In Vitro Evaluation of Esters of Quinoxaline-1,4-di-N-oxide Derivatives as New Antitaeniasis Agents and Their Inhibitory Activity Against Triosephosphate Isomerase
Abstract
1. Introduction
2. Results
2.1. Antitaenia Activity
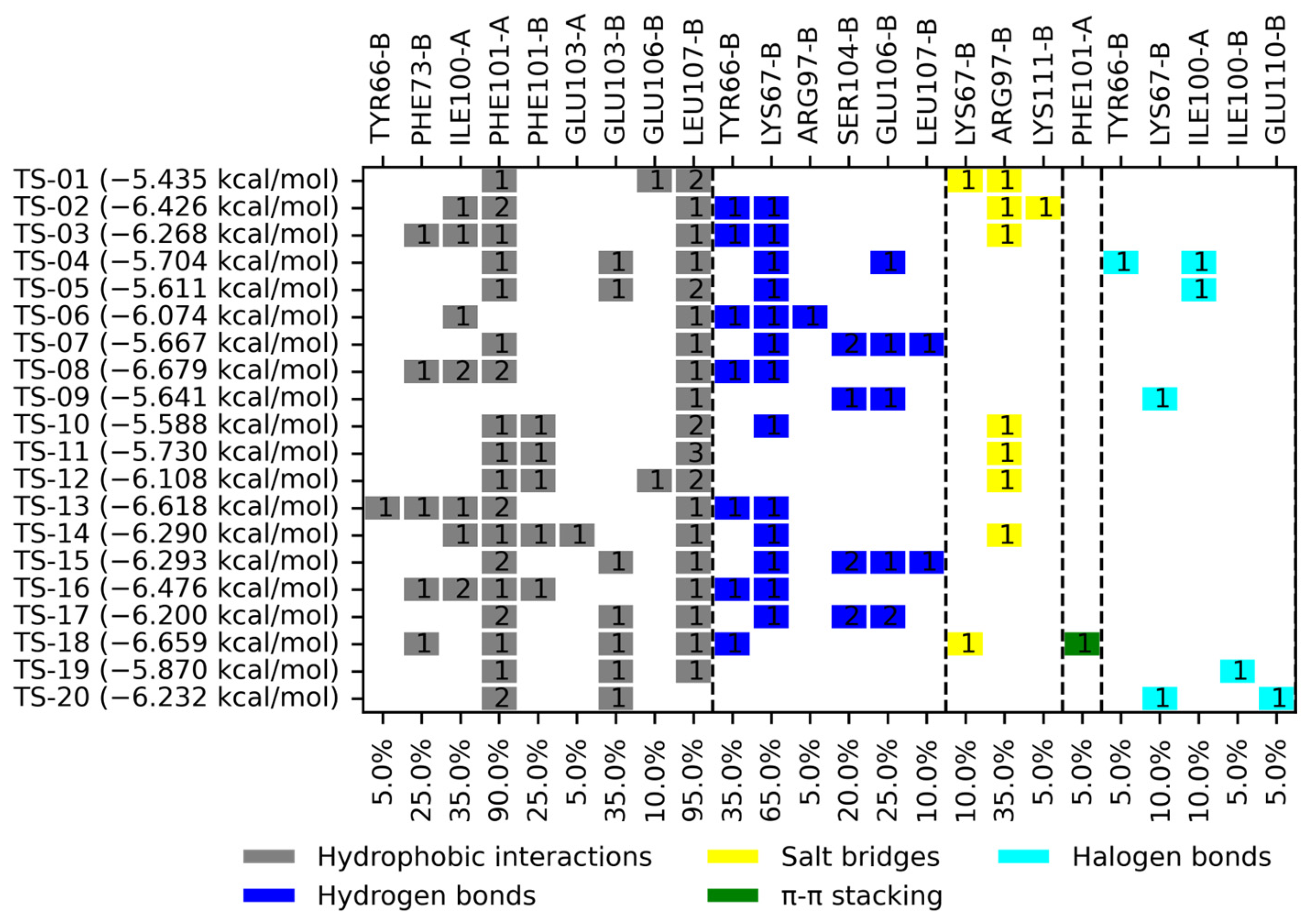
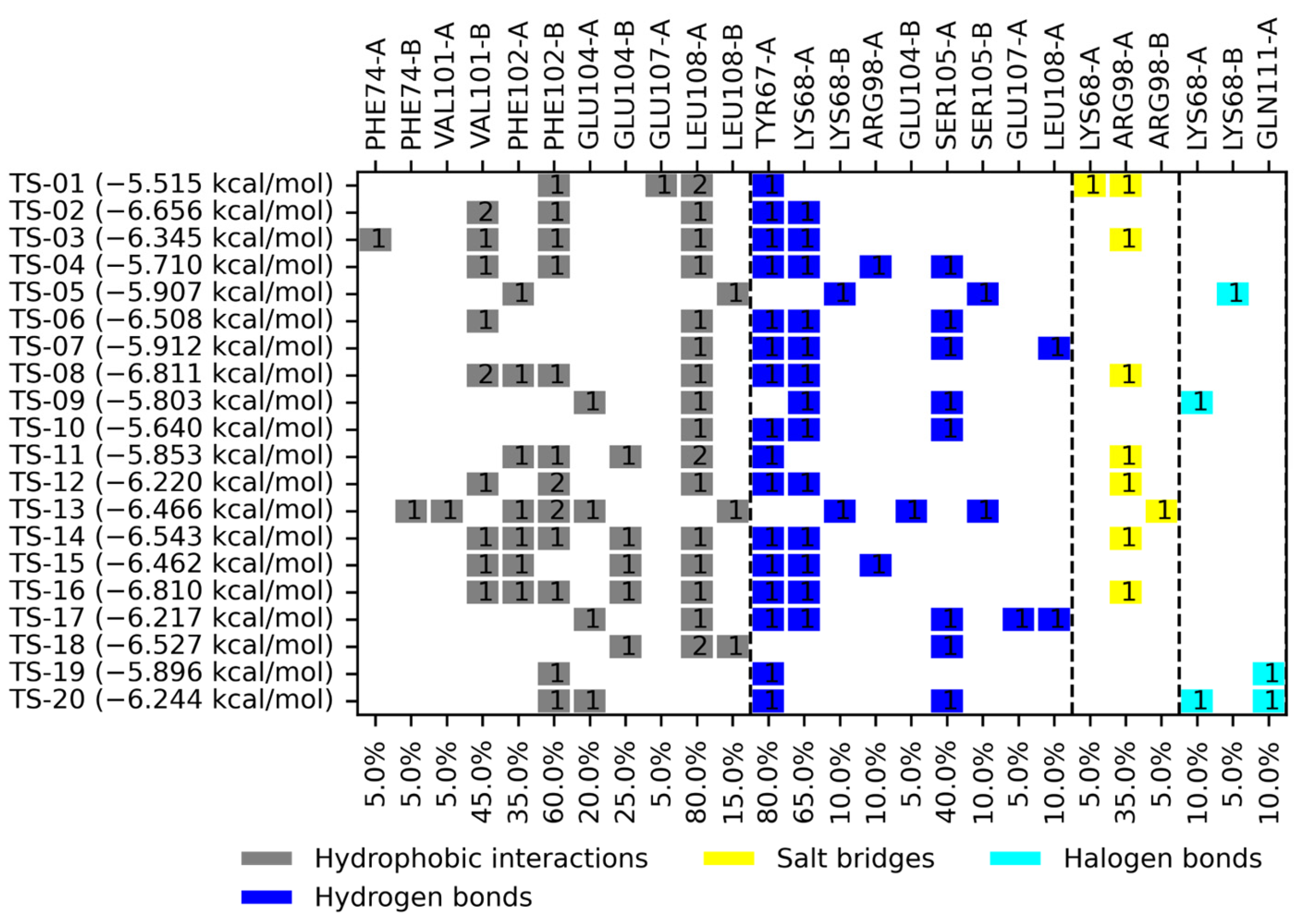
2.2. Molecular Docking
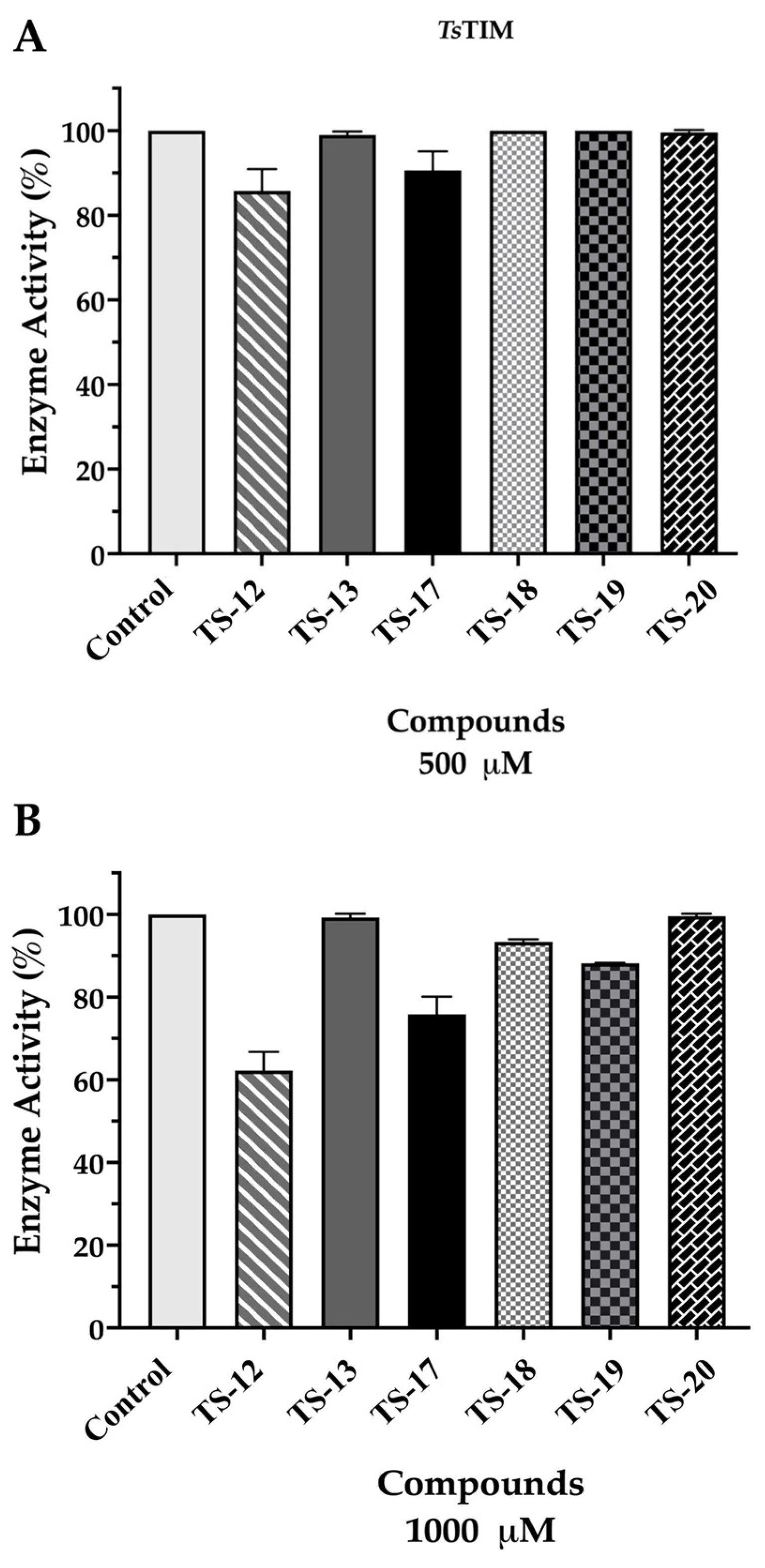
2.3. Enzymatic Activity Evaluation Against TsTIM
3. Discussion
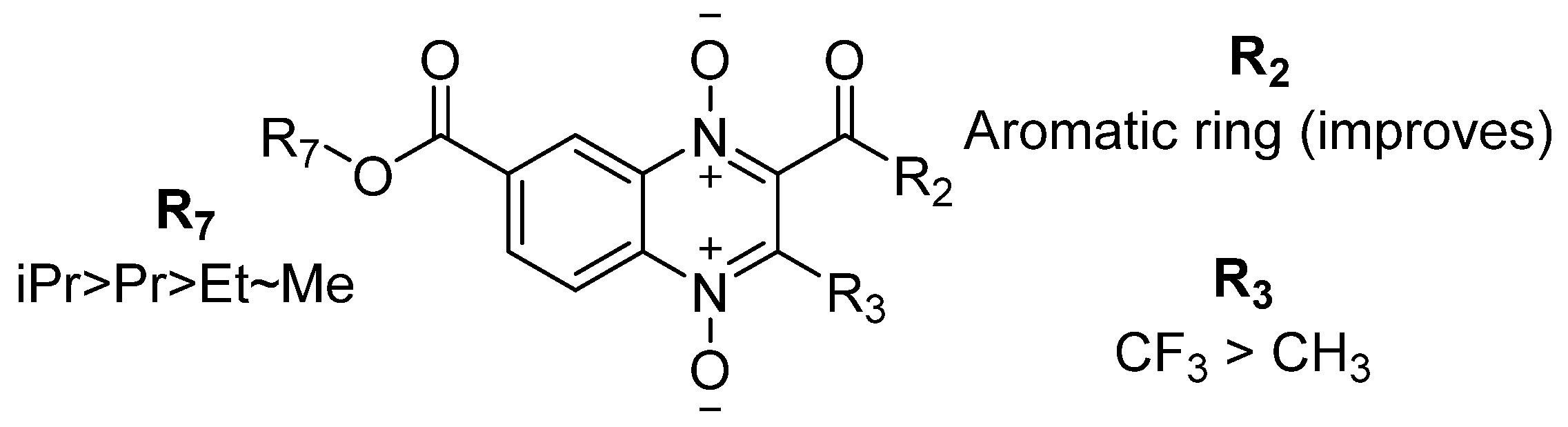
3.1. Structure–Activity Relationship Analysis
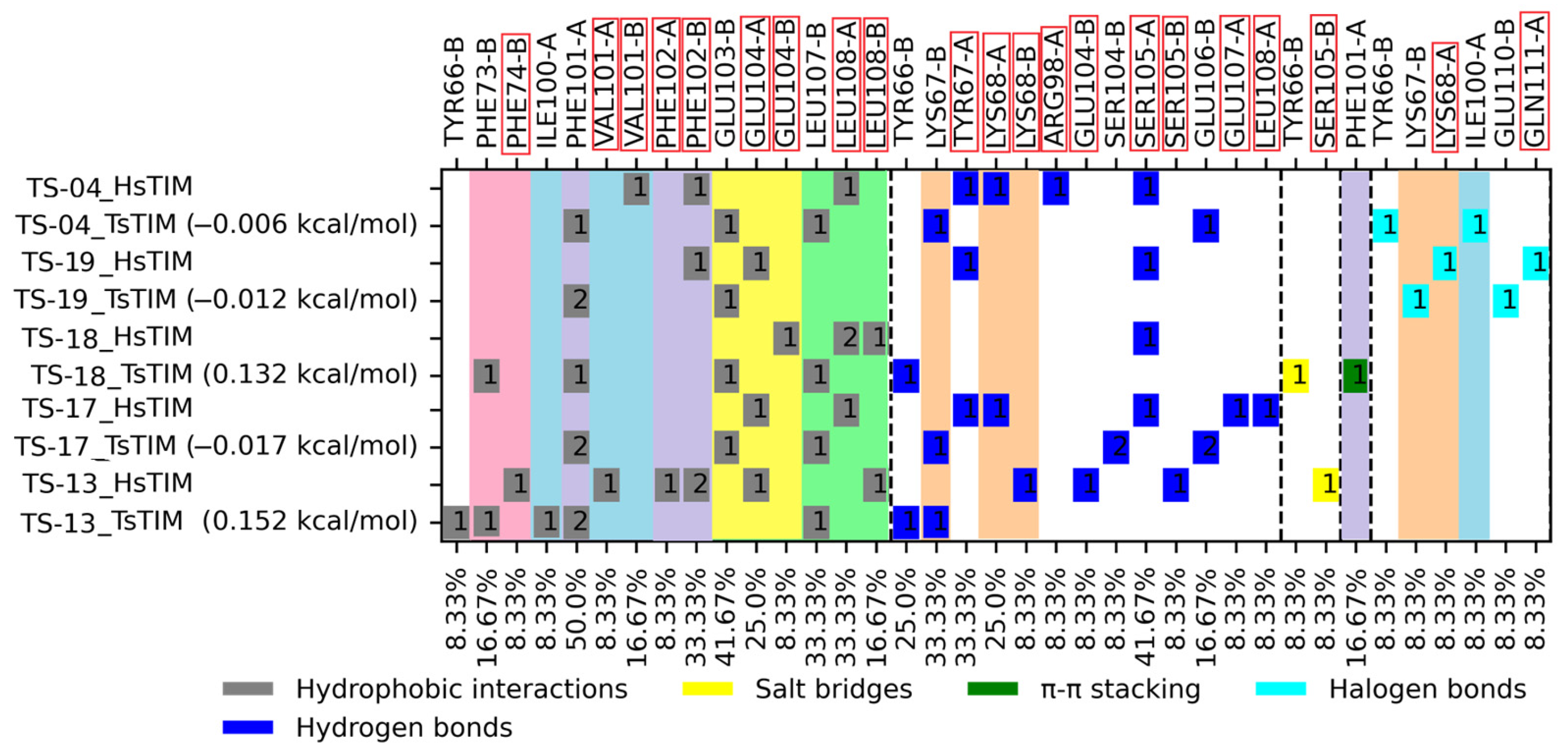
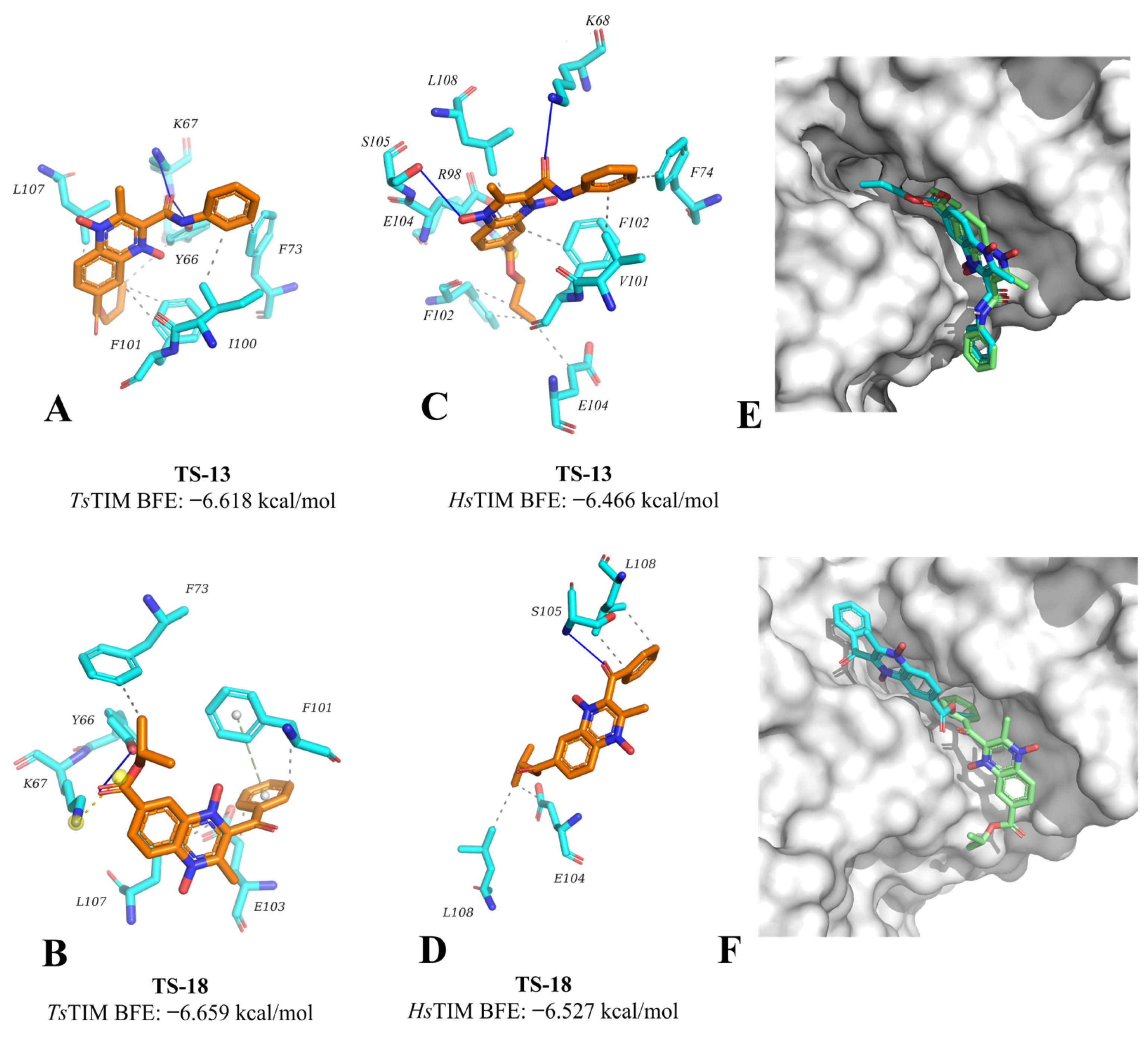
3.2. Molecular Docking on TsTIM
3.3. Enzymatic Activity Evaluation Against TsTIM
