Targeting Hepatic Stellate Cells for the Prevention and Treatment of Liver Cirrhosis and Hepatocellular Carcinoma: Strategies and Clinical Translation
Abstract
1. Introduction
2. The Role of Hepatic Stellate Cells (HSC) in Liver Cirrhosis and HCC
2.1. HSC and Liver Fibrosis
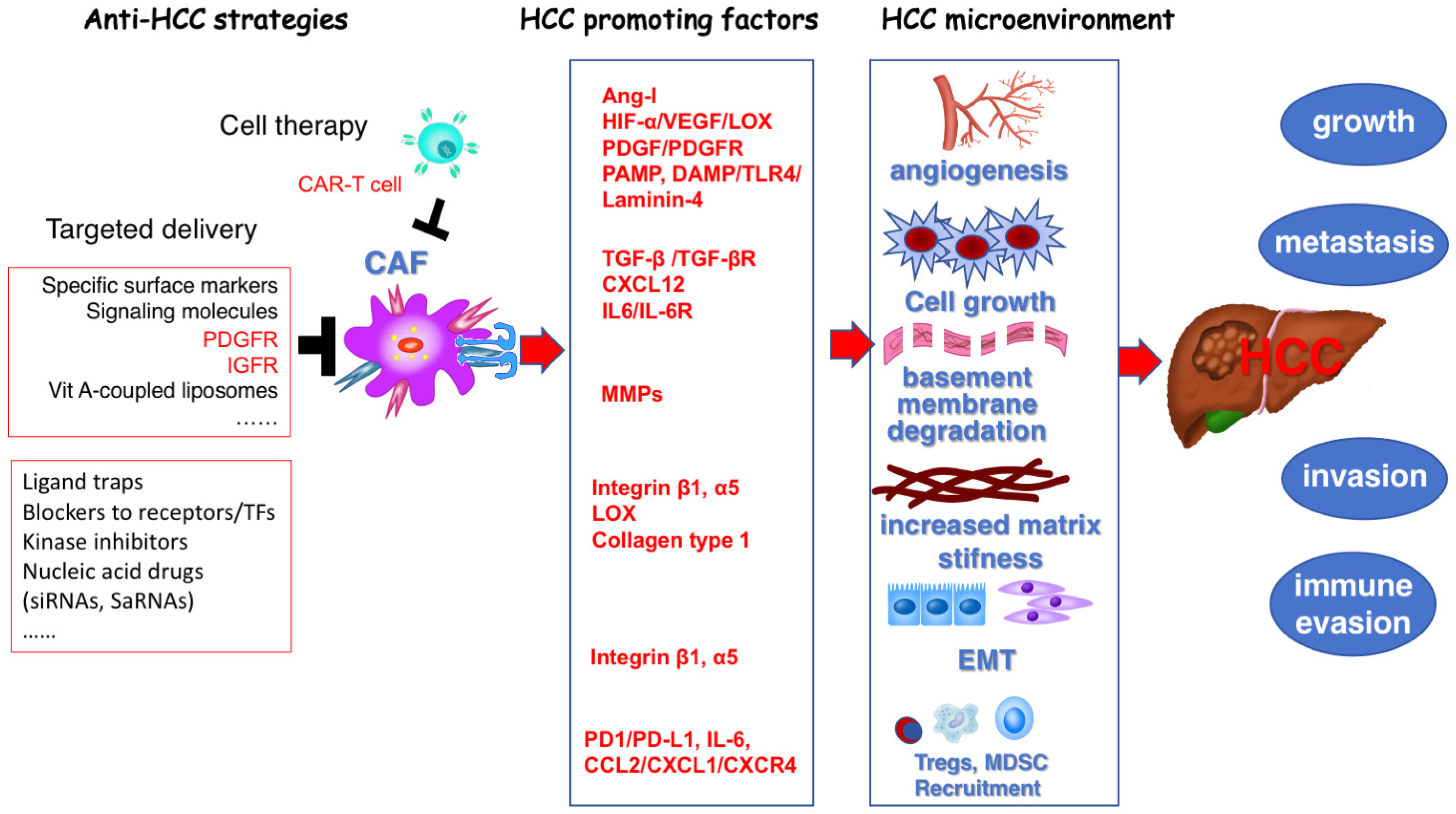
2.2. CAF and HCC
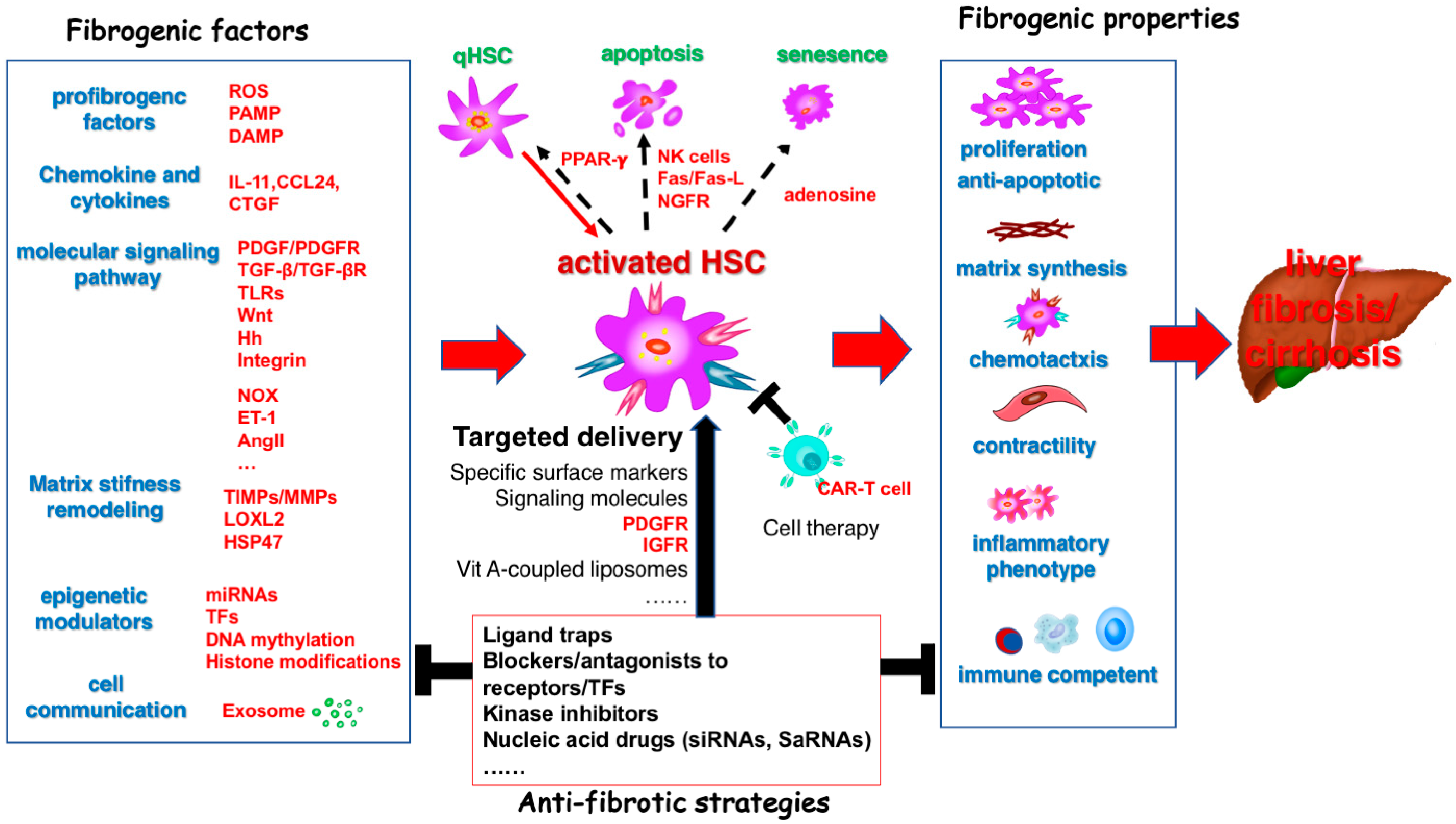
3. Targeting HSC for the Prevention and Treatment of Liver Cirrhosis
3.1. Molecular Signaling Pathways Associated with HSC/MFB Activation
3.1.1. Reactive Oxygen Radicals (ROS)
3.1.2. Toll-like Receptors (TLRs)
3.1.3. Hedgehog Signaling Pathway
3.1.4. Wnt Signaling Pathway
3.1.5. Fibroblast Growth Factor (FGF)/Fibroblast Growth Factor Receptor (FGFR) Signaling Way
3.1.6. Thyroid Hormone Receptor (THR)
3.1.7. Chemokine and Cytokine
3.2. Molecular Signaling Pathways Associated with HSC/MFB Proliferation
3.3. Molecular Signaling Pathways Associated with Pro-Liver Fibrosis
3.3.1. Transforming Growth Factor β (TGF-β)
3.3.2. FAP
3.3.3. Cannabinoid Receptors (CB)
3.4. Molecular Signaling Pathways Associated with HSC/MFB Contraction Responses
3.5. Molecular Signaling Pathways Associated with Reversal of Liver Fibrosis
3.5.1. Activated HSC Return to Resting Molecular Signaling Pathways
3.5.2. Molecular Signaling Pathways That Induce the Apoptosis and Senescence of Activated HSC/MFB
3.6. ECM and Liver Fibrosis
3.7. Epigenetic Regulation Associated with Liver Fibrosis
3.7.1. DNA Methylation and Related Histone Modifications
3.7.2. MicroRNAs
3.8. Cell Therapies to Treat Fibrosis
4. Targeting Hepatic Stellate Cells for the Prevention and Treatment of Hepatocellular Carcinoma
4.1. Angiogenesis Provides Basic Survival and Metastatic Conditions for Tumor Cells
4.2. Matrix Stiffness Promotes Tumor Growth, Invasion, and Metastasis
4.3. Matrix Remodeling via MMP/TIMPs Are Crucial for Tumor Invasion and Metastasis
4.4. Reprogramming of Cancer-Associated Fibroblasts
5. Translational Barriers in Targeting HSC for Anti-Fibrosis and Anti-Tumor Therapy
5.1. Heterogeneity of HSC
5.2. Lack of Specific Targeted Methods for HSC
5.3. Barriers to the Translation of Basic Research into Clinical Practice
6. Promoting Targeted Hepatic Stellate Cell-Based Strategies for the Prevention and Treatment of Liver Fibrosis and Hepatocellular Carcinoma
6.1. Utilizing New Omics Technologies to Identify Markers and Therapeutic Targets for Activated HSC
6.2. Receptor-Mediated Targeted Treatment Strategies for HSC and Clinical Translation
6.3. Design and Progress of Peptide Drugs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Kutala, B.K. Liver Diseases: A Major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. S1), 2–6. [Google Scholar] [CrossRef]
- Wang, S.S.; Tang, X.T.; Lin, M.; Yuan, J.; Peng, Y.J.; Yin, X.; Shang, G.; Ge, G.; Ren, Z.; Zhou, B.O. Perivenous stellate cells are the main source of myofibroblasts and cancer-associated fibroblasts formed after chronic liver injuries. Hepatology 2021, 74, 1578–1594. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Guo, J.; Friedman, S.L. Hepatic fibrogenesis. Semin. Liver Dis. 2007, 27, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Baldeosingh, R.; Lamm, R.; Patel, K.; Zhang, K.; Dominguez, D.A.; Kirton, K.J.; Shah, A.P.; Dang, H. Hepatic Stellate Cells and Hepatocarcinogenesis. Front. Cell Dev. Biol. 2020, 8, 709. [Google Scholar] [CrossRef]
- Bárcena, C.; Stefanovic, M.; Tutusaus, A.; Martinez-Nieto, G.A.; Martinez, L.; García-Ruiz, C.; de Mingo, A.; Caballeria, J.; Fernandez-Checa, J.C.; Marí, M.; et al. Angiogenin secretion from hepatoma cells activates hepatic stellate cells to amplify a self-sustained cycle promoting liver cancer. Sci. Rep. 2015, 5, 7916. [Google Scholar] [CrossRef]
- Santamato, A.; Fransvea, E.; Dituri, F.; Caligiuri, A.; Quaranta, M.; Niimi, T.; Pinzani, M.; Antonaci, S.; Giannelli, G. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin. Sci. 2011, 121, 159–168. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J.; Chen, S.; Zhou, Z.; Lin, N. Lipopolysaccharide promotes angiogenesis in mice model of HCC by stimulating hepatic stellate cell activation via TLR4 pathway. Acta Biochim. Biophys. Sin. 2017, 49, 1029–1034. [Google Scholar] [CrossRef][Green Version]
- Zhao, W.; Zhang, L.; Xu, Y.; Zhang, Z.; Ren, G.; Tang, K.; Kuang, P.; Zhao, B.; Yin, Z.; Wang, X. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab. Investig. 2014, 94, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hu, Y.; Wu, Y.; Qi, Q.; Wang, J.; Chen, L.; Wang, F. The Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma-Current Situation and Outlook. Mol. Immunol. 2022, 151, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Wiering, L.; Subramanian, P.; Hammerich, L. Hepatic Stellate Cells: Dictating Outcome in Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1277–1292. [Google Scholar] [CrossRef]
