α1 Adrenergic Receptors Mediate Panic-like Defensive Behavior in Alcohol-Drinking but Not Alcohol-Naïve Rats
Abstract
1. Introduction
2. Results
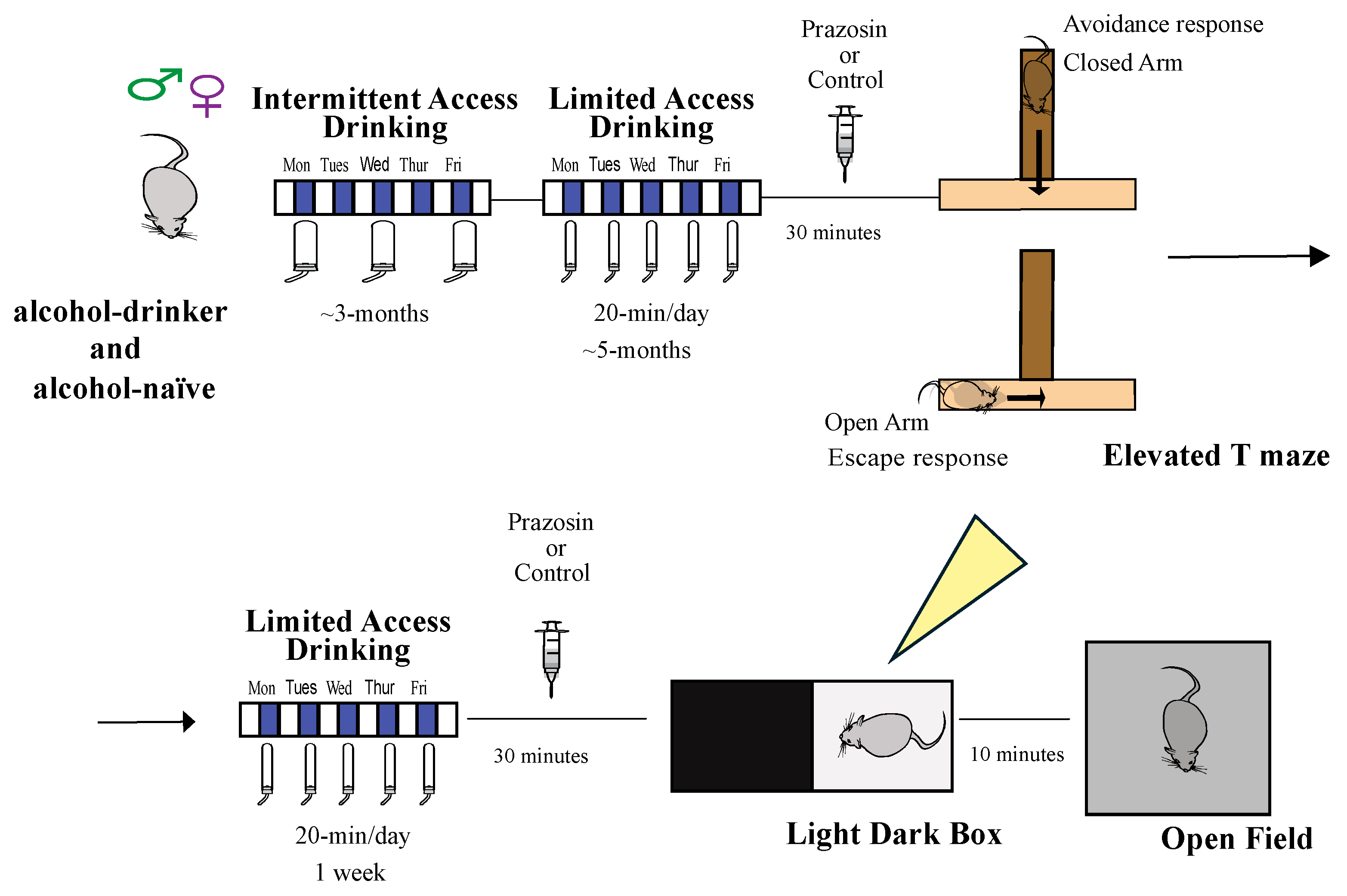
2.1. Experiment 1: Impact of the α1 Adrenergic Receptor Antagonist Prazosin on Avoidance and Escape Behavior in the Elevated T-Maze
2.2. Experiment 2: α1AR Antagonist Effects in the Light-Dark Box Task (LDT)
2.3. Experiment 3: α1AR Antagonist Prazosin in Open Field Test
3. Discussion
4. Methods
4.1. Animals
4.2. Drinking Methods
4.3. Drugs
4.4. Behavioral Sessions
4.5. Elevated T-Maze
4.6. Light Dark Box Test (LDT)
4.7. Open Field
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mobbs, D.; Headley, D.B.; Ding, W.; Dayan, P. Space, Time, and Fear: Survival Computations along Defensive Circuits. Trends Cogn. Sci. 2020, 24, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, S.M.; Zbozinek, T.D.; Mobbs, D. A Decision Architecture for Safety Computations. Trends Cogn. Sci. 2021, 25, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Gioiosa, L.; Parmigiani, S. Social stress in mice: Gender differences and effects of estrous cycle and social dominance. Physiol. Behav. 2001, 73, 411–420. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Coplan, J.D.; Kent, J.M.; Gorman, J.M. The noradrenergic system in pathological anxiety: A focus on panic with relevance to generalized anxiety and phobias. Biol. Psychiatry 1999, 46, 1205–1218. [Google Scholar] [CrossRef]
- Downs, A.M.; McElligott, Z.A. Noradrenergic circuits and signaling in substance use disorders. Neuropharmacology 2022, 208, 108997. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharm 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Puddephatt, J.A.; Irizar, P.; Jones, A.; Gage, S.H.; Goodwin, L. Associations of common mental disorder with alcohol use in the adult general population: A systematic review and meta-analysis. Addiction 2022, 117, 1543–1572. [Google Scholar] [CrossRef]
- Sinha, R.; Wemm, S.; Fogelman, N.; Milivojevic, V.; Morgan, P.M.; Angarita, G.A.; Hermes, G.; Fox, H.C. Moderation of Prazosin’s Efficacy by Alcohol Withdrawal Symptoms. Am. J. Psychiatry 2021, 178, 447–458. [Google Scholar] [CrossRef]
- Raskind, M.A.; Millard, S.P.; Petrie, E.C.; Peterson, K.; Williams, T.; Hoff, D.J.; Hart, K.; Holmes, H.; Hill, J.; Daniels, C.; et al. Higher Pretreatment Blood Pressure Is Associated With Greater Posttraumatic Stress Disorder Symptom Reduction in Soldiers Treated With Prazosin. Biol. Psychiatry 2016, 80, 736–742. [Google Scholar] [CrossRef]
- Datta, D.; Yang, S.T.; Galvin, V.C.; Solder, J.; Luo, F.; Morozov, Y.M.; Arellano, J.; Duque, A.; Rakic, P.; Arnsten, A.F.T.; et al. Noradrenergic alpha1-Adrenoceptor Actions in the Primate Dorsolateral Prefrontal Cortex. J. Neurosci. 2019, 39, 2722–2734. [Google Scholar] [CrossRef]
- Berridge, C.W.; Spencer, R.C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016, 1641, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.; Wang, W.W.; Han, R.; McFadden, K.; Valentino, R.J. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 2012, 37, 520–530. [Google Scholar] [CrossRef]
