Neuroprotective Pharmacological Intervention for the Treatment of Glaucoma. Cureus 2022, 14, e28445. [Google Scholar] [CrossRef]
- Chircop, M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases 2014, 5, e29770. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Kopczynski, C. Effects of netarsudil on actin-driven cellular functions in normal and glaucomatous trabecular meshwork cells: A live imaging study. J. Clin. Med. 2020, 9, 3524. [Google Scholar] [CrossRef]
- Tian, B.; Kaufman, P.L. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert. Rev. Ophthalmol. 2012, 7, 177–187. [Google Scholar] [CrossRef]
- Wang, W.; Halasz, E.; Townes-Anderson, E. Actin Dynamics, Regulated by RhoA-LIMK-Cofilin Signaling, Mediates Rod Photoreceptor Axonal Retraction After Retinal Injury. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2274–2285. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Shibata, M.; Kajiura, S.; Okuno, T.; Tonari, M.; Oku, H.; Ikeda, T. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Investig. Ophthalmol. Vis. Sci. 2011, 52, 64–69. [Google Scholar] [CrossRef]
- Ibrahim, D.G.; Ko, J.A.; Iwata, W.; Okumichi, H.; Kiuchi, Y. An in vitro study of scarring formation mediated by human Tenon fibroblasts: Effect of Y-27632, a Rho kinase inhibitor. Cell Biochem. Funct. 2019, 37, 113–124. [Google Scholar] [CrossRef]
- Ko, J.; Ibrahim, D.G.; Kiuchi, Y. Effects of rho-associated protein kinase inhibitor Y-27632 on scarring formation after glaucoma filtration surgery. Molecular Biology of the Cell. Conference 2017, 28. [Google Scholar] [CrossRef]
- Huang, T.Y.; DerMardirossian, C.; Bokoch, G.M. Cofilin phosphatases and regulation of actin dynamics. Curr. Opin. Cell Biol. 2006, 18, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. [Google Scholar] [CrossRef]
- Johnson, M.; Shapiro, A.; Ethier, C.R.; Kamm, R.D. Modulation of outflow resistance by the pores of the inner wall endothelium. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1670–1675. [Google Scholar]
- Overby, D.R.; Zhou, E.H.; Vargas-Pinto, R.; Pedrigi, R.M.; Fuchshofer, R.; Braakman, S.T.; Gupta, R.; Perkumas, K.M.; Sherwood, J.M.; Vahabikashi, A.; et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 13876–13881. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Tanihara, H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn. J. Ophthalmol. 2018, 62, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp. Eye Res. 2017, 158, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wiederholt, M.; Thieme, H.; Stumpff, F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog. Retin. Eye Res. 2000, 19, 271–295. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef]
- Rao, V.P.; Epstein, D.L. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs 2007, 21, 167–177. [Google Scholar] [CrossRef]
- Challa, P.; Arnold, J.J. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert. Opin. Investig. Drugs 2014, 23, 81–95. [Google Scholar] [CrossRef]
- Hein, T.W.; Rosa, R.H., Jr.; Yuan, Z.; Roberts, E.; Kuo, L. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1583–1590. [Google Scholar] [CrossRef]
- Ohta, Y.; Takaseki, S.; Yoshitomi, T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn. J. Ophthalmol. 2017, 61, 423–432. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Flammer, J. Blood flow in glaucoma. Curr. Opin. Ophthalmol. 2005, 16, 79–83. [Google Scholar] [CrossRef]
- Lee, S.S.; Robinson, M.R.; Weinreb, R.N. Episcleral Venous Pressure and the Ocular Hypotensive Effects of Topical and Intracameral Prostaglandin Analogs. J. Glaucoma 2019, 28, 846–857. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Tanihara, H.; Inatani, M.; Honjo, M.; Tokushige, H.; Azuma, J.; Araie, M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008, 126, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 1 clinical trials of a selective rho kinase inhibitor, k-115. JAMA Ophthalmol. 2013, 131, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, J.; Dubiner, H.B.; Levy, B.; Kopczynski, C.C.; Novack, G.D. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology 2015, 122, 302–307. [Google Scholar] [CrossRef]