- Guo, J.; Loke, J.; Zheng, F.; Hong, F.; Yea, S.; Fukata, M.; Tarocchi, M.; Abar, O.T.; Huang, H.; Sninsky, J.J.; et al. Functional Linkage of Cirrhosis-Predictive Single Nucleotide Polymorphisms of Toll-Like Receptor 4 to Hepatic Stellate Cell Responses. Hepatology 2009, 49, 960–968. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, Y.; Cao, Y.; Yuan, Z.; Zhang, Y.; Zhang, D.Y.; Hasegawa, D.; Friedman, S.L.; Guo, J. Slit2-Robo2 Signaling Modulates the Fibrogenic Activity and Migration of Hepatic Stellate Cells. Life Sci. 2018, 203, 39–47. [Google Scholar] [CrossRef]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Rockey, D.C. Translating an Understanding of the Pathogenesis of Hepatic Fibrosis to Novel Therapies. Clin. Gastroenterol. Hepatol. 2013, 11, 224–231.e5. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, C.; Peng, L.; Ouyang, Y.; Cao, Y.; Wang, J.; Friedman, S.L.; Guo, J. High Mobility Group Box 1 Activates Toll Like Receptor 4 Signaling in Hepatic Stellate Cells. Life Sci. 2012, 91, 207–212. [Google Scholar] [CrossRef]
- Guo, J.; Friedman, S.L. Toll-Like Receptor 4 Signaling in Liver Injury and Hepatic Fibrogenesis. Fibrogenesis Tissue Repair 2010, 3, 21. [Google Scholar] [CrossRef]
- Bansal, M.B.; Chamroonkul, N. Antifibrotics in Liver Disease: Are We Getting Closer to Clinical Use? Hepatol. Int. 2019, 13, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Novo, E.; Parola, M. Therapeutic Pro-Fibrogenic Signaling Pathways in Fibroblasts. Adv. Drug Deliv. Rev. 2017, 121, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kietzmann, T. Reactive Oxygen Species and Fibrosis: Further Evidence of a Significant Liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef]
- Liang, S.; Kisseleva, T.; Brenner, D.A. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front. Physiol. 2016, 7, 17. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Bertran, E.; Fabregat, I. Cross-Talk Between TGF-β and NADPH Oxidases During Liver Fibrosis and Hepatocarcinogenesis. Curr. Pharm. Des. 2015, 21, 5964–5976. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, X.; Yang, L.; Kisseleva, T.; Brenner, D.A.; Seki, E. Transcriptional Repression of the Transforming Growth Factor β (TGF-β) Pseudoreceptor BMP and Activin Membrane-Bound Inhibitor (BAMBI) by Nuclear Factor κB (NF-κB) p50 Enhances TGF-β Signaling in Hepatic Stellate Cells. J. Biol. Chem. 2014, 289, 7082–7091. [Google Scholar] [CrossRef]
- Yang, J.J.; Tao, H.; Li, J. Hedgehog Signaling Pathway as Key Player in Liver Fibrosis: New Insights and Perspectives. Expert Opin. Ther. Targets 2014, 18, 1011–1021. [Google Scholar] [CrossRef]
- Du, K.; Hyun, J.; Premont, R.T.; Choi, S.S.; Michelotti, G.A.; Swiderska-Syn, M.; Dalton, G.D.; Thelen, E.; Rizi, B.S.; Jung, Y.; et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology 2018, 154, 1465–1479.e13. [Google Scholar] [CrossRef]
- El-Agroudy, N.N.; El-Naga, R.N.; El-Razeq, R.A.; El-Demerdash, E. Forskolin, a Hedgehog Signalling Inhibitor, Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Br. J. Pharmacol. 2016, 173, 3248–3260. [Google Scholar] [CrossRef]
- Lian, N.; Jiang, Y.; Zhang, F.; Jin, H.; Lu, C.; Wu, X.; Lu, Y.; Zheng, S. Curcumin Regulates Cell Fate and Metabolism by Inhibiting Hedgehog Signaling in Hepatic Stellate Cells. Lab. Investig. 2015, 95, 790–803. [Google Scholar] [CrossRef]
- Moon, R.T.; Bowerman, B.; Boutros, M.; Perrimon, N. The Promise and Perils of Wnt Signaling Through Beta-Catenin. Science 2002, 296, 1644–1646. [Google Scholar] [CrossRef]
- Kweon, S.M.; Chi, F.; Higashiyama, R.; Lai, K.; Tsukamoto, H. Wnt Pathway Stabilizes MeCP2 Protein to Repress PPAR-γ in Activation of Hepatic Stellate Cells. PLoS ONE 2016, 11, e0156111. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lu, Z.; Huang, K.; Wang, X.; Xu, Z.; Chen, B.; Dong, P.; Zheng, J. MicroRNA-17-5p-Activated Wnt/β-Catenin Pathway Contributes to the Progression of Liver Fibrosis. Oncotarget 2016, 7, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kong, L.N.; Huang, C.; Ma, T.T.; Meng, X.M.; He, Y.; Wang, Q.Q.; Li, J. Hesperetin Derivative-7 Inhibits PDGF-BB-Induced Hepatic Stellate Cell Activation and Proliferation by Targeting Wnt/β-Catenin Pathway. Int. Immunopharmacol. 2015, 25, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wu, C.; Hu, J.; Wang, Q.; Chen, S.; Wang, Z.; Xiong, W. Wnt5a Promotes Cytokines Production and Cell Proliferation in Human Hepatic Stellate Cells Independent of Canonical Wnt Pathway. Clin. Lab. 2015, 61, 537–547. [Google Scholar] [CrossRef]
- Peterová, E.; Podmolíková, L.; Řezáčová, M.; Mrkvicová, A. Fibroblast Growth Factor-1 Suppresses TGF-β-Mediated Myofibroblastic Differentiation of Rat Hepatic Stellate Cells. Acta Medica 2016, 59, 124–132. [Google Scholar] [CrossRef][Green Version]
- El Taghdouini, A.; Najimi, M.; Sancho-Bru, P.; Sokal, E.; van Grunsven, L.A. In Vitro Reversion of Activated Primary Human Hepatic Stellate Cells. Fibrogenesis Tissue Repair 2015, 8, 14. [Google Scholar] [CrossRef]
- Pan, R.L.; Xiang, L.X.; Wang, P.; Liu, X.Y.; Nie, L.; Huang, W.; Shao, J.Z. Low-Molecular-Weight Fibroblast Growth Factor 2 Attenuates Hepatic Fibrosis by Epigenetic down-Regulation of Delta-Like1. Hepatology 2015, 61, 1708–1720. [Google Scholar] [CrossRef]