4. Materials and Methods
4.1. Synthesis
4.2. Parasite Culture
4.3. Drugs and Reagents
4.4. In Vitro Cysticidal Activity Against T. crassiceps
4.5. Molecular Docking
4.6. Expression and Purification of Recombinant TsTIM
4.7. Enzyme Inactivation Assays of Recombinant TsTIM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-GDH | α-Glycerophosphate Dehydrogenase |
| ABZSO | Albendazole sulfoxide |
| BFE | Binding free energy |
| CNS | Central nervous system |
| DHAP | Dihydroxyacetone-3-phosphate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DTT | Dithiothreitol |
| EC50 | Half-maximal effective concentration |
| EDTA | Ethylenediaminetetraacetic acid |
| GAP | Glyceraldehyde-3-phosphate |
| HsTIM | Homo sapiens triosephosphate isomerase |
| IMAC | Immobilized metal affinity chromatography |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| LB medium | Lysogeny broth medium |
| µM | Micromolar |
| NADH | Nicotinamide adenine dinucleotide |
| PLIP | Protein–ligand interaction profiler |
| PMSF | Phenylmethylsulfonyl fluoride |
| QNO | Quinoxaline-1,4-di-N-oxide |
| SAR | Structure–activity relationship |
| T. crassiceps | Taenia crassiceps |
| T. solium | Taenia solium |
| TE buffer | Tris EDTA |
| TIM | Triosephosphate isomerase |
| TsTIM | Taenia solium triosephosphate isomerase |
| WHO | World Health Organization |
References