- Inoue, T.; Tanihara, H.; Tokushige, H.; Araie, M. Efficacy and safety of SNJ-1656 in primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2015, 93, e393–e395. [Google Scholar] [CrossRef]
- Kopczynski, C.; Novack, G.D.; Swearingen, D.; van Haarlem, T. Ocular hypotensive efficacy, safety and systemic absorption of AR-12286 ophthalmic solution in normal volunteers. Br. J. Ophthalmol. 2013, 97, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.D.; Novack, G.D.; Van Haarlem, T.; Kopczynski, C. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol. 2011, 152, 834–841.e831. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 randomized clinical study of a Rho kinase inhibitor, k-115, in primary open-angle glaucoma and ocular hypertension. Am. J. Ophthalmol. 2013, 156, 731–736.e732. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 h in primary open-angle glaucoma and ocular hypertension: A randomized, open-label, crossover study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Fukushima, A.; Suganami, H.; Araie, M. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016, 94, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: A report of 2 randomized clinical trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef]
- Muhlisah, A.; Hirooka, K.; Nurtania, A.; Onoe, H.; Okumichi, H.; Nitta, E.; Baba, T.; Tanito, M.; Matsuoka, Y.; Nakakura, S.; et al. Effect of ripasudil after trabeculectomy with mitomycin C: A multicentre, randomised, prospective clinical study. BMJ Open Ophthalmol. 2024, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Yamamoto, T.; Aihara, M.; Koizumi, N.; Minami, H.; Kojima, S.; Isobe, T.; Kanazawa, M.; Suganami, H.; Group, K.C.S. Crossover Randomized Study of Pharmacologic Effects of Ripasudil-Brimonidine Fixed-Dose Combination Versus Ripasudil or Brimonidine. Adv. Ther. 2023, 40, 3559–3573. [Google Scholar] [CrossRef] [PubMed]
- Araie, M.; Sugiyama, K.; Aso, K.; Kanemoto, K.; Kothapalli, K.; Kopczynski, C.; Senchyna, M.; Hollander, D.A. Phase 2 Randomized Clinical Study of Netarsudil Ophthalmic Solution in Japanese Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. Adv. Ther. 2021, 38, 1757–1775. [Google Scholar] [CrossRef]
- Serle, J.B.; Katz, L.J.; McLaurin, E.; Heah, T.; Ramirez-Davis, N.; Usner, D.W.; Novack, G.D.; Kopczynski, C.C. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients with Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 2018, 186, 116–127. [Google Scholar] [CrossRef]
- Kahook, M.Y.; Serle, J.B.; Mah, F.S.; Kim, T.; Raizman, M.B.; Heah, T.; Ramirez-Davis, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D. Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am. J. Ophthalmol. 2019, 200, 130–137. [Google Scholar] [CrossRef]
- Lewis, R.A.; Levy, B.; Ramirez, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D. Fixed-dose combination of AR-13324 and latanoprost: A double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br. J. Ophthalmol. 2016, 100, 339–344. [Google Scholar] [CrossRef]
- Pharmaceuticals, A. Study of Netarsudil (AR-13324) Ophthalmic Solution in Patients with Glaucoma or Ocular Hypertension (ROCKET-3). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02246764 (accessed on 24 December 2024).
- Peace, J.H.; McKee, H.J.; Kopczynski, C.C. A Randomized, Phase 2 Study of 24-h Efficacy and Tolerability of Netarsudil in Ocular Hypertension and Open-Angle Glaucoma. Ophthalmol. Ther. 2021, 10, 89–100. [Google Scholar] [CrossRef]
- Sit, A.J.; Gupta, D.; Kazemi, A.; McKee, H.; Challa, P.; Liu, K.C.; Lopez, J.; Kopczynski, C.; Heah, T. Netarsudil Improves Trabecular Outflow Facility in Patients with Primary Open Angle Glaucoma or Ocular Hypertension: A Phase 2 Study. Am. J. Ophthalmol. 2021, 226, 262–269. [Google Scholar] [CrossRef]
