Chemosensitization and Molecular Docking Assessment of Dio-NPs on Resistant Breast Cancer Cells to Tamoxifen
Abstract
1. Introduction
2. Results
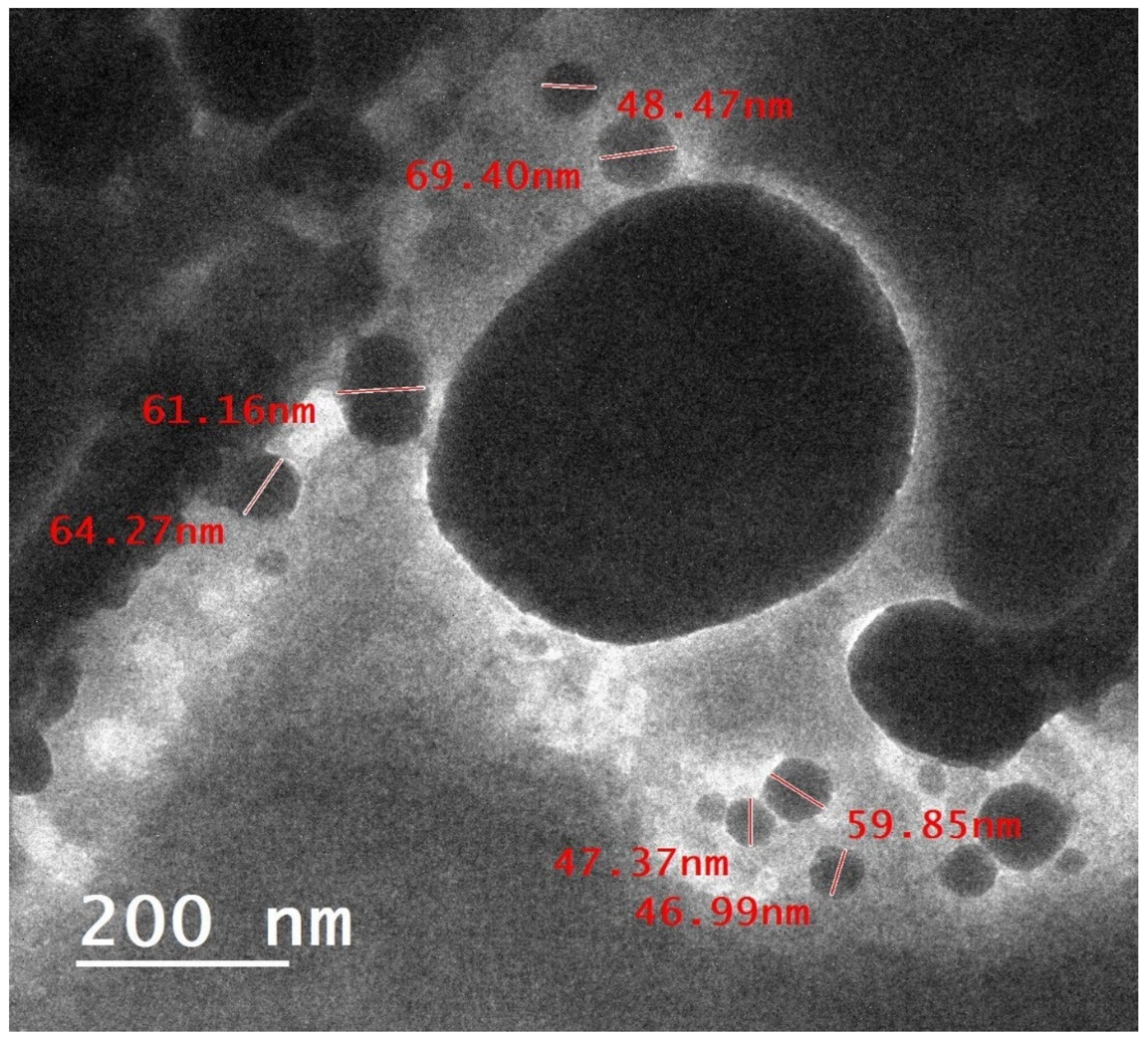
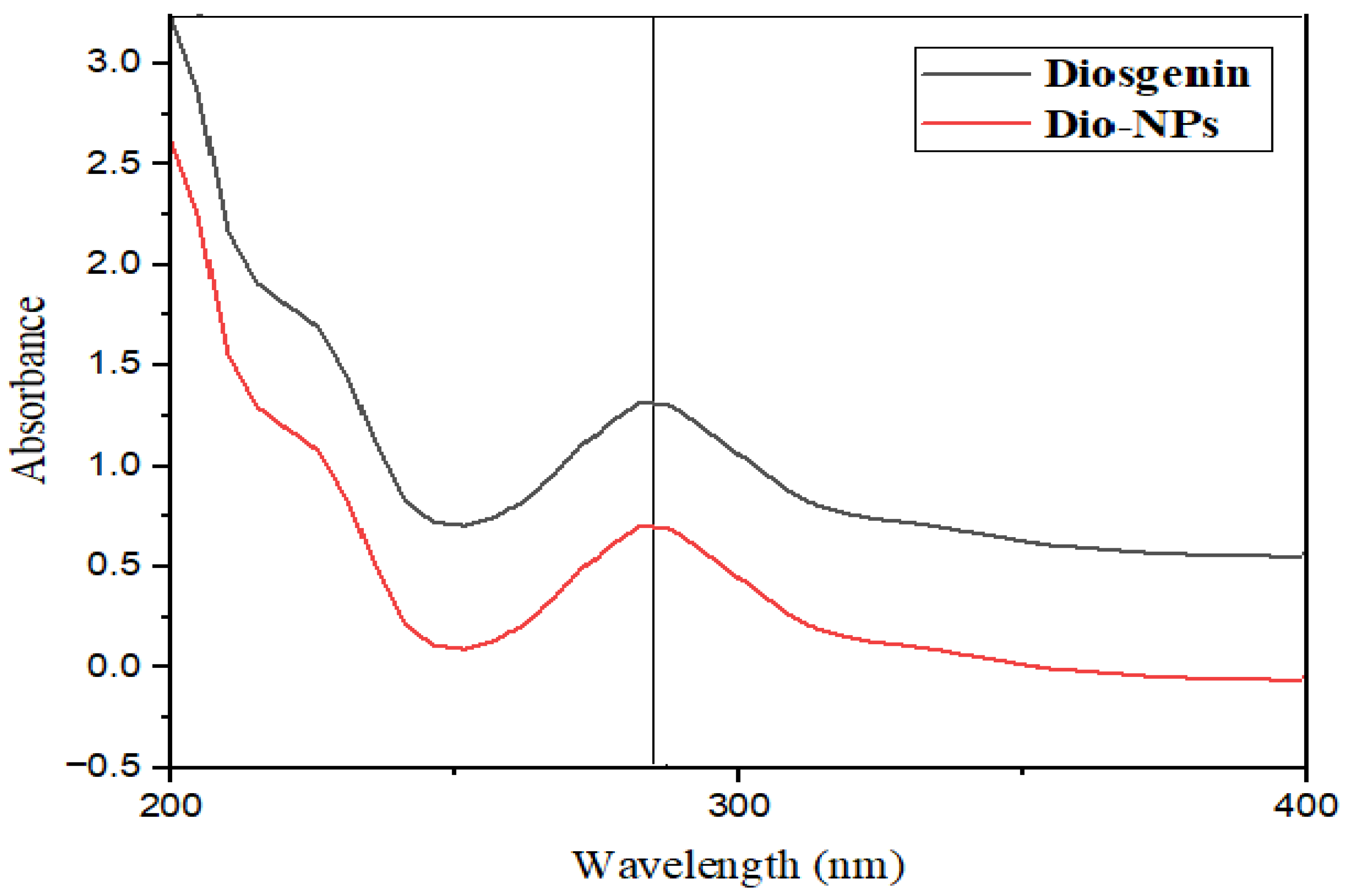
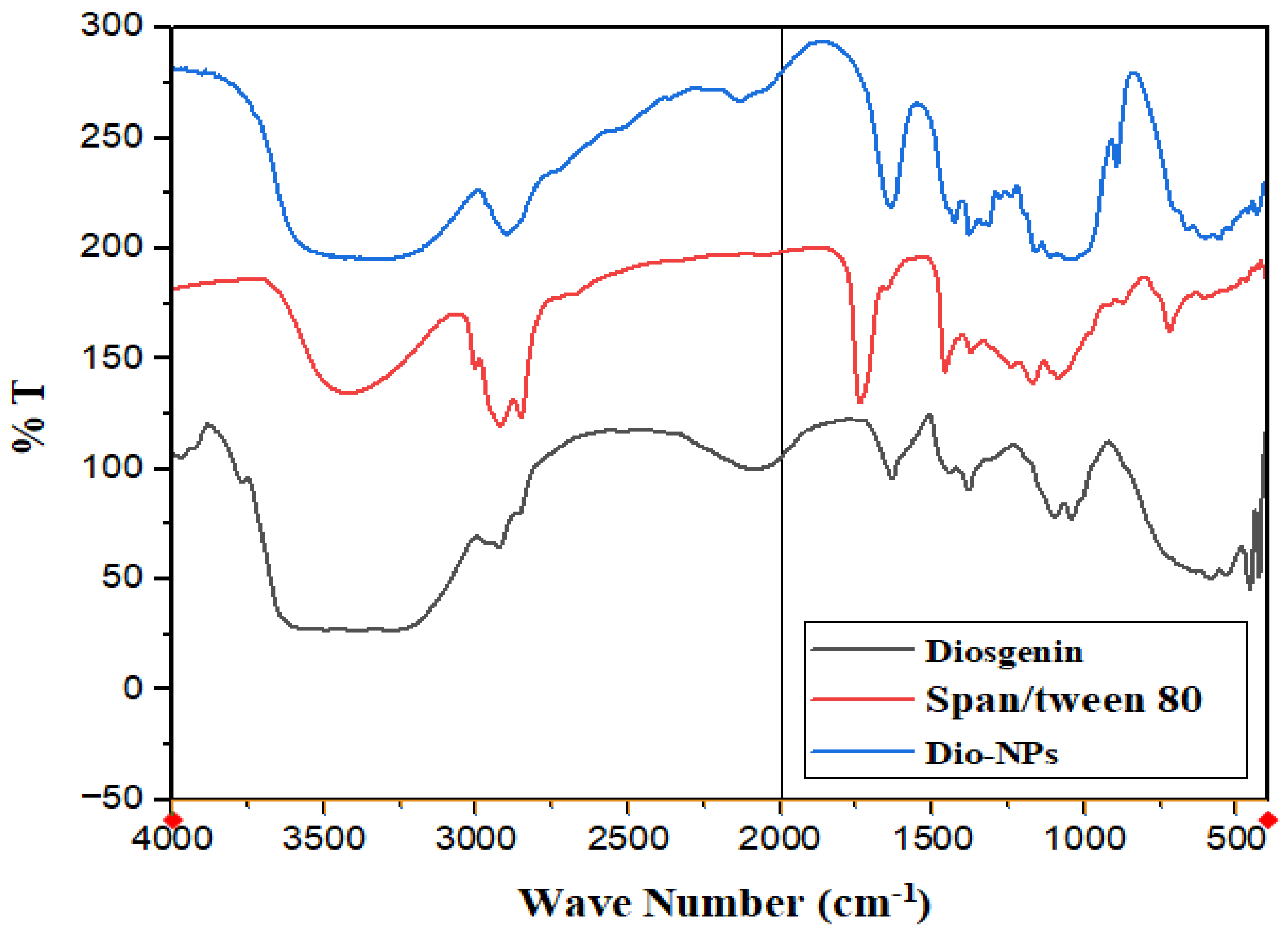
2.1. Characterization of Dio-NPs
2.2. Cytotoxicity of Dio-NPs
2.3. LD50 of Dio-NPs
- LD50 = Dm − [Σ (Z × d)/n]
- LD50 = 3500 − [(11,000)/10] = 2400 mg/Kg.b.w.
2.4. Effect of Dio-NPs and Tamoxifen Administration, Individually and in Combination on Blood Hb, RBCs, WBC, and PLTs Levels in DMBA-Treated Mice
