A Systematic Profiling of the Components of Kukeya Tablets, a Traditional Ethnic Medicine Prescription, by Ultra-High-Performance Liquid Chromatography–Quadrupole/Orbitrap High-Resolution Mass Spectrometry
Abstract
1. Introduction
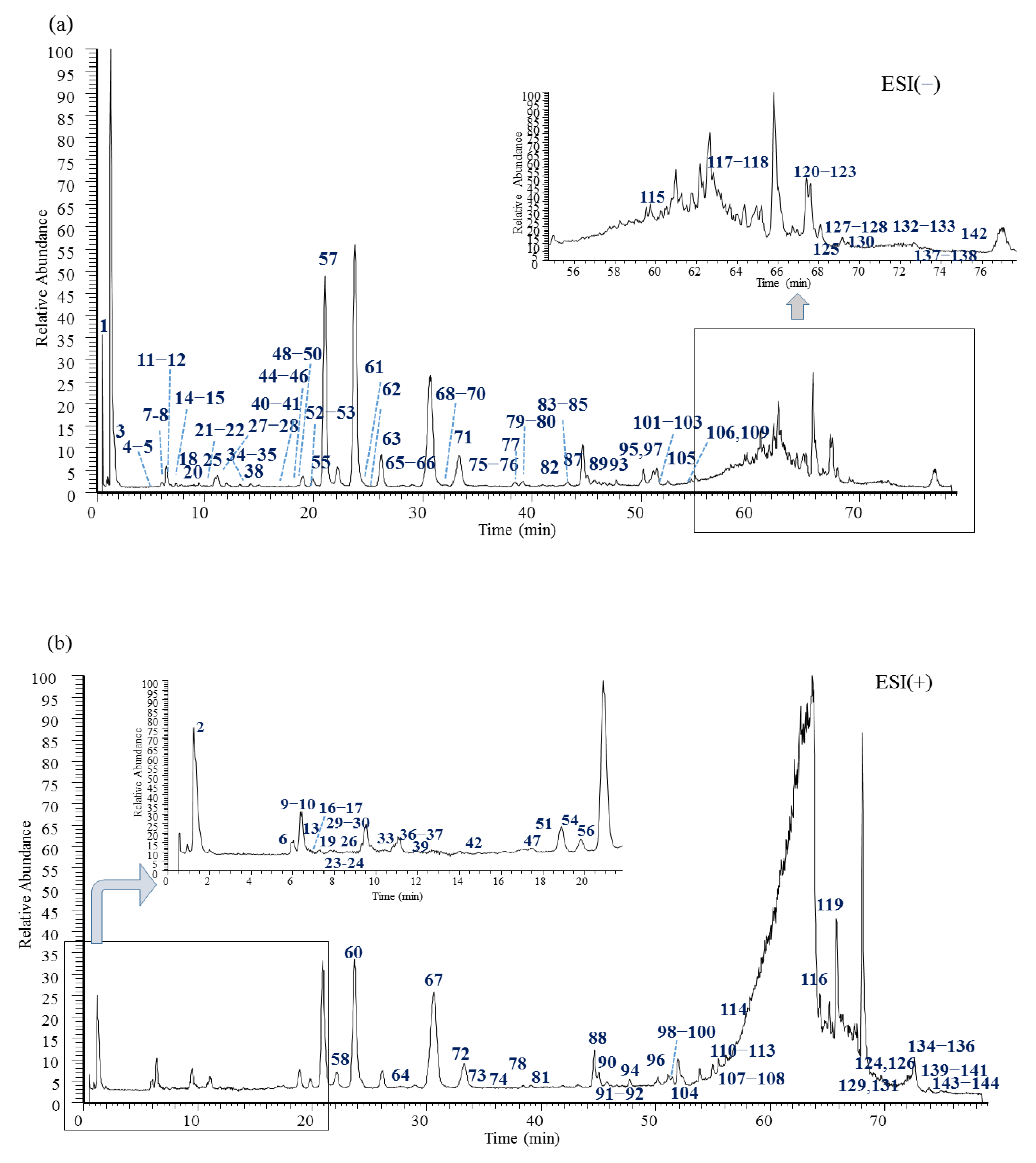
2. Results and Discussion
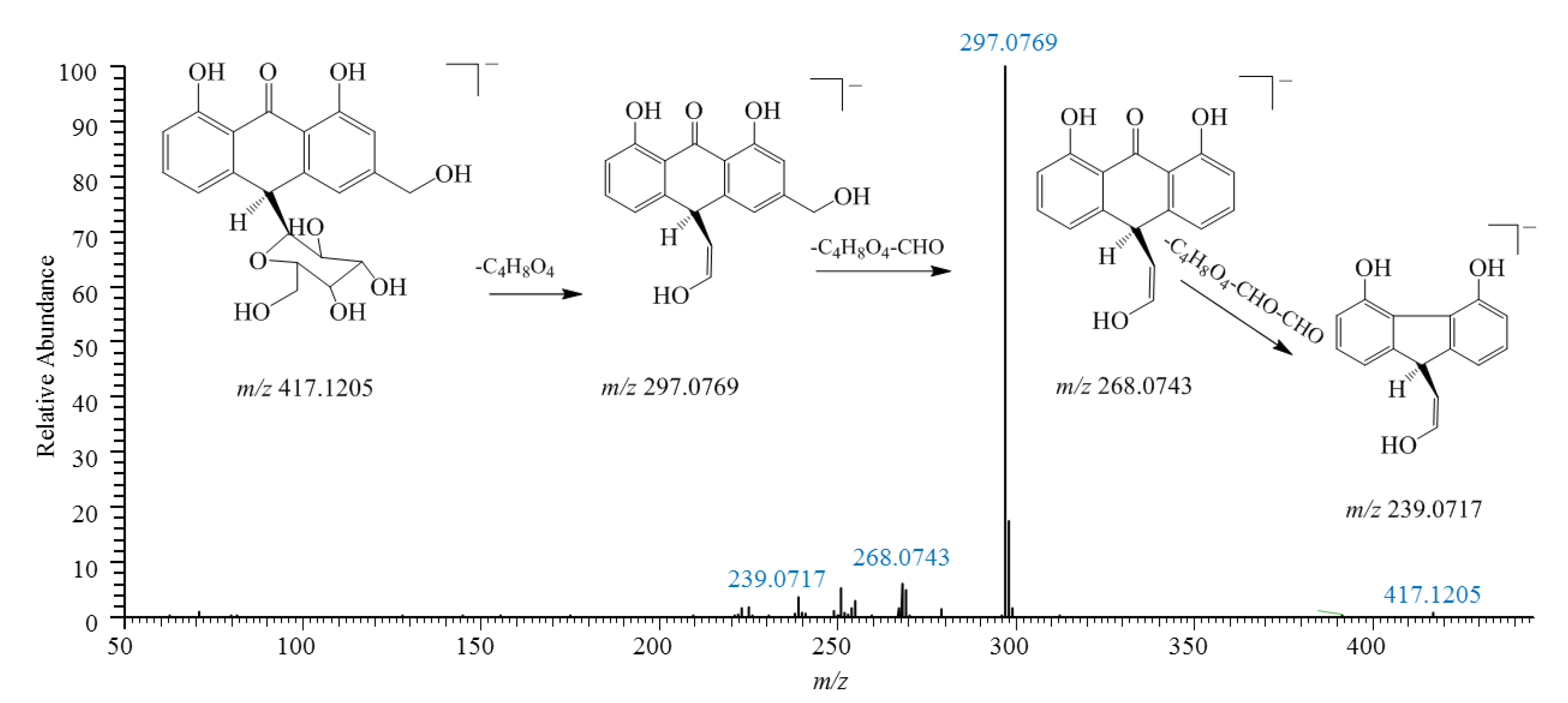
2.1. Identification of Anthrones and Anthraquinones
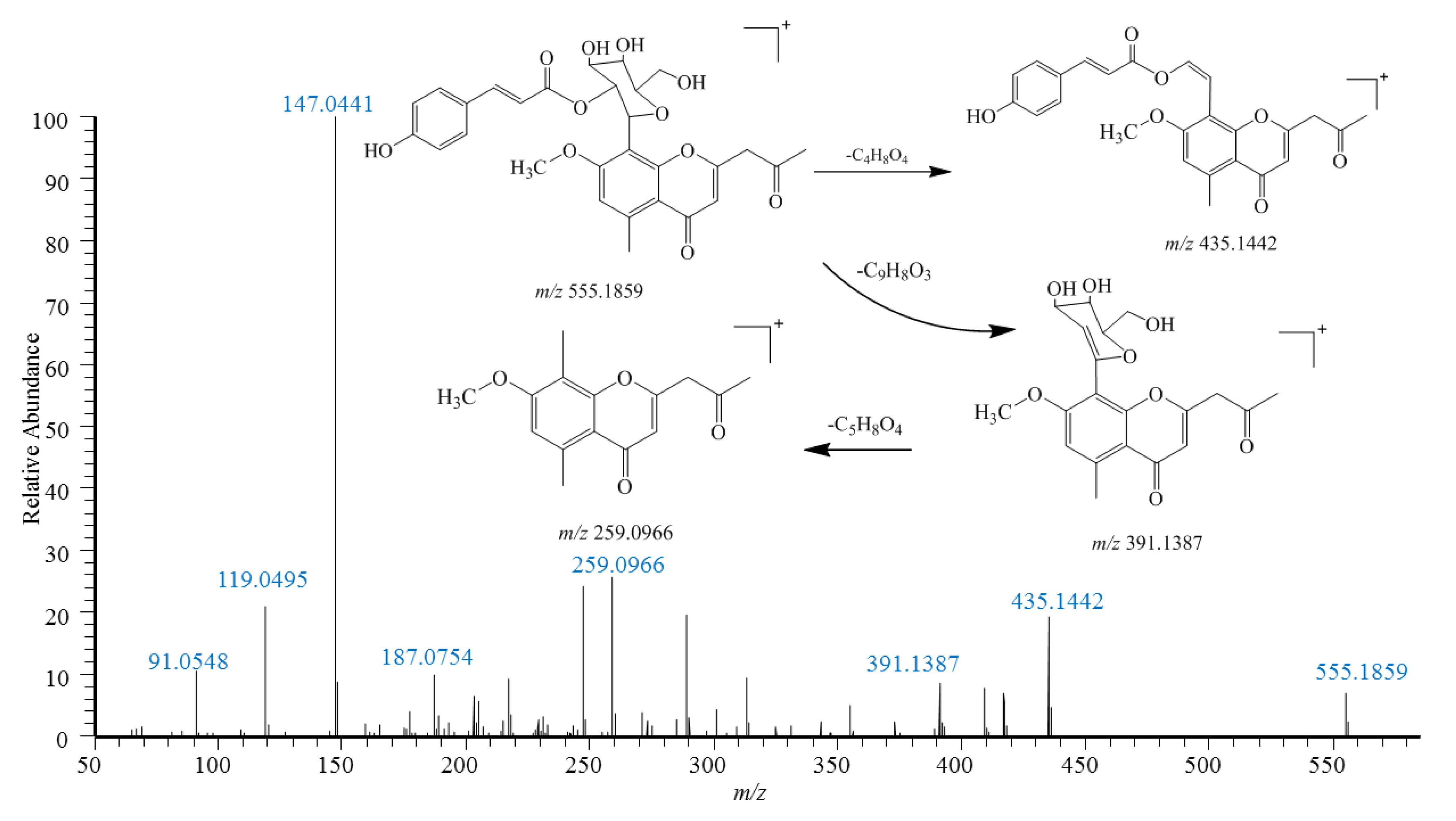
2.2. Identification of Chromones
2.3. Identification of Phenylpyrones
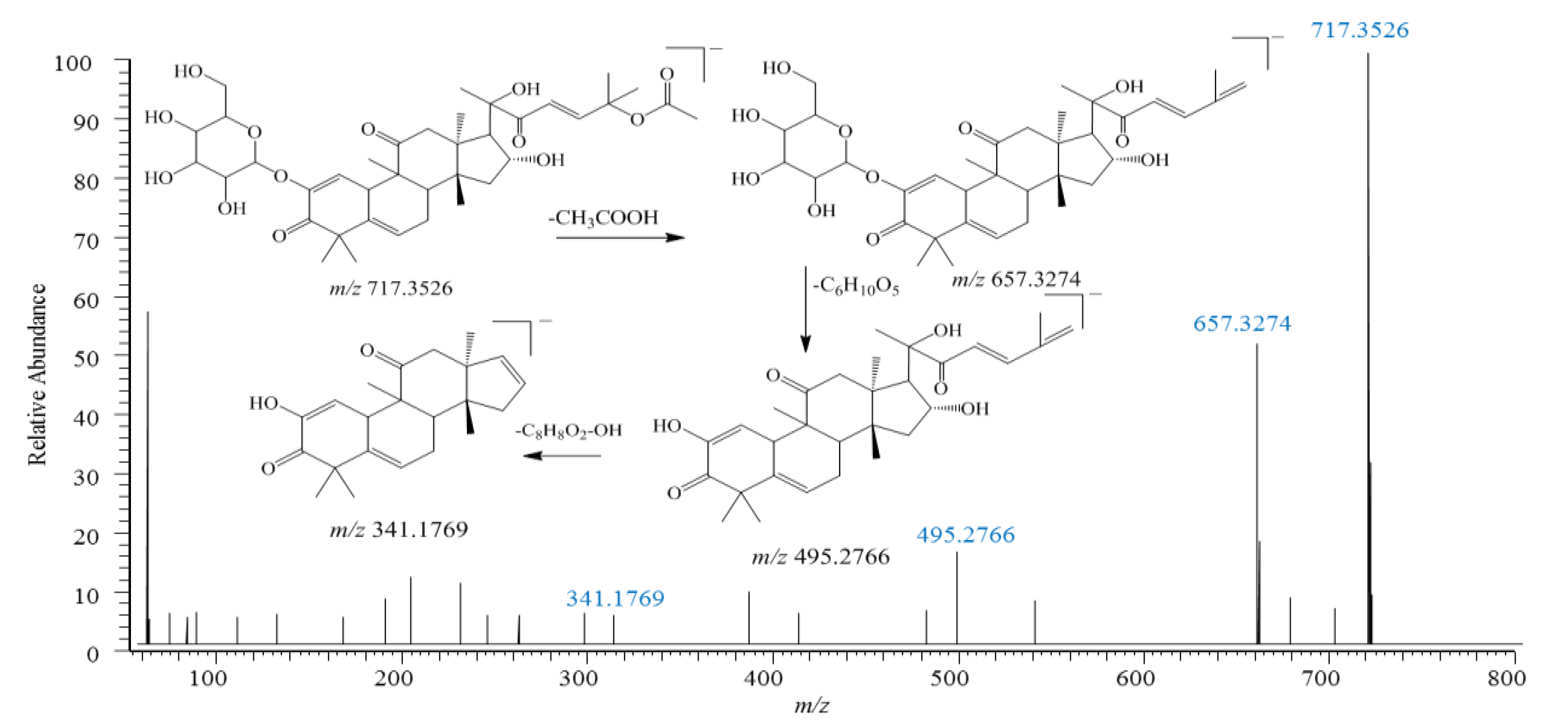
2.4. Identification of Triterpenes
2.4.1. Cucurbitacin-Type Triterpenes
2.4.2. Tirucallane-Type Triterpenes
2.4.3. Olean- and Lupane-Type Triterpenes
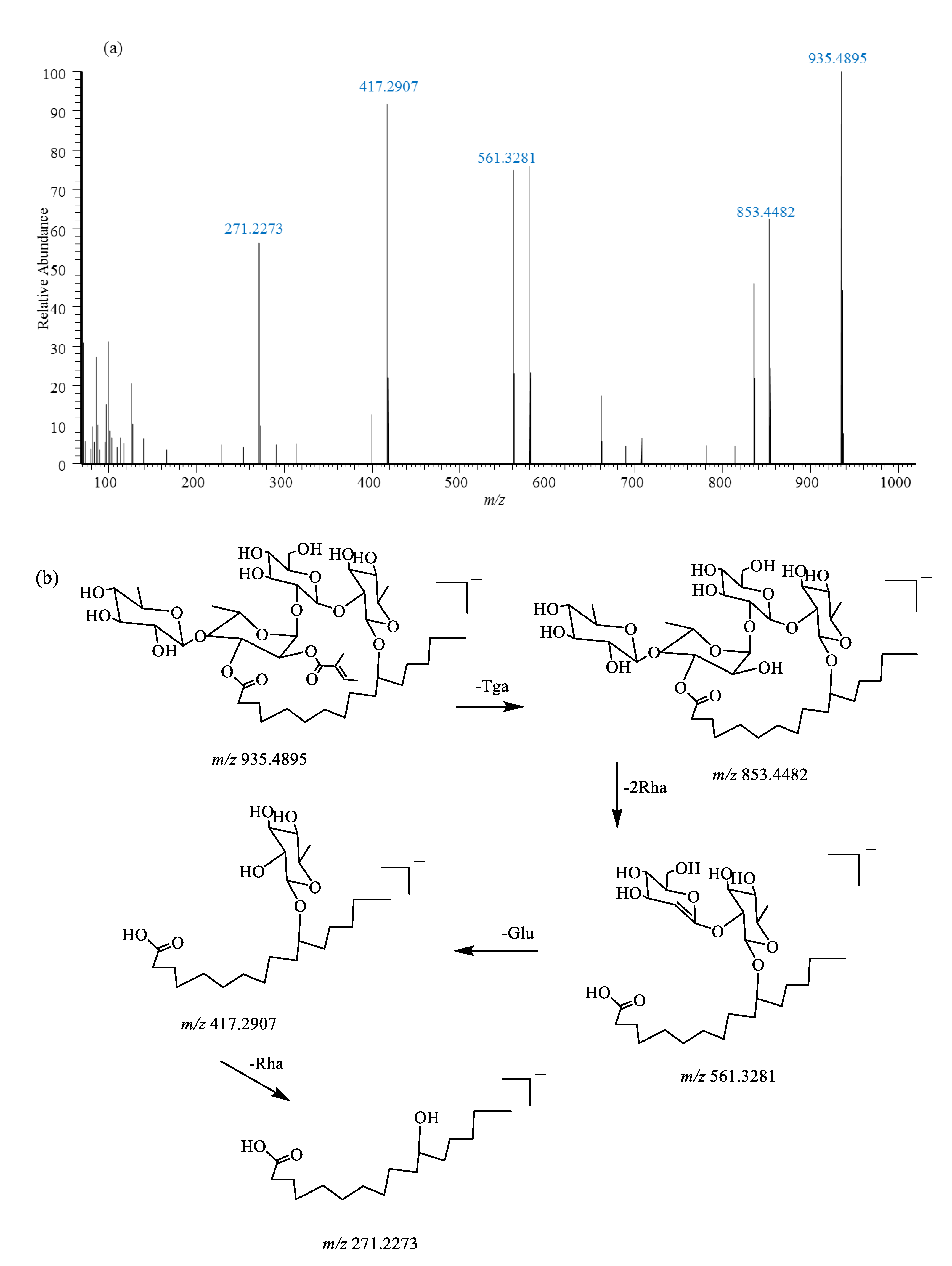
2.5. Identification of Resin Glycosides
2.6. Identification of Phenolic Acids
2.7. Identification of Flavonoids
2.8. Identification of Lignanes
2.9. Identification of Others
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials
3.3. Sample Preparation
3.4. UHPLC and MS Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Administration of Traditional Chinese Herbal Editorial Board. Chinese Materia Medica, 1st ed.; Shang Hai Science and Technology press: Shanghai, China, 2005; pp. 105–125. [Google Scholar]
- Pharmacopoeia Committee of the Ministry of Health of the People’s Republic of China (ChPC). Drug Standards of the Ministry of Health of the People’s Republic of China-Uighur Drug Volume, 1st ed.; Xinjiang Science and Technology Health Publishing House: Urumqi, Xinjiang, China, 1999; 205p. [Google Scholar]
- Zhong, J.S.; Huang, Y.Y.; Ding, W.J.; Wu, X.F.; Wan, J.Z.; Luo, H.B. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia 2013, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Adhami, H.R.; Viljoen, A.M. Preparative isolation of bio-markers from the leaf exudate of Aloe ferox (“aloe bitters”) by high performance counter-current chromatography. Phytochem Let. 2015, 11, 321–325. [Google Scholar] [CrossRef]