- Dolivo, D.M. Anti-fibrotic effects of pharmacologic FGF-2: A review of recent literature. J. Mol. Med. 2022, 100, 847–860. [Google Scholar]
- Xu, P.; Zhang, Y.; Liu, Y.; Yuan, Q.; Song, L.; Liu, M.; Liu, Z.; Yang, Y.; Li, J.; Li, D.; et al. Fibroblast Growth Factor 21 Attenuates Hepatic Fibrogenesis through TGF-β/smad2/3 and NF-κB Signaling Pathways. Toxicol. Appl. Pharmacol. 2016, 290, 43–53. [Google Scholar] [CrossRef]
- Ji, Y.; Duan, Y.; Li, Y.; Lu, Q.; Liu, D.; Yang, Y.; Chang, R.; Tian, J.; Yao, W.; Yin, J.; et al. A Long-Acting FGF21 Attenuates Metabolic Dysfunction-Associated Steatohepatitis-Related Fibrosis by Modulating NR4A1-Mediated Ly6C Phenotypic Switch in Macrophages. Br. J. Pharmacol. 2024, 181, 2923–2946. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Sestito, S.; Runfola, M.; Rapposelli, S.; Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front. Med. 2020, 7, 331. [Google Scholar] [CrossRef]
- Manka, P.; Coombes, J.D.; Sydor, S.; Swiderska-Syn, M.K.; Best, J.; Gauthier, K.; van Grunsven, L.A.; Oo, Y.H.; Wang, C.; Diehl, A.M.; et al. Thyroid Hormone Receptor Alpha Modulates Fibrogenesis in Hepatic Stellate Cells. Liver Int. 2024, 44, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Targher, G.; Romeo, S.; Pajvani, U.B.; Zheng, M.-H.; Aghemo, A.; Valenti, L.V.C. The First MASH Drug Therapy on the Horizon: Current Perspectives of Resmetirom. Liver Int. 2024, 44, 1526–1536. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Singh, B.K.; Adami, E.; Viswanathan, S.; Dong, J.; D’Agostino, G.A.; Ng, B.; Lim, W.W.; Tan, J.; Paleja, B.S.; et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology 2019, 157, 777–792.e14. [Google Scholar] [CrossRef]
- Mor, A.; Friedman, S.; Hashmueli, S.; Peled, A.; Pinzani, M.; Frankel, M.; Safadi, R. Targeting CCL24 in Inflammatory and Fibrotic Diseases: Rationale and Results from Three CM-101 Phase 1 Studies. Drug Saf. 2024, 47, 869–881. [Google Scholar] [CrossRef]
- De Lorenzis, E.; Mor, A.; Ross, R.L.; Di Donato, S.; Aricha, R.; Vaknin, I.; Del Galdo, F. Serum CCL24 as a Biomarker of Fibrotic and Vascular Disease Severity in Systemic Sclerosis. Arthritis Care Res. 2024, 76, 1269–1277. [Google Scholar] [CrossRef]
- Segal-Salto, M.; Barashi, N.; Katav, A.; Edelshtein, V.; Aharon, A.; Hashmueli, S.; George, J.; Maor, Y.; Pinzani, M.; Haberman, D.; et al. A Blocking Monoclonal Antibody to CCL24 Alleviates Liver Fibrosis and Inflammation in Experimental Models of Liver Damage. JHEP Rep. 2020, 2, 100064. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Weiskirchen, R. The PDGF System and Its Antagonists in Liver Fibrosis. Cytokine Growth Factor. Rev. 2016, 28, 53–61. [Google Scholar] [CrossRef]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The Integrin Alpha V Beta 6 Binds and Activates Latent TGF Beta 1: A Mechanism for Regulating Pulmonary Inflammation and Fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Stockis, J.; Liénart, S.; Colau, D.; Collignon, A.; Nishimura, S.L.; Sheppard, D.; Coulie, P.G.; Lucas, S. Blocking Immunosuppression by Human Tregs in Vivo with Antibodies Targeting Integrin αVβ8. Proc. Natl. Acad. Sci. USA 2017, 114, E10161–E10168. [Google Scholar] [CrossRef]
- Guo, W.; Liu, H.; Yan, Y.; Wu, D.; Yao, H.; Lin, K.; Li, X. Targeting the TGF-β Signaling Pathway: An Updated Patent Review (2021–Present). Expert Opin. Ther. Pat. 2024, 34, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.C.; Martin, J.S.; Cousins, F.M.; Kulkarni, A.B.; Karlsson, S.; Akhurst, R.J. Defective Haematopoiesis and Vasculogenesis in Transforming Growth Factor-Beta 1 Knock out Mice. Development 1995, 121, 1845–1854. [Google Scholar] [CrossRef]
- Danielpour, D. Advances and Challenges in Targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective. Pharmaceuticals 2024, 17, 533. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.; Cottin, V.; Ramaswamy, M.; Wuyts, W.A.; Jenkins, R.G.; Scholand, M.B.; Kreuter, M.; Valenzuela, C.; Ryerson, C.J.; Goldin, J.; et al. Bexotegrast in Patients with Idiopathic Pulmonary Fibrosis: The INTEGRIS-IPF Clinical Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 424–434. [Google Scholar] [CrossRef]
- Duan, Z.; Lin, X.; Wang, L.; Zhen, Q.; Jiang, Y.; Chen, C.; Yang, J.; Lee, C.H.; Qin, Y.; Li, Y.; et al. Specificity of TGF-β1 Signal Designated by LRRC33 and Integrin α(V)β(8). Nat. Commun. 2022, 13, 4988. [Google Scholar] [CrossRef]
- Liénart, S.; Merceron, R.; Vanderaa, C.; Lambert, F.; Colau, D.; Stockis, J.; van der Woning, B.; De Haard, H.; Saunders, M.; Coulie, P.G.; et al. Structural Basis of Latent TGF-β1 Presentation and Activation by GARP on Human Regulatory T Cells. Science 2018, 362, 952–956. [Google Scholar] [CrossRef]
- Lack, J.; O’Leary, J.M.; Knott, V.; Yuan, X.; Rifkin, D.B.; Handford, P.A.; Downing, A.K. Solution Structure of the Third TB Domain from LTBP1 Provides Insight into Assembly of the Large Latent Complex That Sequesters Latent TGF-Beta. J. Mol. Biol. 2003, 334, 281–291. [Google Scholar] [CrossRef]
