Chromatographic Analysis and Enzyme Inhibition Potential of Reynoutria japonica Houtt.: Computational Docking, ADME, Pharmacokinetic, and Toxicokinetic Analyses of the Major Compounds
Abstract
1. Introduction
2. Results
2.1. Enzyme Inhibition Results
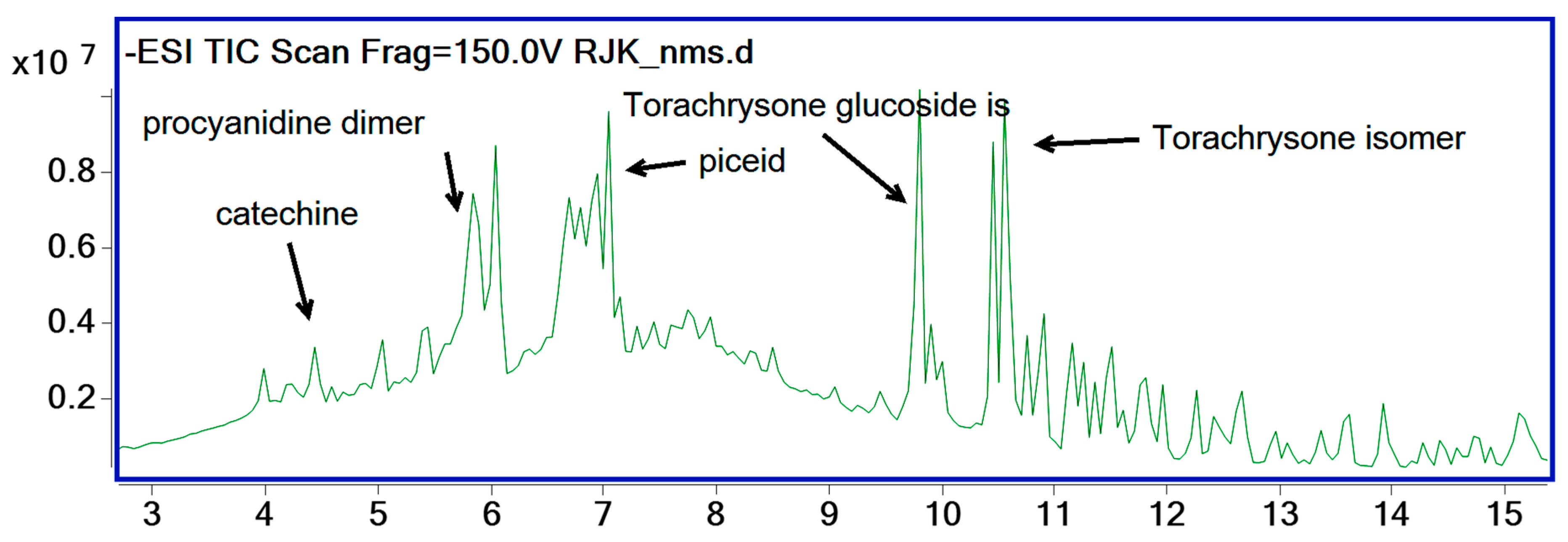
2.2. Results of Phytochemical Analyses