- Kaithwas, G.; Dubey, K.; Pillai, K.K. Effect of aloe vera (Aloe barbadensis Miller) gel on doxorubicin-induced myocardial oxidative stress and calcium overload in albino rats. Indian J. Exp. Biol. 2011, 49, 260–268. [Google Scholar] [PubMed]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arabic J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Chen, T.T. Studies on the Chemical Profile and Pharmacokinetics of Herbal Medicine Aloe; Tian Jin University of Traditional Chinese Medicine: Tianjin, China, 2021. [Google Scholar]
- Darwish, R.S.; Abdulmunem, O.A.; Khairy, A.; Ghareeb, D.A.; Yassin, A.M.; Abdumalek, S.A.; Shawky, E. Comparative metabolomics reveals the cytotoxic and anti-inflammatory discriminatory chemical markers of raw and roasted colocynth fruit (Citrullus colocynthis L.). RSC Adv. 2021, 11, 37049–37062. [Google Scholar] [CrossRef]
- Chowdhury, K.; Sharma, A.; Kumar, S.; Gunjan, G.K.; Nag, A.; Mandal, C.C. Colocynth Extracts Prevent Epithelial to Mesenchymal Transition and Stemness of Breast Cancer Cells. Front. Pharmacol. 2017, 8, 593. [Google Scholar] [CrossRef]
- Shaikh, J.; Shaikh, D.; Rahman, A.B.; Shafi, S. Antimicrobial and toxicological studies on fruit pulp of Citrullus colocynthis L. Pak. J. Pharm. Sci. 2016, 29, 9–15. [Google Scholar]
- Shawkey, A.M.; Rabeh, M.A.; Abdellatif, A.O. Biofunctional molecules from Citrullus colocynthis: An HPLC/MS analysis in correlation to antimicrobial and anticancer activities. Rev. De Neurol. 2014, 38, 924–927. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef]
- Abbas, S.; Vincourt, J.B.; Habib, L.; Netter, P.; Greige-Gerge, H.; Maddalou, J. The cucurbitacins E, D and I: Investigation of their cytotoxicity toward human chondrosarcoma SW 1353 cell line and their biotransformation in man liver. Toxicol. Lett. 2013, 216, 189–199. [Google Scholar] [CrossRef]
- Benariba, N.; Djaziri, R.; Bellakhdar, W.; Belkacem, N.; Kadiaya, M.; Malaisse, W.J.; Sener, A. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extract. Asian Pac. J. Trop. Biomed. 2013, 3, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Lee, S.E.; Yang, J.Y.; Lee, H.S. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues against foodborne bacteria. J. Sci. Food Agric. 2014, 94, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Hussaina, A.I.; Rathorea, H.A.; Sattara, M.Z.A.; Johnsc, E.J. Phenolic profile and antioxidant activity of various extracts from Citrullus colocynthis (L.) from the Pakistani flora. Ind. Crops Prod. 2013, 45, 416–422. [Google Scholar]