2.5. Effect of Dio-NPs and Tamoxifen Administration, Individually and in Combination on Breast GSH, CAT, SOD, and MDA in DMBA-Treated Mice
2.6. Effect of Dio-NPs and Tamoxifen Administration, Individually and in Combination on Breast NF-kB, IL-6, and IL-10 in DMBA-Treated Mice
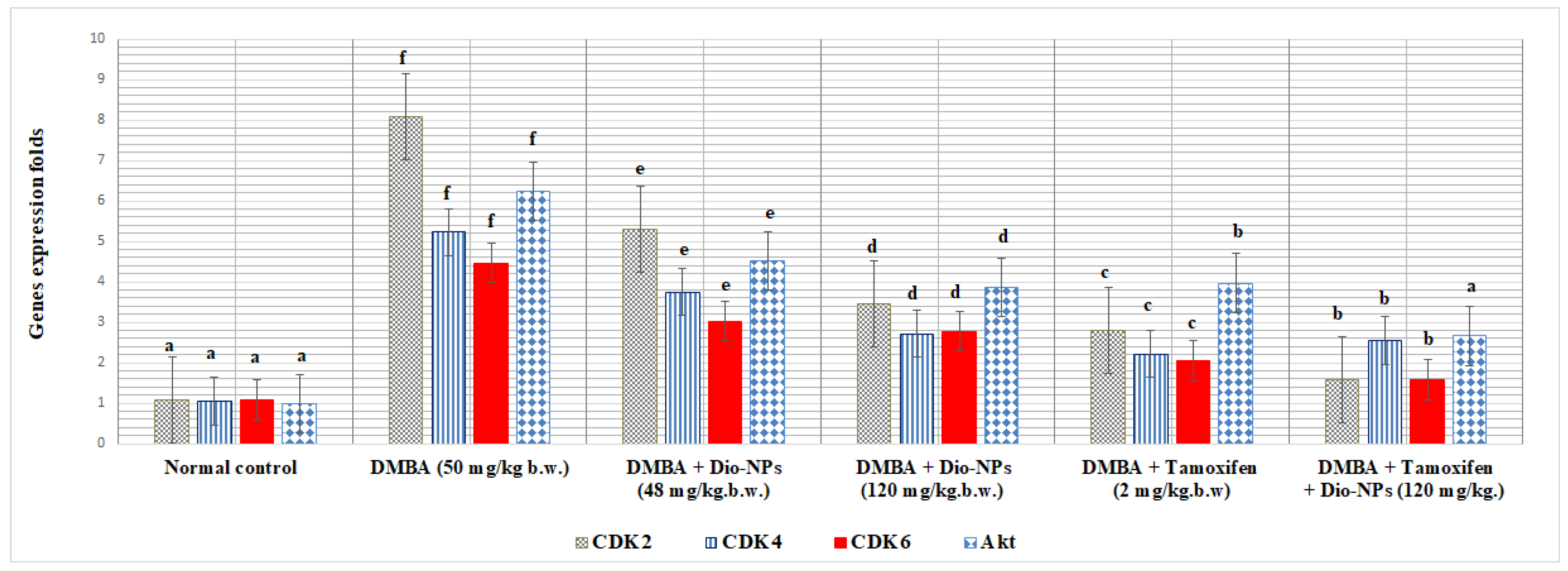
2.7. Effect of Dio-NPs and Tamoxifen Administration, Individually and in Combination, on Breast CDK2, CDK4, CDK6, and Akt in DMBA-Treated Mice
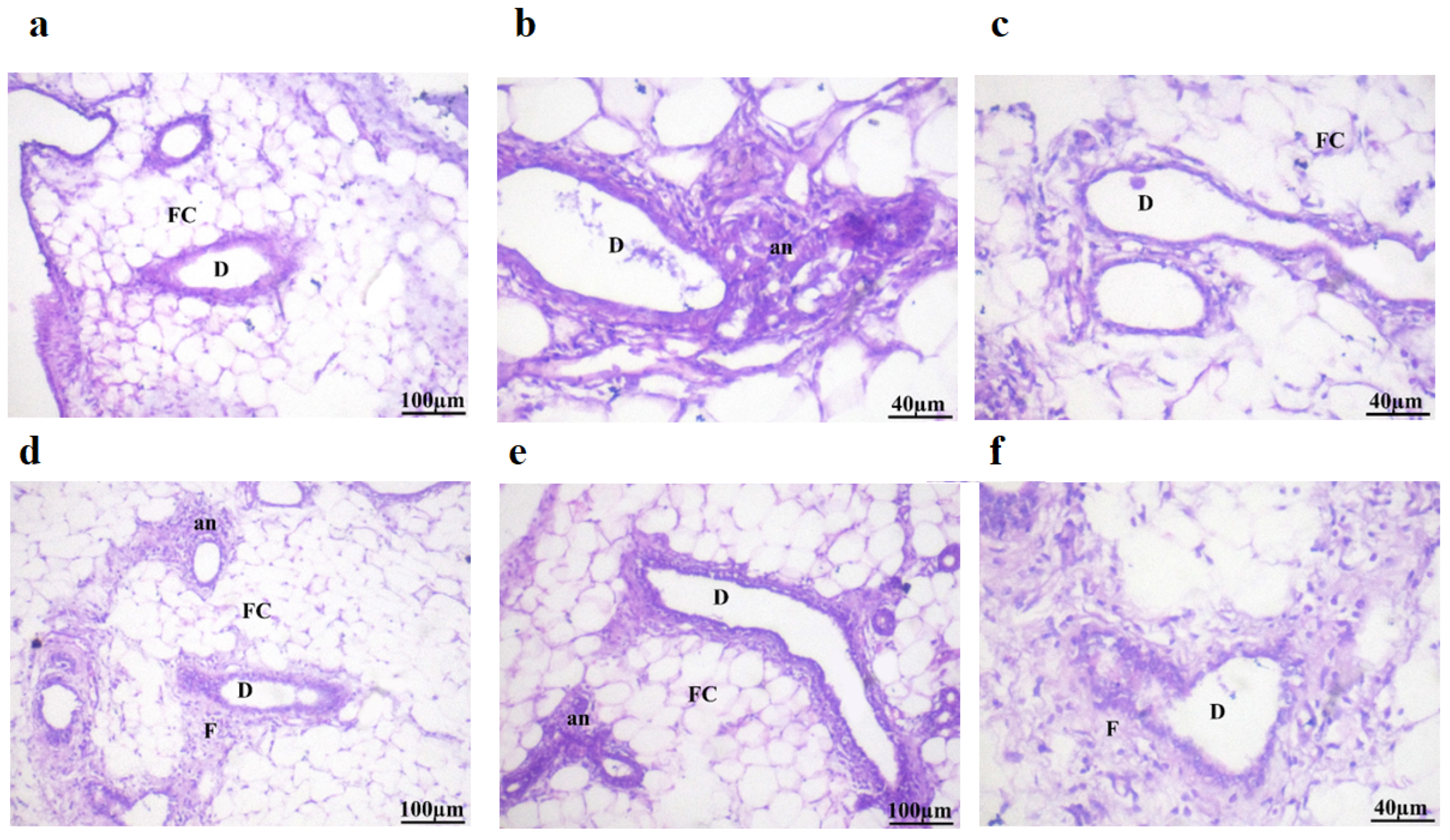
2.8. Histopathological Examination
2.9. Binding Affinities
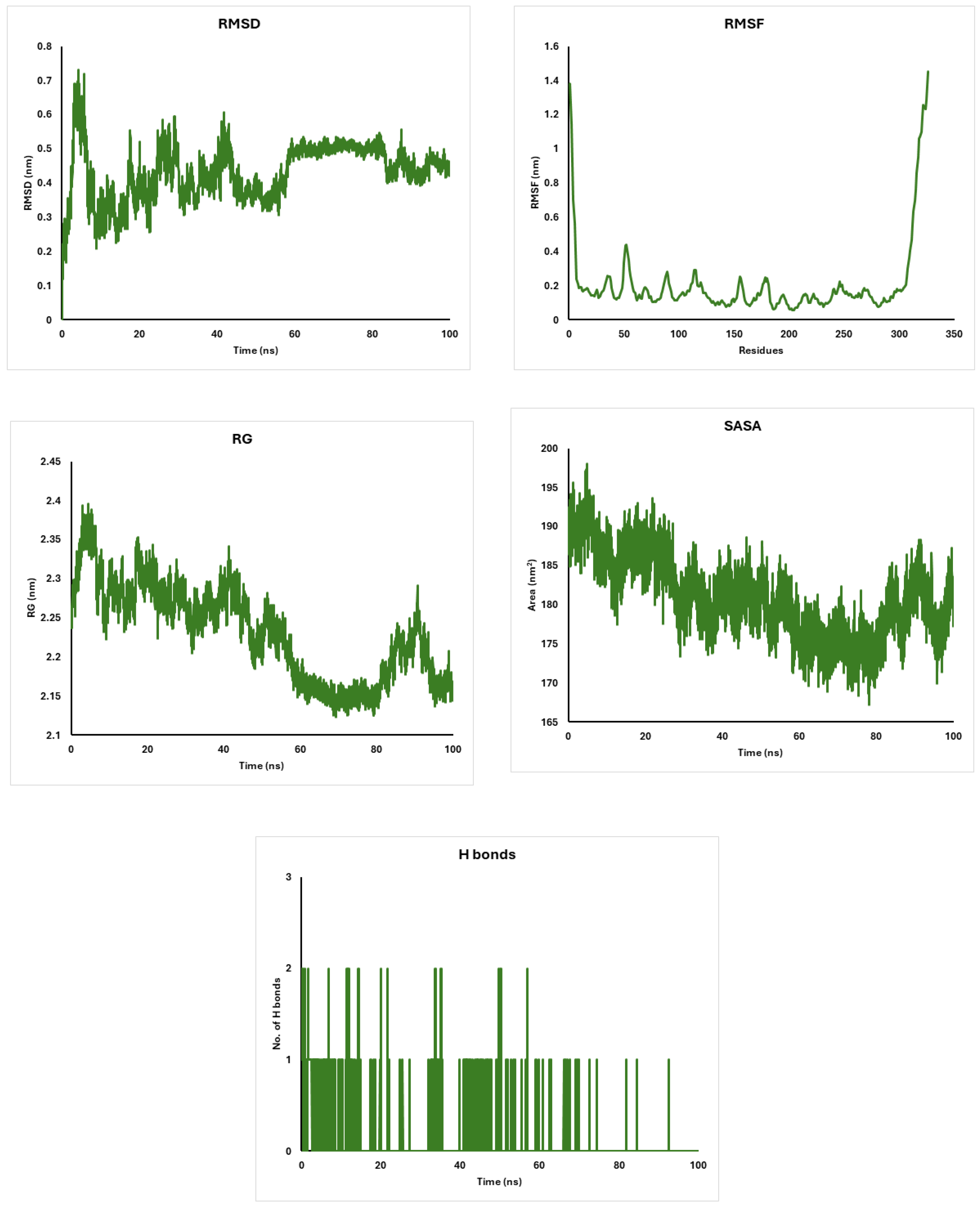
2.10. Molecular Dynamics Simulations of CDK4, AKT, and CDK6 Proteins with Diosgenin: Stability and Structural Insights
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Dio-NPs Preparation
4.2.2. Conditions for Sonication
4.3. Dio-NPs Characterization
4.4. Estimation of Encapsulation Efficiency
Determination of Dio-NPs Cytotoxicity Against MCF-7 Cells
4.5. Determination of the Dio-NPs LD50
- Dm = The largest that kill all animals
- ∑ = The sum of (Z . d).
- Z = Mean of Dead animals between 2 successive groups.
- d = the constant factor between two successive doses.
- n = number of animals in each group.