- Garcia, H.H. Neurocysticercosis. Neurol. Clin. 2018, 36, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H.; Nash, T.E.; Del Brutto, O.H. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014, 13, 1202–1215. [Google Scholar] [CrossRef]
- Garg, R.K.; Rizvi, I.; Nigam, H.; Pandey, S.; Uniyal, R. Treatment outcome in patients with spinal neurocysticercosis: A systematic review of published cases and case series. Future Microbiol. 2025, 20, 45–56. [Google Scholar] [CrossRef]
- Nash, T.E.; Mahanty, S.; Loeb, J.A.; Theodore, W.H.; Friedman, A.; Sander, J.W.; Singh, G.; Cavalheiro, E.; Del Brutto, O.H.; Takayanagui, O.M.; et al. Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia 2015, 56, 177–183. [Google Scholar] [CrossRef]
- Gripper, L.B.; Welburn, S.C. Neurocysticercosis infection and disease–A review. Acta Trop. 2017, 166, 218–224. [Google Scholar] [CrossRef]
- Del Brutto, O.H. Management of calcified cysticerci in the brain parenchyma: Treating the dead parasite. Expert Rev. Anti-Infect. Ther. 2024, 22, 1073–1084. [Google Scholar] [CrossRef]
- Gonzales, I.; Rivera, J.T.; Garcia, H.H. Pathogenesis of Taenia solium taeniasis and cysticercosis. Parasite Immunol. 2016, 38, 136–146. [Google Scholar] [CrossRef]
- WHO. Taeniasis/Cysticercosis. Available online: https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis (accessed on 14 October 2024).