- Jeon, J.H.; Lee, H.S. Bio functional constituent isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues show acaricidal and insecticidal efficacy. J. Agric. Food Chem. 2014, 62, 8663–8667. [Google Scholar] [PubMed]
- Li, C.; Su, Y.L.; Liu, H.; Ji, T.F. Chemical constituents from Citrullus colocynthis. Chin. Experiment Tradit. Med. Form. 2014, 20, 102–105. [Google Scholar]
- Ono, M. Resin glycosides from Convolvulaceae plants. J. Nat. Med. 2017, 71, 591–604. [Google Scholar]
- Rosas-Ramírez, D.; Escandón-Rivera, S.; Pereda-Miranda, R. Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis. Phytochem 2018, 148, 39–47. [Google Scholar]
- Hrichi, S.; Chaabane-Banaoues, R.; Giuffrida, D.; Mangraviti, D.; Majdoub, Y.Q.E.; Rigano, F.; Mondello, L.G.; Babba, H.; Mighir, Z.; Cacciola, F. Effect of seasonal variation on the chemical composition and antioxidant and antifungal activities of Convolvulus althaeoides L. leaf extracts. Arabian J. Chem. 2020, 13, 5651–5668. [Google Scholar]
- Noda, N.; Kogetsu, H.; Kawasaki, T.; Miyahara, K. Scammonins I and II, the resin glycosides of radix scammoniae from Convolvulus scammonia. Phytochem 1990, 29, 3565–3569. [Google Scholar]
- Kogetsu, H.; Noda, N.; Kawasaki, T.; Miyahara, K. Scammonin III–VI, resin glycosides of Convolvulus scammonia. Phytochem 1991, 30, 957–963. [Google Scholar]
- Noda, N.; Kogetsu, H.; Kawasaki, T.; Miyahara, K. Scammonins VII and VIII, two resin glycosides from Convolvulus scammonia. Phytochem 1992, 31, 2761–2766. [Google Scholar]
- Kahar, G.; Abdulla, R.; Liu, Y.Q.; Wu, T.; Aisa, H.A. Chemical composition analysis of scammony resin by UHPLC-Q-Orbitrap-HRMS. Nat. Prod. Res. Dev. 2023, 35, 427–443. [Google Scholar]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)-A curious plant with both neurotoxic and neuroprotective properties. J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [PubMed]
