1. Introduction
Bladder cancer (BC) ranks as the fifth most prevalent malignancy worldwide, with an estimated annual incidence exceeding 573,000 new cases, and its incidence continues to rise [
1,
2]. Current classification stratifies BC into three principal categories: non-muscle-invasive bladder cancer (NMIBC), non-metastatic disease, muscle-invasive bladder cancer (MIBC), and metastatic bladder cancer [
3]. While NMIBC accounts for 75–85% of initial diagnoses and exhibits 5-year survival rates exceeding 90%, its high recurrence rate (50–70%) and progression potential to MIBC (10–15%) necessitate vigilance [
4,
5]. Current intravesical agents include chemotherapeutics such as mitomycin C, epirubicin, gemcitabine, and Bacillus Calmette-Guérin (BCG) [
6,
7]. First-line intravesical therapy with Bacillus Calmette-Guérin (BCG) achieves complete responses in 55–68% of high-risk NMIBC patients, yet 30–40% develop a BCG-unresponsive disease requiring radical cystectomy (RC). Nonetheless, many patients either refuse or are unsuitable for RC [
8,
9]. Despite these interventions, the recurrence rates are significant, with 50–70% of patients experiencing relapse within five years, and 10–15% of NMIBC patients progressing to MIBC [
10]. This therapeutic impasse underscores the urgent need for bladder-preserving alternatives.
The advent of oncolytic virotherapy has reshaped the bladder cancer treatment landscape. FDA approval of nadofaragene firadenovec (Adstiladrin), a non-replicating adenoviral vector delivering interferon-α2b, demonstrated 53.4% complete response rates at 3 months in BCG-unresponsive NMIBC [
11,
12]. Parallel developments include cretostimogene grenadenorepvec (CG0070), a GM-CSF-armed oncolytic adenovirus achieving 75.2% CR in phase III trials [
13]. The achievements of the oncolytic virus underscore its potential as a therapeutic strategy for this challenging patient population, with better therapeutic efficacy than BCG; however, the CR rate is not a satisfactory one when used alone, and the combination places a greater burden on the patient. The oncolytic virus can be modified to carry some genes to increase efficiency further [
14,
15]. The adenovirus is one of the most researched viruses in the field of oncolytic virus therapy, and its oncolytic methods include the direct killing of tumor cells and activation of the body immune reaction to inhibit tumor development [
16,
17]. Recombinant oncolytic adenovirus (KD01) is a type 5 recombinant oncolytic adenovirus that carries the apoptosis gene tBID, which shows conditional replication ability. KD01 can conditionally replicate in ovarian cancer cells, and triggering apoptosis, cisplatin combination therapy improves anti-tumor efficacy in patients with advanced ovarian cancer [
18,
19,
20]. Therefore, we performed preclinical pharmacodynamic evaluations across multiple solid tumor models, revealing that KD01 exhibits significant anti-tumor efficacy in bladder cancer. However, its potential synergistic effects with chemotherapy agents and the precise mechanisms underlying tumor cell death induction remain unclear.
Oncolytic virus monotherapy limitations persist, such as incomplete tumor clearance and insufficient immune activation. The standard of care for patients with MIBC is cystectomy accompanied by adjuvant chemotherapy, but not all patients are candidates for resection therapy. The concern in patients with MIBC is the metastasis of cancer and the difficulty in detecting and effectively treating the deep, tiny cancer foci, so pharmacologic systemic therapy has emerged as an alternative treatment modality. In a phase 2 trial, the combination of gemcitabine, cisplatin, and anti-PD-1 resulted in 43% of patients with MIBC in complete clinical remission [
21], although the rate of complete clinical remission is not a significant one, it provides a promising option for patients who cannot undergo cystectomy. Cisplatin is a widely used chemotherapeutic agent that inhibits cell proliferation by inducing DNA damage, and its therapeutic mechanism also appears to be related to the promotion of apoptosis. This article investigates the therapeutic effect of KD01 in combination with cisplatin in bladder cancer, which adds an additional mode of use to the clinical use of KD01.