- Barrie, U.; Badejo, O.; Aoun, S.G.; Adeyemo, E.; Moler, N.; Christian, Z.K.; Caruso, J.P.; El Ahmadieh, T.Y.; Ban, V.S.; MacAllister, M.C.; et al. Systematic Review and Meta-Analysis of Management Strategies and Outcomes in Adult Spinal Neurocysticercosis. World Neurosurg. 2020, 138, 504–511.e508. [Google Scholar] [CrossRef]
- Prodjinotho, U.F.; Lema, J.; Lacorcia, M.; Schmidt, V.; Vejzagic, N.; Sikasunge, C.; Ngowi, B.; Winkler, A.S.; Prazeres Da Costa, C. Host immune responses during Taenia solium Neurocysticercosis infection and treatment. PLoS Neglected Trop. Dis. 2020, 14, e0008005. [Google Scholar] [CrossRef]
- Mehta, Y.; Kaur, D.; Kaur, U.; Shree, R.; Singh, P.; Modi, M.; Lal, V.; Sehgal, R. Efficiency of Cysticidal Therapy Impacted by the Host’s Immune Response in Neurocysticercosis Patients. Mol. Neurobiol. 2025, 62, 2203–2211. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, S.; Tang, J.; Xing, Y.; Qu, G.; Dai, J.; Jin, X.; Wang, X. Evaluation of protective efficacy induced by different heterologous prime-boost strategies encoding triosephosphate isomerase against Schistosoma japonicum in mice. Parasites Vectors 2017, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Fonseca, L.M.; Trujillo-Ferrara, J.G. Exploring the possible binding sites at the interface of triosephosphate isomerase dimer as a potential target for anti-tripanosomal drug design. Bioorganic Med. Chem. Lett. 2004, 14, 3151–3154. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ramos, G.; Cabrera, N.; Saavedra-Lira, E.; Tuena de Gomez-Puyou, M.; Ostoa-Saloma, P.; Perez-Montfort, R.; Gomez-Puyou, A. Sulfhydryl reagent susceptibility in proteins with high sequence similarity. Triosephosphate isomerase from Trypanosoma brucei, Trypanosoma cruzi and Leishmania mexicana. Eur. J. Biochem. 1998, 253, 684–691. [Google Scholar] [CrossRef]
- Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Nava-Zuazo, C.; Castillo-Villanueva, A.; Méndez, S.T.; Torres-Arroyo, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Ponce-Macotela, M.; Rufino-González, Y.; et al. Novel giardicidal compounds bearing proton pump inhibitor scaffold proceeding through triosephosphate isomerase inactivation. Sci. Rep. 2017, 7, 7810. [Google Scholar] [CrossRef]
- Pérez-Montfort, R.; Garza-Ramos, G.; Alcántara, G.H.; Reyes-Vivas, H.; Gao, X.-g.; Maldonado, E.; Tuena de Gómez-Puyou, M.; Gómez-Puyou, A. Derivatization of the Interface Cysteine of Triosephosphate Isomerase from Trypanosoma brucei and Trypanosoma cruzi as Probe of the Interrelationship between the Catalytic Sites and the Dimer Interface. Biochemistry 1999, 38, 4114–4120. [Google Scholar] [CrossRef]
- Jacqueline, S.-S.; Salvador, P.-M.; Juan David, O.-V.; Lizeth Mariel, Z.-O. Esters of Quinoxaline-7-Carboxylate 1,4-di-N-Oxide as Potential Inhibitors of Glycolytic Enzymes of Entamoeba histolytica: In silico Approach. Curr. Comput.-Aided Drug Des. 2024, 20, 155–169. [Google Scholar] [CrossRef]
- Benítez-Cardoza, C.G.; Brieba, L.G.; Arroyo, R.; Rojo-Domínguez, A.; Vique-Sánchez, J.L. Triosephosphate isomerase as a therapeutic target against trichomoniasis. Mol. Biochem. Parasitol. 2021, 246, 111413. [Google Scholar] [CrossRef]
- Ferraro, F.; Corvo, I.; Bergalli, L.; Ilarraz, A.; Cabrera, M.; Gil, J.; Susuki, B.M.; Caffrey, C.R.; Timson, D.J.; Robert, X.; et al. Novel and selective inactivators of Triosephosphate isomerase with anti-trematode activity. Sci. Rep. 2020, 10, 2587. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Pérez-Silanes, S.; Galiano, S.; Gonzalez, G.; Monge, A.; Deharo, E.; Aldana, I. New Amide Derivatives of Quinoxaline 1,4-di-N-Oxide with Leishmanicidal and Antiplasmodial Activities. Molecules 2013, 18, 4718–4727. [Google Scholar] [CrossRef]
- Benitez, D.; Cabrera, M.; Hernández, P.; Boiani, L.a.; Lavaggi, M.a.L.; Di Maio, R.; Yaluff, G.; Serna, E.; Torres, S.; Ferreira, M.a.E.; et al. 3-Trifluoromethylquinoxaline N, N′-Dioxides as Anti-Trypanosomatid Agents. Identification of Optimal Anti- T. cruzi Agents and Mechanism of Action Studies. J. Med. Chem. 2011, 54, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Ancizu, S.; Villar, R.; Solano, B.; Moreno, E.; Torres, E.; Pérez, S.; Aldana, I.; et al. Synthesis and Biological Evaluation of New Quinoxaline Derivatives as Antioxidant and Anti-Inflammatory Agents. Chem. Biol. Drug Des. 2011, 77, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P.L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Sa, W.; Cao, C.; Guo, L.; Hao, H.; Liu, Z.; Wang, X.; Yuan, Z. Quinoxaline 1,4-di-N-Oxides: Biological Activities and Mechanisms of Actions. Front. Pharmacol. 2016, 7, 64. [Google Scholar] [CrossRef]