- Jackson, J.W.; Frederick, C.S., Jr.; Pal, A.; Coricor, G.; Boston, C.; Brueckner, C.T.; Canonico, K.; Chapron, C.; Cote, S.; Dagbay, K.B.; et al. An antibody That Inhibits TGF-β1 Release from Latent Extracellular Matrix Complexes Attenuates the Progression of Renal Fibrosis. Sci. Signal. 2024, 17, eadn6052. [Google Scholar] [CrossRef]
- Sun, T.; Huang, Z.; Liang, W.C.; Yin, J.; Lin, W.Y.; Wu, J.; Vernes, J.M.; Lutman, J.; Caplazi, P.; Jeet, S.; et al. TGFβ2 and TGFβ3 Isoforms Drive Fibrotic Disease Pathogenesis. Sci. Transl. Med. 2021, 13, eabe0407. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Vander Heiden, J.A.; Gao, X.; Yin, J.; Uttarwar, S.; Liang, W.C.; Jia, G.; Yadav, R.; Huang, Z.; Mitra, M.; et al. Isoform-Selective TGF-β3 Inhibition for Systemic Sclerosis. Med 2024, 5, 132–147.e7. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Maher, T.M. The Future of Clinical Trials in Idiopathic Pulmonary Fibrosis. Curr. Opin. Pulm. Med. 2024, 30, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Fernández Pérez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an Anti-Connective Tissue Growth Factor Therapy, for Idiopathic Pulmonary Fibrosis (PRAISE): A Phase 2, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Respir. Med. 2020, 8, 25–33. [Google Scholar] [CrossRef]
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Müller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast Activation Protein: A Cell Surface Dipeptidyl Peptidase and Gelatinase Expressed by Stellate Cells at the Tissue Remodelling Interface in Human Cirrhosis. Hepatology 1999, 29, 1768–1778. [Google Scholar] [CrossRef]
- Levy, M.T.; McCaughan, G.W.; Marinos, G.; Gorrell, M.D. Intrahepatic Expression of the Hepatic Stellate Cell Marker Fibroblast Activation Protein Correlates with the Degree of Fibrosis in Hepatitis C Virus Infection. Liver 2002, 22, 93–101. [Google Scholar] [CrossRef]
- Yang, A.-T.; Kim, Y.-O.; Yan, X.-Z.; Abe, H.; Aslam, M.; Park, K.-S.; Zhao, X.-Y.; Jia, J.-D.; Klein, T.; You, H.; et al. Fibroblast Activation Protein Activates Macrophages and Promotes Parenchymal Liver Inflammation and Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 841–867. [Google Scholar] [CrossRef]
- Williams, K.H.; Viera de Ribeiro, A.J.; Prakoso, E.; Veillard, A.S.; Shackel, N.A.; Bu, Y.; Brooks, B.; Cavanagh, E.; Raleigh, J.; McLennan, S.V.; et al. Lower Serum Fibroblast Activation Protein Shows Promise in the Exclusion of Clinically Significant Liver Fibrosis Due to Non-Alcoholic Fatty Liver Disease in Diabetes and Obesity. Diabetes Res. Clin. Pract. 2015, 108, 466–472. [Google Scholar] [CrossRef]
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in Liver Disease. Hepatology 2011, 53, 346–355. [Google Scholar]
- Teixeira-Clerc, F.; Julien, B.; Grenard, P.; Tran Van Nhieu, J.; Deveaux, V.; Li, L.; Serriere-Lanneau, V.; Ledent, C.; Mallat, A.; Lotersztajn, S. CB1 Cannabinoid Receptor Antagonism: A New Strategy for the Treatment of Liver Fibrosis. Nat. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef]
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid Signaling and Liver Therapeutics. J. Hepatol. 2013, 59, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Khimji, A.K.; Rockey, D.C. Endothelin and Hepatic Wound Healing. Pharmacol. Res. 2011, 63, 512–518. [Google Scholar] [CrossRef]
- He, C.; Miao, X.; Li, J.; Qi, H. Angiotensin II Induces Endothelin-1 Expression in Human Hepatic Stellate Cells. Dig. Dis. Sci. 2013, 58, 2542–2549. [Google Scholar] [CrossRef]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of Liver Fibrosis: A Translational Success Story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective Effects of the Dual Peroxisome Proliferator-Activated Receptor Alpha/Delta Agonist, GFT505, in Rodent Models of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef]
- Fernández-Álvarez, S.; Gutiérrez-de Juan, V.; Zubiete-Franco, I.; Barbier-Torres, L.; Lahoz, A.; Parés, A.; Luka, Z.; Wagner, C.; Lu, S.C.; Mato, J.M.; et al. TRAIL-Producing NK Cells Contribute to Liver Injury And Related Fibrogenesis in the Context of GNMT Deficiency. Lab. Investig. 2015, 95, 223–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahsan, M.K.; Mehal, W.Z. Activation of Adenosine Receptor A2A Increases HSC Proliferation and Inhibits Death and Senescence by down-Regulation of p53 and Rb. Front. Pharmacol. 2014, 5, 69. [Google Scholar] [CrossRef]
- Yashaswini, C.N.; Qin, T.; Bhattacharya, D.; Amor, C.; Lowe, S.; Lujambio, A.; Wang, S.; Friedman, S.L. Phenotypes and Ontogeny of Senescent Hepatic Stellate Cells in Metabolic Dysfunction-Associated Steatohepatitis. J. Hepatol. 2024, 81, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cai, H.; Ammar, R.; Zhang, Y.; Wang, Y.; Ravi, K.; Thompson, J.; Jarai, G. Molecular Characterization of a Precision-Cut Rat Liver Slice Model for the Evaluation of Antifibrotic Compounds. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G15–G24. [Google Scholar] [CrossRef]