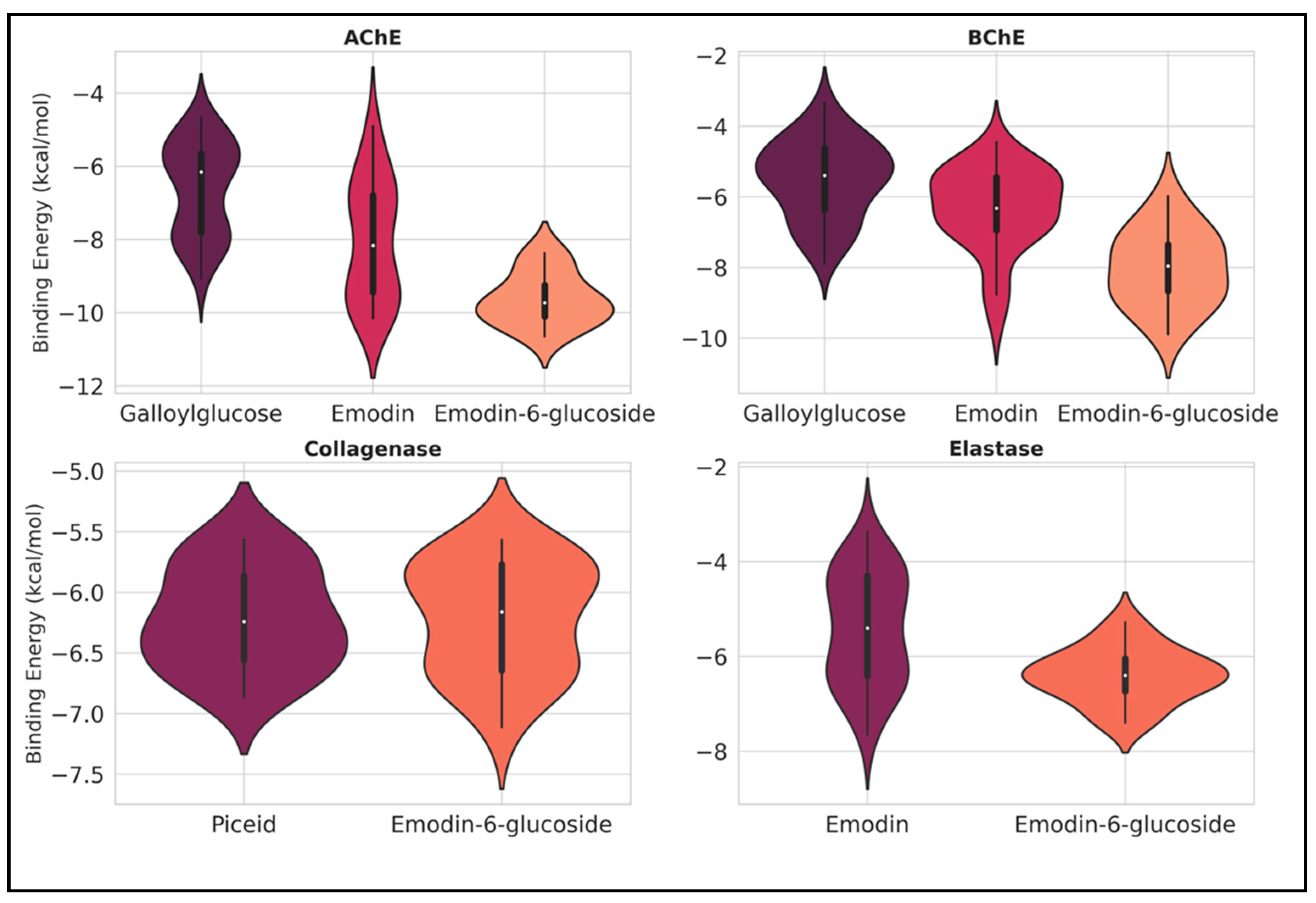
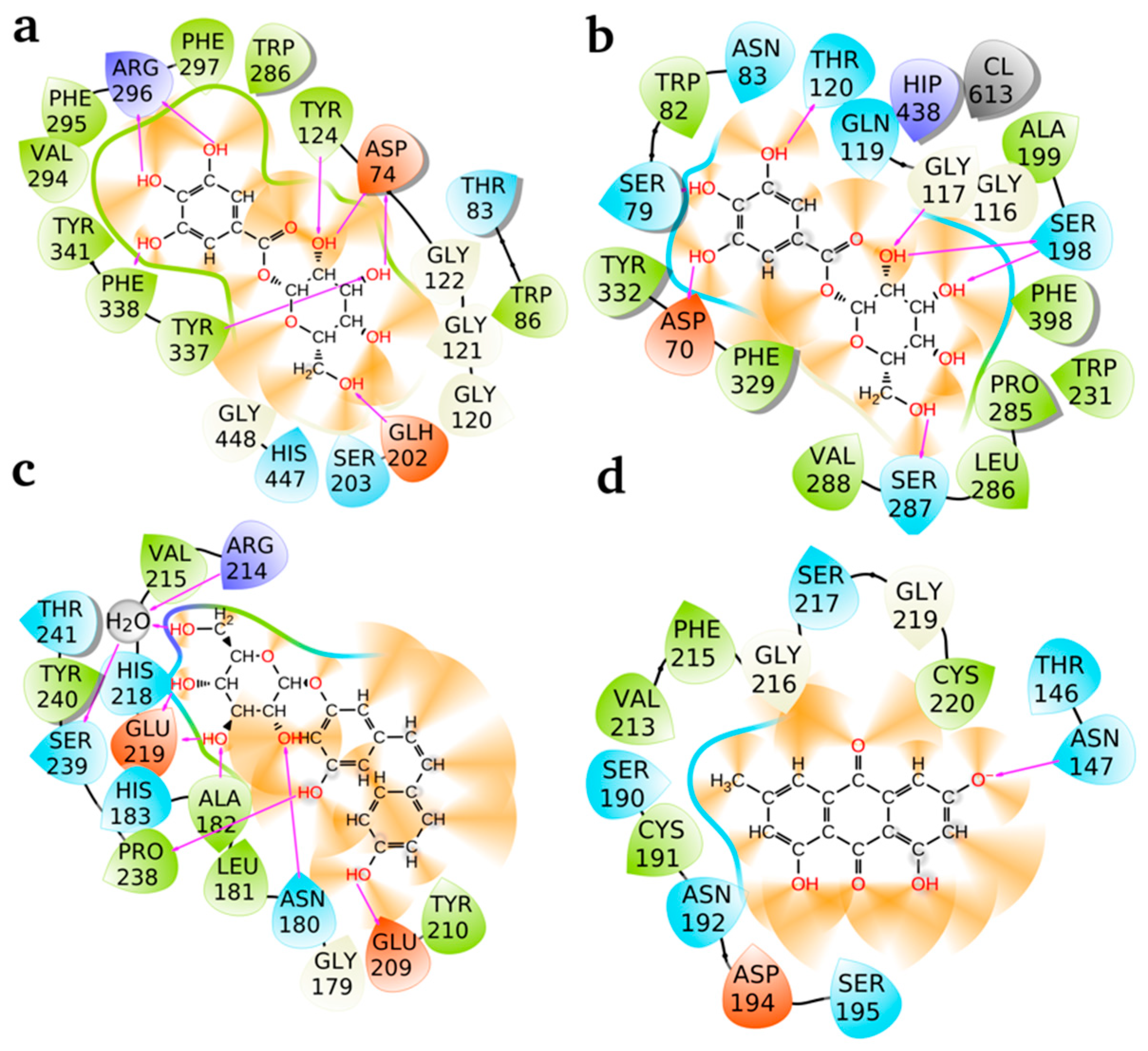
2.3. Ligand Binding Energy Analysis Across Proteins
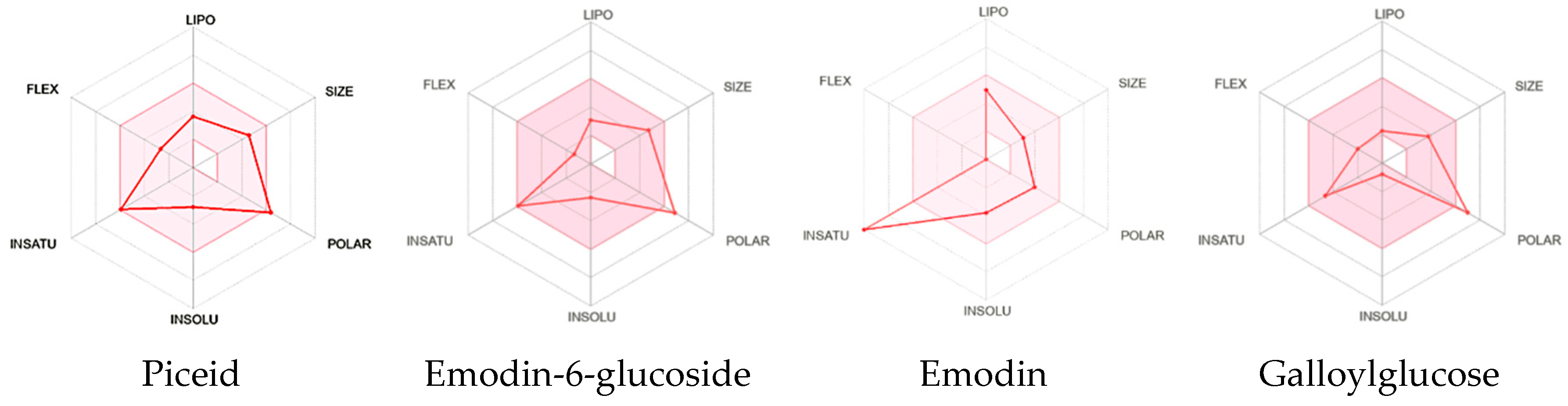
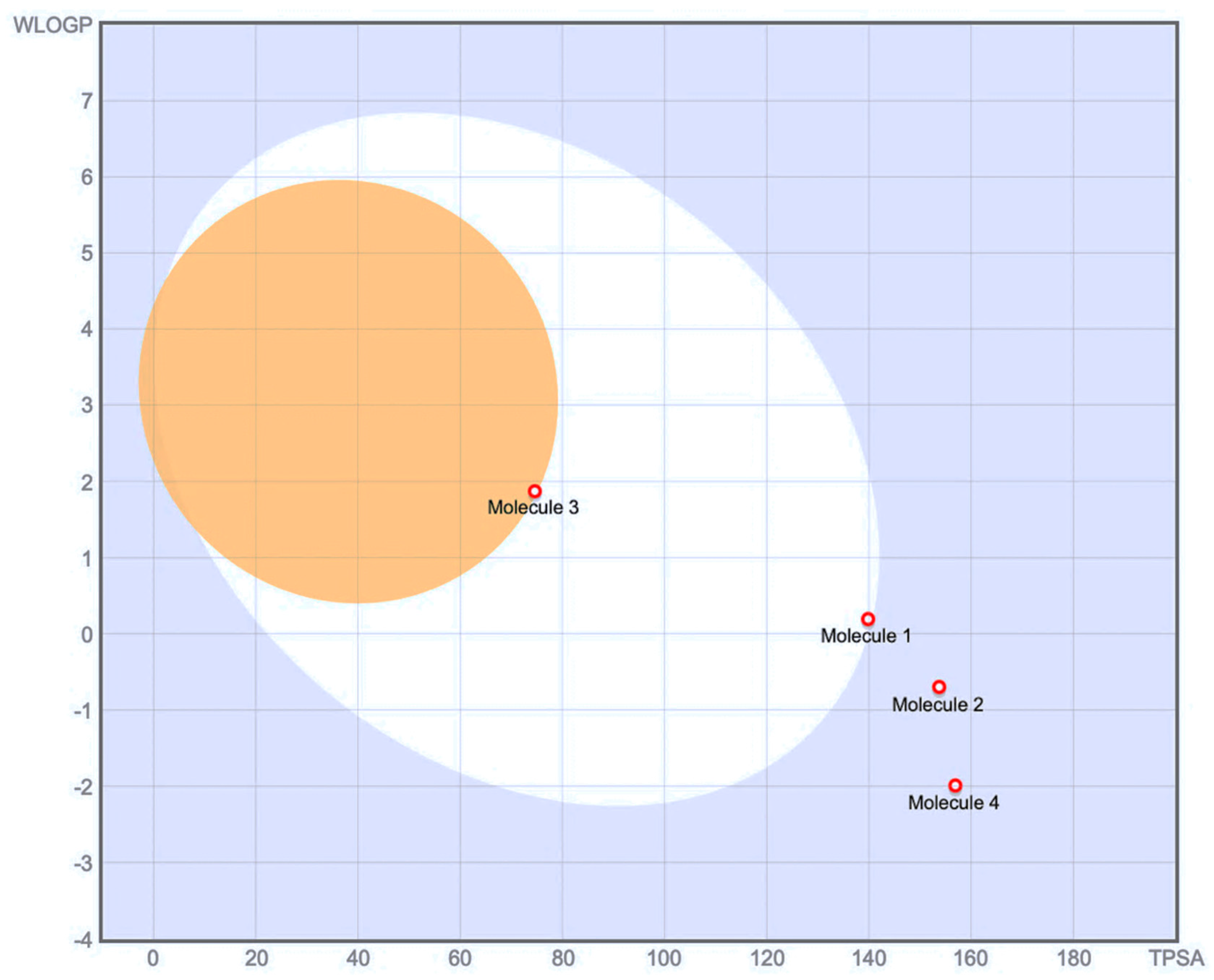
2.4. Drug-Likeness Parameters and ADME Profile Analysis of Galloylglucose, Emodin, Emodin-6-Glucoside, and Piceid
2.5. Cytochrome P450 Inhibition and Potential Drug Interactions
2.6. Synthetic Accessibility Assessment
2.7. Statistical Analyses
3. Discussion
- Quinone_A Alert (emodin and emodin-6-glucoside): Quinone groups can undergo redox cycling, leading to the generation of reactive oxygen species (ROS), which may cause cytotoxicity independent of a specific biological target.
