The Impact of Fentanyl and Morphine on Maternal Hemodynamics in Spinal Anesthesia for Cesarean Section
Abstract
1. Introduction
2. Results
2.1. Blood Pressure and Ventricular Rate
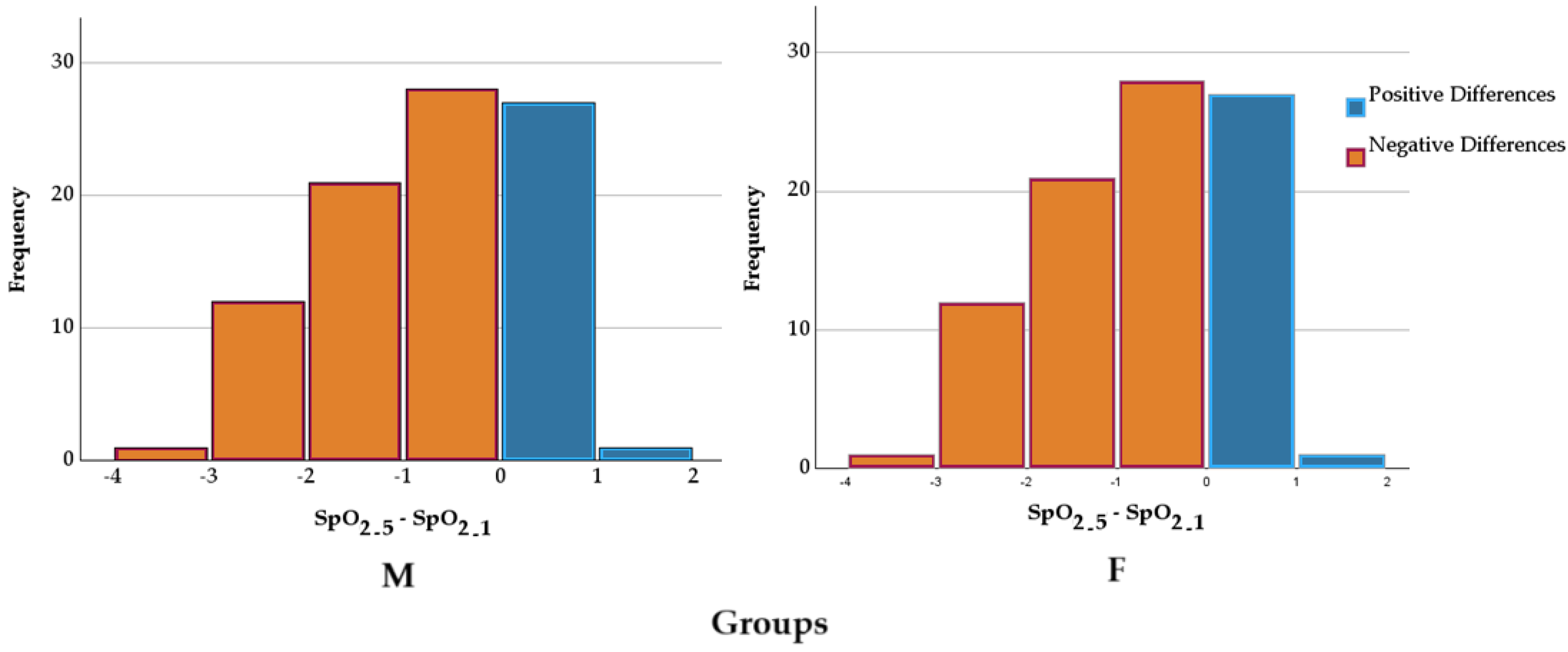
2.2. Peripheral Capillary Oxygen Saturation
2.3. Administration of Ephedrine and Atropine Based on the Type of Anesthetic Used
3. Discussion