4.6. Test Animals
4.7. The Experimental Configuration
4.8. Collection of Samples
4.9. Hematological Evaluation
4.10. Preparation of Breast Samples
4.11. qRT-PCR Assays
4.12. Histological Evaluation
4.13. Quantitative Analysis
4.14. Ethics Statement
4.15. Protein Preparation
4.16. Ligand Preparation
4.17. Active Site Prediction
4.18. Molecular Docking
4.19. Molecular Dynamics Simulations
5. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Van Mechelen, M.; Van Herck, A.; Punie, K.; Nevelsteen, I.; Smeets, A.; Neven, P.; Weltens, C.; Han, S.; Vanderstichele, A.; Floris, G. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res. Treat. 2020, 181, 115–125. [Google Scholar] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Morrison, L.; Loibl, S.; Turner, N.C. The CDK4/6 inhibitor revolution—A game-changing era for breast cancer treatment. Nat. Rev. Clin. Oncol. 2024, 21, 89–105. [Google Scholar]
- Kim, M.K.; Shin, H.-C. Risk factors for tamoxifen-induced ovarian hyperstimulation in breast cancer patients. Clin. Breast Cancer 2020, 20, 408–412. [Google Scholar]
- Al-Awaida, W.; Al-Hourani, B.J.; Akash, M.; Talib, W.H.; Zein, S.; Falah, R.R.; Aburubaiha, Z. In vitro anticancer, anti-inflammatory, and antioxidant potentials of Ephedra aphylla. J. Cancer Res. Ther. 2018, 14, 1350–1354. [Google Scholar]
- Mukherjee, A.K.; Basu, S.; Sarkar, N.; Ghosh, A.C. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef]
- Mondal, S.; Bandyopadhyay, S.; Ghosh, M.K.; Mukhopadhyay, S.; Roy, S.; Mandal, C. Natural products: Promising resources for cancer drug discovery. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2012, 12, 49–75. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Giordano, C.; De Amicis, F.; Lanzino, M.; Ando, S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016, 16, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Pazhouhi, M. Antiproliferative effect of Trifolium pratens L. extract in human breast cancer cells. Nutr. Cancer 2019, 71, 128–140. [Google Scholar] [CrossRef]
- Raju, J.; Mehta, R. Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr. Cancer 2008, 61, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, S.; Zhou, S.; Dong, S.; Du, J. Prognostic value of lncRNA DRAIC and miR-3940-3p in lung adenocarcinoma and their effect on lung adenocarcinoma cell progression. Cancer Manag. Res. 2021, 13, 8367–8376. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeon, B.K.; Lee, Y.E.; Woo, W.H.; Mun, Y.J. Diosgenin induces apoptosis in HepG2 cells through generation of reactive oxygen species and mitochondrial pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 981675. [Google Scholar] [CrossRef]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Silvestre, S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef]
- Conrad, M.; Angeli, J.P.F.; Vandenabeele, P.; Stockwell, B.R. Regulated necrosis: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2016, 15, 348–366. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Vengaimaran, M.; Dhamodharan, K.; Sankaran, M. Diosgenin nanoparticles competes plain diosgenin on reviving biochemical and histopathological alterations in DMBA induced rat mammary carcinoma via modulating the AhR-Nrf-2 signaling cascades. J. Pharm. Res. Int. 2021, 33, 141–157. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Yue, Y.; Wang, L.; He, Q.; Xu, S.; Li, J.; Liao, Y.; Chen, Y.; Wang, S. The immunotoxin targeting PRLR increases tamoxifen sensitivity and enhances the efficacy of chemotherapy in breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 173. [Google Scholar] [PubMed]
- Ahn, E.-Y.; Park, Y. Anticancer prospects of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C 2020, 116, 111253. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: In vitro characterization and ex vivo evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Duodenum-triggered delivery of pravastatin sodium via enteric surface-coated nanovesicular spanlastic dispersions: Development, characterization and pharmacokinetic assessments. Int. J. Pharm. 2015, 483, 77–88. [Google Scholar] [PubMed]
- Salah, A.; El-Laban, N.M.; Alam, S.M.; Islam, M.S.; Hussein, M.A.; Roshdy, T. Optimization of Naringenin-loaded nanoparticles for targeting of Vanin-1, iNOS, and MCP-1 signaling pathway in HFD-induced obesity. Int. J. Pharm. 2024, 654, 123967. [Google Scholar] [CrossRef]
- Mohamad, E.A.; Mohamed, Z.N.; Hussein, M.A.; Elneklawi, M.S. GANE can improve lung fibrosis by reducing inflammation via promoting p38MAPK/TGF-β1/NF-κB signaling pathway downregulation. ACS Omega 2022, 7, 3109–3120. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; Abo-Salem, H.M.; Ali, A.A.; Hussein, M.A. Phenolic profiling and therapeutic potential of certain isolated compounds from parkia roxburghii against AChE activity as well as GABAA α5, GSK-3β, and p38α MAP-kinase genes. ACS Omega 2021, 6, 20492–20511. [Google Scholar]
- Mostafa, M.M.; Amin, M.M.; Zakaria, M.Y.; Hussein, M.A.; Shamaa, M.M.; Abd El-Halim, S.M. Chitosan surface-modified PLGA nanoparticles loaded with cranberry powder extract as a potential oral delivery platform for targeting colon cancer cells. Pharmaceutics 2023, 15, 606. [Google Scholar] [CrossRef]
- Soliman, S.M.; Mosallam, S.; Mamdouh, M.A.; Hussein, M.A.; Abd El-Halim, S.M. Design and optimization of cranberry extract loaded bile salt augmented liposomes for targeting of MCP-1/STAT3/VEGF signaling pathway in DMN-intoxicated liver in rats. Drug Deliv. 2022, 29, 427–439. [Google Scholar] [CrossRef]