- Catechol_A Alert (galloylglucose): Catechol moieties have a known tendency to chelate metal ions and inhibit various enzymes non-selectively, potentially leading to misleading bioactivity results.
- Stilbene Alert (piceid): Stilbene derivatives can exhibit aggregation behavior or auto-fluorescence, which may interfere with fluorescence-based assays, distorting the interpretation of biological activity.
4. Material and Methods
4.1. Plant Material and Extraction Method
4.2. Enzyme Inhibition Assays
4.2.1. AChE/BChE Inhibition Assay
4.2.2. Elastase Inhibition Assay
4.2.3. Collagenase Inhibition Assay
4.2.4. TYR Inhibition Assay
4.2.5. Calculation of Data for Enzyme Assays
4.3. Conditions of LC-MS QTOF Analysis
4.4. Optimization Pipeline for Protein Crystal Structures and Ligands
4.5. Ligand Binding Energy Analysis Using Induced Fit Docking
4.6. Computational ADME, Pharmacokinetic and Toxicokinetic Predictions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Frąc, M.; Oszust, K.; Woch, M.W.; Zubek, S. Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci. Total Environ. 2021, 767, 145439. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tuck, T.; Ji, X.; Zhou, X.; Kelly, G.; Cuerrier, A.; Zhang, J. Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J. Agric. Food Chem. 2013, 61, 6383–6392. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J. Profile of bioactive compounds in the morphological parts of wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and their antioxidative activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical diversity in rhizomes of three Reynoutria species and their antioxidant activity correlations elucidated by LC-ESI-MS/MS analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum-A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cucu, A.A.; Baci, G.M.; Dezsi, Ş.; Nap, M.E.; Beteg, F.I.; Bonta, V.; Bobiş, O.; Caprio, E.; Dezmirean, D.S. New approaches on Japanese knotweed (Fallopia japonica) bioactive compounds and their potential of pharmacological and beekeeping activities: Challenges and future directions. Plants 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nam, Y.; Song, J.; Kim, H. Gastroprotective and healing effects of Polygonum cuspidatum root on experimentally induced gastric ulcers in rats. Nutrients 2020, 12, 2241. [Google Scholar] [CrossRef]
- Karaer, F.; Terzıoglu, S.; Kutbay, H.G. A new genus record for the flora of Turkey: Reynoutria (Polygonaceae). J. Agric. Nat. Sci. 2020, 23, 606–610. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health–a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Abacı, N.; Senol Deniz, F.S.; Ekhteiari Salmas, R.; Uysal Bayar, F.; Turgut, K.; Orhan, I.E. In vitro and in silico cholinesterase inhibitory and antioxidant effects of essential oils and extracts of two new Salvia fruticosa mill. cultivars (Turgut and Uysal) and GC-MS analysis of the essential oils. Int. J. Environ. Health Res. 2024, 34, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.M.; Branco, P.S.; Ferreira, L.M. Enhancing the therapeutic effect in Alzheimer’s disease drugs: The role of polypharmacology and cholinesterase inhibitors. ChemistrySelect 2023, 8, e202300461. [Google Scholar] [CrossRef]