- Khouri, A.S.; Serle, J.B.; Bacharach, J.; Usner, D.W.; Lewis, R.A.; Braswell, P.; Kopczynski, C.C.; Heah, T.; Benza, R.; Boyle, J.W.; et al. Once-Daily Netarsudil Versus Twice-Daily Timolol in Patients With Elevated Intraocular Pressure: The Randomized Phase 3 ROCKET-4 Study. Am. J. Ophthalmol. 2019, 204, 97–104. [Google Scholar] [CrossRef]
- Asrani, S.; Robin, A.L.; Serle, J.B.; Lewis, R.A.; Usner, D.W.; Kopczynski, C.C.; Heah, T.; Ackerman, S.L.; Alpern, L.M.; Bashford, K.; et al. Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure: Three-Month Data from a Randomized Phase 3 Trial. Am. J. Ophthalmol. 2019, 207, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.R.; Ahmed, I.I.K.; Lewis, R.A.; Usner, D.W.; Lopez, J.; Kopczynski, C.C.; Heah, T.; Group, M.-S. Once-Daily Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure in the Randomized Phase 3 MERCURY-2 Study. Ophthalmol. Glaucoma 2019, 2, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, I.; Lim, K.S.; Oddone, F.; Fichtl, M.; Belda, J.I.; Hommer, A.; Laganovska, G.; Schweitzer, C.; Voykov, B.; Zarnowski, T.; et al. MERCURY-3: A randomized comparison of netarsudil/latanoprost and bimatoprost/timolol in open-angle glaucoma and ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zaman, F.; Gieser, S.C.; Schwartz, G.F.; Swan, C.; Williams, J.M. A multicenter, open-label study of netarsudil for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension in a real-world setting. Curr. Med. Res. Opin. 2021, 37, 1011–1020. [Google Scholar] [CrossRef]
- Shahid, S.; Rizvi, S.W.A.; Khan, A.A.; Ashraf, H.; Akhter, A. Comparison of safety and efficacy of Netarsudil 0.02% and Bimatoprost 0.01% monotherapy and combination therapy in primary open-angle glaucoma and ocular hypertension. Indian. J. Ophthalmol. 2024, 72, 427–431. [Google Scholar] [CrossRef]
- Clement Freiberg, J.; von Spreckelsen, A.; Kolko, M.; Azuara-Blanco, A.; Virgili, G. Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. 2022, 6, CD013817. [Google Scholar] [CrossRef]
- Ramakrishnan, M.S.; Addis, V.M.; Lehman, A.Y.; Sankar, P.S. Netarsudil-associated epithelial keratopathy. Am. J. Ophthalmol. Case Rep. 2020, 19, 100800. [Google Scholar] [CrossRef]
- Ha, A.; Kim, Y.K.; Jeoung, J.W.; Satyal, S.; Kim, J.; Kim, S.; Park, K.H. Sovesudil (locally acting rho kinase inhibitor) for the treatment of normal-tension glaucoma: The randomized phase II study. Acta Ophthalmol. 2022, 100, e470–e477. [Google Scholar] [CrossRef]
- Agency, E.M. Roclanda: EPAR—Product information. Available online: https://www.ema.europa.eu/en/documents/product-information/roclanda-epar-product-information_en.pdf (accessed on 12 February 2025).
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular benzalkonium chloride exposure: Problems and solutions. Eye 2022, 36, 361–368. [Google Scholar] [CrossRef]
- Futakuchi, A.; Inoue, T.; Fujimoto, T.; Inoue-Mochita, M.; Kawai, M.; Tanihara, H. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp. Eye Res. 2016, 149, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ko, J.A.; Yanai, R.; Kimura, K.; Chikama, T.; Sagara, T.; Nishida, T. Induction by latanoprost of collagen gel contraction mediated by human tenon fibroblasts: Role of intracellular signaling molecules. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1429–1436. [Google Scholar] [CrossRef]
- Mizuno, Y.; Okada, N.; Onoe, H.; Tokumo, K.; Okumichi, H.; Hirooka, K.; Kiuchi, Y. Effect of the rho-kinase inhibitor ripasudil in needling with mitomycin C for the failure of filtering bleb after trabeculectomy: A cross-sectional study. BMC Ophthalmol. 2022, 22, 433. [Google Scholar] [CrossRef]