- Duque-Montaño, B.E.; Gómez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernández-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide against Entamoeba histolytica. Bioorganic Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef]
- Estevez, Y.; Quiliano, M.; Burguete, A.; Cabanillas, B.; Zimic, M.; Málaga, E.; Verástegui, M.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; et al. Trypanocidal properties, structure–activity relationship and computational studies of quinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2011, 127, 745–751. [Google Scholar] [CrossRef]
- Torres, E.; Moreno-Viguri, E.; Galiano, S.; Devarapally, G.; Crawford, P.W.; Azqueta, A.; Arbillaga, L.; Varela, J.; Birriel, E.; Di Maio, R.; et al. Novel quinoxaline 1,4-di-N-oxide derivatives as new potential antichagasic agents. Eur. J. Med. Chem. 2013, 66, 324–334. [Google Scholar] [CrossRef]
- Vieira, M.; Pinheiro, C.; Fernandes, R.; Noronha, J.P.; Prudêncio, C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2- and 3-substituted derivatives. Microbiol. Res. 2014, 169, 287–293. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, G.; Hao, H.; Wang, Y.; Wang, X.; Chen, D.; Peng, D.; Liu, Z.; Yuan, Z.; Dai, M. Mechanisms of Antibacterial Action of Quinoxaline 1,4-di-N-oxides against Clostridium perfringens and Brachyspira hyodysenteriae. Front. Microbiol. 2016, 7, 1948. [Google Scholar] [CrossRef]
- Silva, L.; Coelho, P.; Soares, R.; Prudêncio, C.; Vieira, M. Quinoxaline-1,4-dioxide derivatives inhibitory action in melanoma and brain tumor cells. Future Med. Chem. 2019, 11, 645–657. [Google Scholar] [CrossRef]
- Ross, F.; Hernández, P.; Porcal, W.; López, G.V.; Cerecetto, H.; González, M.; Basika, T.; Carmona, C.; Fló, M.; Maggioli, G.; et al. Identification of Thioredoxin Glutathione Reductase Inhibitors That Kill Cestode and Trematode Parasites. PLoS ONE 2012, 7, e35033. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; Sánchez-Sánchez, O.; Yépez-Mulia, L.; Delgado-Maldonado, T.; Vázquez-Jiménez, L.K.; López-Velázquez, G.; de la Mora-de la Mora, J.I.; Pacheco-Gutierrez, S.; Chino-Ríos, L.; Arias, D.; et al. Expanding the antiprotozoal activity and the mechanism of action of n-butyl and iso-butyl ester of quinoxaline-1,4-di-N-oxide derivatives against Giardia lamblia, Trichomonas vaginalis, and Entamoeba histolytica. An in vitro and in silico approach. J. Enzym. Inhib. Med. Chem. 2024, 39, 2413018. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules 2017, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Palos, I.; Luna-Herrera, J.; Lara-Ramírez, E.; Loera-Piedra, A.; Fernández-Ramírez, E.; Aguilera-Arreola, M.; Paz-González, A.; Monge, A.; Wan, B.; Franzblau, S.; et al. Anti-Mycobacterium tuberculosis Activity of Esters of Quinoxaline 1,4-Di-N-Oxide. Molecules 2018, 23, 1453. [Google Scholar] [CrossRef]
- Villalobos-Rocha, J.C.; Sánchez-Torres, L.; Nogueda-Torres, B.; Segura-Cabrera, A.; García-Pérez, C.A.; Bocanegra-García, V.; Palos, I.; Monge, A.; Rivera, G. Anti-Trypanosoma cruzi and anti-leishmanial activity by quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives. Parasitol. Res. 2014, 113, 2027–2035. [Google Scholar] [CrossRef]
- Gómez-Caro, L.C.; Sánchez-Sánchez, M.; Bocanegra-García, V.; Rivera, G.; Monge, A. Synthesis of quinoxaline 1,4-di-n-oxide derivatives on solid support using room temperature and microwave-assisted solvent-free procedures. Quim. Nova 2011, 34, 1147–1151. [Google Scholar] [CrossRef]
- Willms, K.; Zurabian, R. Taenia crassiceps: In vivo and in vitro models. Parasitology 2010, 3, 12. [Google Scholar] [CrossRef]
- Ancarola, M.E.; García, L.C.A.; Mourglia-Ettlin, G.; Cucher, M.A. Chapter Two—Using the model cestode Taenia crassiceps for the study of cysticercosis. In Methods in Cell Biology; Bravo-San Pedro, J.M., Aranda, F., Buqué, A., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 185, pp. 19–33. [Google Scholar]
- Palomares-Alonso, F.; Palencia Hernández, G.; Rojas-Tomé, I.S.; Jung-Cook, H.; Pinzón-Estrada, E. Murine cysticercosis model: Influence of the infection time and the time of treatment on the cysticidal efficacy of albendazole and praziquantel. Exp. Parasitol. 2015, 149, 1–6. [Google Scholar] [CrossRef]
- López-Méndez, L.J.; Palomares-Alonso, F.; González-Hernández, I.; Jung-Cook, H.; Cabrera-Quiñones, N.C.; Guadarrama, P. β-cyclodextrin dendritic derivatives as permeation mediators to enhance the in vitro albendazole cysticidal activity by the improvement of the diffusion component. RSC Adv. 2022, 12, 23153–23161. [Google Scholar] [CrossRef]