KD01 has received Investigational New Drug (IND) approval from the National Medical Products Administration (NMPA) and is currently undergoing Phase I clinical trials targeting over ten advanced solid tumors (Clinical Trial Acceptance Number: CXSL2300531). Our findings establish a pharmacodynamic foundation for ongoing investigator-initiated trials exploring intravesical KD01 in BCG-refractory NMIBC, while informing rational combination strategies for MIBC patients ineligible for cystectomy.
3. Discussion
In recent years, the oncolytic virus, as a new cancer therapeutic agent, has demonstrated its advantages in treating bladder cancer, with intravesical delivery allowing easy access to the disease site while bypassing the systemic antiviral humoral response to ensure adequate drug delivery [
24,
25]. Based on the efficacy of oncolytic viruses against bladder cancer, in this article, we found that the modified oncolytic adenovirus KD01 exhibited an effective killing effect on bladder cancer cells both in vivo and in vitro, which was more pronounced compared with the unmodified oncolytic adenovirus. Oncolytic viruses destroy tumor cells and activate immune responses, replicating within these cells to cause lysis and release new particles [
26]. This process induces cell death and the release of signaling molecules, including PAMPs and DAMPs, which can restore the immune response against tumors [
27,
28].
A major problem facing oncolytic viruses currently lies in the heterogeneity of tumor cells leading to their inefficient replication in specific tumor cells. In some cases, viral infection induces autophagy, while infected cells die before virus release, which limits further lysis of the virus [