- Buarque, F.S.; Monteiro e Silva, S.A.; Ribeiro, B.D. Choline chloride-based deep eutectic solvent as an inhibitor of metalloproteases (collagenase and elastase) in cosmetic formulation. Biotech 2023, 13, 219. [Google Scholar] [CrossRef]
- Figueiredo, C.C.M.; da Costa Gomes, A.; Zibordi, L.C.; Granero, F.O.; Ximenes, V.F.; Pavan, N.M.; Silva, L.P.; Sonvesso, C.d.S.M.; Job, A.E.; Nicolau-Junior, N. Biosynthesis of silver nanoparticles of Tribulus terrestris food supplement and evaluated antioxidant activity and collagenase, elastase and tyrosinase enzyme inhibition: In vitro and in silico approaches. Food Bioprod. Process. 2023, 138, 150–161. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase inhibitors naturally present in plants and synthetic modifications of these natural products as anti-melanogenic agents: A review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Xue, S.; Li, Z.; Ze, X.; Wu, X.; He, C.; Shuai, W.; Marlow, M.; Chen, J.; Scurr, D.; Zhu, Z. Design, synthesis, and biological evaluation of novel hybrids containing dihydrochalcone as tyrosinase ınhibitors to treat skin hyperpigmentation. J. Med. Chem. 2023, 66, 5099–5117. [Google Scholar] [CrossRef]
- Božin, B.; Gavrilović, M.; Kladar, N.; Rat, M.; Anačkov, G.; Gavarić, N. Highly invasive alien plant Reynoutria japonica Houtt. represents a novel source for pharmaceutical industry–Evidence from phenolic profile and biological activity. J. Serb. Chem. Soc. 2017, 82, 803–813. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Chen, M.; Hu, H.; Cao, J.; Chai, T.; Wang, H. A study on tissue-specific metabolite variations in Polygonum cuspidatum by high-resolution mass spectrometry-based metabolic profiling. Molecules 2019, 24, 1058. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Grabowski, K.; Baringhaus, K.-H.; Schneider, G. Scaffold diversity of natural products: Inspiration for combinatorial library design. Nat. Prod. Rep. 2008, 25, 892–904. [Google Scholar] [CrossRef] [PubMed]
- DeGoey, D.A.; Chen, H.J.; Cox, P.B.; Wendt, M.D. Beyond the rule of 5: Lessons learned from AbbVie’s drugs and compound collection: Mini perspective. J. Med. Chem. 2017, 61, 2636–2651. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as potential enzyme inhibitors from medicinal plants. Enzyme Inhib. Activators 2017, 165, 177. [Google Scholar]
- Yan, T.C.; Cao, J.; Ye, L.H. Recent advances on discovery of enzyme inhibitors from natural products using bioactivity screening. J. Sep. Sci. 2022, 45, 2766–2787. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Palumbo, F.; Costantini, M. Marine sponges and bacteria as challenging sources of enzyme inhibitors for pharmacological applications. Mar. Drugs 2017, 15, 173. [Google Scholar] [CrossRef]
- Vrchotová, N.; Sera, B.; Triska, J. The stilbene and catechin content of the spring sprouts of Reynoutria species. Acta Chromatogr. 2007, 19, 21. [Google Scholar]