- Yamagishi, R.; Kamachi, F.; Nakamura, M.; Yamazaki, S.; Kamiya, T.; Takasugi, M.; Cheng, Y.; Nonaka, Y.; Yukawa-Muto, Y.; Thuy, L.T.T.; et al. Gasdermin D-Mediated Release of IL-33 from Senescent Hepatic Stellate Cells Promotes Obesity-Associated Hepatocellular Carcinoma. Sci. Immunol. 2022, 7, eabl7209. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-Approved Disulfiram Inhibits Pyroptosis by Blocking Gasdermin D Pore Formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of Matrix Metalloproteinases (MMPs) and Inflammasome Pathway in Molecular Mechanisms of Fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef]
- Urtasun, R.; Lopategi, A.; George, J.; Leung, T.M.; Lu, Y.; Wang, X.; Ge, X.; Fiel, M.I.; Nieto, N. Osteopontin, an Oxidant Stress Sensitive Cytokine, up-Regulates Collagen-I via Integrin α(V)β(3) Engagement and PI3K/pAkt/NFκB Signaling. Hepatology 2012, 55, 594–608. [Google Scholar] [CrossRef]
- Chen, W.; Yang, A.; Jia, J.; Popov, Y.V.; Schuppan, D.; You, H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology 2020, 72, 729–741. [Google Scholar] [CrossRef]
- Meissner, E.G.; McLaughlin, M.; Matthews, L.; Gharib, A.M.; Wood, B.J.; Levy, E.; Sinkus, R.; Virtaneva, K.; Sturdevant, D.; Martens, C.; et al. Simtuzumab Treatment of Advanced Liver Fibrosis in HIV and HCV-Infected Adults: Results of a 6-Month Open-Label Safety Trial. Liver Int. 2016, 36, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Bian, E.B.; Zhao, B.; Huang, C.; Wang, H.; Meng, X.M.; Wu, B.M.; Ma, T.T.; Zhang, L.; Lv, X.W.; Li, J. New Advances of DNA Methylation in Liver Fibrosis, with Special Emphasis on the Crosstalk between microRNAs and DNA Methylation Machinery. Cell. Signal. 2013, 25, 1837–1844. [Google Scholar] [CrossRef]
- Kuang, Y.; El-Khoueiry, A.; Taverna, P.; Ljungman, M.; Neamati, N. Guadecitabine (SGI-110) Priming Sensitizes Hepatocellular Carcinoma Cells to Oxaliplatin. Mol. Oncol. 2015, 9, 1799–1814. [Google Scholar] [CrossRef]
- Hardy, T.; Mann, D.A. Epigenetics in Liver Disease: From Biology to Therapeutics. Gut 2016, 65, 1895–1905. [Google Scholar] [CrossRef]
- Hyun, J.; Park, J.; Wang, S.; Kim, J.; Lee, H.H.; Seo, Y.S.; Jung, Y. MicroRNA Expression Profiling in CCl4-Induced Liver Fibrosis of Mus Musculus. Int. J. Mol. Sci. 2016, 17, 961. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Urban, G.W.; Bettermann, K.; Vucur, M.; Zimmermann, H.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA Profiling Reveals a Role for miR-29 in Human and Murine Liver Fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Zheng, X.; Li, H.; Cao, Z.; Chang, H.; Luan, S.; Zhu, J.; Chen, J.; Zang, Y.; Zhang, J. MicroRNA-30 Protects against Carbon Tetrachloride-Induced Liver Fibrosis by Attenuating Transforming Growth Factor Beta Signaling in Hepatic Stellate Cells. Toxicol. Sci. 2015, 146, 157–169. [Google Scholar] [CrossRef]
- Høydahl, L.S.; Berntzen, G.; Løset, G. Engineering T-Cell Receptor-Like Antibodies for Biologics and Cell Therapy. Curr. Opin. Biotechnol. 2024, 90, 103224. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T Cells Reverse Senescence-Associated Pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting Cardiac Fibrosis with Engineered T Cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Amrute, J.M.; Luo, X.; Penna, V.; Yang, S.; Yamawaki, T.; Hayat, S.; Bredemeyer, A.; Jung, I.H.; Kadyrov, F.F.; Heo, G.S.; et al. Targeting Immune-Fibroblast Cell Communication in Heart Failure. Nature 2024, 635, 423–433. [Google Scholar] [CrossRef]
- Friedman, S.L. Fighting Cardiac Fibrosis with CAR T Cells. N. Engl. J. Med. 2022, 386, 1576–1578. [Google Scholar] [CrossRef]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T Cells Produced in Vivo to Treat Cardiac Injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef]
- Basalova, N.; Alexandrushkina, N.; Grigorieva, O.; Kulebyakina, M.; Efimenko, A. Fibroblast Activation Protein Alpha (FAPα) in Fibrosis: Beyond a Perspective Marker for Activated Stromal Cells? Biomolecules 2023, 13, 1718. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, J.; Liu, M.; Jiang, D.; Zhang, Y.; Li, X.; Shi, B.; Jiang, J.; Ma, C.; Shao, H.; et al. Targeting FAP-Positive Chondrocytes in Osteoarthritis: A Novel Lipid Nanoparticle siRNA Approach to Mitigate Cartilage Degeneration. J. Nanobiotechnol. 2024, 22, 659. [Google Scholar] [CrossRef]
- Eriksson, O.; Velikyan, I. Radiotracers for Imaging of Fibrosis: Advances during the Last Two Decades and Future Directions. Pharmaceuticals 2023, 16, 1540. [Google Scholar] [CrossRef]
- Trinh, V.Q.; Lee, T.F.; Lemoinne, S.; Ray, K.C.; Ybanez, M.D.; Tsuchida, T.; Carter, J.K.; Agudo, J.; Brown, B.D.; Akat, K.M.; et al. Hepatic Stellate Cells Maintain Liver Homeostasis Through Paracrine Neurotrophin-3 Signaling That Induces Hepatocyte Proliferation. Sci. Signal. 2023, 16, eadf6696. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, B.; Li, L.; Lv, C.; Tian, Y. Reprogramming of Cancer-Associated Fibroblasts Combined with Immune Checkpoint Inhibitors: A Potential Therapeutic Strategy for Cancers. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188945. [Google Scholar] [CrossRef]