29,
30,
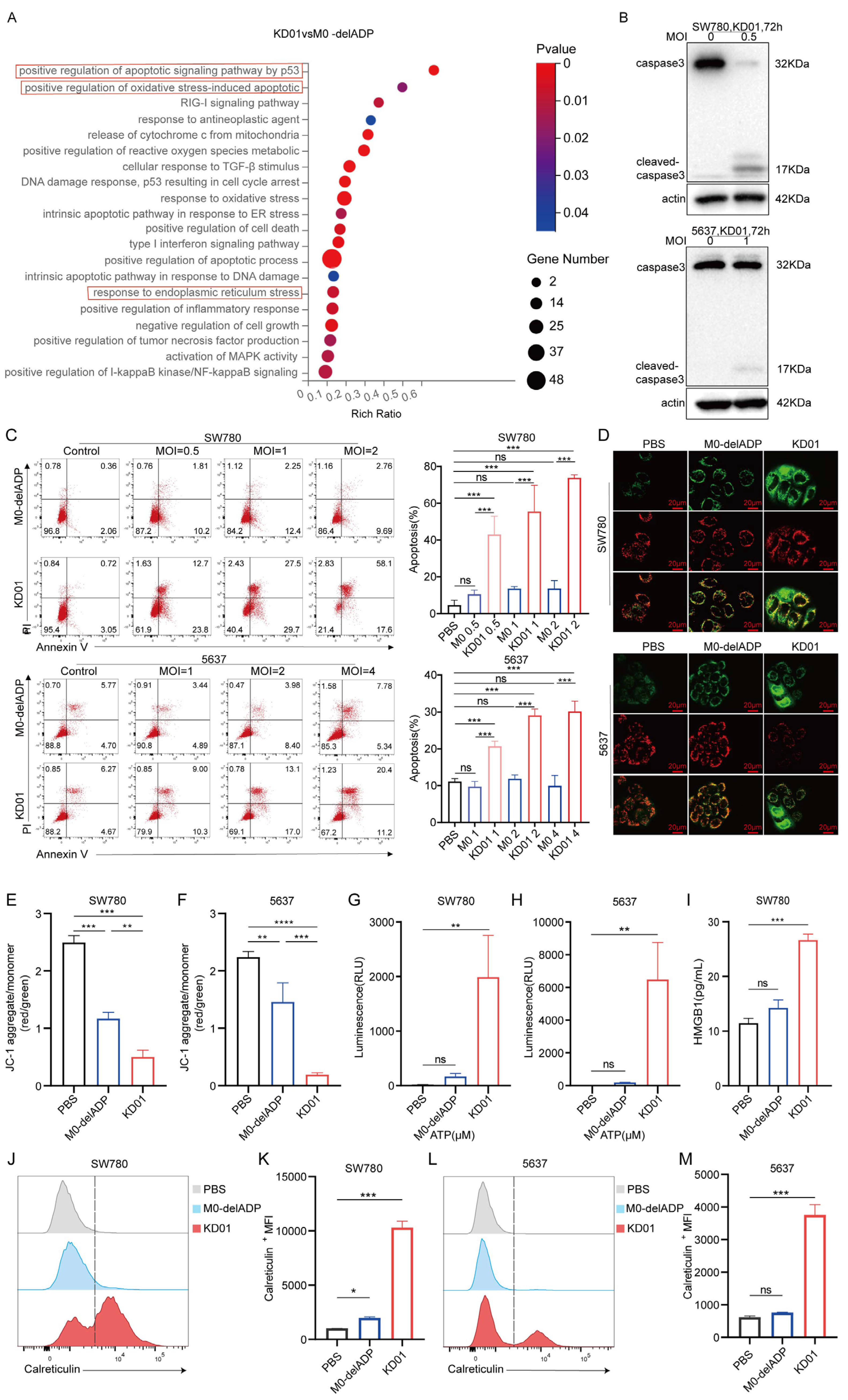
31]. Elevating the expression of apoptotic genes may be the key to addressing this problem; however, this may raise concerns about the reduced ability of the virus to replicate within the cell. Our study showed that the expression of the tBID gene did not compromise the replication capability of KD01 within bladder tumor cells and the modification of KD01 did not affect tumor cell death due to viral replication; however, tBID induced the formation of pores in the mitochondrial membrane, enhancing its permeability and elevating the apoptotic rate of the bladder cancer cells, particularly at the late stage of apoptosis. The increase in tBID expression accelerated tumor cell death, suggesting that our modification improved the efficiency of oncolytic viruses.
Moreover, the RNA-seq results in our study indicate that KD01 infection intensifies endoplasmic reticulum stress and oxidative stress in tumor cells—two biological processes capable of inducing immunogenic cell death—while also amplifying the inflammatory response of the tumor cells [
32]. Additional investigations have demonstrated that KD01 induces upregulation of calreticulin expression on the cellular membrane and facilitates the extrusion of ATP and HMGB1 from the cells. These findings substantiate that KD01 infection elicits ICD in bladder cancer cells, a process characterized by activating an immune response to the antigens of deceased cells [
33,
34]. This indicates that KD01 directly kills bladder cancer cells and enhances their immunogenicity, potentially improving the anti-tumor immunity in vivo and thereby killing tumor cells through a dual mechanism.
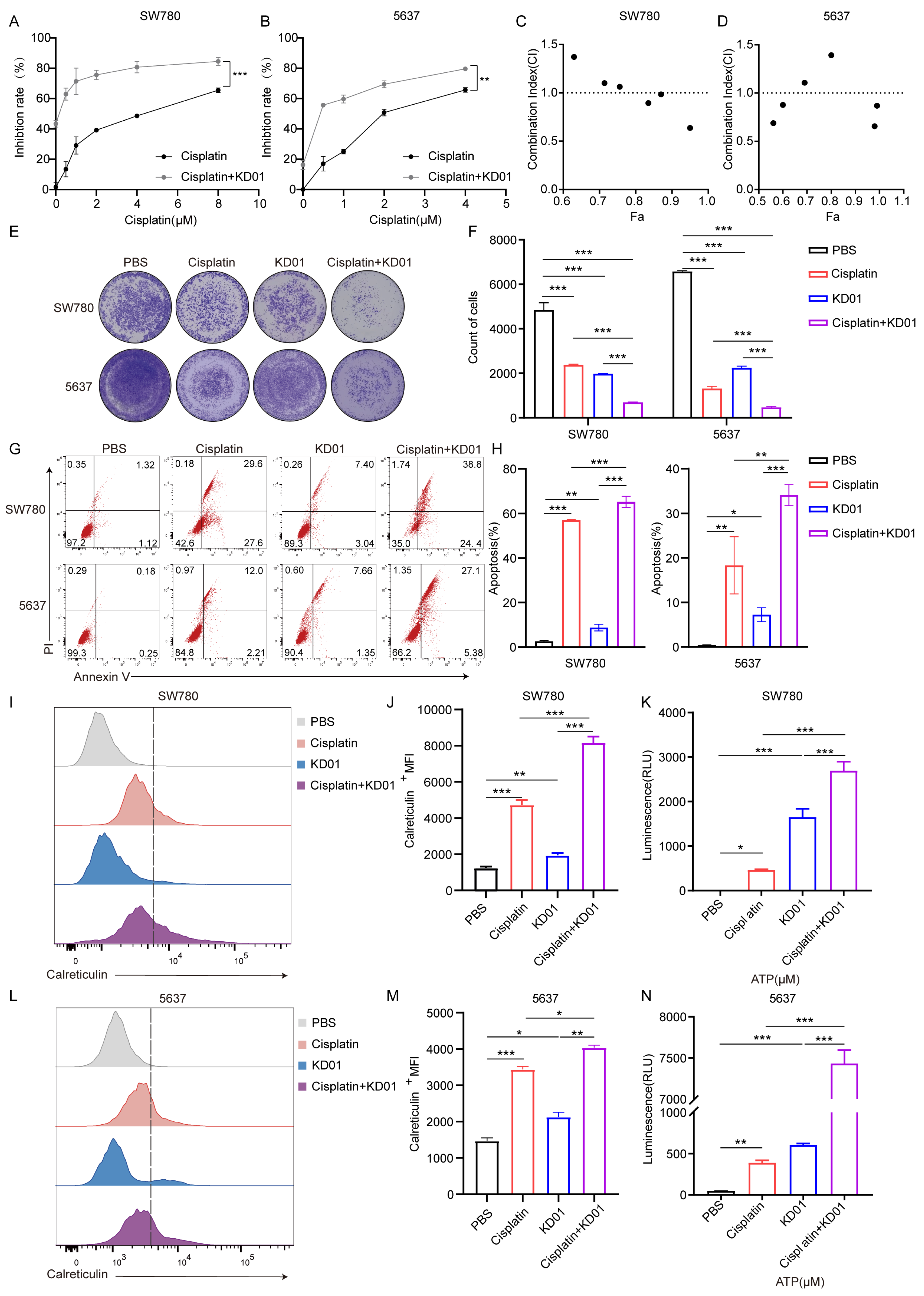