3.1. Blood Pressure and Ventricular Rate
3.2. Peripheral Capillary Oxygen Saturation
3.3. Administration of Ephedrine and Atropine Based on the Type of Anesthetic Used
3.4. Study Limitations and Clinical Implications
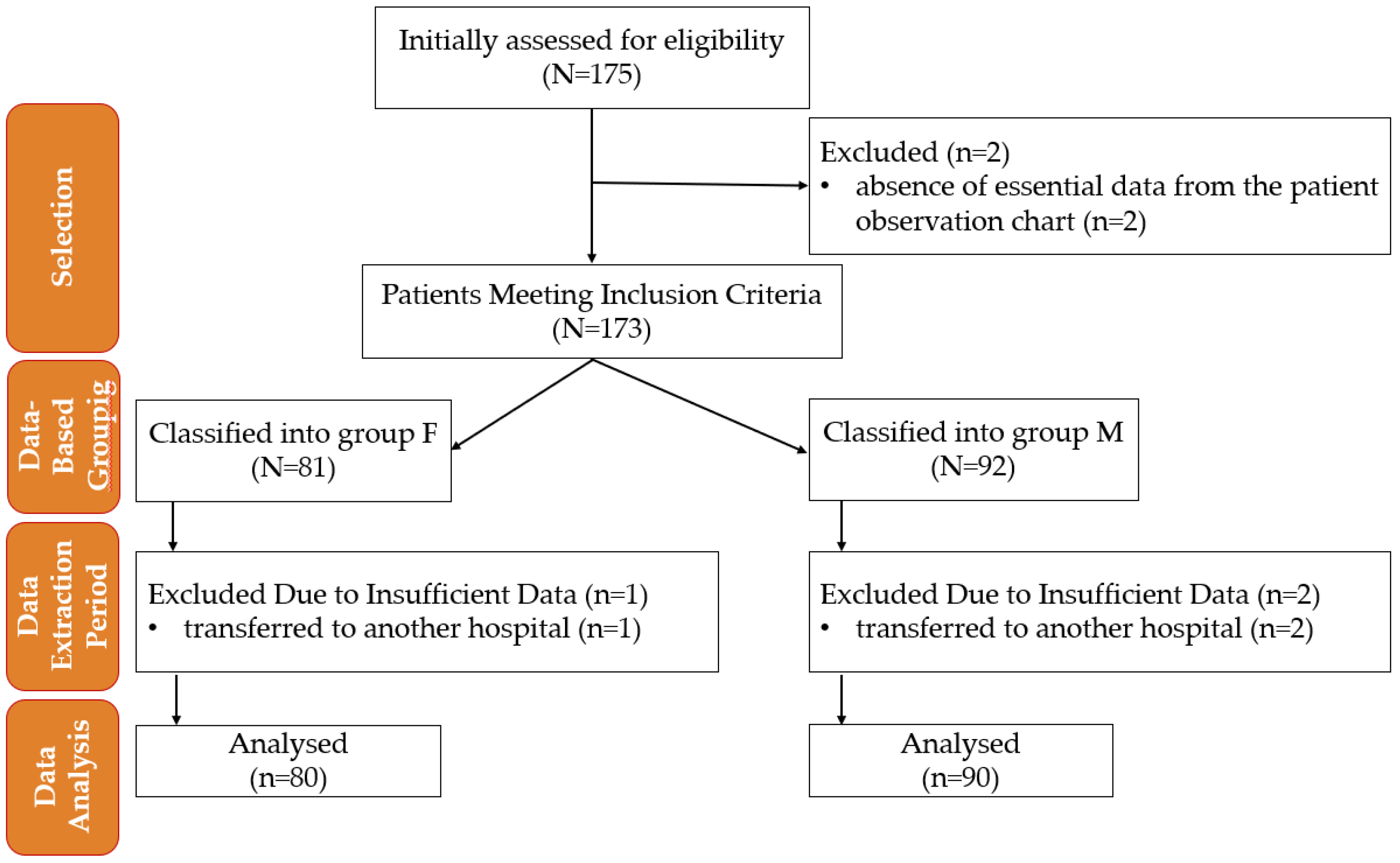
4. Materials and Methods
4.1. Study Design
- Vasopressor requirement (total dose of ephedrine administered for BP correction).