- Krenkel, O.; Hundertmark, J.; Ritz, T.P.; Weiskirchen, R.; Tacke, F. Single Cell RNA Sequencing Identifies Subsets of Hepatic Stellate Cells and Myofibroblasts in Liver Fibrosis. Cells 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Fischer, J.; Harrer, L.; Schwiering, F.; Groneberg, D.; Friebe, A. Hepatic Stellate Cells in Zone 1 Engage in Capillarization Rather Than Myofibroblast Formation in Murine Liver Fibrosis. Sci. Rep. 2024, 14, 18840. [Google Scholar] [CrossRef]
- Yamagata, K.; Takasuga, S.; Tatematsu, M.; Fuchimukai, A.; Yamada, T.; Mizuno, M.; Morii, M.; Ebihara, T. FoxD1 Expression Identifies a Distinct Subset of Hepatic Stellate Cells Involved in Liver Fibrosis. Biochem. Biophys. Res. Commun. 2024, 734, 150632. [Google Scholar] [CrossRef]
- Rosenthal, S.B.; Liu, X.; Ganguly, S.; Dhar, D.; Pasillas, M.P.; Ricciardelli, E.; Li, R.Z.; Troutman, T.D.; Kisseleva, T.; Glass, C.K.; et al. Heterogeneity of HSCs in a Mouse Model of NASH. Hepatology 2021, 74, 667–685. [Google Scholar] [CrossRef]
- Cogliati, B.; Yashaswini, C.N.; Wang, S.; Sia, D.; Friedman, S.L. Friend or Foe? The Elusive Role of Hepatic Stellate Cells in Liver Cancer. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 647–661. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Pickholz, E.; Dobie, R.; Matchett, K.P.; Henderson, N.C.; Carrico, C.; Driver, I.; Borch Jensen, M.; Chen, L.; et al. An Autocrine Signaling Circuit in Hepatic Stellate Cells Underlies Advanced Fibrosis in Nonalcoholic Steatohepatitis. Sci. Transl. Med. 2023, 15, eadd3949. [Google Scholar] [CrossRef]
- Filliol, A.; Saito, Y.; Nair, A.; Dapito, D.H.; Yu, L.X.; Ravichandra, A.; Bhattacharjee, S.; Affo, S.; Fujiwara, N.; Su, H.; et al. Opposing Roles of Hepatic Stellate Cell Subpopulations in Hepatocarcinogenesis. Nature 2022, 610, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Merens, V.; Knetemann, E.; Gürbüz, E.; De Smet, V.; Messaoudi, N.; Reynaert, H.; Verhulst, S.; van Grunsven, L.A. Hepatic Stellate Cell Single Cell Atlas Reveals a Highly Similar Activation Process across Liver Disease Aetiologies. JHEP Rep. 2025, 7, 101223. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.A. Therapeutic Targets in Liver Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G709–G715. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013, 5, 167sr1. [Google Scholar] [CrossRef]
- Ramachandran, P.; Matchett, K.P.; Dobie, R.; Wilson-Kanamori, J.R.; Henderson, N.C. Single-Cell Technologies in Hepatology: New Insights into Liver Biology and Disease Pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Dobie, R.; Wilson-Kanamori, J.R.; Henderson, B.E.P.; Smith, J.R.; Matchett, K.P.; Portman, J.R.; Wallenborg, K.; Picelli, S.; Zagorska, A.; Pendem, S.V.; et al. Single-Cell Transcriptomics Uncovers Zonation of Function in the Mesenchyme during Liver Fibrosis. Cell Rep. 2019, 29, 1832–1847.e8. [Google Scholar] [CrossRef]
- Su, T.; Yang, Y.; Lai, S.; Jeong, J.; Jung, Y.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Single-Cell Transcriptomics Reveals Zone-Specific Alterations of Liver Sinusoidal Endothelial Cells in Cirrhosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1139–1161. [Google Scholar] [CrossRef]
- Saviano, A.; Henderson, N.C.; Baumert, T.F. Single-Cell Genomics and Spatial Transcriptomics: Discovery of Novel Cell States and Cellular Interactions in Liver Physiology and Disease biology. J. Hepatol. 2020, 73, 1219–1230. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the Fibrotic Niche of Human Liver Cirrhosis at Single-Cell Level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef]
- Chen, Z.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Targeted Drug Delivery to Hepatic Stellate Cells for the Treatment of Liver Fibrosis. J. Pharmacol. Exp. Ther. 2019, 370, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Poelstra, K. Hepatic Stellate Cell Targeting Using Peptide-Modified Biologicals. Methods Mol. Biol. 2023, 2669, 269–284. [Google Scholar] [CrossRef]
- Qian, T.; Fujiwara, N.; Koneru, B.; Ono, A.; Kubota, N.; Jajoriya, A.K.; Tung, M.G.; Crouchet, E.; Song, W.M.; Marquez, C.A.; et al. Molecular Signature Predictive of Long-Term Liver Fibrosis Progression to Inform Antifibrotic Drug Development. Gastroenterology 2022, 162, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, W.; Zeng, Z.; Lin, J.; Zhang, X.; Chen, L. Tgfb3 and Mmp13 Regulated the Initiation of Liver Fibrosis Progression as Dynamic Network Biomarkers. J. Cell. Mol. Med. 2021, 25, 867–879. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in Wound Healing, Fibrosis and Tumor Stroma: High Potential Targets for Therapeutics and Drug Delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.W.; Booijink, R.; Pater, L.; Wols, I.; Vrynas, A.; Storm, G.; Prakash, J.; Bansal, R. Fibroblast Growth Factor 2 Conjugated Superparamagnetic Iron Oxide Nanoparticles (FGF2-SPIONs) Ameliorate Hepatic Stellate Cells Activation In Vitro and Acute Liver Injury In Vivo. J. Control Release 2020, 328, 640–652. [Google Scholar] [CrossRef]