We found that the combination of KD01 and cisplatin enhanced the sensitivity of bladder cancer cells to KD01, and the combination treatment further promoted apoptosis, and there was a synergistic effect between the two at high inhibition rates, suggesting that the combination could enhance the anti-tumor effect. We detected that the combination of KD01 and cisplatin also promoted ICD in bladder cancer cells. Therefore, we believe that cisplatin can enhance the efficacy of KD01, and that it is also helpful in terms of enhancing the anti-tumor immunity, which adds an additional mode of use to the clinical use of KD01. Currently there are a variety of choices of chemotherapeutic and immunotherapeutic agents used for the treatment of bladder cancer, and when used clinically, they are not only limited to the combination with cisplatin. The ideal way of administration of KD01 for NMIBC patients is bladder perfusion therapy, which can easily reach the site of the tumor and adequately deliver the drug. The therapeutic effect of KD01 bladder perfusion is limited for patients with muscle invasive bladder cancer. The enhanced anti-tumor effect of the combination of KD01 and cisplatin suggests that KD01 can be used in combination with other chemotherapeutic drugs in our clinical practice, and that the combination of KD01 bladder perfusion with systemic chemotherapeutic drugs adjuvant treatment, in the enhancement of oncolytic virus tumor lysis effect at the same time, perhaps can inhibit the metastasis of bladder cancer and other progress. For the treatment of patients with muscle invasive bladder cancer, this provides a new idea, and the future is a therapeutic modality that can be explored.