- Adjei, K.; Ali, A.A. PSS4 A Cost Effectiveness Analysis of Latanoprost Monotherapy, Netarsudil Monotherapy and Fixed Dose Combination Latanoprost/Netarsudil in Management of Primary Open Angle Glaucoma in USA. Value Health 2021, 24 (Suppl. S1), S220–S221. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.G.; Greene, L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e218348. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, G.; Kolko, M.; Iester, M.; Crabb, D.P.; Educational Club of Ocular, S.; Glaucoma, M. A Scoping Review of Quality of Life Questionnaires in Glaucoma Patients. J. Glaucoma 2021, 30, 732–743. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Yin, Y.; Cameron, M.D.; Lin, L.; Khan, S.; Schroter, T.; Grant, W.; Pocas, J.; Chen, Y.T.; Schurer, S.; Pachori, A.; et al. Discovery of Potent and Selective Urea-Based ROCK Inhibitors and Their Effects on Intraocular Pressure in Rats. ACS Med. Chem. Lett. 2010, 1, 175–179. [Google Scholar] [CrossRef]
- Bahr, T.; Woolf, S.; Favre, H.; Waldman, C. Comparison of netarsudil and latanoprostene bunod as adjuncts to maximum medical therapy in primary open-angle glaucoma. Can. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Inoue, K.; Okayama, R.; Shiokawa, M.; Ishida, K.; Tomita, G. Efficacy and safety of adding ripasudil to existing treatment regimens for reducing intraocular pressure. Int. Ophthalmol. 2018, 38, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Enomoto, N.; Ishida, K.; Anraku, A.; Tomita, G. One-Year Efficacy and Safety Assessment of Ripasudil, a Rho Kinase Inhibitor, in an Addition to or Replacing Existing Treatment Regimens: A Retrospective Study. J. Ocul. Pharmacol. Ther. 2020, 36, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Villegas, N.C.; Lee, W.S. Effectiveness of netarsudil as an additional therapy for glaucoma in patients already on maximally tolerated medical therapy. Clin. Ophthalmol. 2021, 15, 4367–4372. [Google Scholar] [CrossRef]
- Andres-Guerrero, V.; Garcia-Feijoo, J.; Konstas, A.G. Targeting Schlemm’s Canal in the Medical Therapy of Glaucoma: Current and Future Considerations. Adv. Ther. 2017, 1–21. [Google Scholar] [CrossRef] [PubMed]




| Study | Phase | Indication | Study Design | Drug | Study Size | Duration of Treatment |
|---|---|---|---|---|---|---|
| SNJ-1656 | ||||||
| Tanihara et al. [87] | I | Healthy volunteers | Randomized, double-masked, group-comparison, placebo–control | Placebo (OD) SNJ-1656 0.003% (OD) SNJ-1656 0.01% (OD) SNJ-1656 0.03% (OD) SNJ-1656 0.05% (OD) SNJ-1656 0.1% (OD) | 45 patients | 7 days followed by 7 days of repeated instillation |
| Inoue et al. [90] | II | POAG OHT | Randomized, double-masked, multicenter, placebo–control | Placebo (BD) SNJ-1656 0.03% (BD) SNJ-1656 0.05% (BD) SNJ-1656 0.1% (BD) | 63 (initially 66 patients) | 7 days |
| AR-12286 | ||||||
| Kopczynski et al. [91] | I | Healthy volunteers | Randomized, double-masked, crossover study | AR-12286 0.5% with 0.0065% BC (OD) AR-12286 0.5% with 0.015% BC (OD) | 18 patients | 2 periods of 8 days with a 7-day washout period in between |
| Williams et al. (NCT00902200) [92] | II | POAG OHT | Randomized, double-masked, multicenter, vehicle–control | Vehicle (OD for 14 days, then BD for 7 days) AR-12286 0.05% (OD for 14 days, then BD for 7 days) AR-12286 0.1% (OD for 14 days, then BD for 7 days) AR-12286 0.25% (OD for 14 days, then BD for 7 days) | 89 patients | 3 consecutive 7-day periods |
| Ripasudil | ||||||
| Tanihara et al. [88] | I | Healthy volunteers | Randomized, double-masked, placebo–control, group comparison | Placebo (OD) Ripasudil 0.05% (OD) Ripasudil 0.1% (OD) Ripasudil 0.2% (OD) Ripasudil 0.4% (OD) Ripasudil 0.8% (OD) | 50 patients | 7 days followed by 7 days of repeated instillation |
| Tanihara et al. [93] | II | POAG OHT | Randomized, double-masked, multicenter, placebo–control, group comparison | Placebo (BD) Ripasudil 0.1% (BD) Ripasudil 0.2% (BD) Ripasudil 0.4% (BD) | 210 patients | 8 weeks |
| Tanihara et al. [94] | III | POAG OHT | Randomized, open label, Latin-square crossover | Placebo (BD) Ripasudil 0.2% (BD) Ripasudil 0.4% (BD) | 28 (initially 43 patients) | 3 periods of 2 days each |
