Inhibition of GSK-3β Restores Differentiation Potential of Late-Passage Mesenchymal Stem Cells
Abstract
1. Introduction
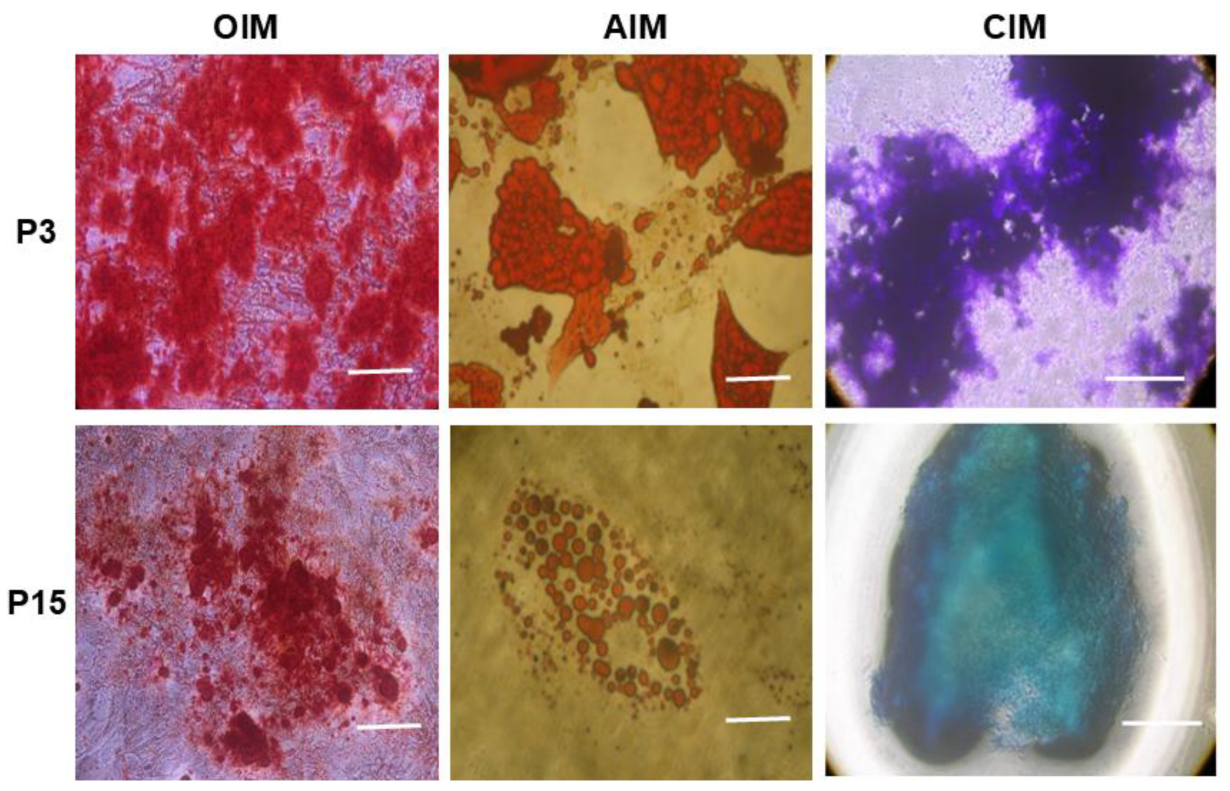
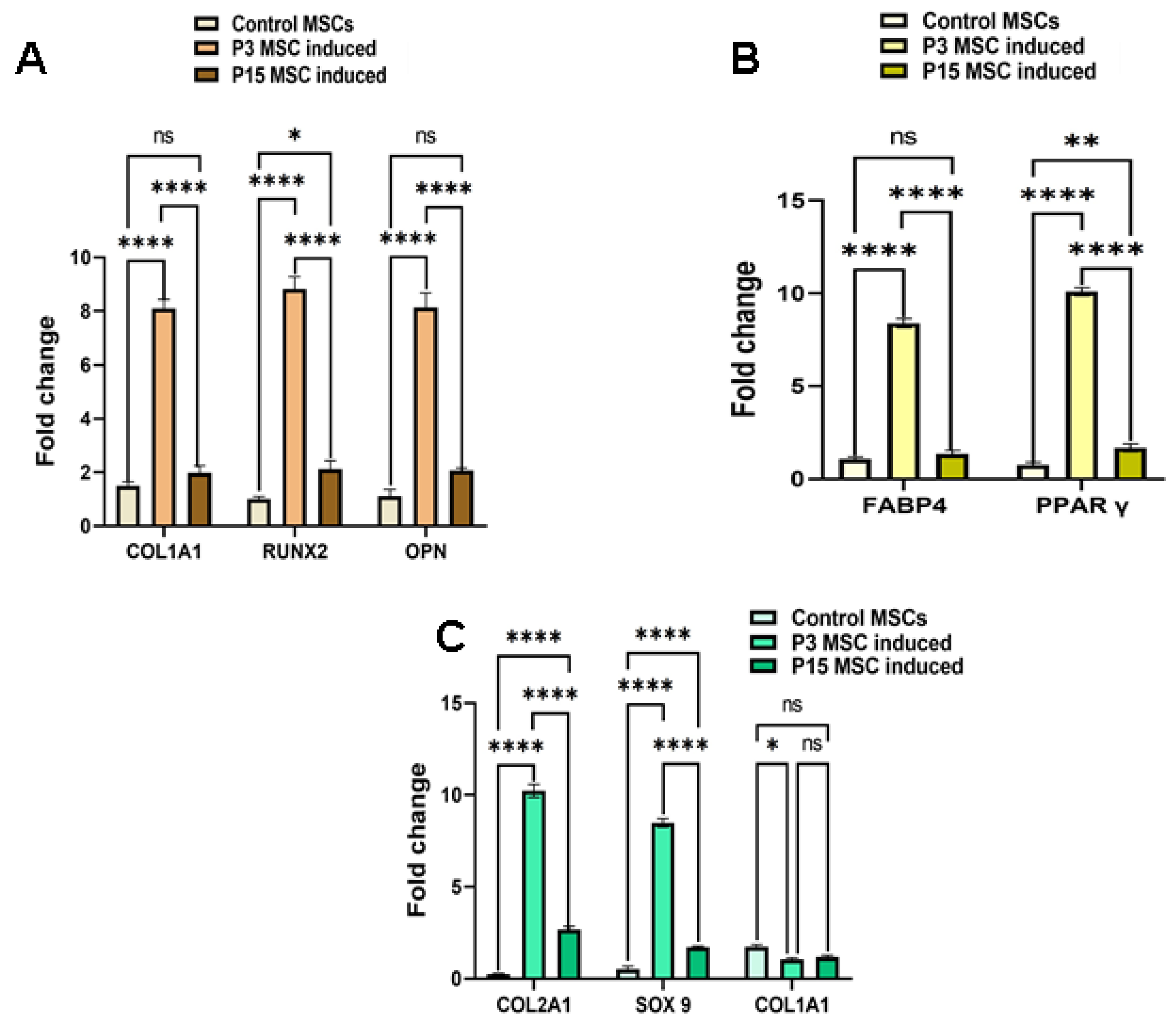
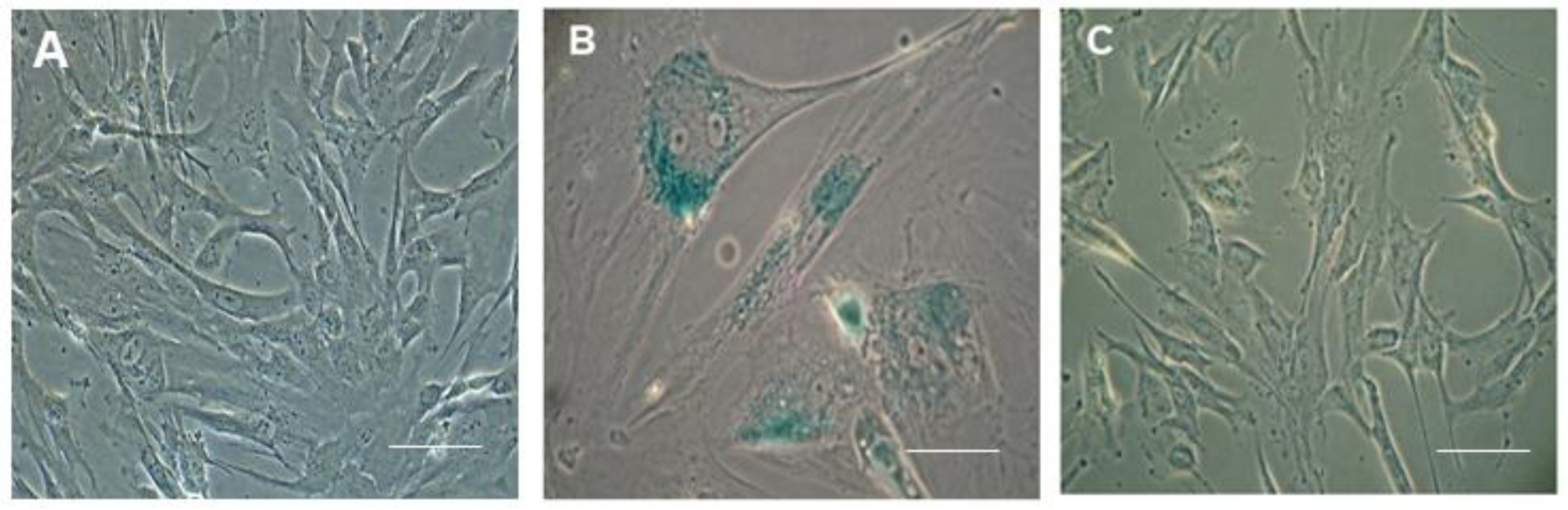
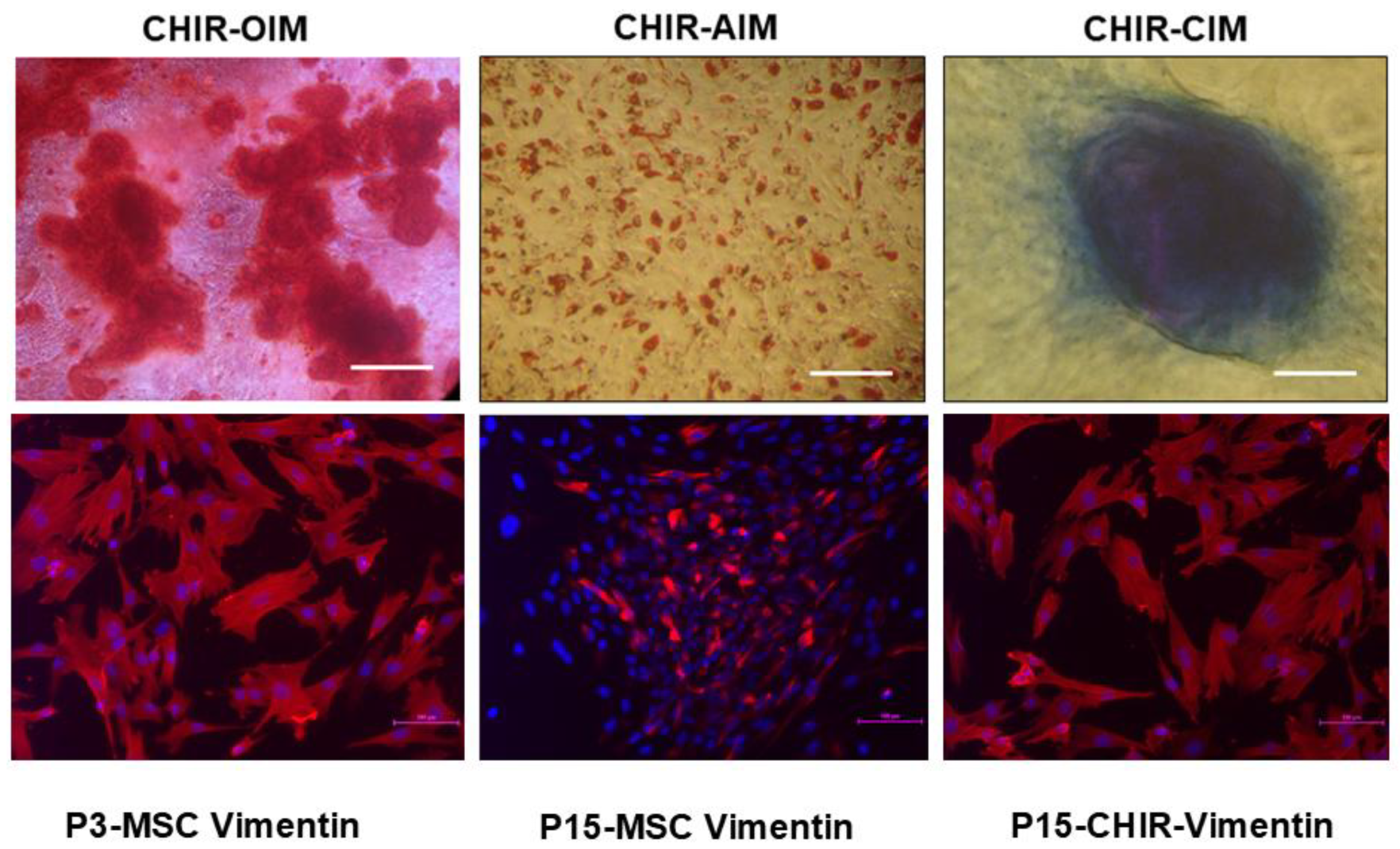
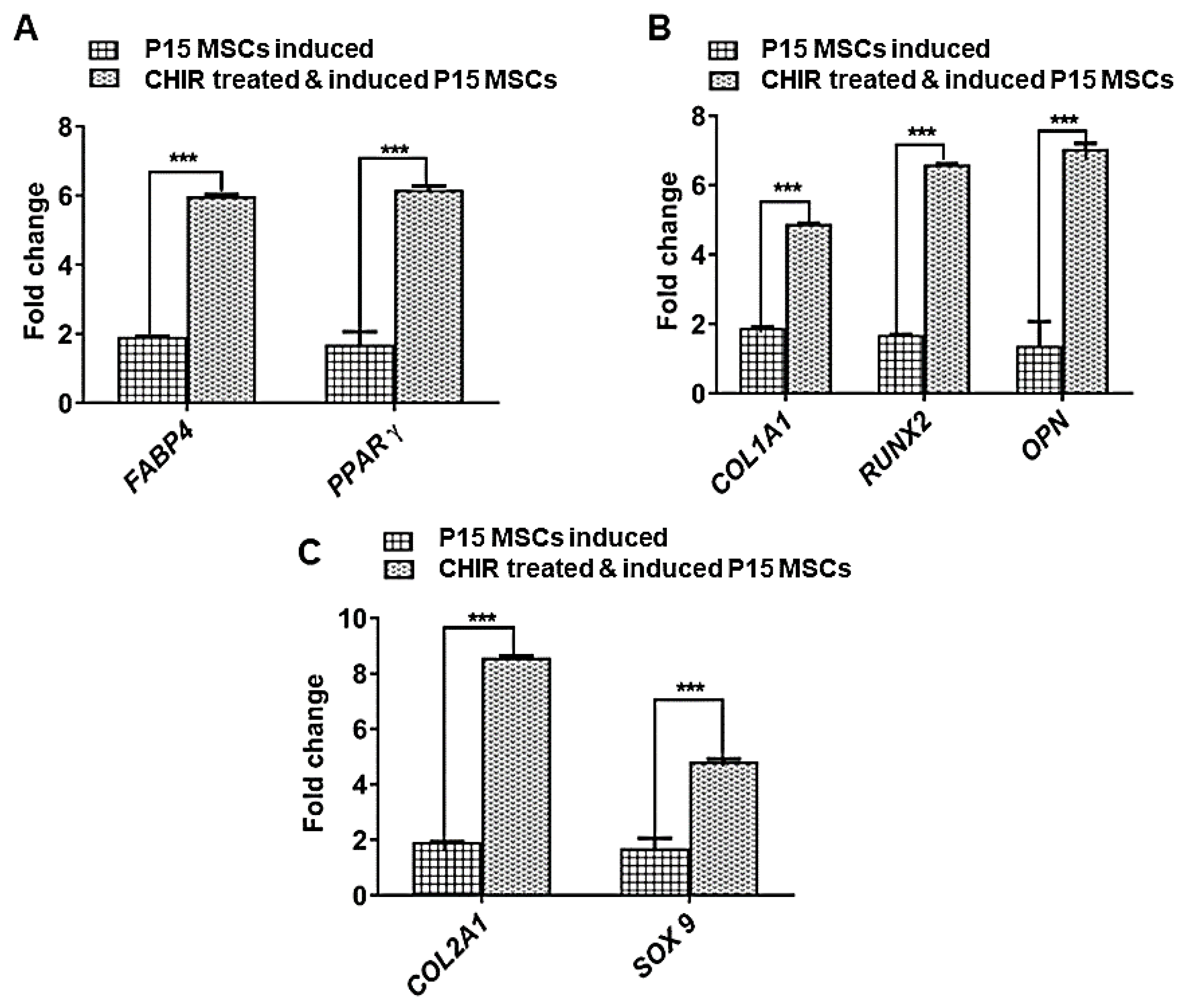
2. Results
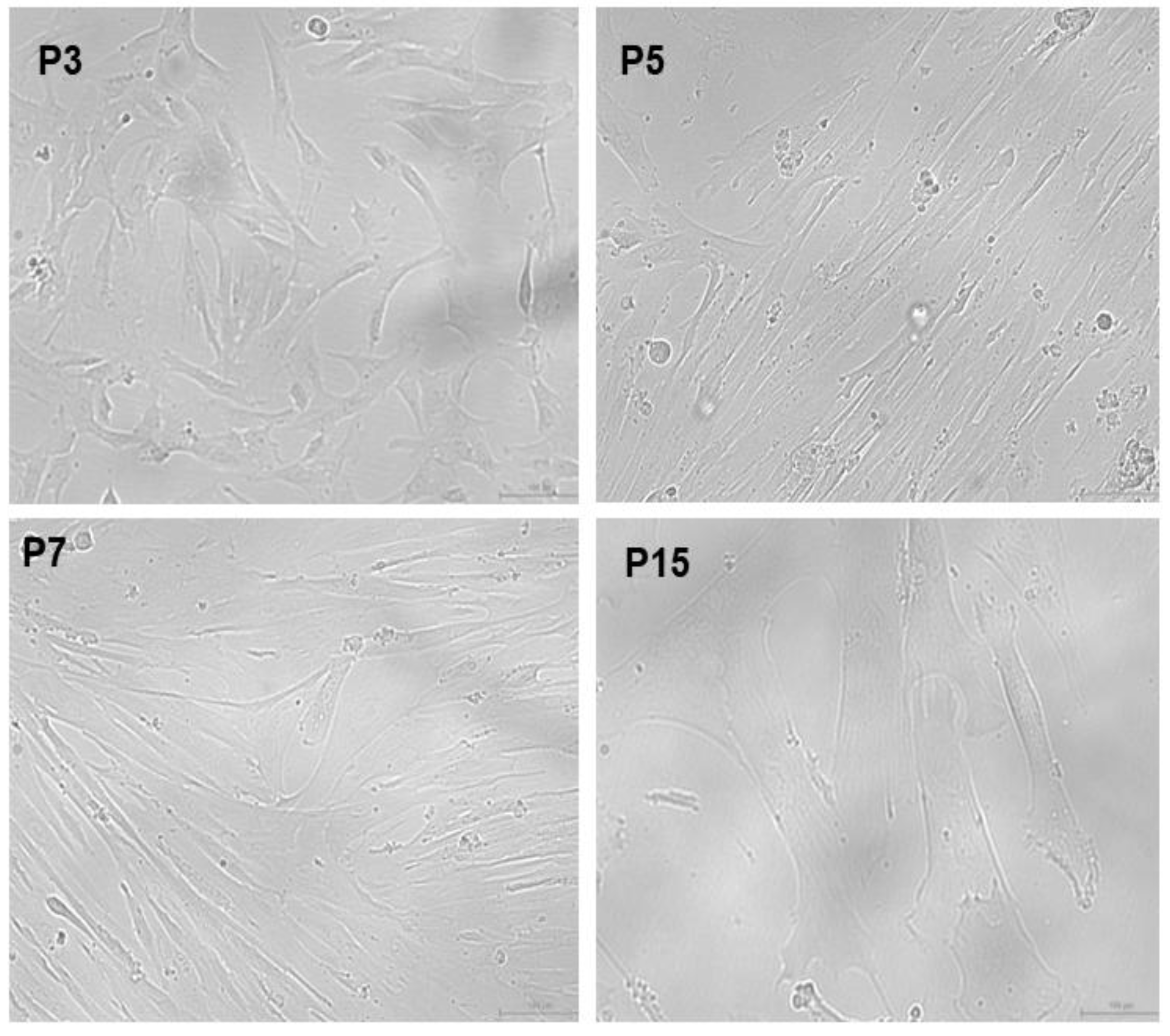
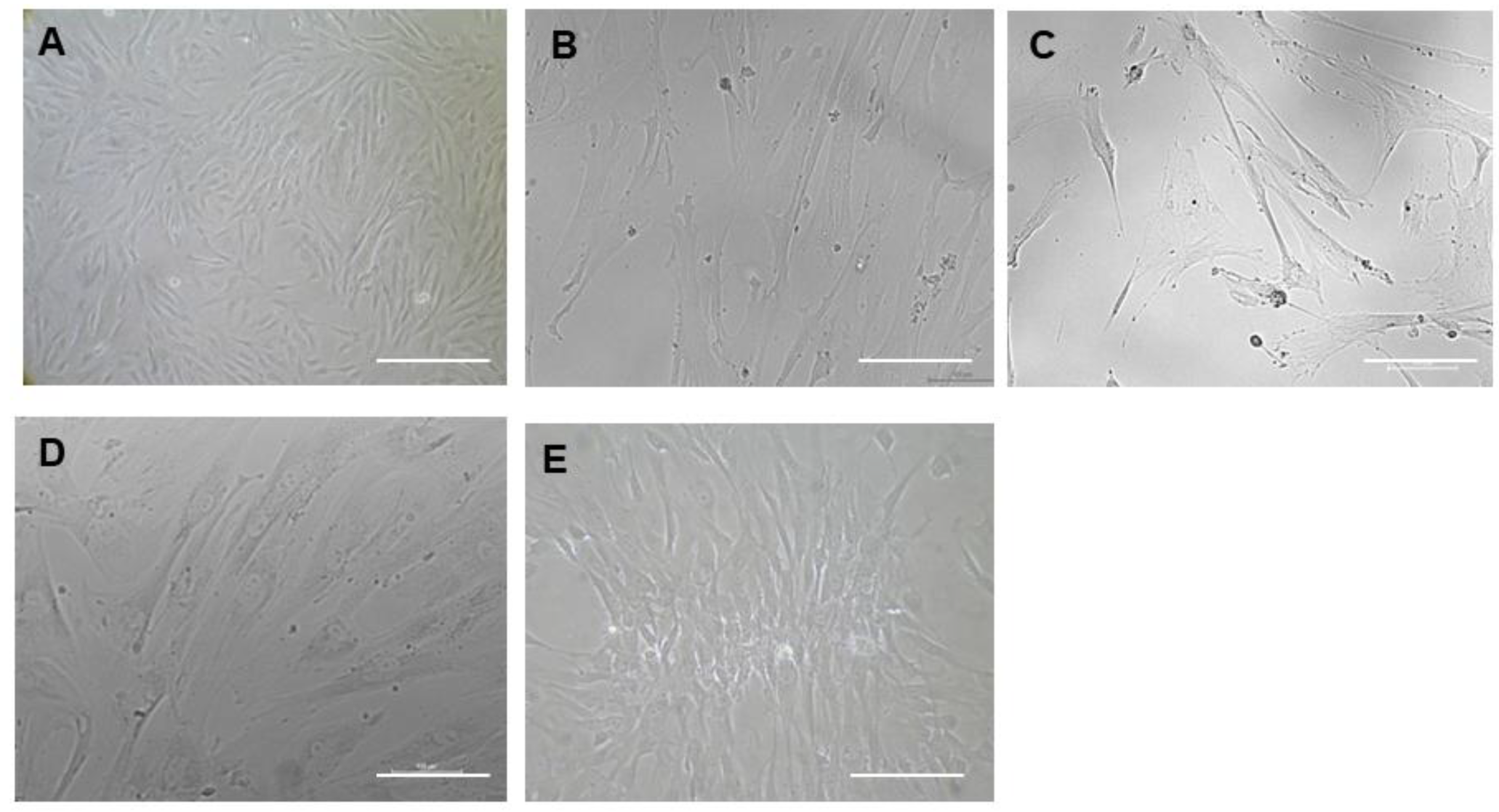
Isolation of Mesenchymal Stem Cells from Wharton’s Jelly (WJ-MSCs)
3. Discussion
4. Materials and Methods
4.1. Isolation of WJ-MSCs
4.2. Pre-Treatment of WJ-MSCs with CHIR 99021
4.3. Tri-Lineage Differentiation Potential of MSCs
4.4. Senescence Assay
4.5. G-Banded Karyotyping
4.6. RNA Extraction and cDNA Synthesis
Gene Expression Analysis
4.7. Immunocytochemistry (ICC)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [PubMed]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Camardelli, F.; Cazzola, M.; Rogliani, P.; Chetta, A. Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis. Cells 2022, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi Sundaram, R.; Kadapakkam Nandabalan, S.; Rupert, S.; Srinivasan, P.; Sankar, P.; Patra, B.; Verma, R.S.; Vennila, R.; Sathyanesan, J.; Rajagopal, S. Differential immunomodulation of human mesenchymal stromal cells from various sources in an inflammation mimetic milieu. Cytotherapy 2022, 24, 110–123. [Google Scholar]
- Govarthanan, K.; Vidyasekar, P.; Gupta, P.K.; Lenka, N.; Verma, R.S. Glycogen synthase kinase 3β inhibitor-CHIR 99021 augments the differentiation potential of mesenchymal stem cells. Cytotherapy 2020, 22, 91–105. [Google Scholar]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef]
- Huang, C.; Dai, J.; Zhang, X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1261–1266. [Google Scholar]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar]
- Noronha Nc, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar]
- Chen, J.Y.; Mou, X.Z.; Du, X.C.; Xiang, C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac. J. Trop. Med. 2015, 8, 739–746. [Google Scholar] [PubMed]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.; Zhao, D.; Jin, H.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 226. [Google Scholar] [PubMed]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of mesenchymal stem cell: Machinery, markers, and strategies of fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Yujia, W.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar]
- Cleary, M.A.; Van Osch, G.J.V.M.; Brama, P.A.; Hellingman, C.A.; Narcisi, R. FGF, TGFβ and Wnt crosstalk: Embryonic to in vitro cartilage development from mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 332–342. [Google Scholar]