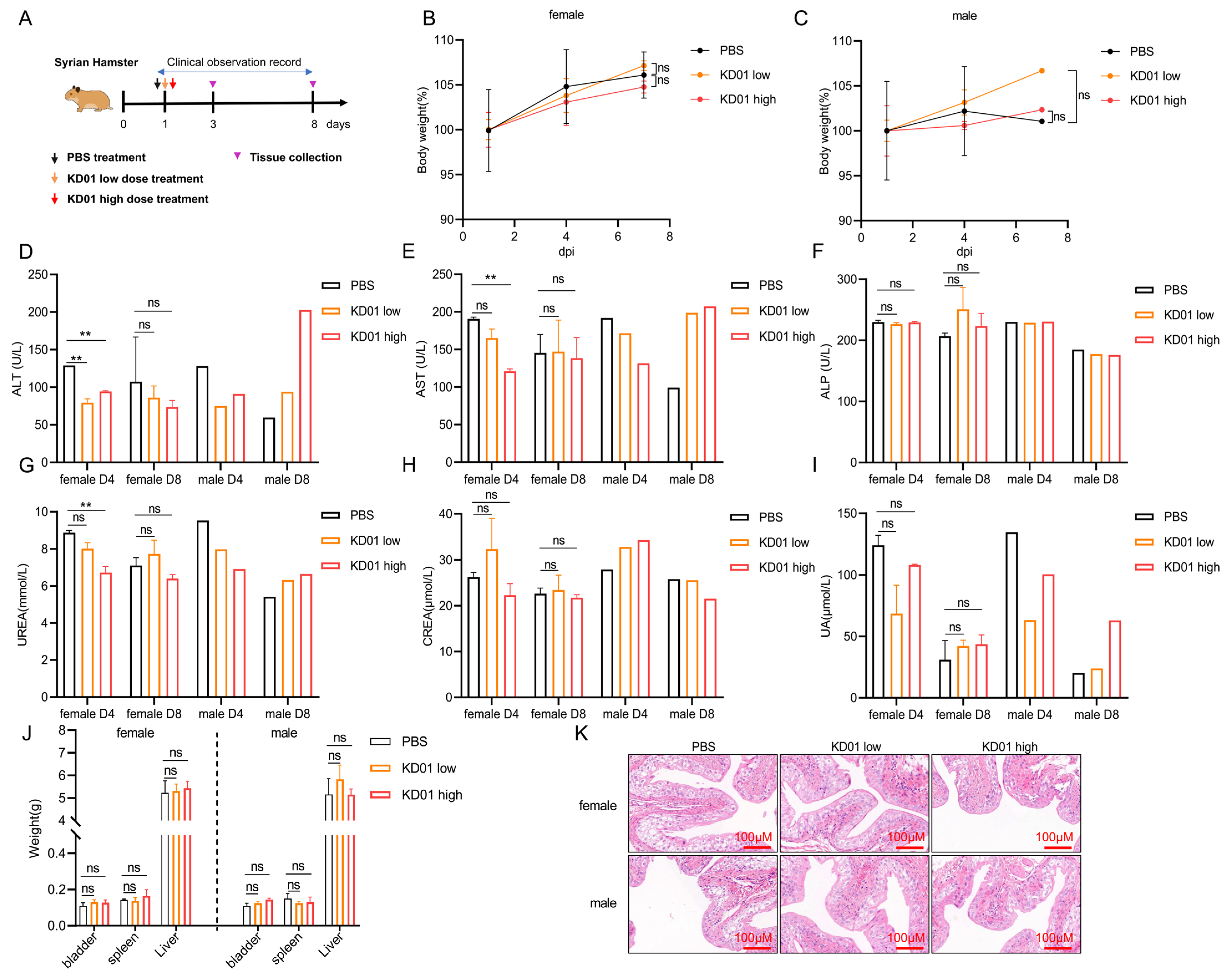
To assess the in vivo killing effect of KD01, we selected BALB/c nude mice to establish the SW780 bladder cancer model for our experiments, as mouse tumor cells are not an ideal model for verifying the efficacy of oncolytic adenovirus [
35,
36]. After intra-tumoral administration, we found that unmodified adenovirus had a slight inhibitory effect on tumor growth. In contrast, modified KD01 had a more significant inhibitory effect on tumor growth, inhibited tumor proliferation, and promoted apoptosis of tumor cells in vivo. After treatment of Syrian hamsters with KD01 bladder perfusion, no abnormalities were found in their clinical examination, body weight, and blood tests, and there were no signs of inflammation or injury in the bladder, indicating that KD01 bladder perfusion was well tolerated and had a favorable safety profile. Supported by the data in this post, the clinical trial for KD01 in treating bladder cancer has completed review and has been approved for IND, with a clinical trial to be conducted soon (Clinical Trial Acceptance Number: MR-42-24-038517).