- Valentino, R.J.; Van Bockstaele, E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008, 583, 194–203. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Jahn, C.I.; Gilardeau, S.; Varazzani, C.; Blain, B.; Sallet, J.; Walton, M.E.; Bouret, S. Dual contributions of noradrenaline to behavioural flexibility and motivation. Psychopharmacology 2018, 235, 2687–2702. [Google Scholar] [CrossRef]
- Verplaetse, T.L.; Rasmussen, D.D.; Froehlich, J.C.; Czachowski, C.L. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res. 2012, 36, 881–886. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, C.R.; Blandino, K.L.; Nash, M.L.; Sjogren, E.R.; Grampetro, M.A.; Moorman, D.E.; Vazey, E.M. Noradrenergic tone mediates marble burying behavior after chronic stress and ethanol. Psychopharmacology 2020, 237, 3021–3031. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Lei, K.; Kwok, C.; Ghotra, S.; Wegner, S.A.; Walsh, M.; Waal, J.; Darevsky, D.; Hopf, F.W. The role of anterior insula-brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking. Neuropsychopharmacology 2021, 46, 1918–1926. [Google Scholar] [CrossRef]
- Epstein, D.H.; Kowalczyk, W.J. Compulsive Seekers: Our take. Two Clinicians’ Perspective on a New Animal Model of Addiction. Neuropsychopharmacology 2018, 43, 677–679. [Google Scholar] [CrossRef]
- Hopf, F.W.; Lesscher, H.M. Rodent models for compulsive alcohol intake. Alcohol 2014, 48, 253–264. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Frasier, R.M.; Hopf, F.W. Animal models of compulsion alcohol drinking: Why we love quinine-resistant intake and what we learned from it. Front. Psychiatry 2023, 14, 1116901. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Jane Smith, R.; Wean, S.E.; Engleman, E.A.; Hopf, F.W. Greater inhibition of female rat binge alcohol intake by adrenergic receptor blockers using a novel Two-Shot rat binge drinking model. Sci. Rep. 2024, 14, 14029. [Google Scholar] [CrossRef]
- Zangrossi, H., Jr.; Graeff, F.G. Serotonin in anxiety and panic: Contributions of the elevated T-maze. Neurosci. Biobehav. Rev. 2014, 46, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.B.; Nizhnikov, M.E.; Molina, J.C.; Pautassi, R.M. Relationship between ethanol-induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behav. Brain Res. 2014, 265, 203–215. [Google Scholar] [CrossRef]
- Amodeo, L.R.; Wills, D.N.; Sanchez-Alavez, M.; Nguyen, W.; Conti, B.; Ehlers, C.L. Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol 2018, 73, 57–66. [Google Scholar] [CrossRef]
- Becker, J.B.; McClellan, M.L.; Reed, B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017, 95, 136–147. [Google Scholar] [CrossRef]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef]
- Radke, A.K.; Sneddon, E.A.; Frasier, R.M.; Hopf, F.W. Recent Perspectives on Sex Differences in Compulsion-Like and Binge Alcohol Drinking. Int. J. Mol. Sci. 2021, 22, 3788. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Darevsky, D.; Kellner, J.; de Paula Soares, V.; de Cassia Albino, M.; Maulucci, D.; Wean, S.; Hopf, F.W. Sex- and estrous-related response patterns for alcohol depend critically on the level of compulsion-like challenge. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 133, 111008. [Google Scholar] [CrossRef]