- Louis, K.S.; Siegel, A.C. Cell Viability Analysis Using Trypan Blue: Manual and Automated Methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar]
- Cherinka, B.; Andrews, B.H.; Sánchez-Gallego, J.; Brownstein, J.; Argudo-Fernández, M.; Blanton, M.; Bundy, K.; Jones, A.; Masters, K.; Law, D.R.; et al. Marvin: A Tool Kit for Streamlined Access and Visualization of the SDSS-IV MaNGA Data Set. Astron. J. 2019, 158, 74. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Jimenez-Sandoval, P.; Castro-Torres, E.; González-González, R.; Díaz-Quezada, C.; Gurrola, M.; Camacho-Manriquez, L.D.; Leyva-Navarro, L.; Brieba, L.G. Crystal structures of Triosephosphate Isomerases from Taenia solium and Schistosoma mansoni provide insights for vaccine rationale and drug design against helminth parasites. PLOS Neglected Trop. Dis. 2020, 14, e0007815. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Volkamer, A.; Griewel, A.; Grombacher, T.; Rarey, M. Analyzing the Topology of Active Sites: On the Prediction of Pockets and Subpockets. J. Chem. Inf. Model. 2010, 50, 2041–2052. [Google Scholar] [CrossRef]
- Volkamer, A.; Kuhn, D.; Grombacher, T.; Rippmann, F.; Rarey, M. Combining Global and Local Measures for Structure-Based Druggability Predictions. J. Chem. Inf. Model. 2012, 52, 360–372. [Google Scholar] [CrossRef]
- Graef, J.; Ehrt, C.; Rarey, M. Binding Site Detection Remastered: Enabling Fast, Robust, and Reliable Binding Site Detection and Descriptor Calculation with DoGSite3. J. Chem. Inf. Model. 2023, 63, 3128–3137. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Olivares-Illana, V.; Pérez-Montfort, R.; López-Calahorra, F.; Costas, M.; Rodríguez-Romero, A.; Tuena de Gómez-Puyou, M.; Gómez Puyou, A. Structural Differences in Triosephosphate Isomerase from Different Species and Discovery of a Multitrypanosomatid Inhibitor. Biochemistry 2006, 45, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
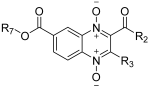 | |||||
|---|---|---|---|---|---|
| Code | R7 | R2 | R3 | EC50 (µM) | SD |
| TS-01 | CH3 | CH3CH2O | CH3 | >10 | |
| TS-02 | CH3 | C6H5 | CH3 | >10 | |
| TS-03 | CH3 | NHC6H5 | CH3 | >10 | |
| TS-04 | CH3 | CH3CH2 | CF3 | >10 | |
| TS-05 | CH3 | CH(CH3)2 | CF3 | >10 | |
| TS-06 | CH3 | C4H3S | CF3 | 5.34 | 1.06–2.11 |
| TS-07 | CH3CH2 | CH3 | CH3 | >10 | |
| TS-08 | CH3CH2 | C6H5 | CH3 | >10 | |
| TS-09 | CH3CH2 | CH3 | CF3 | >10 | |
| TS-10 | CH3CH2 | CH3CH2 | CF3 | >10 | |
| TS-11 | CH3CH2 | CH(CH3)2 | CF3 | >10 | |
| TS-12 | CH3CH2 | C4H3S | CF3 | 0.58 | 0.24–1.46 |
| TS-13 | CH3CH2CH2 | NHC6H5 | CH3 | >10 | |
| TS-14 | CH3CH2CH2 | C4H3O | CF3 | >10 | |
| TS-15 | CH3CH2CH2 | C4H3S | CF3 | 3.87 | 0.76–1.47 |
| TS-16 | CH3CH2CH2 | C6H5 | CF3 | >10 | |
| TS-17 | (CH3)2CH | NH2 | CH3 | 2.69 | 1.54–5.24 |
| TS-18 | (CH3)2CH | C6H5 | CH3 | 1.75 | 1.04–3.25 |
| TS-19 | (CH3)2CH | CF2CF3 | CF3 | 1.02 | 0.50–2.62 |
| TS-20 | (CH3)2CH | C4H3S | CF3 | 0.80 | 0.42–1.85 |
| ABZSO | 0.68 | 0.39–1.85 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomares-Alonso, F.; González-González, A.; Paz-González, A.D.; Ortiz-Pérez, E.; Martínez-Vázquez, A.V.; García-Torres, I.; López-Velázquez, G.; Jung-Cook, H.; Rivera, G. In Vitro Evaluation of Esters of Quinoxaline-1,4-di-N-oxide Derivatives as New Antitaeniasis Agents and Their Inhibitory Activity Against Triosephosphate Isomerase. Pharmaceuticals 2025, 18, 406. https://doi.org/10.3390/ph18030406
Palomares-Alonso F, González-González A, Paz-González AD, Ortiz-Pérez E, Martínez-Vázquez AV, García-Torres I, López-Velázquez G, Jung-Cook H, Rivera G. In Vitro Evaluation of Esters of Quinoxaline-1,4-di-N-oxide Derivatives as New Antitaeniasis Agents and Their Inhibitory Activity Against Triosephosphate Isomerase. Pharmaceuticals. 2025; 18(3):406. https://doi.org/10.3390/ph18030406
Chicago/Turabian StylePalomares-Alonso, Francisca, Alonzo González-González, Alma D. Paz-González, Eyra Ortiz-Pérez, Ana Verónica Martínez-Vázquez, Itzhel García-Torres, Gabriel López-Velázquez, Helgi Jung-Cook, and Gildardo Rivera. 2025. "In Vitro Evaluation of Esters of Quinoxaline-1,4-di-N-oxide Derivatives as New Antitaeniasis Agents and Their Inhibitory Activity Against Triosephosphate Isomerase" Pharmaceuticals 18, no. 3: 406. https://doi.org/10.3390/ph18030406
APA StylePalomares-Alonso, F., González-González, A., Paz-González, A. D., Ortiz-Pérez, E., Martínez-Vázquez, A. V., García-Torres, I., López-Velázquez, G., Jung-Cook, H., & Rivera, G. (2025). In Vitro Evaluation of Esters of Quinoxaline-1,4-di-N-oxide Derivatives as New Antitaeniasis Agents and Their Inhibitory Activity Against Triosephosphate Isomerase. Pharmaceuticals, 18(3), 406. https://doi.org/10.3390/ph18030406