- Chi, N.J.; Liu, Y.Q.; Aisa, H.A. Tetrahydrofuran lignans and flavonoids from Artemisia absinthium. Chem. Nat. Compound 2012, 48, 666–667. [Google Scholar]
- Boukhalkhal, S.; Gourine, N.; Pinto, D.C.G.A.; Silva, A.M.S.; Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocatal. Agric. Biotech. 2020, 23, 101483. [Google Scholar]
- Celebi, O.; Cinisli, T.K.; Celebi, D. Antimicrobial activity of the combination (Nano-Bio) of Artemisia absinthium with copper nanoparticles. Mater. Today Proceed. 2021, 45, 3809–3813. [Google Scholar]
- Yazdani, M.; Hallaj, A.; Salek, F.; Baharara, J. Potential of the combination of Artemisia absinthium extract and cisplatin in inducing apoptosis cascades through the expression of p53, BAX, caspase 3 ratio, and caspase 9 in lung cancer cells (Calu-6). Europ. J. Integr. Med. 2022, 56, 102193. [Google Scholar]
- Papageorgiou, V.P.; Bakola-Christianopoulou, M.N.; Apazidou, K.K.; Psarros, E.E. Gas chromatographic-mass spectroscopic analysis of the acidic triterpenic fraction of mastic gum. J. Chromatogar. A 1997, 769, 263–273. [Google Scholar]
- Doelen, G.A.V.D.; Berg, K.J.V.D.; Boon, J.J.; Shibayama, N.; Genuit, R.W.J.L. Analysis of fresh triterpenoid resins and aged triterpenoid varnishes by high-performance liquid chromatography-atmospheric pressure chemical ionisation (tandem) mass spectrometry. J. Chromatogar. A 1998, 809, 21–37. [Google Scholar]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Chromatogar. A 2020, 254, 112485. [Google Scholar]
- Svingou, D.; Mikropoulou, E.V.; Pachi, V.K.; Smyrnioudis, I.; Halabalaki, M. Chios mastic gum: A validated method towards authentication. J. Food Compos. Anal. 2023, 115, 104997. [Google Scholar]
- Liu, W. Studies on the Chemical constituents and bioactivities of mastic Pistacia lentiscus. Ph.D. Thesis, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Science, Beijing, China, 2021. [Google Scholar]
- Liu, W.; Gao, J.; Li, M.; Aisa, H.A.; Yuan, T. Tirucallane triterpenoids from the mastic (Pistacia lentiscus) and their anti-inflammatory and cytotoxic activities. Phytochem 2021, 182, 112596. [Google Scholar]