- Cao, Y.; Yang, X.Y.; Lin, S.S.; Liu, S.J.; Xu, X.W.; Huang, L.F. In vitro screening of cholinesterase inhibitory activities using traditional Chinese medicine. Phytother. Res. 2020, 34, 1135–1142. [Google Scholar]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M.A.; Ahmad, W.; Shah, M.R.; Imran, M.; Ahmad, S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A preliminary anti-Alzheimer’s study. Lipids Health Dis. 2015, 14, 141. [Google Scholar] [CrossRef]
- Miyazaki, Y. Immune effects and antiacetylcholinesterase activity of Polygonum hydropiper L. Biosci. Microbiota Food Health 2016, 35, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Q.; He, Z.; Tan, G.; Zou, Y.; Xie, J.; Qian, Z. Screening of tyrosinase, xanthine oxidase, and α-glucosidase inhibitors from Polygoni Cuspidati Rhizoma et Radix by ultrafiltration and HPLC analysis. Molecules 2023, 28, 4170. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Hwang, T.L.; Hu, J.W.; Fang, J.Y. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother. Res. 2008, 22, 552–556. [Google Scholar] [CrossRef]
- Jeong, E.T.; Jin, M.H.; Kim, M.-S.; Chang, Y.H.; Park, S.G. Inhibition of melanogenesis by piceid isolated from Polygonum cuspidatum. Arch. Pharm. Res. 2010, 33, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chen, Y.T.; Chiu, C.C.; Liao, W.T.; Liu, Y.C.; Wang, H.M.D. Polygonum cuspidatum extracts as bioactive antioxidation, anti-tyrosinase, immune stimulation and anticancer agents. J. Biosci. Bioeng. 2015, 119, 464–469. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and evaluation of dermo-cosmetic activities of the invasive plant species Polygonum cuspidatum. Plants 2022, 12, 83. [Google Scholar] [CrossRef]
- Xiao-jing, L.; Pei-dong, S.; Jing-ru, Q.; Guang-qun, C.; Cheng, Y. Elastase inhibitory activity of extracts from Polygonum cuspidatum. Nat. Prod. Res. Dev. 2012, 24, 378. [Google Scholar]
- Yan, H.; Jianyu, Z.; Wei, N.; Changxiang, C. Studies on the constituents of Reynoutria japonica Houtt. Nat. Prod. Res. Dev. 2001, 13, 16–18. [Google Scholar]
- Dong, J.; Wang, H.; Wan, L.; Hashi, Y.; Chen, S. Identification and determination of major constituents in Polygonum cuspidatum Sieb. et Zucc. by high performance liquid chromatography/electrospray ionization-ion trap-time-of-flight mass spectrometry. Se Pu Chin. J. Chromatogr. 2009, 27, 425–430. [Google Scholar]
- Huang, W.-Y.; Cai, Y.-Z.; Xing, J.; Corke, H.; Sun, M. Comparative analysis of bioactivities of four Polygonum species. Planta Med. 2008, 74, 43–49. [Google Scholar] [CrossRef]
- Haiwu, X.; Liuxin, L. Study on the content of resveratrol in some fruits. Zhongguo Nong Xue Tong Bao Chin. Agric. Sci. Bull. 2005, 21, 99–100, 131. [Google Scholar]
- Martin, H.; Lázaro, L.R.; Gunnlaugsson, T.; Scanlan, E.M. Glycosidase activated prodrugs for targeted cancer therapy. Chem. Soc. Rev. 2022, 51, 9694–9716. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kraunsoe, J.A.; Claridge, T.D.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sandesh, S.; Shruti, S.; Seo, S. Potent antielastase and antityrosinase activities of Astilbe chinensis. Am. J. Pharmacol. Toxicol. 2009, 4, 127–129. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Steinbrink, D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef]