- Sneddon, E.A.; White, R.D.; Radke, A.K. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcohol. Clin. Exp. Res. 2019, 43, 243–249. [Google Scholar] [CrossRef]
- Frasier, R.M.; De Oliveira Sergio, T.; Starski, P.A.; Grippo, A.J.; Hopf, F.W. Heart rate variability measures indicating sex differences in autonomic regulation during anxiety-like behavior in rats. Front. Psychiatry 2023, 14, 1244389. [Google Scholar] [CrossRef] [PubMed]
- Frasier, R.M.; Starski, P.A.; de Oliveira Sergio, T.; Grippo, A.J.; Hopf, F.W. Sex differences in heart rate variability measures that predict alcohol drinking in rats. Addict. Biol. 2024, 29, e13387. [Google Scholar] [CrossRef]
- Fox, H.C.; Anderson, G.M.; Tuit, K.; Hansen, J.; Kimmerling, A.; Siedlarz, K.M.; Morgan, P.T.; Sinha, R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcohol. Clin. Exp. Res. 2012, 36, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, S.I.; Aspley, S.; Butler, S.; Beckett, S.; Marsden, C.A. Effects of lesioning noradrenergic neurones in the locus coeruleus on conditioned and unconditioned aversive behaviour in the rat. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1307–1321. [Google Scholar] [CrossRef]
- Doze, V.A.; Handel, E.M.; Jensen, K.A.; Darsie, B.; Luger, E.J.; Haselton, J.R.; Talbot, J.N.; Rorabaugh, B.R. alpha(1A)- and alpha(1B)-adrenergic receptors differentially modulate antidepressant-like behavior in the mouse. Brain Res. 2009, 1285, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Haass-Koffler, C.L.; Swift, R.M.; Leggio, L. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology 2018, 235, 1625–1634. [Google Scholar] [CrossRef]
- Simpson, T.L.; Saxon, A.J.; Meredith, C.W.; Malte, C.A.; McBride, B.; Ferguson, L.C.; Gross, C.A.; Hart, K.L.; Raskind, M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol. Clin. Exp. Res. 2009, 33, 255–263. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Wean, S.; Katner, S.N.; Hopf, F.W. The role of beta- and alpha-adrenergic receptors on alcohol drinking. Neuropharmacology 2023, 234, 109545. [Google Scholar] [CrossRef]
- Kang, S.; Li, J.; Zuo, W.; Fu, R.; Gregor, D.; Krnjevic, K.; Bekker, A.; Ye, J.H. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology 2017, 42, 1813–1824. [Google Scholar] [CrossRef]
- Quadir, S.G.; Arleth, G.M.; Jahad, J.V.; Echeveste Sanchez, M.; Effinger, D.P.; Herman, M.A. Sex differences in affective states and association with voluntary ethanol intake in Sprague-Dawley rats. Psychopharmacology 2022, 239, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, P.; Han, X.; Zuo, W.; Mei, Q.; Bian, E.Y.; Umeugo, J.; Ye, J. Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 163–176. [Google Scholar] [PubMed]
- Albrechet-Souza, L.; Schratz, C.L.; Gilpin, N.W. Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol. Sex. Differ. 2020, 11, 27. [Google Scholar] [CrossRef]