- Kahaer, G.; Abdulla, R.; Wu, T.; Aisa, H.A. Systematic qualitative analysis of terpenes in mastic (Pistacia lentiscus L.) extract and their fragmentations by UHPLC-Q-Orbitarp-HRMS. Phytochem. Anal. 2024, 35, 1072–1087. [Google Scholar] [PubMed]
- Yan, Y.H.; Abdulla, R.; Liu, X.Y.; Li, S.P.; Aisa, H.A. Comprehensive chemical profile and quantitative analysis of the Shabyar tablet, a traditional ethnic medicine prescription, by ultra-high-performance liquid chromatography with quadrupole-orbitrap high-resolution mass spectrometry. J. Sep. Sci. 2022, 45, 2148–2160. [Google Scholar]
- Kang, J.; Sun, J.; Xue, L.P.; Li, Z.L.; Bao, X.Y.; Wang, Z.H.; Fan, F.; Li, S.J.; Hu, D.; Zhang, X.J.; et al. Chemical profiling of Zilong Jin tablets using ultra high performance liquid chromatography- quadrupole/orbitrap high resolution mass spectrometry. J Sep. Sci. 2021, 44, 3562–3579. [Google Scholar]
- Kaur, J.; Dhiman, V.; Bhadada, S.; Katare, O.P.; Ghoshal, G. LC/MS guided identification of metabolites of different extracts of Cissus quadrangularis. Food Chem. Adv. 2022, 1, 100084. [Google Scholar]
- Huang, C.C.; Zhao, Y.; Huang, S.Y.; Li, L.; Yuan, Z.Y.; Xu, G.M. Screening of anti- thrombin active components from Ligusticum chuanxiong by affinity-ultrafiltration coupled with HPLC-Q-Orbitrap-MSn. Phytochem. Anal. 2023, 34, 443–452. [Google Scholar]
- Zuo, L.H.; Sun, Z.; Hu, Y.R.; Sun, Y.; Xue, W.H.; Zhou, L.; Zhang, J.; Bao, X.Y.; Zhu, Z.F.; Suo, G.L.; et al. Rapid determination of 30 bioactive constituents in Xue Bi Jing injection using ultra high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry coupled with principal component analysis. J. Pharm. Bio. Med. Anal. 2017, 137, 220–228. [Google Scholar]
- Zhang, J.; Hao, B.Q.; Li, Y.Q. Qualitative analysis of chemical constituents of aloe made from Aloe barbadensis by liquid chromatography-mass spectrometry. Guihaia 2024, 44, 313–326. [Google Scholar]