| Tanihara et al. [95] | III | POAG OHT Exfoliation glaucoma Pigmentary glaucoma | Non-randomized, multicenter, open-label | Ripasudil 0.4% (BD) Ripasudil 0.4% with PGA (BD) Ripasudil 0.4% with BB (BD) Ripasudil 0.4% with FC PGA and BB (BD) | 388 patients | 52 weeks |
| Tanihara et al. (JAPIC111700) [96] | III | POAG OHT | Randomized, double-masked, multicenter, placebo–control | Placebo (BD) Ripasudil 0.4% (BD) | 208 patients | 8 weeks |
| Tanihara et al. (JAPIC111700) [96] | III | POAG OHT | Randomized, double-masked, multicenter, placebo–control | Placebo (BD) Ripasudil 0.4% (BD) | 205 patients | 8 weeks |
| Muhlisah et al. (UMIN000019017) [97] | III | POAG | Randomized, multicenter, open-label | Ripasudil (BD) | 90 (initially 122 patients) | 3 months |
| Tanihara et al. (Crossover Study) (jRCT2080225220) [98] | III | Healthy volunteers | Randomized, single-center, open-label | RBFC (BD) Ripasudil (BD) Brimonidine | 18 patients | 8 days |
| Netarsudil | ||||||
| Bacharach et al. (NCT01731002) [89] | III | OAG OHT | Randomized, double-masked, multicenter | Latanoprost 0.005% (OD) Netarsudil 0.01% (OD) Netarsudil 0.02% (OD) | 213 (initially 224 patients) | 28 days |
| Aerie (NCT03310580) [99] | II | OAG OHT | Randomized, double-masked, multicenter | Netarsudil 0.02% (OD) Netarsudil 0.04% (OD) | 42 patients | 28 days |
| Serle et al. (NCT02207491) [100] | III | OAG OHT | Randomized, double-masked, multicenter | Timolol 0.5% (BD) Netarsudil 0.02% (OD) | 411 patients | 3 months |
| Kahook et al. (NCT02207621) [101] | III | OAG OHT | Randomized, double-masked, multicenter | Timolol 0.5% (BD) Netarsudil 0.02% (OD) Netarsudil 0.02% (BD) | 756 patients | 3-month data reported for 12-month trial |
| Lewis et al. (NCT02057575) [102] | II | OAG OHT | Randomized, double-masked, multicenter | Latanoprost 0.005% and Netarsudil 0.01% (OD) Latanoprost 0.005% and Netarsudil 0.02% (OD) Latanoprost 0.005% (OD) Netarsudil 0.02% (OD) | 292 (initially 298 patients) | 28 days |
| Aerie (NCT02246764) [103] | III | OAG OHT | Randomized, double-masked, multicenter | Netarsudil 0.02% and Placebo (OD) Netarsudil 0.02% (BD) Timolol 0.5% (BD) | 83 patients | 12 months |
| Peace et al. (NCT02874846) [104] | II | OAG OHT | Randomized, double-masked, vehicle-control | Vehicle (OD) Netarsudil 0.02% (OD) | 12 patients | 9 days |
| Sit et al. (NCT03233308) [105] | II | POAG OHT | Randomized, double-masked, vehicle–control | Vehicle (OD) Netarsudil 0.02% (OD) | 40 patients | 7 days |
| Araie et al. (NCT03844945) [99] | II | POAG OHT | Randomized, double-masked, parallel-group, placebo–control | Placebo (OD) Netarsudil 0.01% (OD) Netarsudil 0.02% (OD) Netarsudil 0.04% (OD) | 207 (initially 215 patients) | 4 weeks |
| Khouri et al. (NCT02558374) [106] | III | OAG OHT | Randomized, double-masked | Timolol 0.5% (BD) Netarsudil 0.02% (OD) | 708 patients | 6 months |
| Asrani et al. (NCT02558400) [107] | III | OAG OHT | Randomized, double-masked | Latanoprost 0.005%and Netarsudil 0.02% FC (OD) Latanoprost 0.005% (OD) Netarsudil 0.02% (OD) | 718 patients | 3-month data reported for 12-month trial |
| Walters et al. (NCT02674854) [108] | III | OAG OHT | Randomized, double-masked | Latanoprost 0.005% FC and Netarsudil 0.02% (OD) Latanoprost 0.005% (OD) Netarsudil 0.02% (OD) | 750 patients | 3 months |
| Stalmans et al. (NCT03284853) [109] | III | OAG OHT | Randomized, double-masked, parallel-group, multicenter, active-control | Latanoprost 0.005% and netarsudil 0.02% FC (OD) Bimatoprost 0.03% and timolol 0.5% FC (OD) | 430 patients | 6 months |
| Zaman et al. (NCT03808688) [110] | IV | OAG OHT | Prospective, interventional, open-label, multicenter | Netarsudil 0.02% (OD) | 242 (initially 262 patients) | 12 weeks |
| Shahid et al. [111] | III | OAG OHT | Randomized, parallel-group, open-label | Netarsudil 0.02% (OD) Netarsudil 0.02% and bimatoprost 0.01% (OD) Bimatoprost 0.01% (OD) | 109 (initially 133 patients) | 12 weeks |
| Study | Duration of Follow-Up | Baseline IOP Inclusion Range | Drug (Number of Participants) | Baseline IOP mmHg (SD) | Efficacy mmHg (SD) | Frequent Adverse Events |