- Huang, L.; Xie, J.; Bi, Q.; Li, Z.; Liu, S.; Shen, Q.; Li, C. Highly Selective Targeting of Hepatic Stellate Cells for Liver Fibrosis Treatment Using a D-Enantiomeric Peptide Ligand of Fn14 Identified by Mirror-Image mRNA Display. Mol. Pharm. 2017, 14, 1742–1753. [Google Scholar] [CrossRef]
- Moreno, M.; Gonzalo, T.; Kok, R.J.; Sancho-Bru, P.; van Beuge, M.; Swart, J.; Prakash, J.; Temming, K.; Fondevila, C.; Beljaars, L.; et al. Reduction of Advanced Liver Fibrosis by Short-Term Targeted Delivery of an Angiotensin Receptor Blocker to Hepatic Stellate Cells in Rats. Hepatology 2010, 51, 942–952. [Google Scholar] [CrossRef]
- Lee, J.; Byun, J.; Shim, G.; Oh, Y.K. Fibroblast Activation Protein Activated Antifibrotic Peptide Delivery Attenuates Fibrosis in Mouse Models of Liver Fibrosis. Nat. Commun. 2022, 13, 1516. [Google Scholar] [CrossRef]
- Klein, S.; Van Beuge, M.M.; Granzow, M.; Beljaars, L.; Schierwagen, R.; Kilic, S.; Heidari, I.; Huss, S.; Sauerbruch, T.; Poelstra, K.; et al. HSC-Specific Inhibition of Rho-Kinase Reduces Portal Pressure in Cirrhotic Rats Without Major Systemic Effects. J. Hepatol. 2012, 57, 1220–1227. [Google Scholar] [CrossRef]
- Bansal, R.; Prakash, J.; Post, E.; Beljaars, L.; Schuppan, D.; Poelstra, K. Novel Engineered Targeted Interferon-Gamma Blocks Hepatic Fibrogenesis in Mice. Hepatology 2011, 54, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murase, K.; Kato, J.; Kobune, M.; Sato, T.; Kawano, Y.; Takimoto, R.; Takada, K.; Miyanishi, K.; Matsunaga, T.; et al. Resolution of Liver Cirrhosis Using Vitamin A—Coupled Liposomes to Deliver siRNA Against a Collagen-Specific Chaperone. Nat. Biotechnol. 2008, 26, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Poelstra, K. Drug Targeting and Nanomedicine: Lessons Learned from Liver Targeting and Opportunities for Drug Innovation. Pharmaceutics 2022, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Prakash, J.; De Ruiter, M.; Poelstra, K. Targeted Recombinant Fusion Proteins of IFNγ and Mimetic IFNγ with PDGFβR Bicyclic Peptide Inhibits Liver Fibrogenesis in Vivo. PLoS ONE 2014, 9, e89878. [Google Scholar] [CrossRef]
- Yazdani, S.; Bansal, R.; Prakash, J. Drug Targeting to Myofibroblasts: Implications for Fibrosis and Cancer. Adv. Drug Deliv. Rev. 2017, 121, 101–116. [Google Scholar] [CrossRef]
- Pringle, T.A.; Ramon-Gil, E.; Leslie, J.; Oakley, F.; Wright, M.C.; Knight, J.C.; Luli, S. Synthesis and Preclinical Evaluation of a (89)Zr-Labelled Human Single Chain Antibody for Non-Invasive Detection of Hepatic Myofibroblasts in Acute Liver Injury. Sci. Rep. 2024, 14, 633. [Google Scholar] [CrossRef]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic Targets, Novel Drugs, and Delivery Systems for Diabetes Associated NAFLD and Liver Fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113888. [Google Scholar] [CrossRef]
- Lin, C.Y.; Mamani, U.F.; Guo, Y.; Liu, Y.; Cheng, K. Peptide-Based siRNA Nanocomplexes Targeting Hepatic Stellate Cells. Biomolecules 2023, 13, 448. [Google Scholar] [CrossRef]
- Jain, A.; Barve, A.; Zhao, Z.; Fetse, J.P.; Liu, H.; Li, Y.; Cheng, K. Targeted Delivery of an siRNA/PNA Hybrid Nanocomplex Reverses Carbon Tetrachloride-Induced Liver Fibrosis. Adv. Ther. 2019, 2, 1900046. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Jain, A.; Chen, Z.; Liu, H.; Jin, W.; Cheng, K. Development of a Peptide-Modified siRNA Nanocomplex for Hepatic Stellate Cells. Nanomedicine 2018, 14, 51–61. [Google Scholar] [CrossRef]
- Lawitz, E.J.; Shevell, D.E.; Tirucherai, G.S.; Du, S.; Chen, W.; Kavita, U.; Coste, A.; Poordad, F.; Karsdal, M.; Nielsen, M.; et al. BMS-986263 in Patients with Advanced Hepatic Fibrosis: 36-Week Results From a Randomized, Placebo-Controlled Phase 2 Trial. Hepatology 2022, 75, 912–923. [Google Scholar] [CrossRef]
- Hill, T.A.; Shepherd, N.E.; Diness, F.; Fairlie, D.P. Constraining Cyclic Peptides to Mimic Protein Structure Motifs. Angew. Chem. Int. Ed. Engl. 2014, 53, 13020–13041. [Google Scholar] [CrossRef] [PubMed]
- Petta, I.; Lievens, S.; Libert, C.; Tavernier, J.; De Bosscher, K. Modulation of Protein-Protein Interactions for the Development of Novel Therapeutics. Mol. Ther. 2016, 24, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Targher, G. Efficacy of Peroxisome Proliferator-Activated Receptor Agonists, Glucagon-Like Peptide-1 Receptor Agonists, or Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Lancet Gastroenterol. Hepatol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Malm-Erjefält, M.; Bjørnsdottir, I.; Vanggaard, J.; Helleberg, H.; Larsen, U.; Oosterhuis, B.; van Lier, J.J.; Zdravkovic, M.; Olsen, A.K. Metabolism and Excretion of the Once-Daily Human Glucagon-Like Peptide-1 Analog Liraglutide In Healthy Male Subjects and Its In Vitro Degradation by Dipeptidyl Peptidase IV and Neutral Endopeptidase. Drug Metab. Dispos. 2010, 38, 1944–1953. [Google Scholar] [CrossRef]