- Issa, R.; Al-Akayleh, F.; Alnsour, L.; Al-Sammarraie, T.R.; Omari, K.W.; Awwad, S.H. Antioxidant Activity and UHPLC-MS/MS Characterization of Polyphenol and Nicotine Content in Nicotiana glauca Leaf Extracts: A Comparative Study of Conventional and Deep Eutectic Solvent Extraction Methods. Plants 2024, 13, 2240. [Google Scholar] [CrossRef]
- Tenório, C.J.L.; Dantas, T.d.S.; Abreu, L.S.; Ferreira, M.R.A.; Soares, L.A.L. Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation. Molecules 2024, 29, 2761. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Li, X.; Liang, H.; Chen, S.; Huang, Y.; Chai, H.; Lin, R.; Jiang, G. High-Resolution LC-MS Simultaneous quantification of forty-six compounds from Jatropha podagrica fruit recommends four top antioxidant contributors as Q-Markers. Molecules 2025, 30, 722. [Google Scholar] [CrossRef] [PubMed]
- Siegle, J.; Pietsch, J. Determionation of Veratrum alkaloid contents in three Veraturum species by HPLC-MS/MS. Phytochem. Anal. 2024, 35, 1577–1586. [Google Scholar] [CrossRef]
- Durì, L.; Morelli, C.F.; Crippa, S.; Speranza, G. 6-Phenylpyrones and 5-methylchromones from Kenya aloe. Fitoterapia 2004, 75, 520–522. [Google Scholar] [CrossRef]
- Bisrat, D.; Dagne, E.; van Wyk, B.-E.; Viljoen, A. Chromones and anthrones from Aloe marlothii and Aloe rupestris. Phytochem 2000, 55, 949–952. [Google Scholar] [CrossRef]
- Okamura, N.; Hine, N.; Tateyama, Y.; Nakazawa, M.; Fujioka, T.; Mihashi, K. Five chromones from Aloe vera leaves. Phytochem 1998, 49, 219–223. [Google Scholar] [CrossRef]
- Heerden, F.R.V.; Wyk, B.E.V.; Viljoen, A.M. Aloeresin E and F, two chromone derivatives from Aloe peglerae. Phytochem 1996, 43, 867–869. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, A.S.; Zeshan, B.; Riaz, M.; Ejaz, U.; Saleem, A.; Zaineb, R. Characterization of Bioactive Compounds and Novel Proteins Derived from Promising Source Citrullus colocynthis along with In-Vitro and In-Vivo Activities. Molecules 2023, 28, 1743. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zou, Q.P.; Yook, C.S. Spectral characteristics of Lupane-type triterpenoids from natural products. J. Hunan Univ. Chin. Med. 2013, 33, 10–30. [Google Scholar]