- Boshra, S.; Hussein, M. Cranberry extract as a supplemented food in treatment of oxidative stress and breast cancer induced by N-Methyl-N-Nitrosourea in female virgin rats. Int. J. Phytomed. 2016, 8, 217–227. [Google Scholar]
- Lo, Y.; Lester, S.C.; Ellis, I.O.; Lanjewar, S.; Laurini, J.; Patel, A.; Bhattarai, A.; Ustun, B.; Harmon, B.; Kleer, C.G. Identification of Glandular (Acinar)/Tubule Formation in Invasive Carcinoma of the Breast: A Study to Determine Concordance Using the World Health Organization Definition. Arch. Pathol. Lab. Med. 2024, 148, 1119–1125. [Google Scholar] [PubMed]
- Mohammed Abdalla, H., Jr.; Soad Mohamed, A.G. In vivo hepato-protective properties of purslane extracts on paracetamol-induced liver damage. Malays. J. Nutr. 2010, 16, 161–170. [Google Scholar]
- El-Gizawy, H.; Hussein, M. Fatty acids profile, nutritional values, anti-diabetic and antioxidant activity of the fixed oil of Malva parviflora growing in Egypt. Int. J. Phytomed. 2015, 7, 219–230. [Google Scholar]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar]
- Mazyed, E.A.; Helal, D.A.; Elkhoudary, M.M.; Abd Elhameed, A.G.; Yasser, M. Formulation and optimization of nanospanlastics for improving the bioavailability of green tea epigallocatechin gallate. Pharmaceuticals 2021, 14, 68. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Layek, B.; Mukherjee, A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro study. Int. J. Nanomed. 2007, 2, 213–225. [Google Scholar]
- Khanal, P.; Patil, V.S.; Bhandare, V.V.; Patil, P.P.; Patil, B.; Dwivedi, P.S.; Bhattacharya, K.; Harish, D.R.; Roy, S. Systems and in vitro pharmacology profiling of diosgenin against breast cancer. Front. Pharmacol. 2023, 13, 1052849. [Google Scholar]
- Huang, H.; Li, M.; Gu, J.; Roy, S.; Jin, J.; Kuang, T.; Zhang, Y.; Hu, G.; Guo, B. Bright NIR-II emissive cyanine dye-loaded lipoprotein-mimicking nanoparticles for fluorescence imaging-guided and targeted NIR-II photothermal therapy of subcutaneous glioblastoma. J. Nanobiotechnol. 2024, 22, 788. [Google Scholar] [CrossRef]
- Balas, M.; Ciobanu, C.S.; Burtea, C.; Stan, M.S.; Bezirtzoglou, E.; Predoi, D.; Dinischiotu, A. Synthesis, characterization, and toxicity evaluation of dextran-coated iron oxide nanoparticles. Metals 2017, 7, 63. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.G.; Gao, J.L.; Lyu, G.Y.; Su, J.; Zhang, Q.; Ji, X.; Yan, J.-Z.; Qiu, Q.L.; Zhang, Y.L. Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncol. Lett. 2015, 10, 754–760. [Google Scholar] [PubMed]
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; MaitiChoudhury, S.; Kundu, S.C.; Mandal, M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS ONE 2012, 2012, e0046641. [Google Scholar]
- Jiang, S.; Fan, J.; Wang, Q.; Ju, D.; Feng, M.; Li, J.; Guan, Z.-b.; An, D.; Wang, X.; Ye, L. Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells. Phytomedicine 2016, 23, 243–252. [Google Scholar]
- Raju, J.; Patlolla, J.M.; Swamy, M.V.; Rao, C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1392–1398. [Google Scholar]
- Léger, D.Y.; Liagre, B.; Cardot, P.J.P.; Beneytout, J.-L.; Battu, S. Diosgenin dose-dependent apoptosis and differentiation induction in human erythroleukemia cell line and sedimentation field-flow fractionation monitoring. Anal. Biochem. 2004, 335, 267–278. [Google Scholar] [PubMed]
- Corbiere, C.; Liagre, B.; Bianchi, A.; Bordji, K.; Dauça, M.; Netter, P.; Beneytout, J.-L. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int. J. Oncol. 2003, 22, 899–905. [Google Scholar]
- Hussein, M.A.; Ismail, N.E.M.; Mohamed, A.H.; Borik, R.M.; Ali, A.A.; Mosaad, Y.O. Plasma phospholipids: A promising simple biochemical parameter to evaluate COVID-19 infection severity. Bioinform. Biol. Insights 2021, 15, 11779322211055891. [Google Scholar]
- Gobba, N.A.E.K.; Hussein Ali, A.; El Sharawy, D.E.; Hussein, M.A. The potential hazardous effect of exposure to iron dust in Egyptian smoking and nonsmoking welders. Arch. Environ. Occup. Health 2018, 73, 189–202. [Google Scholar]
- Mosaad, Y.O.; Hussein, M.A.; Ateyya, H.; Mohamed, A.H.; Ali, A.A.; Ramadan Youssuf, A.; Wink, M.; El-Kholy, A.A. Vanin 1 gene role in modulation of iNOS/MCP-1/TGF-β1 signaling pathway in obese diabetic patients. J. Inflamm. Res. 2022, 15, 6745–6759. [Google Scholar]
- Khamis, A.A.; Ali, E.M.; Abd El-Moneim, M.A.; Abd-Alhaseeb, M.M.; El-Magd, M.A.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar]
- Abouzed, T.K.; Sadek, K.M.; Ayoub, M.M.; Saleh, E.A.; Nasr, S.M.; El-Sayed, Y.S.; Shoukry, M. Papaya extract upregulates the immune and antioxidants-related genes, and proteins expression in milk somatic cells of Friesian dairy cows. J. Anim. Physiol. Anim. Nutr. 2019, 103, 407–415. [Google Scholar]
- Lebda, M.A.; Sadek, K.M.; Abouzed, T.K.; Tohamy, H.G.; El-Sayed, Y.S. Melatonin mitigates thioacetamide-induced hepatic fibrosis via antioxidant activity and modulation of proinflammatory cytokines and fibrogenic genes. Life Sci. 2018, 192, 136–143. [Google Scholar]
- Sadek, K.M. Antioxidant and immunostimulant effect of Carica papaya Linn. aqueous extract in acrylamide intoxicated rats. Acta Inform. Medica 2012, 20, 180. [Google Scholar]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Combined efficacy of tamoxifen and coenzyme Q 10 on the status of lipid peroxidation and antioxidants in DMBA induced breast cancer. Mol. Cell. Biochem. 2005, 273, 151–160. [Google Scholar]