|---|---|---|---|---|---|---|
| SNJ-1656 | ||||||
| Tanihara et al. [87] | 7 days | - | Change in IOP from baseline after 2 h | Conjunctival hyperemia | ||
| Placebo (15) | 14.1 (2.5) | −0.9 | 0.0% | |||
| SNJ-1656 0.003% (6) | 14.1 (1.4) | −1.2 | 16.7% | |||
| SNJ-1656 0.01% (6) | 13.7 (1.5) | −1.5 | 0.0% | |||
| SNJ-1656 0.03% (6) | 13.7 (2.2) | −2.2 | 33.3% | |||
| SNJ-1656 0.05% (6) | 13.2 (1.4) | −1.5 | 83.3% | |||
| SNJ-1656 0.1% (6) | 13.4 (2.7) | −2.0 | 100.0% | |||
| Inoue et al. [90] | 7 days | 22 ≤ IOP ≤ 31 mmHg | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| Placebo (16) | ~22.5 | −1.5 (2.2) | Not reported | |||
| SNJ-1656 0.03% (15) | −5.0 (2.4) | 60.0% | ||||
| SNJ-1656 0.05% (14) | −4.4 (2.7) | 100.0% | ||||
| SNJ-1656 0.1% (18) | −4.5 (1.9) | 83.0% | ||||
| AR-12286 | ||||||
| Kopczynski et al. [91] | 8 days | 14 ≤ IOP ≤ 20 mmHg | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| AR-12286 0.5% with 0.015% BC (9) | 17.0 (2.3) | −7.2 (2.0) | 6.0% | |||
| AR-12286 0.5% with 0.0065% BC (9) | −6.9 (1.8) | 0.0% | ||||
| Williams et al. (NCT00902200) [92] | 22 days | 21 ≤ IOP ≤ 24 mmHg | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| Vehicle (22) | 26.3 (2.5) | −2.4 | 9.1% | |||
| AR-12286 0.05% (22) | 26.0 (2.2) | −4.1 | 27.3% | |||
| AR-12286 0.1% (23) | 27.3 (3.2) | −5.0 | 39.1% | |||
| AR-12286 0.25% (22) | 26.9 (2.0) | −6.0 | 59.1% | |||
| Ripasudil | ||||||
| Tanihara et al. [88] | 7 days | ≥13 mmHg | Change in IOP from baseline after 2 h | Conjunctival hyperemia | ||
| Placebo (9) | 15.1 (2.3) | −1.6 | 0.0% | |||
| 0.05% Ripasudil (8) | 14.3 (2.5) | −3.4 | 62.5% | |||
| Ripasudil 0.1% (7) | 13.1 (1.8) | −2.2 | 57.1% | |||
| Ripasudil 0.2% (8) | 14.5 (2.5) | −2.6 | 62.5% | |||
| Ripasudil 0.4% (7) | 13.9 (1.5) | −4.0 | 28.6% | |||
| Ripasudil 0.8% (8) | 13.1 (1.4) | −4.3 | 87.5% | |||
| Tanihara et al. [93] | 8 weeks | 21 < IOP < 35 mmHg | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| Placebo (54) | 23.0 (2.1) | −2.5 | 13% | |||
| Ripasudil 0.1% (53) | 23.3 (2.4) | −3.7 | 43% | |||
| Ripasudil 0.2% (54) | 23.2 (2.0) | −4.2 | 57% | |||
| Ripasudil 0.4% (49) | 23.2 (1.9) | −4.5 | 65% | |||
| Tanihara et al. [94] | 2 days | 21 < IOP < 30 mmHg | Change in IOP from baseline after 2 h | Conjunctival hyperemia | ||
| Placebo (28) | 22.0 (2.1) | −4.1 | 10.7% | |||
| Ripasudil 0.2% (28) | −6.8 | 78.6% | ||||
| Ripasudil 0.4% (28) | −7.3 | 96.4% | ||||
| Tanihara et al. [95] | 52 weeks | 15 < IOP < 35 mmHg | Change in IOP from baseline at peak | Conjunctival hyperemia (74.6%) Blepharitis (20.6%) Allergic conjunctivitis (17.2%) | ||
| Ripasudil 0.4% (173) | 19.3 (2.7) | −3.7 | ||||
| Ripasudil 0.4% + with PGA (62) | 17.6 (2.0) | −2.4 | ||||
| Ripasudil 0.4% + BB (60) | 18.2 (2.3) | −2.0 | ||||
| Ripasudil 0.4% + FC PGA + BB (59) | 17.6 (2.0) | −1.7 | ||||
| Tanihara et al. (JAPIC111700) [96] | 8 weeks | IOP ≥ 18 mmHg on Timolol | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| Placebo (104) | 19.7 (1.7) | −1.3 | 5.8% | |||
| Ripasudil 0.4% (104) | 19.9 (1.9) | −2.9 | 65.4% | |||
| Tanihara et al. (JAPIC111701) [96] | 8 weeks | IOP ≥ 18 mmHg on Latanoprost | Change in IOP from baseline at peak | Conjunctival hyperemia | ||
| Placebo (103) | 19.6 (1.9) | −1.8 | 8.7% | |||
| Ripasudil 0.4% (102) | 20.1 (1.9) | −3.2 | 55.9% | |||
| Muhlisah et al. (UMIN000019017) [97] | 3 months | - | Change in IOP from baseline at end of study | Hypotony, choroidal detachment | ||
| Control (52) | 16.2 (4.4) | −5.3 | 19.2%, 3.8% | |||
| Ripasudil (38) | 16.8 (5.0) | −6.2 | 18.4%, 10.5% | |||
| Tanihara et al. (Crossover Trial) (jRCT2080225220) [98] | 8 days | IOP > 15 mmHg | Change in IOP from baseline at end of study | Conjunctival hyperemia | ||
| RBFC (6) | 12.7 (2.4) | −3.7 | 100.0% | |||
| Ripasudil (6) | Not recorded | Not recorded | 100.0% | |||
| Brimonidine (6) | Not recorded | Not recorded | 58.8% | |||