The shortcomings of this study include the following: The mechanism of KD01 oncolytic needs to be refined; we mainly found that KD01 causes bladder cancer cells to undergo apoptosis and ICD; whether KD01 also promotes other modes of cell death in tumor cells was not fully explored; the mechanism of KD01 oncolytic may be elucidated in subsequent studies. In addition, the RNA sequencing results suggest that KD01 infection enhances the inflammatory response of bladder cancer cells, and whether the enhanced inflammatory response has a positive or negative inhibitory role against the tumor immune response in vivo has not been clarified.
In conclusion, this study demonstrates that KD01, a tumor-specific oncolytic adenovirus carrying tBID, promotes apoptosis and induces ICD in bladder cancer cells, killing them through dual pathways. Combination with the chemotherapeutic agent cisplatin enhances the efficacy of KD01, providing data to support the efficacy of sensitizing oncolytic viruses in conjunction with systemic chemotherapy using cisplatin in small doses. Safety assessment in healthy Syrian hamsters showed that KD01 was well tolerated by bladder perfusion. This provides a new option for the treatment of bladder cancer patients, as well as new evidence and reference for the clinical use and efficacy of KD01, and it supports pharmacological and safety data for conducting clinical trials of KD01 bladder instillation therapy in bladder cancer.
4. Materials and Methods
4.1. Cell Lines, Viruses, and Compounds
Human bladder metastatic carcinoma cells (SW780) and mouse bladder carcinoma cells (MB49) were obtained from Wuhan Punosai Biotechnology Co. Ltd. (Wuhan, China), while human bladder carcinoma cells (5637) were purchased from Applied Biological Materials Inc (ABM, Richmond, BC, Canada). SW780 and MB49 cells were cultured in DMEM supplemented with 15% and 10% fetal bovine serum (FBS), respectively, and 10,000 U/mL Penicillin-Streptomycin Solution. A total of 5637 cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 10,000 U/mL Penicillin-Streptomycin Solution. All cell cultures were maintained in an incubator at 37 °C with 5% CO2.
Two adenoviruses were used in this study: M0-delADP, an E1A CR2 and E3 ADP deletion adenovirus mutant, and KD01, which contains the pro-apoptotic tBID gene inserted into the ADP region of M0-delADP. Both M0-delADP and KD01 were provided by Wuhan Kadwise Biotechnology Co., Ltd. (Wuhan, China). Cisplatin (HY-17394) was purchased from MedChemExpress (MCE, Shanghai, China), dissolved in pure water to prepare a 2 mM stock solution, and stored at −80 °C until use.
4.2. Real-Time qPCR (RT-qPCR) Analysis
Total RNA or DNA was extracted from cultured cells using the FastPure Cell/Tissue Total RNA Isolation Kit V2/Tissue DNA Kit D3396 kit according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA using the ABScript Neo RT Master Mix for qPCR with gDNA Remover kit and stored at −20 °C or used directly for qPCR. SYBR Green-based qPCR was performed using 2× Universal SYBR Green Fast qPCR Mix (ABclonal, Wuhan, China). The primer sequences used were as follows. BID, forward: 5′-ATGGACCGTAGCATCCCTCC-3′, reverse: 5′-GTAGGTGCGTAGGTTCTGGT-3′; Fiber, forward: 5′-ACTATATGGACAACGTCAACCCATT-3′, reverse: 5′-ACCTTCTGAGGCACCTGGATGT-3′; GAPDH, forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3′. Data were normalized to GAPDH when calculating the relative expression of the target gene. qPCR was performed using a CFX96 real-time PCR system (Bio-Rad, Hercules, CA, USA). Data were analyzed by the Bio-Rad CFX Manager using the normalized expression pattern (2−ΔΔCq). Three measurements were performed.