- Anticholinergic requirement for bradycardia correction (total dose of atropine administered).
- Incidence of adverse effects (nausea and vomiting).
- Ventricular rate (VR) variability after anesthesia.
- Peripheral capillary oxygen saturation (SpO2) changes after anesthesia.
4.2. Inclusion Criteria
- American Society of Anesthesiologists (ASA) classification II;
- Age between 18 and 50 years;
- No significant medical history, including cardiovascular, respiratory, neurological, endocrine, or hematological disorders;
- No known drug or food allergies;
- No chronic medication use, including antihypertensive drugs or treatment for any condition, particularly preeclampsia;
- No history of chronic pain or regular use of analgesics (opioids or non-opioids)
- No contraindications to spinal anesthesia, such as coagulopathy, local infection, or anatomical abnormalities;
- Body weight over 50 kg;
- Singleton, viable fetus confirmed via ultrasound;
- Gestational age of at least 37 weeks at the time of elective cesarean section;
- Indication for elective cesarean section, excluding emergency procedures or cases with maternal or fetal distress.
4.3. Exclusion Criteria
- Acute or chronic fetal distress, diagnosed pre- or postnatally;
- Fetal malformations, detected intrauterine or postpartum;
- Development of preeclampsia during pregnancy or peripartum;
- Need for surgical reintervention within 72 h postpartum, regardless of the cause;
- Requirement for opioid administration or other intravenous anesthetics during the procedure;
- Spinal anesthesia failure, necessitating conversion to general anesthesia;
- Absence of essential data in the patient observation chart;
- Blood loss of more than 500 mL;
- Sensory block maximum at T4 level;
- Transferred to another hospital.
4.4. Anesthetic Protocol and Monitoring
4.5. Data Collection
4.6. Statistical Analysis
- p—the probability of the occurrence of the studied phenomenon (0 ≤ p ≤ 1);
- q—the complementary probability, q = 1 − p;
- t—the probability factor;
- Δx—the allowable margin of error;
- N—the total population (the total number of cesarean deliveries during the analyzed period).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| BPD | Diastolic Blood Pressure |
| BPS | Systolic Blood Pressure |
| MAP | Mean Arterial Pressure |
| SpO2 | Peripheral Capillary Oxygen Saturation |
| VR | Ventricular Rate |
References
- Yu, C.; Gu, J.; Liao, Z.; Feng, S. Prediction of spinal anesthesia-induced hypotension during elective cesarean section: A systematic review of prospective observational studies. Int. J. Obstet. Anesth. 2021, 47, 103175. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Rashid, A.M. Knowledge, fear and acceptance rate of spinal anesthesia among pregnant women scheduled for cesarean section: A cross-sectional study from a tertiary care hospital in Karachi. BMC Anesthesiol. 2024, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Chooi, C.; Cox, J.J.; Lumb, R.S.; Middleton, P.; Chemali, M.; Emmett, R.S.; Simmons, S.W.; Cyna, A.M. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst. Rev. 2017, 8, Cd002251. [Google Scholar] [CrossRef] [PubMed]
- Klöhr, S.; Roth, R.; Hofmann, T.; Rossaint, R.; Heesen, M. Definitions of hypotension after spinal anaesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol. Scand. 2010, 54, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Butwick, A.J.; Columb, M.O.; Carvalho, B. Preventing spinal hypotension during caesarean delivery: What is the latest? Br. J. Anaesth. 2015, 114, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Khan, S.; Khan, R. Frequency of post spinal hypotension in elective cesarean section after Spinal Anesthesia. J. Popul. Ther. Clin. Pharmacol. 2024, 6, 778. [Google Scholar] [CrossRef]
- Ebrie, A.M.; Woldeyohanis, M.; Abafita, B.J.; Ali, S.A.; Zemedkun, A.; Yimer, Y.; Ashebir, Z.; Mohammed, S. Hemodynamic and analgesic effect of intrathecal fentanyl with bupivacaine in patients undergoing elective cesarean section; a prospective Cohort Study. PLoS ONE 2022, 17, e0268318. [Google Scholar] [CrossRef] [PubMed]
- Hamber, E.A.; Viscomi, C.M. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg. Anesth. Pain Med. 1999, 24, 255–263. [Google Scholar] [CrossRef]
- Cummings, A.; Orgill, B.D.; Fitzgerald, B.M. Intrathecal Morphine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499880/ (accessed on 2 February 2025).