- Hopf, F.W.; Chang, S.J.; Sparta, D.R.; Bowers, M.S.; Bonci, A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol. Clin. Exp. Res. 2010, 34, 1565–1573. [Google Scholar] [CrossRef]
- Seif, T.; Chang, S.J.; Simms, J.A.; Gibb, S.L.; Dadgar, J.; Chen, B.T.; Harvey, B.K.; Ron, D.; Messing, R.O.; Bonci, A.; et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci. 2013, 16, 1094–1100. [Google Scholar]
- De Oliveira Sergio, T.; Frias, A.T.; Vilela-Costa, H.H.; De Oliveira, D.C.; Zuardi, A.W.; Zangrossi, H., Jr. Serotonin mediates the panicolytic-like effect of oxytocin in the dorsal periaqueductal gray. J. Psychopharmacol. 2020, 34, 383–390. [Google Scholar] [CrossRef]
- Hernandes, P.M.; Batistela, M.F.; Vilela-Costa, H.H.; Sant’Ana, A.B.; Kumpel, V.D.; Tirapelle, M.C.; Bom, A.O.P.; de Andrade, T.; Zangrossi, H., Jr. Role of 5-HT1A receptors in the ventral hippocampus in the regulation of anxiety- and panic-related defensive behaviors in rats. Behav. Brain Res. 2021, 408, 113296. [Google Scholar] [CrossRef]
- Teixeira, R.C.; Zangrossi, H.; Graeff, F.G. Behavioral effects of acute and chronic imipramine in the elevated T-maze model of anxiety. Pharmacol. Biochem. Behav. 2000, 65, 571–576. [Google Scholar] [CrossRef]
- Maraschin, J.C.; Sestile, C.C.; Yabiku, C.T.; Roncon, C.M.; de Souza Fiaes, G.C.; Graeff, F.G.; Audi, E.A.; Zangrossi, H., Jr. Effects of the adjunctive treatment of antidepressants with opiorphin on a panic-like defensive response in rats. Behav. Brain Res. 2020, 378, 112263. [Google Scholar] [CrossRef]
- Vicente, M.A.; Zangrossi, H., Jr. Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharmacology 2014, 79, 127–135. [Google Scholar] [CrossRef]


| Sex | Groups | Distance | Velocity |
|---|---|---|---|
| Naïve Control | 1806.3 ± 213.3 | 10.2 ± 1.9 | |
| Males | Naïve Prazosin | 1821.7 ± 441.3 | 10.1 ± 3.1 |
| Drinker Control | 1960.9 ± 724.7 | 7.6 ± 10.1 | |
| Drinker Prazosin | 1491.9 ± 220.4 | 8.5 ± 1.1 | |
| Naïve Control | 2845.7 ± 589.1 | 6.2 ± 1.1 | |
| Females | Naïve Prazosin | 3993.7 ± 1509.5 | 8.8 ± 3.3 |
| Drinker Control | 3134.0 ± 781.9 | 7.6 ± 1.6 | |
| Drinker Prazosin | 2179.0 ± 414.8 | 6.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira Sergio, T.; Kellner, J.; Wean, S.; Hopf, F.W. α1 Adrenergic Receptors Mediate Panic-like Defensive Behavior in Alcohol-Drinking but Not Alcohol-Naïve Rats. Pharmaceuticals 2025, 18, 484. https://doi.org/10.3390/ph18040484
De Oliveira Sergio T, Kellner J, Wean S, Hopf FW. α1 Adrenergic Receptors Mediate Panic-like Defensive Behavior in Alcohol-Drinking but Not Alcohol-Naïve Rats. Pharmaceuticals. 2025; 18(4):484. https://doi.org/10.3390/ph18040484
Chicago/Turabian StyleDe Oliveira Sergio, Thatiane, Jacob Kellner, Sarah Wean, and Frederic W. Hopf. 2025. "α1 Adrenergic Receptors Mediate Panic-like Defensive Behavior in Alcohol-Drinking but Not Alcohol-Naïve Rats" Pharmaceuticals 18, no. 4: 484. https://doi.org/10.3390/ph18040484
APA StyleDe Oliveira Sergio, T., Kellner, J., Wean, S., & Hopf, F. W. (2025). α1 Adrenergic Receptors Mediate Panic-like Defensive Behavior in Alcohol-Drinking but Not Alcohol-Naïve Rats. Pharmaceuticals, 18(4), 484. https://doi.org/10.3390/ph18040484