| Netarsudil | ||||||
| Bacharach et al. (NCT01731002) [89] | 28 days | 24 ≤ IOP ≤ 36 mmHg | Change in IOP from baseline after 28 days | Conjunctival hyperemia | ||
| Netarsudil 0.01% (74) | 25.8 | −5.4 | 52.0% | |||
| Netarsudil 0.02% (72) | 25.6 | −5.9 | 57.0% | |||
| Latanoprost 0.005% (77) | 25.5 | −6.8 | 16.0% | |||
| Aerie (NCT03310580) [99] | 28 days | 15 ≤ IOP < 30 mmHg | Mean diurnal iop at 28 days | Conjunctival hyperemia | ||
| Placebo (15) | 17.3 | 0.0% | ||||
| Netarsudil 0.02% (15) | 14.4 | 66.7% | ||||
| Netarsudil 0.04% (12) | 14.3 | 71.4% | ||||
| Serle et al. (NCT02207491) [100] | 3 months | 17 < IOP < 27 mmHg | Change in IOP from baseline after 3 months (diurnal range) | Conjunctival hyperemia, conjunctival hemorrhage | ||
| Netarsudil 0.02% (202) | 22.5 | −3.3 to −5.0 | 53.0%, 13.0% | |||
| Timolol 0.5% (209) | 22.3 | −3.7 to −5.1 | 7.0%, 0.5% | |||
| Kahook et al. (NCT02207621) [101] | 3 months | 17 < IOP < 27 mmHg | Change in IOP from baseline after 3 months (diurnal range) | Conjunctival hyperemia, conjunctival hemorrhage, corneal verticillata | ||
| Netarsudil 0.02% (251) | 21.4 | −3.3 to −4.6 | 50.0%, 15.0%, 9.0% | |||
| Netarsudil 0.02% (254) | 21.5 | −4.1 to −5.4 | 59.0%, 17.0%, 15.0% | |||
| Timolol 0.5% (251) | 21.5 | −3.7 to −5.1 | 10.0%, 0.0%, 0.4% | |||
| Lewis et al. (NCT02057575) [102] | 28 days | 24 ≤ IOP < 36 mmHg | Change in IOP from baseline after 28 days | Conjunctival hyperemia | ||
| Latanoprost and netarsudil 0.01% (74) | 25.1 (2.3) | −7.8 | 41.0% | |||
| Latanoprost and netarsudil 0.02% (73) | 25.1 (2.4) | −8.6 | 40.0% | |||
| Latanoprost (73) | 26.0 (2.8) | −7.6 | 14.0% | |||
| Netarsudil 0.02% (78) | 25.4 (2.7) | −6.3 | 40.0% | |||
| Peace et al. (NCT02874846) [104] | 9 days | 17 < IOP < 30 mmHg | Change in IOP from nocturnal baseline | None | ||
| Vehicle (4) | 22.9 (1.3) | −3.5 | ||||
| Netarsudil 0.2% (8) | 22.4 (2.1) | −0.4 | ||||
| Sit et al. (NCT03233308) [105] | 7 days | 17 < IOP < 30 mmHg | Change in IOP from baseline after 7 days | Conjunctival hyperemia | ||
| Vehicle (18) | 23.0 (1.4) | −4.5 | 0.0% | |||
| Netarsudil 0.2% (18) | 22.9 (1.6) | −1.0 | 72.2% | |||
| Araie et al. (NCT03844945) [99] | 4 weeks | 14 < IOP < 30 mmHg | Change in IOP from baseline after 4 weeks | Conjunctival hyperemia | ||
| Placebo (55) | 21.1 (3.7) | −1.7 (1.8) | 1.8% | |||
| Netarsudil 0.01% (54) | 20.5 (2.8) | −4.1 (2.1) | 23.6% | |||
| Netarsudil 0.02% (51) | 20.3 (2.8) | −4.8 (1.8) | 37.0% | |||
| Netarsudil 0.04% (55) | 20.8 (3.2) | −4.8 (2.2) | 56.9% | |||
| Khouri et al. (NCT02558374) [106] | 6 months | 20 < IOP < 30 mmHg | Change in IOP from baseline after 6 months | Conjunctival hyperemia | ||
| Netarsudil 0.02% OD (351) | 22.4 | −3.9 to −4.7 | 47.9% | |||
| Timolol 0.5% BD (357) | 22.4 | −3.8 to −5.2 | 9.2% | |||
| Asrani et al. (NCT02558400) [107] | 3 months | 20 < IOP < 36 mmHg | Change in IOP from baseline after 3 months (mean diurnal) | Conjunctival hyperemia | ||
| Latanoprost 0.005% and netarsudil 0.02% FC OD (238) | 23.7 | −8.1 | 53.4% | |||
| Latanoprost 0.005% OD (243) | 23.6 | −5.5 | 41.0% | |||
| Netarsudil 0.02% OD (237) | 23.5 | −6.4 | 14.0% | |||
| Walters et al. (NCT02674854) [108] | 3 months | 20 < IOP < 36 mmHg | Change in IOP from baseline after 3 months (mean diurnal) | Conjunctival hyperemia | ||
| Latanoprost 0.005% and netarsudil 0.02% FC OD (245) | 23.5 | −7.6 | 54.5% | |||
| Latanoprost 0.005% OD (255) | 23.6 | −5.0 | 42.7% | |||
| Netarsudil 0.02% OD (250) | 23.5 | −6.0 | 22.3% | |||
| Stalmans et al. (NCT03284853) [109] | 6 months | IOP ≥ 17 mmHg in at least one eye IOP < 28 mmHg in both eyes | Change in IOP from baseline after 3 months | Conjunctival hyperemia, cornea verticillata | ||
| Latanoprost 0.005% and netarsudil 0.02% FC OD (218) | 25.1 (3.4) | −9.9 | 30.7%, 11.0% | |||
| Bimatoprost 0.03% and timolol 0.5% FC OD (212) | 24.8 (3.3) | −10.4 | 9.0%, 0.0% | |||