- Grape, S.; El-Boghdadly, K.; Albrecht, E. Management of adverse effects of intrathecal opioids in acute pain. Best Pract. Res. Clin. Anaesthesiol. 2023, 37, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Šklebar, I.; Bujas, T.; Habek, D. Spinal anaesthesia-induced hypotension in obstetrics: Prevention and therapy. Acta Clin. Croat. 2019, 58, 90–95. [Google Scholar] [CrossRef]
- Ranjan, A.; Prasad, A. Effectiveness of intravenous lidocaine and fentanyl on attenuation of hemodynamic response to extubation for ear, nose and throat surgeries at JLNMCH, Bhagalpur, Bihar. Int. J. Health Sci. 2022, 6 (Suppl. S1), 13157–13164. [Google Scholar] [CrossRef]
- Rosow, C.E.; Moss, J.; Philbin, D.M.; Savarese, J.J. Histamine release during morphine and fentanyl anesthesia. Anesthesiology 1982, 56, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Goss, S.; Jedlicka, J.; Strinitz, E.; Niedermayer, S.; Chappell, D.; Hofmann-Kiefer, K.; Hinske, L.C.; Groene, P. Association between intraoperative hypotension and postoperative nausea and vomiting: A retrospective cohort study. Curr. Med. Res. Opin. 2024, 40, 1439–1448. [Google Scholar] [CrossRef]
- DeSousa, K.A. Intrathecal morphine for postoperative analgesia: Current trends. World J. Anesthesiol. 2014, 3, 191. [Google Scholar] [CrossRef]