- Pereda-Miranda, R.; Hernández-Carlos, B. HPLC Isolation and structural elucidation of diastereomeric niloyl ester tetrasaccharides from Mexican scammony root. Tetrahedron 2002, 58, 3145–3154. [Google Scholar] [CrossRef]
- Noda, N.; Ono, M.; Miyahara, K.; Kawasaki, T. Resin glycosides. Ⅰ. Isolation and structure elucidation of orizabin-Ⅰ, Ⅱ, Ⅲ and Ⅳ, genuine resin glycosides from the root of the Ipomoea orizabensis. Tetrahedron 1987, 43, 3889–3902. [Google Scholar]
- Nagy, A.; Abrankό, L. Profiling of hydroxycinnamoylquinic acids in plant extracts using in-source CID fragmentation. J. Mass Spectrom. 2016, 51, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, J.; Deng, S.M.; Dai, B. A new flavone C-glycoside from Citrullus colocynthis. Chin. Herb. Med. 2012, 4, 1–3. [Google Scholar] [CrossRef]
- Iwashina, T.; Kamenosono, K.; Ueno, T. Hispidulin and nepetin 4’-glucosides from Cirsium oligophyllum. Phytochem 1999, 51, 1109–1111. [Google Scholar] [CrossRef]
- Iwashina, T.; Rahayu, S.; Nakane, T.; Devkota, H.P.; Mizuno, T.; Tsutsumi, C.; Widyatmoko, D. Flavonoids and phenylethanoids from the flowers and leaves of Aeschynanthus species and cultivars (Gesneriaceae). Phytochem 2022, 203, 113367. [Google Scholar]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita L. Rauschert). Phytochem 2004, 65, 2323–2332. [Google Scholar]
- Liu, S.; Zhao, Z.Y.; Zhao, S.B.; Wang, H.Y. Determination of five sugars and mannitol in Da qu by UPLC-Q/Orbitrap HRMS. China Brew. 2020, 39, 197–200. [Google Scholar]
- Torri, G.; Urso, E.; Naggy, A.; Coletti, A.; Valerio, A.; Vismara, E. Anti-metastatic semi-synthetic sulfated maltotriose C-C linked dimers, synthesis and characterization. Molecules 2012, 17, 9912–9930. [Google Scholar]
- Meng, Y. Study on the Chemical Constituents of Aloe: Isolation, Structure Characterization and Anti-Oxygenic Property. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2004. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gomez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Sultan, M.H.; Zuwaiel, A.A.; Moni, S.S.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M. Bioactive and potentiality of hot methanolic extract of the leaves of Artemisia absinthium L. “in vitro cytotoxicity against human MCF-7 breast cancer celles, antibacterial study and wound healing activity”. Curr. Pharma Biotech 2020, 21, 1711–1721. [Google Scholar]