| HSC Activation Target | Drug Name | Drug Category | Mechanism | Population (n) | Study Start Year | Highest Status (Phase) | NCT | Status |
|---|---|---|---|---|---|---|---|---|
| TGF-β/TGFβR | Hydronidone | Small molecule | Inhibitor | Liver fibrosis (248) | 2021 | III | NCT05115942 | Completed |
| αvβ6 Integrin | PLN-74809 | Small molecule | Inhibitor | Primary sclerosing cholangitis(121) | 2020 | II | NCT04480840 | Completed |
| FGF21 | BIO89-100 | Fusion proteins | Activator | MASH (101) | 2019 | II | NCT04048135 | Completed |
| Efruxifermin | Fusion proteins | Activator | MASH (110) | 2019 | II | NCT03976401 | Completed | |
| Pergbelfermin | Fusion proteins | Activator | Liver cirrhosis and MASH (155) | 2018 | II | NCT03486912 | Completed | |
| FGF19 | Aldafermin | Fusion proteins | Activator | MASH (171) | 2019 | II | NCT03912532 | Completed |
| PDGF/PDGFR | Imatinib | Small molecule | Inhibitor | Liver fibrosis (20) | 2021 | II | NCT05224128 | Unknown status |
| WNT/β-catenin | PRI-724 | Small molecule | Inhibitor | Liver cirrhosis (27) | 2018 | II | NCT03620474 | Completed |
| LOXL2 | PXS-5382A | Small molecule | Inhibitor | MASH (18) | 2019 | I | NCT04183517 | Completed |
| PPARα/γ | Saroglitazar | Small molecule | Activator | MASH (20) | 2019 | II | NCT03639623 | Completed |
| PPARα/δ/γ | Lanifibranor | Small molecule | Activator | MASH (1000) | 2021 | III | NCT04849728 | Recruiting |
| PPARα | Pemafibrate | Small molecule | Activator | MASH (118) | 2017 | II | NCT03350165 | Completed |
| PPARα/δ | ZSP0678 | Small molecule | Activator | MASH (104) | 2019 | I | NCT04137055 | Completed |
| TLR4 | JKB-121 | Small molecule | Inhibitor | MASH (65) | 2015 | II | NCT02442687 | Completed |
| JKB-122 | Small molecule | Inhibitor | MASH (300) | 2020 | II | NCT04255069 | Unknown | |
| LOXL2, PDE3/4 | Epeleuton | Small molecule | Inhibitor | MAFLD (96) | 2016 | II | NCT02941549 | Completed |
| AMPK | PXL-770 | Small molecule | Activator | MAFLD (211) | 2019 | II | NCT03763877 | Completed |
| MMP(MMP2,MMP9, VEGF-A) | ALS-L1023 | Small molecule | Inhibitor | MASH (60) | 2019 | II | NCT04342793 | Completed |
| FXR | Obeticholic Acid | Small molecule | Inhibitor | MASH (2477) | 2015 | III | NCT02548351 | Terminated |
| Tropifexor | Small molecule | Inhibitor | MASH (234) | 2019 | II | NCT04065841 | Terminated | |
| HSP47 | BMS-986263 | siRNA | Inhibitor | MASH (124) | 2021 | II | NCT04267393 | Terminated |
| GLP-1 receptor | Semaglutide | Small molecule | Activator | MASH (1200) | 2021 | III | NCT04822181 | Active, not recruiting |
| GLP-1/GIP receptor | Trizepatide | Small molecule | Activator | MASH (190) | 2019 | II | NCT04166773 | Completed |
| GLP-1/Glucagon receptor | Cotadutide | Small molecule | Activator | MASH (54) | 2019 | II | NCT05364931 | Completed |
| GLP-1/GIP/Glucagon | HM-15211 | Small molecule | Activator | MASH (240) | 2020 | II | NCT04505436 | Recruiting |
| THRβ | Resmetirom | Small molecule | Activator | MASH (1759) | 2019 | III | NCT03900429 | Active, not recruiting |
| VK2809 | Small molecule | Activator | MASH (248) | 2019 | II | NCT04173065 | Completed | |
| MPC | Azemiglitazone | Small molecule | Activator | MASH (1800) | 2022 | III | NCT03970031 | Unknown |
| Deuterium-stabilized (R)-Piglitazone | Small molecule | Activator | MASH (117) | 2022 | II | NCT04321343 | Completed | |
| PDEs (mainly PED2) | NZSP1601 | Small molecule | Inhibitor | MASH (37) | 2020 | II | NCT04140123 | Completed |
| A3AR | Namodenoson | Small molecule | Activator | MASH (60) | 2017 | II | NCT02927314 | Completed |
| FASN | TVB-2640 | Small molecule | Inhibitor | MASH and MAFLD (2000) | 2025 | III | NCT06692283 | Not yet recruiting |
| Stem cell | HepaStem | Cell transplant therapies | Activator | MASH (23) | 2019 | II | NCT03963921 | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Guo, J. Targeting Hepatic Stellate Cells for the Prevention and Treatment of Liver Cirrhosis and Hepatocellular Carcinoma: Strategies and Clinical Translation. Pharmaceuticals 2025, 18, 507. https://doi.org/10.3390/ph18040507
Xiong H, Guo J. Targeting Hepatic Stellate Cells for the Prevention and Treatment of Liver Cirrhosis and Hepatocellular Carcinoma: Strategies and Clinical Translation. Pharmaceuticals. 2025; 18(4):507. https://doi.org/10.3390/ph18040507
Chicago/Turabian StyleXiong, Hao, and Jinsheng Guo. 2025. "Targeting Hepatic Stellate Cells for the Prevention and Treatment of Liver Cirrhosis and Hepatocellular Carcinoma: Strategies and Clinical Translation" Pharmaceuticals 18, no. 4: 507. https://doi.org/10.3390/ph18040507
APA StyleXiong, H., & Guo, J. (2025). Targeting Hepatic Stellate Cells for the Prevention and Treatment of Liver Cirrhosis and Hepatocellular Carcinoma: Strategies and Clinical Translation. Pharmaceuticals, 18(4), 507. https://doi.org/10.3390/ph18040507