- Vengaimaran, M.; Dhamodharan, K.; Sankaran, M. Nano diosgenin abates DMBA induced renal and hepatic toxicities: Biochemical and histopathological evaluation on the Breast Cancer Model. Curr. Bioact. Compd. 2023, 19, 47–67. [Google Scholar]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar]
- Srivastava, S.; Matsuda, M.; Hou, Z.; Bailey, J.P.; Kitazawa, R.; Herbst, M.P.; Horseman, N.D. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 2003, 278, 46171–46178. [Google Scholar]
- Shishodia, S.; Aggarwal, B. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene 2006, 25, 1463–1473. [Google Scholar]
- Franco, J.; Witkiewicz, A.K.; Knudsen, E.S. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 2014, 5, 6512. [Google Scholar]
- Herrera-Abreu, M.T.; Palafox, M.; Asghar, U.; Rivas, M.A.; Cutts, R.J.; Garcia-Murillas, I.; Pearson, A.; Guzman, M.; Rodriguez, O.; Grueso, J. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor–positive breast cancer. Cancer Res. 2016, 76, 2301–2313. [Google Scholar]
- deGraffenried, L.; Chandrasekar, B.; Friedrichs, W.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.; Weiss, G. NF-κB inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann. Oncol. 2004, 15, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar]
- Saab, R.; Bills, J.L.; Miceli, A.P.; Anderson, C.M.; Khoury, J.D.; Fry, D.W.; Navid, F.; Houghton, P.J.; Skapek, S.X. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol. Cancer Ther. 2006, 5, 1299–1308. [Google Scholar] [PubMed]
- Wang, L.; Wang, J.; Blaser, B.W.; Duchemin, A.-M.; Kusewitt, D.F.; Liu, T.; Caligiuri, M.A.; Briesewitz, R. Pharmacologic inhibition of CDK4/6: Mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2007, 110, 2075–2083. [Google Scholar]
- Cetin, B.; Wabl, C.A.; Gumusay, O. CDK4/6 inhibitors: Mechanisms of resistance and potential biomarkers of responsiveness in breast cancer. Future Oncol. 2022, 18, 1143–1157. [Google Scholar]
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; McLeod, H.L.; Ratain, M.J.; Zembutsu, H. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 2018, 103, 770–777. [Google Scholar]
- Skaar, T.C.; Desta, Z. CYP2D6 and endoxifen in tamoxifen therapy: A tribute to David A. Flockhart. Clin. Pharmacol. Ther. 2018, 103, 755–757. [Google Scholar]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 419–433. [Google Scholar]
- Lundgren, C.; Tutzauer, J.; Church, S.E.; Stål, O.; Ekholm, M.; Forsare, C.; Nordenskjöld, B.; Fernö, M.; Bendahl, P.-O.; Rydén, L. Tamoxifen-predictive value of gene expression signatures in premenopausal breast cancer: Data from the randomized SBII: 2 trial. Breast Cancer Res. 2023, 25, 110. [Google Scholar]
- Frasor, J.; Chang, E.C.; Komm, B.; Lin, C.-Y.; Vega, V.B.; Liu, E.T.; Miller, L.D.; Smeds, J.; Bergh, J.; Katzenellenbogen, B.S. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006, 66, 7334–7340. [Google Scholar]
- Schild-Hay, L.J.; Leil, T.A.; Divi, R.L.; Olivero, O.A.; Weston, A.; Poirier, M.C. Tamoxifen Induces Expression of Immune Response–Related Genes in Cultured Normal Human Mammary Epithelial Cells. Cancer Res. 2009, 69, 1150–1155. [Google Scholar] [CrossRef]
- Shen, M.; Qi, C.; Kuang, Y.-P.; Yang, Y.; Lyu, Q.-F.; Long, H.; Yan, Z.-G.; Lu, Y.-Y. Observation of the influences of diosgenin on aging ovarian reserve and function in a mouse model. Eur. J. Med. Res. 2017, 22, 42. [Google Scholar] [PubMed]
- Naruse, M.; Ishigamori, R.; Imai, T. The unique genetic and histological characteristics of DMBA-induced mammary tumors in an organoid-based carcinogenesis model. Front. Genet. 2021, 12, 765131. [Google Scholar]
- Sobinoff, A.P.; Mahony, M.; Nixon, B.; Roman, S.D.; McLaughlin, E.A. Understanding the Villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol. Sci. 2011, 123, 563–575. [Google Scholar] [PubMed]
- Chagaleti, B.K.; Saravanan, V.; Kathiravan, M. Discovery of novel CDK2 inhibitors for cancer treatment: Integrating ligand-based pharmacophore modelling, molecular docking, DFT, ADMET, and molecular dynamics simulation studies. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 118. [Google Scholar]
- Zhang, J.; Wang, W.; Tian, Y.; Ma, L.; Zhou, L.; Sun, H.; Ma, Y.; Hou, H.; Wang, X.; Ye, J. Design, synthesis and biological evaluation of novel diosgenin–benzoic acid mustard hybrids with potential anti-proliferative activities in human hepatoma HepG2 cells. J. Enzym. Inhib. Med. Chem. 2022, 37, 1299–1314. [Google Scholar]
- Baker, S.J.; Poulikakos, P.I.; Irie, H.Y.; Parekh, S.; Reddy, E.P. CDK4: A master regulator of the cell cycle and its role in cancer. Genes Cancer 2022, 13, 21. [Google Scholar]
- Abdel-Gawad, S.M.; Ghorab, M.; El-Sharief, A.S.; El-Telbany, F.; Abdel-Alla, M. Design, synthesis, and antimicrobial activity of some new pyrazolo [3, 4-d] pyrimidines. Heteroat. Chem. Int. J. Main Group Elem. 2003, 14, 530–534. [Google Scholar]
- Shehata, M.R.; Mohamed, M.M.; Shoukry, M.M.; Hussein, M.A.; Hussein, F.M. Synthesis, characterization, equilibria and biological activity of dimethyltin (IV) complex with 1, 4-piperazine. J. Coord. Chem. 2015, 68, 1101–1114. [Google Scholar] [CrossRef]
- Li, J.; Gong, X. Harnessing pre-trained models for accurate prediction of protein-ligand binding affinity. BMC Bioinform. 2025, 26, 55. [Google Scholar]