| Zaman et al. (NCT03808688) [110] | 12 weeks | - | Change in IOP from Baseline after 12 weeks | Conjunctival hyperemia | ||
| Netarsudil 0.02% (24) | 19.4 (4.5) | −3.9 (3.6) | 22.2% | |||
| Netarsudil 0.02% replacing PGA (57) | −0.6 (3.1) | |||||
| Netarsudil 0.02% replacing 2 concomitant therapies (6) | 0.0 (1.6) | |||||
| Netarsudil 0.02% replacing non-PGA monotherapy (4) | 0.5 (1.7) | |||||
| Netarsudil 0.02% and PGA (55) | 20.3 (5.0) | −5.5 (5.0) | 19.9% | |||
| Netarsudil 0.02% and BB (2) | −4.3 (2.8) | |||||
| Netarsudil 0.02% replacing ≥ 1 classes of concomitant therapy (64) | −0.4 (2.5) | |||||
| Netarsudil 0.02% and ≥ 2 classes of concomitant therapy (64) | −4.5 (4.1) | |||||
| Shahid et al. [111] | 12 weeks | 21 < IOP < 32 mmHg | Change in IOP from baseline after 12 weeks | Conjunctival hyperemia | ||
| Netarsudil 0.02% (43) | 26.6 (4.8) | - | 71.4% | |||
| Netarsudil 0.02% and bimatoprost 0.01% (32) | 24.6 (3.0) | - | 83.3% | |||
| Bimatoprost 0.01% (34) | 24.6 (4.6) | - | 15.3% | |||
| Study | Randomization Process | Deviation from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Conflict of Interest | Overall |
|---|---|---|---|---|---|---|---|
| Tanihara et al. [87] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Inoue et al. [90] | Low risk | Low risk | Low risk | Low risk | Some concerns | High risk | Some concerns |
| Williams et al. [92] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Kopczynski et al. [91] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [88] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [93] | Low risk | Some concerns | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [94] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [95] | High risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [96] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Tanihara et al. [96] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Muhlisah et al. [97] | Low risk | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Tanihara et al. (Crossover Study) [98] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Bacharach et al. [89] | Some concerns | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Serle et al. [100] | Low risk | High risk | High risk | Low risk | Low risk | High risk | High risk |
| Kahook et al. [101] | Low risk | High risk | High risk | Low risk | Low risk | High risk | High risk |
| Lewis et al. [102] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Peace et al. [104] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Sit et al. [105] | Low risk | Some concerns | Low risk | Low risk | Low risk | High risk | Some concerns |
| Araie et al. [99] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Khouri et al. [106] | Low risk | High risk | High risk | Low risk | Low risk | High risk | High risk |
| Asrani et al. [107] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Walters et al. [108] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Stalmans et al. [109] | Some concerns | Low risk | Low risk | Low risk | Low risk | High risk | Some concerns |
| Shahid et al. [111] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zaman et al. [110] | High risk | Low risk | High risk | Low risk | Low risk | High risk | High risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.K.; Khaw, P.T.; Henein, C. Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials. Pharmaceuticals 2025, 18, 523. https://doi.org/10.3390/ph18040523
Tan JK, Khaw PT, Henein C. Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials. Pharmaceuticals. 2025; 18(4):523. https://doi.org/10.3390/ph18040523
Chicago/Turabian StyleTan, Jit Kai, Peng Tee Khaw, and Christin Henein. 2025. "Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials" Pharmaceuticals 18, no. 4: 523. https://doi.org/10.3390/ph18040523
APA StyleTan, J. K., Khaw, P. T., & Henein, C. (2025). Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials. Pharmaceuticals, 18(4), 523. https://doi.org/10.3390/ph18040523