- Hernández-Carlos, B.; Bye, R.; Pereda-Miranda, R. Tetrasaccharide glycolipids from the Mexican scammony root (Ipomoea orizabensis). J. Nat. Prod. 1999, 62, 1096–1100. [Google Scholar] [CrossRef]
- Chawech, R.; Jarraya, R.; Girardi, C.; Vansteelandt, M.; Marti, G.; Nasir, I.; Racaud-Sultan, C. Cucurbitacins from the leaves of Citrullus colocynthis (L.) Schard. Molecules 2015, 20, 180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahaer, G.; Muhetaer, M.; Abdulla, R.; Wu, T.; Luo, Y.; Aisa, H.A. A Systematic Profiling of the Components of Kukeya Tablets, a Traditional Ethnic Medicine Prescription, by Ultra-High-Performance Liquid Chromatography–Quadrupole/Orbitrap High-Resolution Mass Spectrometry. Pharmaceuticals 2025, 18, 457. https://doi.org/10.3390/ph18040457
Kahaer G, Muhetaer M, Abdulla R, Wu T, Luo Y, Aisa HA. A Systematic Profiling of the Components of Kukeya Tablets, a Traditional Ethnic Medicine Prescription, by Ultra-High-Performance Liquid Chromatography–Quadrupole/Orbitrap High-Resolution Mass Spectrometry. Pharmaceuticals. 2025; 18(4):457. https://doi.org/10.3390/ph18040457
Chicago/Turabian StyleKahaer, Gulimire, Muhebaiti Muhetaer, Rahima Abdulla, Tao Wu, Yuqin Luo, and Haji Akber Aisa. 2025. "A Systematic Profiling of the Components of Kukeya Tablets, a Traditional Ethnic Medicine Prescription, by Ultra-High-Performance Liquid Chromatography–Quadrupole/Orbitrap High-Resolution Mass Spectrometry" Pharmaceuticals 18, no. 4: 457. https://doi.org/10.3390/ph18040457
APA StyleKahaer, G., Muhetaer, M., Abdulla, R., Wu, T., Luo, Y., & Aisa, H. A. (2025). A Systematic Profiling of the Components of Kukeya Tablets, a Traditional Ethnic Medicine Prescription, by Ultra-High-Performance Liquid Chromatography–Quadrupole/Orbitrap High-Resolution Mass Spectrometry. Pharmaceuticals, 18(4), 457. https://doi.org/10.3390/ph18040457