- Hussein, M.A. Prophylactic effect of resveratrol against ethinylestradiol-induced liver cholestasis. J. Med. Food 2013, 16, 246–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.E.; Cao, J.; Zeng, G.; Shen, C.; Li, Y.; Zhou, M.; Chen, Y.; Pu, W.; Potters, L. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15, 5161–5169. [Google Scholar] [CrossRef]
- Salar, R.K.; Kumar, N. Synthesis and characterization of vincristine loaded folic acid–chitosan conjugated nanoparticles. Resour.-Effic. Technol. 2016, 2, 199–214. [Google Scholar]
- Van de Loosdrecht, A.; Beelen, R.; Ossenkoppele, g.; Broekhoven, M.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Acute oral toxicity of tetrodotoxin in mice: Determination of lethal dose 50 (LD50) and no observed adverse effect level (NOAEL). Toxins 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Steele, G., Jr.; Rodrick, M.L.; Milford, E.; Bleday, R.S.; Lahey, S.J.; Deasy, J.M.; Rayner, A.A.; Wilson, R.E. The effect of 1, 2-dimethylhydrazine (DMH) carcinogenesis on peripheral T cell subsets in the wistar furth rat. J. Surg. Oncol. 1984, 26, 238–244. [Google Scholar] [CrossRef]
- Sabaa, M.; Sharawy, M.H.; El-Sherbiny, M.; Said, E.; Salem, H.A.; Ibrahim, T.M. Canagliflozin interrupts mTOR-mediated inflammatory signaling and attenuates DMBA-induced mammary cell carcinoma in rats. Biomed. Pharmacother. 2022, 155, 113675. [Google Scholar]
- Akerboom, T.P.; Sies, H. [48] Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1981; Volume 77, pp. 373–382. [Google Scholar]
- Tampa, M.; Nicolae, I.; Ene, C.D.; Sarbu, I.; Matei, C.; Georgescu, S.R. Vitamin C and thiobarbituric acid reactive substances (TBARS) in psoriasis vulgaris related to psoriasis area severity index (PASI). Rev. Chim. 2017, 68, 43–47. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological stains: A literature review and case study. Glob. J. Health Sci. 2015, 8, 72. [Google Scholar] [CrossRef]
- Borik, R.M.; Hussein, M.A. Synthesis, molecular docking, biological potentials, and structure activity relationship of new quinazoline and quinazoline-4-one derivatives. Asian J. Chem. 2021, 33, 423–438. [Google Scholar]
- Borik, R.M.; Hussein, M.A. A novel quinazoline-4-one derivatives as a promising cytokine inhibitors: Synthesis, Molecular docking, and structure-activity relationship. Curr. Pharm. Biotechnol. 2022, 23, 1179–1203. [Google Scholar] [PubMed]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017, 33, 3036–3042. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed]
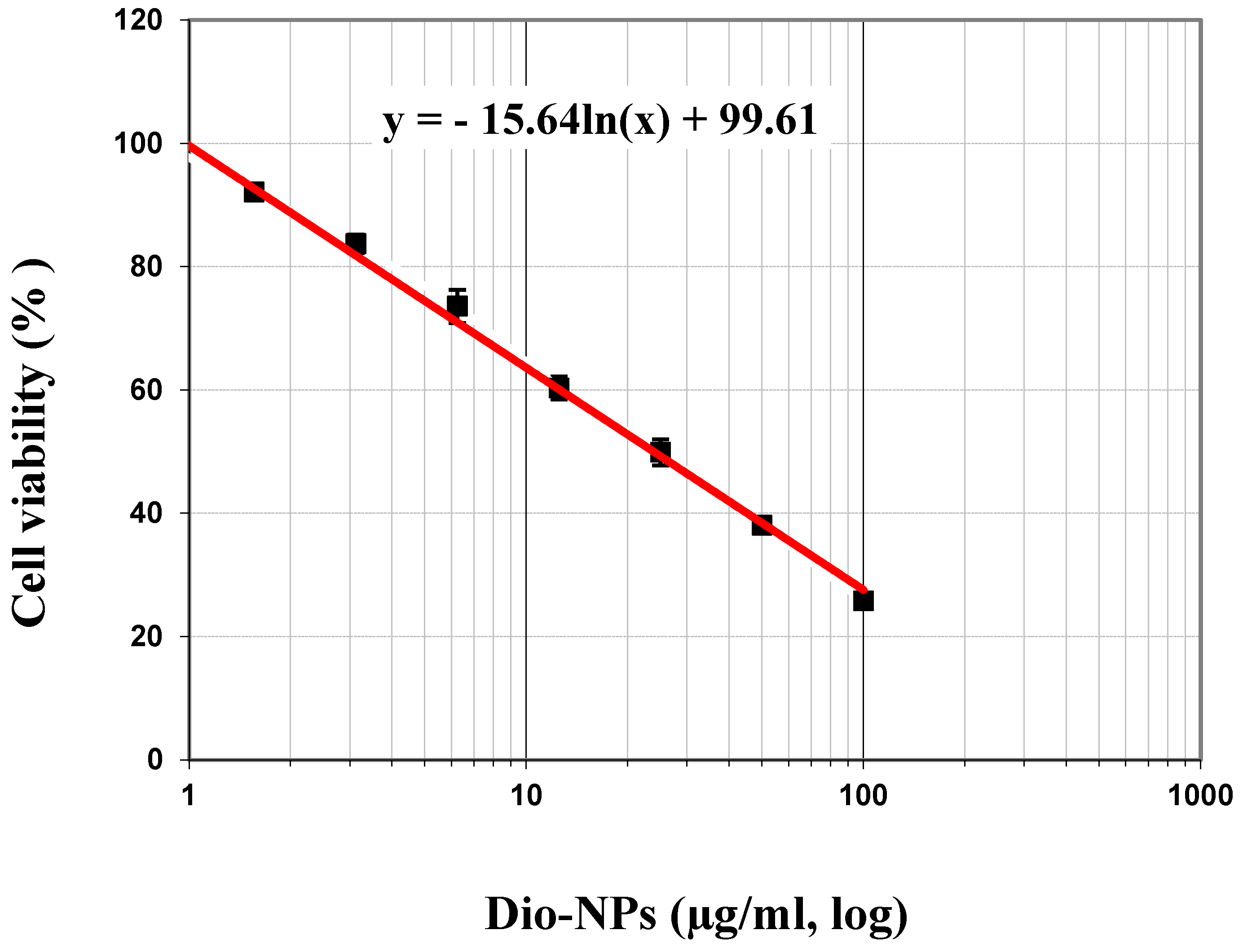


| Doses (µg/mL) | Mean of Viability % | The Mean of Inhibitory % | S.D. (±) |
|---|---|---|---|
| 100 | 25.76 | 74.24 | 0.27 |
| 50 | 38.05 | 61.95 | 0.21 |
| 25 | 49.87 | 50.13 | 2.13 |
| 12.5 | 60.3 | 39.70 | 1.86 |
| 6.25 | 73.57 | 26.43 | 2.70 |
| 3.125 | 83.71 | 16.29 | 1.47 |
| 1.56 | 92.08 | 7.92 | 3.12 |
| 0.8 | 100.0 | 0.0 | 0.07 |
| IC50 | 47.96 ± 1.48 µg/mL | ||
| Group Number | Dose (mg/kg) | No. of Animals/Group | No. of Dead Animals | (Z) | (d) | (Z.d) |
|---|---|---|---|---|---|---|
| 1 | 800 | 10 | 0 | 0.5 | 800 | 400 |
| 2 | 1600 | 10 | 1 | 1.5 | 400 | 600 |
| 3 | 2000 | 10 | 2 | 3.5 | 500 | 1750 |
| 4 | 2500 | 10 | 5 | 7.0 | 500 | 3500 |
| 5 | 3000 | 10 | 9 | 9.5 | 500 | 4750 |
| 6 | 3500 | 10 | 10 | 000 | 00 | 0 |
| Σ (Z.d) = 11,000 | ||||||
| No. | Groups | Hb% (g/dL) | RBCs (106/mm3) | WBCs (103/mm3) | PLT (103/mm3) |
|---|---|---|---|---|---|
| I | Normal control | 13.12 ±0.57 d | 4.36 ±0.20 | 5.79 ±0.60 | 499.21 ±15.88 |
| II | DMBA (50 mg/kg.b.w.) | 8.57 ±0.31 c | 2.77 ±0.31 | 11.67 ±0.68 | 207.88 ±10.65 |
| III | DMBA + Dio-NPs (48 mg/kg.b.w.) | 11.30 ±0.77 b | 3.76 ±0.28 b | 8.33 ±0.68 b | 360.55 ±9.98 |
| IV | DMBA + Dio-NPs (120 mg/kg.b.w.) | 12.73 ±0.25 a | 4.26 ±0.06 | 7.67 ±0.52 | 447.94 ±19.62 |
| V | DMBA + Tamoxifen (2 mg/kg.b.w.) | 8.49 ±0.86 | 2.83 ±0.29 | 8.33 ±0.75 | 175.59 ±13.17 |
| VI | DMBA + Tamoxifen +Dio-NPs (120 mg/kg.b.w.) | 11.71 ±0.84 a | 4.02 ±0.28 | 6.20 ±0.57 | 328.60 ±19.84 |
| No. | Groups | GSH (mmol/g Tissue) | CAT (U/g Tissue) | SOD (U/g Tissue) | MDA (nmol/g Tissue) |
|---|---|---|---|---|---|
| I | Normal control | 46.37 ±4.38 e | 22.12 ±1.93 e | 146.76 ±10.08 e | 322.18 ±15.76 a |
| II | DMBA (50 mg/kg.b.w.) | 16.24 ±1.36 b | 6.59 ±0.52 a | 97.55 ±7.17 a | 838.10 ±26.65 f |
| III | DMBA + Dio-NPs (48 mg/kg.b.w.) | 36.78 ±0.32 d | 12.82 ±1.20 c | 120.56 ±6.58 c | 653.39 ±14.34 c |
| IV | DMBA + Dio-NPs (120 mg/kg.b.w.) | 43.70 ±3.71 e | 20.17 ±0.95 e | 135.82 ±4.75 d | 448.02 ±18.71 b |
| V | DMBA + Tamoxifen (2 mg/kg.b.w.) | 11.95 ±1.05 a | 9.54 ±0.55 b | 108.38 ±6.08 b | 778.51 ±32.78 e |
| VI | DMBA + Tamoxifen +Dio-NPs (120 mg/kg.b.w.) | 32.84 ±2.93 c | 17.46 ±2.02 d | 136.88 ±13.83 d | 730.68 ±28.27 d |
| No. | Groups | NF-kB (pg/g Tissue) | IL-6 (pg/g Tissue) | IL-10 (pg/g Tissue) |
|---|---|---|---|---|
| I | Normal control | 29.20 ±2.27 a | 3.09 ±0.08 a | 61.75 ±4.93 f |
| II | DMBA (50 mg/kg.b.w.) | 74.11 ±6.96 f | 9.16 ±0.31 e | 24.85 ±1.40 a |
| III | DMBA + Dio-NPs (48 mg/kg.b.w.) | 52.78 ±5.31 e | 7.37 ±0.32 d | 35.19 ±1.71 e |
| IV | DMBA + Dio-NPs (120 mg/kg.b.w.) | 44.83 ±3.54 d | 5.74 ±0.36 d | 50.89 ±4.36 d |
| V | DMBA + Tamoxifen (2 mg/kg.b.w.) | 38.63 ±4.02 c | 5.02 ±0.32 c | 15.27 ±1.18 b |
| VI | DMBA + Tamoxifen +Dio-NPs (120 mg/kg.b.w.) | 32.98 ±2.65 b | 4.46 ±0.28 b | 39.91 ±3.10 c |