- Barrantes, E.; Guinea, M.a. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003, 72, 843–850. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Košak, U.; Brus, B.; Knez, D.; Šink, R.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šlenc, J.; Gobec, M.; Živin, M. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef]
- Lovejoy, B.; Cleasby, A.; Hassell, A.M.; Longley, K.; Luther, M.A.; Weigl, D.; McGeehan, G.; McElroy, A.B.; Drewry, D.; Lambert, M.H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science 1994, 263, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Søndergaard, C.R.; Olsson, M.H.; Rostkowski, M.; Jensen, J.H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Johnston, R.C.; Yao, K.; Kaplan, Z.; Chelliah, M.; Leswing, K.; Seekins, S.; Watts, S.; Calkins, D.; Chief Elk, J.; Jerome, S.V. Epik: pKa and protonation state prediction through machine learning. J. Chem. Theory Comput. 2023, 19, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. BMCL 2006, 14, 3160–3173. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
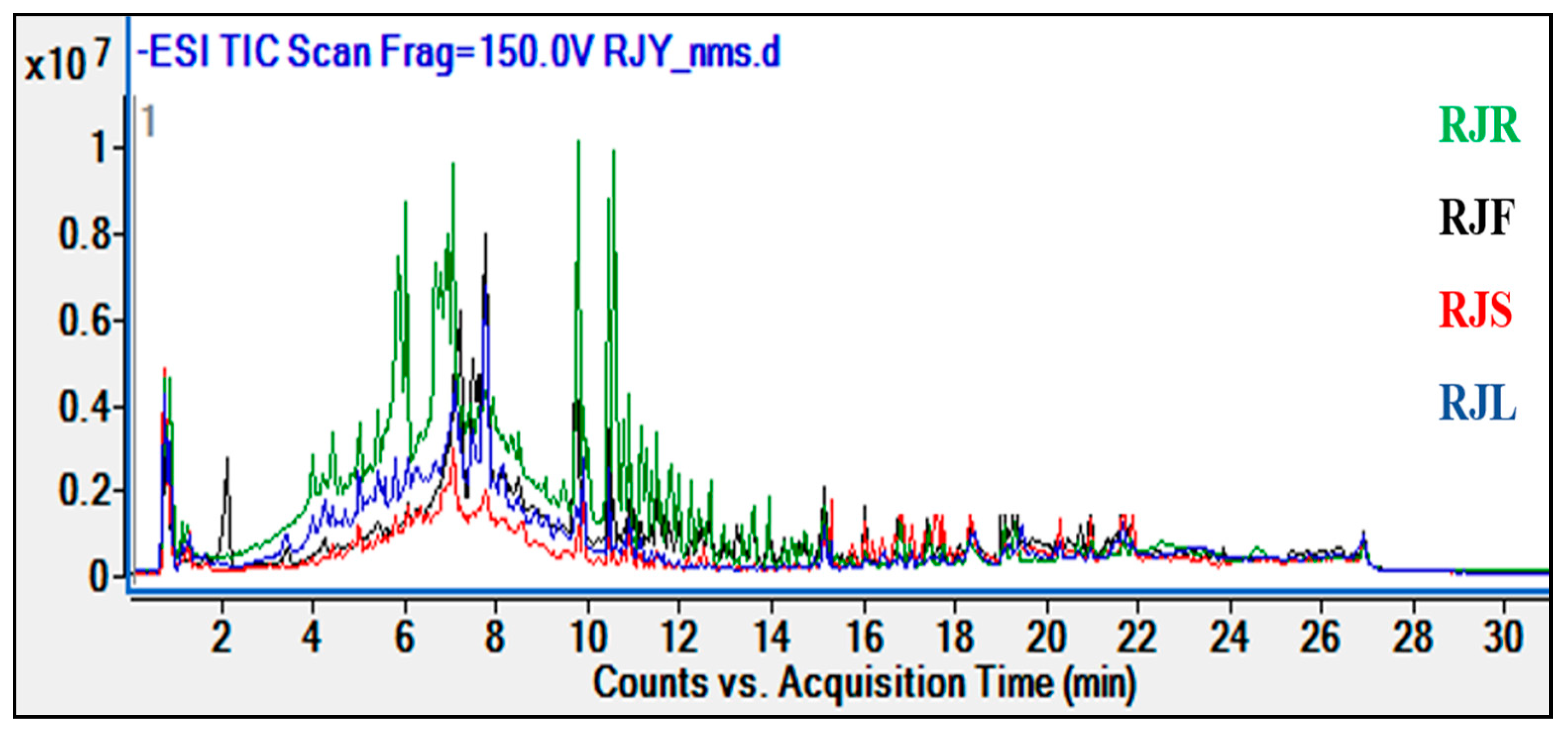
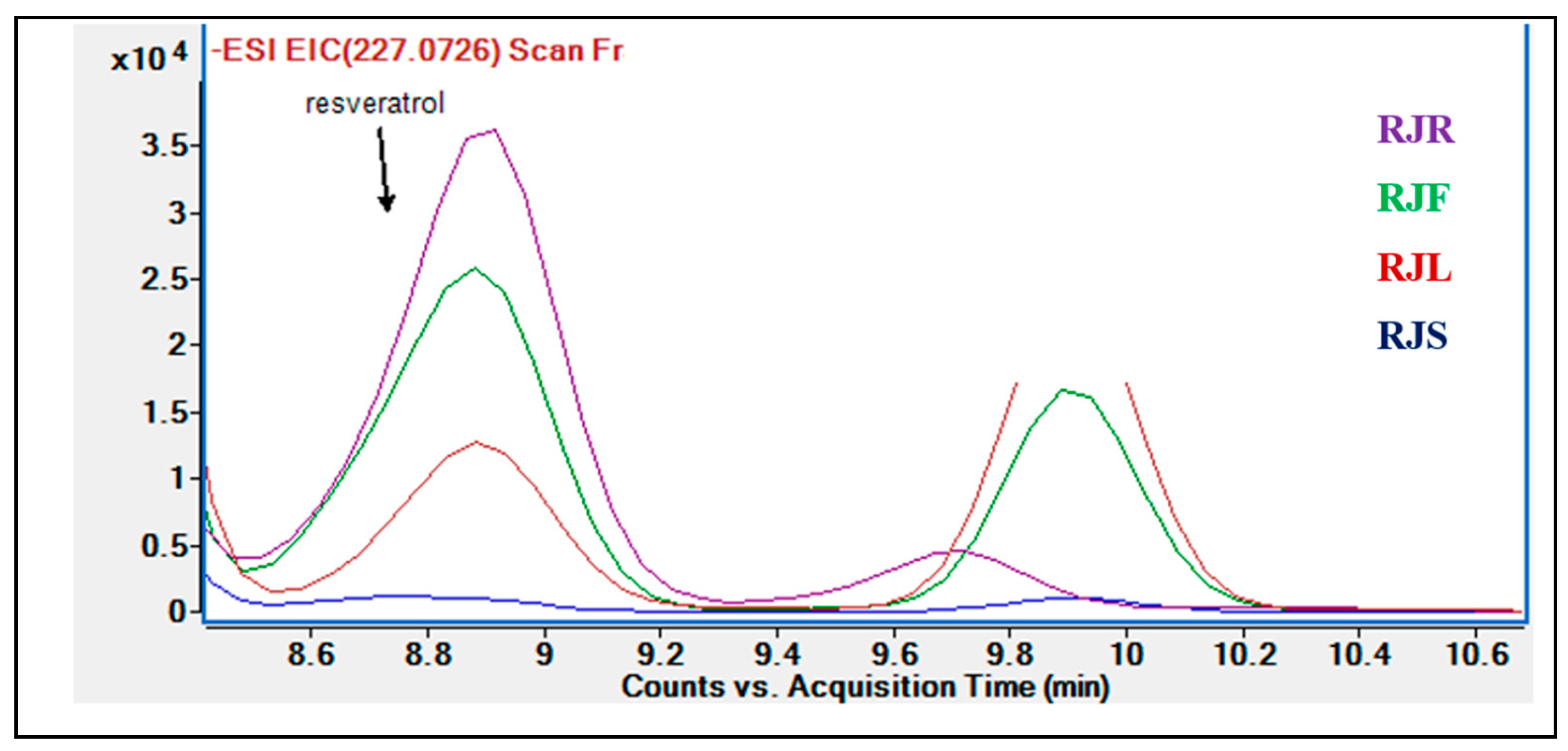

| Samples | Cholinesterase Inhibition (Inhibition% ± S.D. a) at 100 µg/mL b | |
|---|---|---|
| AChE | BChE | |
| RJF | 60.89 ± 4.12 **** (IC50 = 64.44 ± 4.69 µg/mL) | 41.13 ± 1.61 **** |
| RJS | 64.24 ± 3.09 c **** (IC50 = 25.06 ± 0.52 µg/mL) | 31.86 ± 4.17 **** (IC50 = 159.78 ± 3.50 µg/mL) |
| RJL | 63.57 ± 2.50 **** (IC50 = 53.31 ± 3.38 µg/mL) | 20.81 ± 3.38 **** (IC50 = 202.50 ± 1.41 µg/mL) |
| RJR | 49.93 ± 4.18 **** (IC50 = 120.10 ± 0.14 µg/mL) | 20.15 ± 1.77 **** |
| Galanthamine hydrobromide d | 90.47 ± 0.76 (IC50 = 0.91 ± 0.03 µg/mL) | 70.95 ± 1.51 (IC50 = 37.94 ± 3.17 µg/mL) |
| Samples | Inhibition% ± S.D. a at 333 µg/mL b | ||
|---|---|---|---|
| TYR | Elastase | Collagenase | |
| RJF | - c | 70.06 ± 2.12 **** (IC50 = 111.40 ± 1.45 µg/mL) | 68.82 ± 5.57 **** (IC50 = 231.90 ± 4.12 µg/mL) |
| RJS | 26.03 ± 1.68 **** | 65.22 ± 3.21 **** (IC50 = 253.03 ± 3.05 µg/mL) | 78.68 ± 6.08 (IC50 = 236.80 ± 7.34 µg/mL) |
| RJL | - | 80.97 ± 1.70 **** (IC50 = 117.20 ± 4.84 µg/mL) | 81.02 ± 6.73 (IC50 = 171.00 ± 6.76 µg/mL) |
| RJR | 19.46 ± 2.37 **** | 9.31 ± 1.38 **** | 69.68 ± 2.23 *** (IC50 = 160.00 ± 6.81 µg/mL) |
| Reference | 84.56 ± 0.27 d (IC50 = 0.68 ± 0.05 µg/mL) | 99.65 ± 0.08 e (IC50 = 2.65 ± 0.36 µg/mL) | 87.39 ± 2.85 f (IC50 = 27.95 ± 0.37 µg/mL) |
| Chemical Name | Structure | Rt (min) | Formula | M-H Ion | Product Ions |
|---|---|---|---|---|---|
| Galloyl glucose isomer | Phenolic acid | 1.27 | C13H16O10 | 331.0666 | 211.0251, 169.0147 |
| Chlorogenic acid isomer | Polyphenol | 3.4 | C16H17O9 | 331.0863 | 191.0546, 135.0442 |
| Procyanidin B1 | Polyphenol | 4 | C30H26O12 | 577.134 | 559.1228, 289.0718 |
| Catechin | Flavonoid | 4.43 | C15H14O6 | 289.0725 | 245.0827, 109.0298 |
| Procyanidin B1 | Polyphenol | 5.04 | C30H26O12 | 577.1346 | 425.0864, 289.0718 |