- Narcisi, R.; Arikan, O.H.; Lehmann, J.; Ten Berge, D.; Van Osch, G.J.V.M. Differential Effects of Small Molecule WNT Agonists on the Multilineage Differentiation Capacity of Human Mesenchymal Stem Cells. Tissue Eng.-Part A 2016, 22, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- D Ten, B.; Kurek, D.; Blauwkamp, T.; Koole, W.; Maas, A.; Eroglu, E.; Siu, R.K.; Nusse, R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011, 13, 1070–1077. [Google Scholar]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Lehmann, J.; Narcisi, R.; Franceschini, N.; Chatzivasileiou, D.; Boer, C.G.; Koevoet, W.J.L.M.; Putavet, D.; Drabek, D.; van Haperen, R.; de Keizer, P.L.J.; et al. WNT/beta-catenin signalling interrupts a senescence-induction cascade in human mesenchymal stem cells that restricts their expansion. Cell. Mol. Life Sci. 2022, 79, 82. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F. Activator or inhibitor? GSK-3 as a new drug target. Biochem. Pharmacol. 2013, 86, 191–199. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O.; Baudoux, E.; Beguin, Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016, 14, 145. [Google Scholar] [CrossRef]
- Chen, H.; Liu, O.; Chen, S.; Zhou, Y. Aging and Mesenchymal Stem Cells: Therapeutic Opportunities and Challenges in the Older Group. Gerontology 2022, 68, 339–352. [Google Scholar] [CrossRef]
- El-Sayed, M.; El-Feky, M.A.; El-Amir, M.I.; Hasan, A.S.; Tag-Adeen, M.; Urata, Y.; Goto, S.; Luo, L.; Yan, C.; Li, T.-S. Immunomodulatory effect of mesenchymal stem cells: Cell origin and cell quality variations. Mol. Biol. Rep. 2019, 46, 1157–1165. [Google Scholar] [CrossRef]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [PubMed]