4.3. Western Blot
Intact cell contents were obtained using cold RIPA lysates containing cocktail, phosphorylated protease inhibitor A solution and B solution, using the BCATM protein assay kit assay the protein concentration. The proteins were separated by 10% SDS-PAGE gel. The separated proteins were transferred onto a 0.45 µm PVDF membrane, which was blocked with a protein-free rapid-blocking solution for 1 h. Then, they were incubated with the primary antibodies related to the target proteins overnight. The following antibodies were used: BID Antibody (CST, #2002, Shanghai, China), Caspase-3 Antibody (Proteintech, 82202-1, Wuhan, China), Anti-beta Actin (ab6276, abcam, Shanghai, China). After incubation, continue to incubate the corresponding secondary antibodies. After incubation, corresponding secondary antibodies. Finally, the proteins were visualized using the BeyoECL Plus chemiluminescence kit (Beyotime, Shanghai, China). Development results were analyzed in fixed grayscale using Image-pro-plus Version 6.0, and the analyzed data were normalized to β-actin.
4.4. Crystalline Violet Staining Assay
Cells were inoculated in 96-well (7 × 103/well) dishes overnight, infected with viral particles at different MOIs, or treated with cisplatin. After 72 h of inoculation, the supernatant containing viral particles or cisplatin was removed. Cells were fixed and then stained with a crystal violet staining solution, leaving them to dry for photographic recording. The number of clones was counted using Image J 1.54p.
4.5. Apoptosis and Flow Cytometry Analysis
Cells were inoculated in 6-well dishes (2 × 105/well) for overnight growth, co-incubated by adding M0-delADP/KD01, and treated with different concentrations of cisplatin in the viral solution when the drugs were used in combination, and the cells were collected after 72 h of co-incubation, the cells were assayed for apoptosis using the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, Franklin Lakes, NJ, USA) for apoptosis detection. Samples for calreticulin assay were prepared as above and stained using calreticulin antibody (CST, #19780, Shanghai, China) and assayed on the machine. Data using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) were analyzed.
4.6. RNA Seq Analysis
During the sample processing stage, SW780 cells were inoculated into 6-well (2 × 105/well) culture dishes overnight, and 0.5 MOI of virus was added. After co-incubation for 48 h, the supernatant was aspirated, trypsin digestion was performed to collect the cells in cell freezing tubes, and the supernatant was aspirated and placed in liquid nitrogen for storage. Transcriptomics sequencing and analysis were assigned to BGI Genomics.
4.7. JC-1 Mitochondrial Membrane Potential Assay
Different viral particle treatments were added to the cells inoculated in confocal Petri dishes, and the cellular mitochondrial membrane potential was detected using the JC-1 (Beyotime Biotechnology Co., Shanghai, China) kit after 72 h of infection. The cells in the dish were washed once with PBS, 1 mL of cell complete medium was added, then 1 mL of prepared JC-1 working solution was added, and the cells were incubated at 37 °C. The supernatant was aspirated, washed twice, and 2 mL of cell culture medium was added, observing the cells under a laser confocal microscope.
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
SW780 cells were treated with KD01/M0-delADP with MOI = 1 when combined with cisplatin. A total of 2 µM of cisplatin was added to the viral solution simultaneously. After 72 h, the supernatant was stored at −80 °C in a refrigerator, and it was melted on ice before the assay. HMGB1 levels were determined using the Human High Mobility Group Protein B1 ELISA Kit (Sangon Biotech, D711210, Shanghai, China).
4.9. Immunogenic Cell Death Detection Assay
Cells were inoculated with virus particles of different MOIs or treated by adding cisplatin. The supernatants in the well plates were collected after 72 h of treatment, and then ATP levels in the supernatants of the cell culture plates were detected by using the ATP Assay Kit (Beyotime). The remaining supernatants were stored at −80 °C and HMGB1 levels were measured using ELISA. Calreticulin assay was analyzed using flow cytometry as previously described.
4.10. Cell Growth Inhibition Assay with CI Index Analysis
The half inhibitory concentration (IC50) of KD01 and the cell inhibitory assay in combination with cisplatin were determined using the CCK8 assay (Vazyme, Nanjing, China). Various multiplicities of infection (MOI) of M0-delADP or KD01, diluted in complete cell culture medium, were co-incubated with SW780 or 5637 cells seeded in 96-well plates for 72 h in a 37 °C incubator, with a blank cell negative control.