| Epicatechin | Flavonoid | 5.39 | C15H14O6 | 289.0726 | 245.0833, 109.0306 |
| Galloylprocyanidin B1/B2 | Polyphenol | 5.94 | C37H30O16 | 729.1458 | 289.0712 |
| Catechin/epicatechin gallate | Flavonoid | 6.54 | C22H18O10 | 441.0825 | 289.0716, 169.014 |
| Piceid | Stilbenoid glucoside | 6.94 | C20H21O8 | 389.1221 | 227.0718 |
| Catechin/Epicatechin gallate | Flavonoid | 7.04 | C22H18O10 | 441.082 | 289.0710, 169.0147 |
| Torachrysone 8-glucoside | Naphthalene glucoside | 9.85 | C20H23O9 | 407.1335 | 269.0451, 245.0802 |
| Emodin-6-O-glucoside | Anthraquinone glucoside | 9.9 | C21H19O10 | 431.0974 | 269.0462 |
| Malonylgenistin | Flavonoid | 10.46 | C24H21O13 | 517.1026 | 473.1056, 269.0467 |
| Torachrysone glucoside isomer | Naphthalene glucoside | 10.56 | C22H25O10 | 449.144 | 245.0804 |
| Emodin | Anthraquinone | 15.2 | C15H9O5 | 269.0461 | 225.0570, 197.0602 |
| Column | Agilent Poroshell SB C-18 (3.0 mm × 100 mm × 2.7 µm) |
|---|---|
| Column temperature | 35 °C |
| Injection volume | 10 µL |
| Run time | 32 min |
| Mobile phase A | 0.1% formic acid in water |
| Mobile phase B | Acetonitrile |
| Flow rate | 0.6 mL/min |
| Gradient (time-B%) | 0 min-5% B |
| 2 min-5% B | |
| 6 min-20% B | |
| 18 min-70% B | |
| 20 min-90% B | |
| 26 min-90% B | |
| 26.1 min-5% B | |
| Ionization mode | Negative ESI |
| Drying gas temperature | 325 °C |
| Drying gas flow | 11 L/min |
| Nebulizer | 35 psi |
| Capillary voltage | 3000 V |
| Fragmentor voltage | 150 V |
| Mass range | 30–1700 amu |
| Reference ions | 112.98587, 1033.988109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyukyildirim, T.; Senol Deniz, F.S.; Tugay, O.; Salmas, R.E.; Ulutas, O.K.; Aysal, I.A.; Orhan, I.E. Chromatographic Analysis and Enzyme Inhibition Potential of Reynoutria japonica Houtt.: Computational Docking, ADME, Pharmacokinetic, and Toxicokinetic Analyses of the Major Compounds. Pharmaceuticals 2025, 18, 408. https://doi.org/10.3390/ph18030408
Buyukyildirim T, Senol Deniz FS, Tugay O, Salmas RE, Ulutas OK, Aysal IA, Orhan IE. Chromatographic Analysis and Enzyme Inhibition Potential of Reynoutria japonica Houtt.: Computational Docking, ADME, Pharmacokinetic, and Toxicokinetic Analyses of the Major Compounds. Pharmaceuticals. 2025; 18(3):408. https://doi.org/10.3390/ph18030408
Chicago/Turabian StyleBuyukyildirim, Tugsen, Fatma Sezer Senol Deniz, Osman Tugay, Ramin Ekhteiari Salmas, Onur Kenan Ulutas, Ibrahim Ayhan Aysal, and Ilkay Erdogan Orhan. 2025. "Chromatographic Analysis and Enzyme Inhibition Potential of Reynoutria japonica Houtt.: Computational Docking, ADME, Pharmacokinetic, and Toxicokinetic Analyses of the Major Compounds" Pharmaceuticals 18, no. 3: 408. https://doi.org/10.3390/ph18030408
APA StyleBuyukyildirim, T., Senol Deniz, F. S., Tugay, O., Salmas, R. E., Ulutas, O. K., Aysal, I. A., & Orhan, I. E. (2025). Chromatographic Analysis and Enzyme Inhibition Potential of Reynoutria japonica Houtt.: Computational Docking, ADME, Pharmacokinetic, and Toxicokinetic Analyses of the Major Compounds. Pharmaceuticals, 18(3), 408. https://doi.org/10.3390/ph18030408