- Khanh, V.C.; Yamashita, T.; Ohneda, K.; Tokunaga, C.; Kato, H.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Rejuvenation of mesenchymal stem cells by extracellular vesicles inhibits the elevation of reactive oxygen species. Sci. Rep. 2020, 10, 17315. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weismann, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar]
- Rethineswaran, V.K.; Kim, D.Y.; Kim, Y.J.; Jang, W.; Ji, S.T.; Van, L.T.H.; Giang, L.T.T.; Ha, J.S.; Yun, J.; Jung, J.; et al. Chir99021 augmented the function of late endothelial progenitor cells by preventing replicative senescence. Int. J. Mol. Sci. 2021, 22, 4796. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Gao, F.; Liu, T.; Ren, W.; Chen, L.; Cao, Y.; Chen, W.; Guo, S.; Zhang, Q.; Chen, W.; et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow–derived mesenchymal stem cells and slow age-related degeneration. Sci. Transl. Med. 2021, 13, eaaz8697. [Google Scholar]
- Vennila, R.; Sundaram, R.S.M.; Selvaraj, S.; Srinivasan, P.; Pathak, S.; Rupert, S.; Rajagopal, S. Effect of Human Platelet Lysate in Differentiation of Wharton’s Jelly Derived Mesenchymal Stem Cells. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 1177–1191. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govarthanan, K.; Meenakshi Sundaram, R.S.; Richard, A.S.; Chabathula, S.C.; Rupert, S.; Sathyanesan, J.; Verma, R.S.; Jeyaraman, N.; Jeyaraman, M.; Rajendran, R.L.; et al. Inhibition of GSK-3β Restores Differentiation Potential of Late-Passage Mesenchymal Stem Cells. Pharmaceuticals 2025, 18, 483. https://doi.org/10.3390/ph18040483
Govarthanan K, Meenakshi Sundaram RS, Richard AS, Chabathula SC, Rupert S, Sathyanesan J, Verma RS, Jeyaraman N, Jeyaraman M, Rajendran RL, et al. Inhibition of GSK-3β Restores Differentiation Potential of Late-Passage Mesenchymal Stem Cells. Pharmaceuticals. 2025; 18(4):483. https://doi.org/10.3390/ph18040483
Chicago/Turabian StyleGovarthanan, Kavitha, Raja Sundari Meenakshi Sundaram, Arthi Sunil Richard, Siva Chander Chabathula, Secunda Rupert, Jeswanth Sathyanesan, Rama Shanker Verma, Naveen Jeyaraman, Madhan Jeyaraman, Ramya Lakshmi Rajendran, and et al. 2025. "Inhibition of GSK-3β Restores Differentiation Potential of Late-Passage Mesenchymal Stem Cells" Pharmaceuticals 18, no. 4: 483. https://doi.org/10.3390/ph18040483
APA StyleGovarthanan, K., Meenakshi Sundaram, R. S., Richard, A. S., Chabathula, S. C., Rupert, S., Sathyanesan, J., Verma, R. S., Jeyaraman, N., Jeyaraman, M., Rajendran, R. L., Gangadaran, P., & Ahn, B.-C. (2025). Inhibition of GSK-3β Restores Differentiation Potential of Late-Passage Mesenchymal Stem Cells. Pharmaceuticals, 18(4), 483. https://doi.org/10.3390/ph18040483