| No. | Groups | Inflammation | Fat Cell | Lactiferous Ducts | Anaplastic | Fibrous Tissue |
|---|---|---|---|---|---|---|
| I | Normal control | - | - | - | - | - |
| II | DMBA (50 mg/kg.b.w.) | +++ | ++ | +++ | +++ | +++ |
| III | DMBA + Dio-NPs (48 mg/kg.b.w.) | +++ | + | ++ | ++ | ++ |
| IV | DMBA + Dio-NPs (120 mg/kg.b.w.) | ++ | + | ++ | + | ++ |
| V | DMBA + Tamoxifen (2 mg/kg.b.w.) | ++ | ++ | ++ | ++ | + |
| VI | DMBA + Tamoxifen +Dio-NPs (120 mg/kg.b.w.) | + | + | + | + | + |
| Protein/Molecule | Diosgenin | Tamoxifen |
|---|---|---|
| CDK2 | −9.7 | −8.7 |
| CDK4 | −9.6 | −8.4 |
| CDK6 | −10.1 | −8.3 |
| Akt | −9.7 | −7.2 |
| Ligand | 2D/Interaction |
|---|---|
| Diosgenin |  |
| DAIGO =Alkyl (ALA31-4.62A), =Alkyl (VAl18-4.84A), =Alkyl (ILE10-5.31A), =Alkyl(LEU134-4.96, -4.53A) Carbon Hydrogen Bond (LYS129-3.23A) Conventional Hydrogen Bond (LEU83-3.11A) | |
| Tamoxifen | 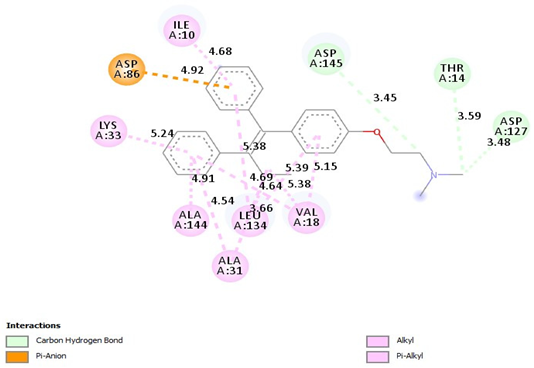 |
| =Carbon Hydrogen Bond (Asp145-3.45A) =Carbon Hydrogen Bond (THR14-359A), =Carbon Hydrogen Bond (ASP127-3.48A), =Pi-Alkyl(ILE10-4.68A), = Pi-Alkyl (LYS33-5.24A), =Pi-Alkyl (ALA144-4.91A), =Pi-Alkyl (ALA31-4.54A), =Pi-Alkyl (LEU134-3.66, 5.38A), =Pi-Alkyl (VAl18-4.64, 4.69A), Pi-Alkyl (VAl18-5.38, -5.39, -5.15A), =Pi-Anion(ASP86-4.92A) |
| Ligand | 2D/Interaction |
|---|---|
| Diosgenin | 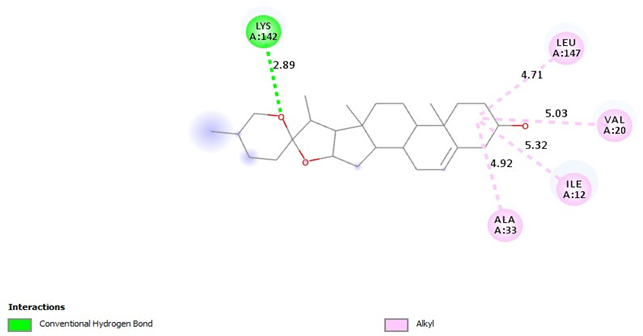 |
| =Alkyl (LEU147-4.71A), =Alkyl (VAl20-5.03A), =Alkyl (ILE12-5:32A), =Alkyl (ALA33-4.92A) =Conventional Hydrogen Bond (LYS142-2.89A) | |
| Tamoxifen | 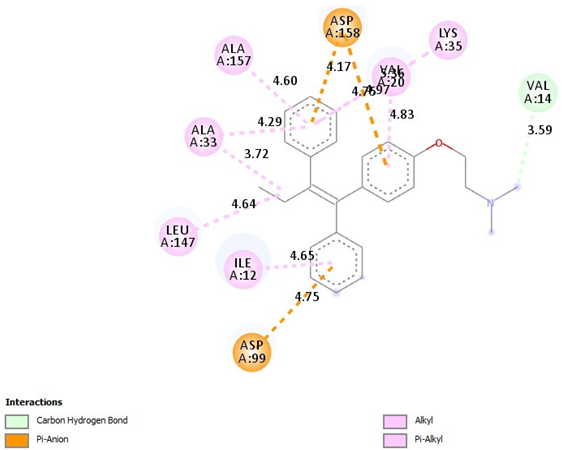 |
| =Pi-Alkyl(ILE12-4.65A), =Pi-Alkyl(LEU147-4.64A), =Pi-Alkyl(ALA33-3.72, -4.29A), =Pi-Alkyl(ALA157-4.60A), =Pi-Alkyl(LYS35-5.36A), =Pi-Alkyl(VAL20-4.83A), =Pi-Anion (ASP158-4.17, -4.76A), =Pi-Anion (ASP99-4.75A), =Carbon Hydrogen Bond, (VAL14-3.59A) |
| Ligand | 2D/Interaction |
|---|---|
| Diosgenin |  |
| =Alkyl (ALA41-4.74A), =Alkyl (VAL27-5.44A), =Alkyl (LEU152-4.75A), =Conventional Hydrogen Bond(VAL101-3.11A) | |
| Tamoxifen | 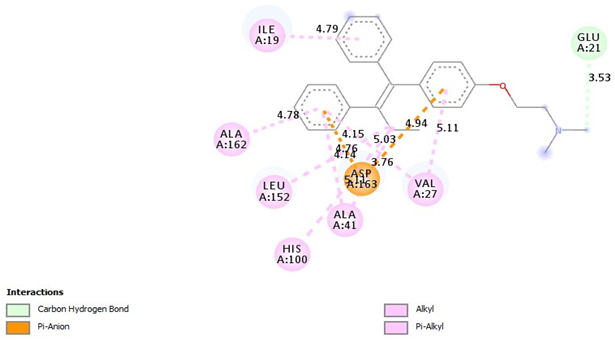 |
| =Pi-Alkyl (ILE19-4.79A), =Pi-Alkyl(VAL27-5.11, 4.15A), =Pi-Alkyl(ALA41-5.11A), =Pi-Alkyl(HIS100-5.03A), =Pi-Alkyl(LEU152-4.14A), =Pi-Alkyl(ALA162-4.78A), =Pi-Anion (ASP163-3.76, -4.94A), =Pi-Anion (ASP163-4.76A)=Carbon Hydrogen Bond (GLU21-3.53A) |
| Groups | Group Description | Description of Treatment |
|---|---|---|
| I | Normal control | 60 days of taking 3 milliliters of distilled water orally. |
| II | DMBA | DMBA (7.5 mg/kg) was injected subcutaneously near the mammary gland area twice weekly for 4 weeks [86]. |
| III | DMBA + Dio-NPs (48 mg/kg.b.w.) | DMBA was injected subcutaneously near the mammary gland twice weekly for 4 weeks [87]; after that, animals were treated with Dio-NPs (48 mg/kg.b.w.) daily for 4 weeks from weeks 5 to 8. |
| IV | DMBA + Dio-NPs (120 mg/kg.b.w.) | DMBA was given subcutaneously near the mammary gland twice weekly for 4 weeks; after that, animals were treated with Dio-NPs (120 mg/kg.b.w.) daily for 4 weeks from weeks 5 to 8. |
| V | DMBA + Tamoxifen | DMBA was injected subcutaneously near the mammary gland twice weekly for 4 weeks; after that, animals were treated with tamoxifen (2 mg/kg.b.w.) [87] on a single weekly dosage for 4 weeks from weeks 5 to 8. |
| VI | DMBA + Tamoxifen + Dio-NPs (120 mg/kg.b.w.) | DMBA was injected subcutaneously near the mammary gland twice weekly for 4 weeks; after that, animals were treated with tamoxifen (2 mg/kg.b.w.) daily for 4 weeks from weeks 5 to 8 plus tamoxifen (2 mg/kg.b.w.) [87] on a single weekly dosage for 4 weeks from weeks 5 to 8. |
| Gene | Primer’s Sequence |
|---|---|
| CDK2 | F:5′-TGGATGCCTCTGCTCTCACT-3′ R:5′-ATATTTCGAGCCCAGGAGGA-3′ |
| CDK4 | F:5′-ATGGCTGCCACTCGATATGAA-3′ R:5′-TCCTCCATTAGGAACTCTCACAC-3′ |
| CDK6 | F:5′-CCAGGCAGGCTTTTCATTCA-3′ R:5′-AGGTCCTGGAAGTATGGGTG-3′ |
| Akt | 5′-TGTGGGAAGATGTGTATGAGAA-′3 5′-TTGATGAGGCGGTGTGATGGTGA-′3 |
| GAPDH (a housekeeping gene) | F:5′-AACTTTGGCATTGTGGAAGG-3′ R:5′-ACACATTGGGGGTAGGAACA-3 |
| Protein | Active Centers |
|---|---|
| CDK2 | center_x = 3.227 center_y = 5.277 center_z = −1.914 |
| CDK4 | center_x = 2.805 center_y = 0.586 center_z = −7.307 |
| CDK6 | center_x = 8.584 center_y = 5.89 center_z = −10.78 |
| AKT | center_x = −9.917 center_y = −6.971 center_z = 12.476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elghany, A.A.; Mohamad, E.A.; Alqarni, A.; Hussein, M.A.; Mansour, M.S. Chemosensitization and Molecular Docking Assessment of Dio-NPs on Resistant Breast Cancer Cells to Tamoxifen. Pharmaceuticals 2025, 18, 452. https://doi.org/10.3390/ph18040452
Abd-Elghany AA, Mohamad EA, Alqarni A, Hussein MA, Mansour MS. Chemosensitization and Molecular Docking Assessment of Dio-NPs on Resistant Breast Cancer Cells to Tamoxifen. Pharmaceuticals. 2025; 18(4):452. https://doi.org/10.3390/ph18040452
Chicago/Turabian StyleAbd-Elghany, Amr A., Ebtesam A. Mohamad, Abdullah Alqarni, Mohammed A. Hussein, and Mohamed S. Mansour. 2025. "Chemosensitization and Molecular Docking Assessment of Dio-NPs on Resistant Breast Cancer Cells to Tamoxifen" Pharmaceuticals 18, no. 4: 452. https://doi.org/10.3390/ph18040452
APA StyleAbd-Elghany, A. A., Mohamad, E. A., Alqarni, A., Hussein, M. A., & Mansour, M. S. (2025). Chemosensitization and Molecular Docking Assessment of Dio-NPs on Resistant Breast Cancer Cells to Tamoxifen. Pharmaceuticals, 18(4), 452. https://doi.org/10.3390/ph18040452