For the drug combination inhibition assay, KD01 at MOI = 1 was added to SW780 cells, and KD01 at MOI = 2 was added to 5637 cells. Cisplatin treatment was applied at various concentrations, and the cells were incubated for 72 h. Following incubation, the supernatant was discarded, and CCK8 working solution was added. Cell viability was measured using a microplate reader, which reflects the amount of reduced methazolene dye. The inhibition rate and IC50 values were calculated using GraphPad Prism 8.0. The combination index (CI) was calculated using Compusyn software 1.0. The experiment was repeated three times with at least three parallel measurements per experiment to obtain mean and standard deviation (SD) values.
4.11. In Vivo Therapeutic Effect Evaluation Experiment
The study used 6–8-week-old female BALB/c-nude mice, purchased from Mouse Lai Bao (Wuhan) Biotechnology Co., Ltd. (Wuhan, China). A total of 1 × 107 SW780 cells were subcutaneously inoculated into the right axilla of the animals. After 10 days, the tumor-bearing animals were screened for subsequent experiments, with the researchers blinded to the group assignments. The mice were randomly put into three groups: PBS control, M0-delADP treatment, and KD01 treatment, with three animals in each group. Intratumoral injections of 50 μL of oncolytic viruses (OVs) or PBS were administered every two days starting from the 10th day of inoculation, with a viral dose of 1 × 108 PFU per injection, for five treatments.
From the day of treatment, weight was recorded, and tumor volume was measured using vernier calipers (volume = length × width × width × 0.5). The animals were continuously observed for 30 days following cell inoculation. At the end of the observation period, tumors were dissected, weighed, and fixed in 4% paraformaldehyde for further analysis. The experimental animals were housed in the pathogen-free animal facility at Tongji Medical College.
4.12. Safety Evaluation Test
The safety evaluation of KD01 in vivo was conducted using 18 Syrian hamsters (12 females and 6 males) aged 6–8 weeks, purchased from Beijing Viton Lihua Laboratory Animal Co. Ltd. (Beijing, China). The hamsters’ body weights were measured, and they were divided into three groups: the control group, the low-dose group, and the high-dose group, with 6 hamsters (4 females and 2 males) in each group. The bladder was perfused with a single dose of 100 μL of PBS in the control group, 100 μL of virus solution at 8.6 × 109 VP per pup in the low-dose group, and 100 μL of virus solution at 2.6 × 1010 VP per pup in the high-dose group.
On D1, D4, andD8 after administration, 3 animals per group (2 females and 1 male) were euthanized for analysis. Throughout the testing period, clinical observations, body weight measurements, clinicopathological tests (blood routine, blood biochemistry), and organ dissection for weight assessment were performed.
4.13. H&E Pathological Experiment
Mouse tissues used for pathological examination were fixed in 4% paraformaldehyde fixative for at least 24 h. Subsequently, they were processed by paraffin embedding and randomly sliced, which were deparaffinized by xylene and then hydrated by immersion in ethanol. Finally, the tissue sections were stained by using hematoxylin-eosin and then dried for observation and recording by using a light microscope.
4.14. Immunohistochemical Assay with TUNEL Staining
Tissue sections were dewaxed with xylene and rehydrated in ethanol. Antigen retrieval was performed by autoclaving the sections in the citrate buffer. Endogenous peroxidase activity was then blocked by 0.3% H2O2 in the dark after washing three times with PBS, blocked with 5% BSA, and overnight incubation with Ki67 primary antibody. After washing and incubation with a biotinylated secondary antibody (Abcam, Shanghai, China), the sections were stained with DAB and visualized with microscope.
For TUNEL staining, the tissue sections were rehydrated in ethanol, permeabilized with proteinase K, and equilibrated in Equilibration Buffer for 30 min. Terminal deoxyribonucleotidyl transferase (TdT)-labeling buffer was added, followed by PBS washes. Then, it was dyed with DAPI and observed. Ki67 histochemistry and TUNEL positivity were quantified using Image J 1.54p.
4.15. Statistical Analysis
GraphPad Prism (version 8.0; GraphPad Software, La Jolla, CA, USA) was used for analysis, employing an unpaired Student’s t-test or one-way ANOVA for statistical significance, with Tukey’s multiple comparison test used when comparing multiple groups. The results are presented as means and SD from three independent experiments, with a p-value of less than 0.05 considered statistically significant.