- Weigl, W.; Bieryło, A.; Wielgus, M.; Krzemień-Wiczyńska, Ś.; Kołacz, M.; Dąbrowski, M.J. Perioperative analgesia after intrathecal fentanyl and morphine or morphine alone for cesarean section: A randomized controlled study. Medicine 2017, 96, e8892. [Google Scholar] [CrossRef] [PubMed]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11 (Suppl. S2), S133–S153. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M. Hypotension and bradycardia during spinal anesthesia: Significance, prevention, and treatment. Tech. Reg. Anesth. Pain Manag. 2000, 4, 148–154. [Google Scholar] [CrossRef]
- Sibanyoni, M.; Biyase, N.; Motshabi Chakane, P. The use of intrathecal morphine for acute postoperative pain in lower limb arthroplasty surgery: A survey of practice at an academic hospital. J. Orthop. Surg. Res. 2022, 17, 323. [Google Scholar] [CrossRef] [PubMed]
- Palkovic, B.; Marchenko, V.; Zuperku, E.J.; Stuth, E.A.; Stucke, A.G. Multi-level regulation of opioid-induced respiratory depression. Physiology 2020, 35, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Maria, S.; Micalos, P.; Ahern, L. Effect of fentanyl compared to morphine on pain score and cardiorespiratory vital signs in out-of-hospital adult STEMI patients. Int. J. Paramed. 2024, 10–22. [Google Scholar] [CrossRef]
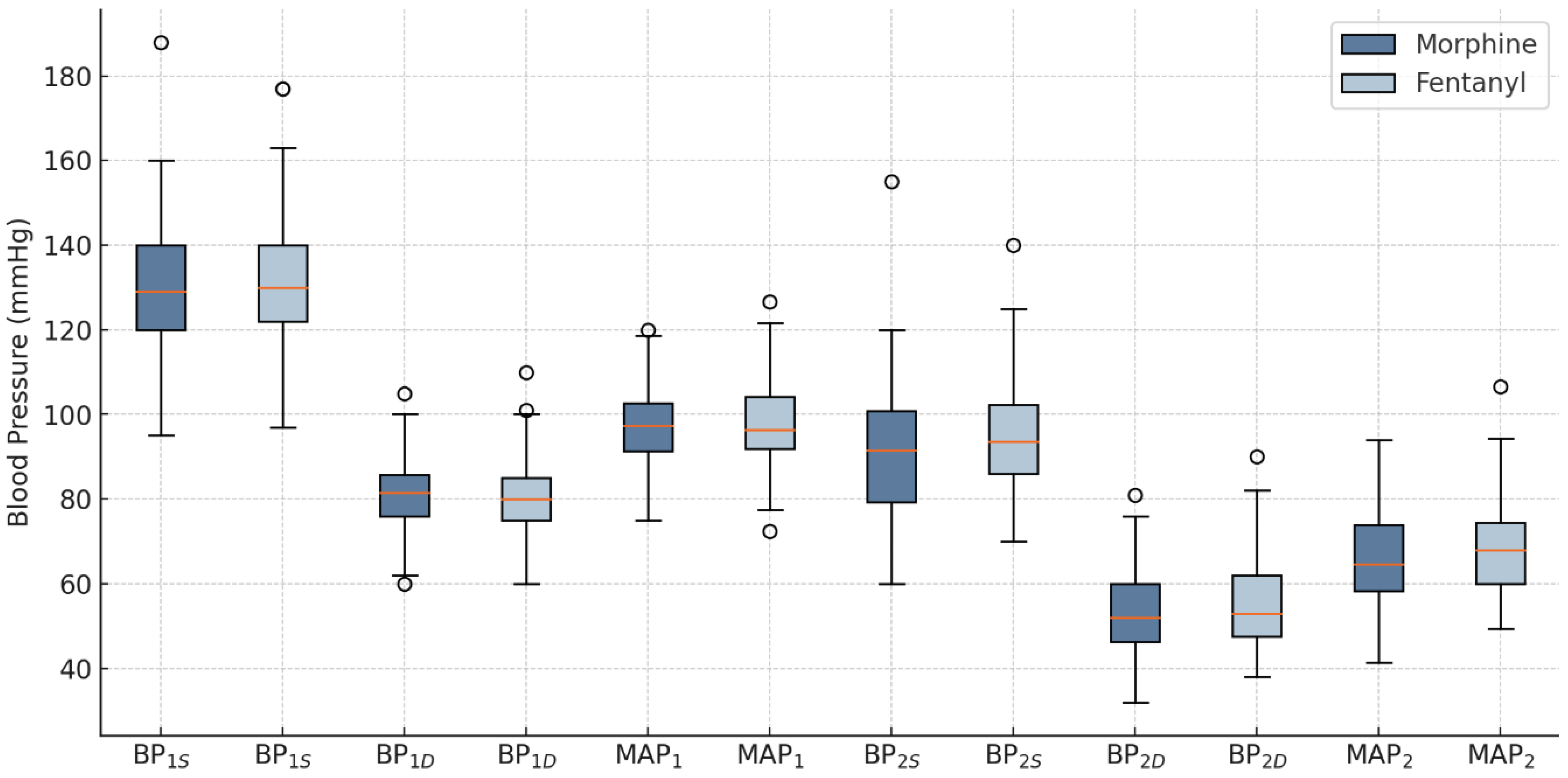
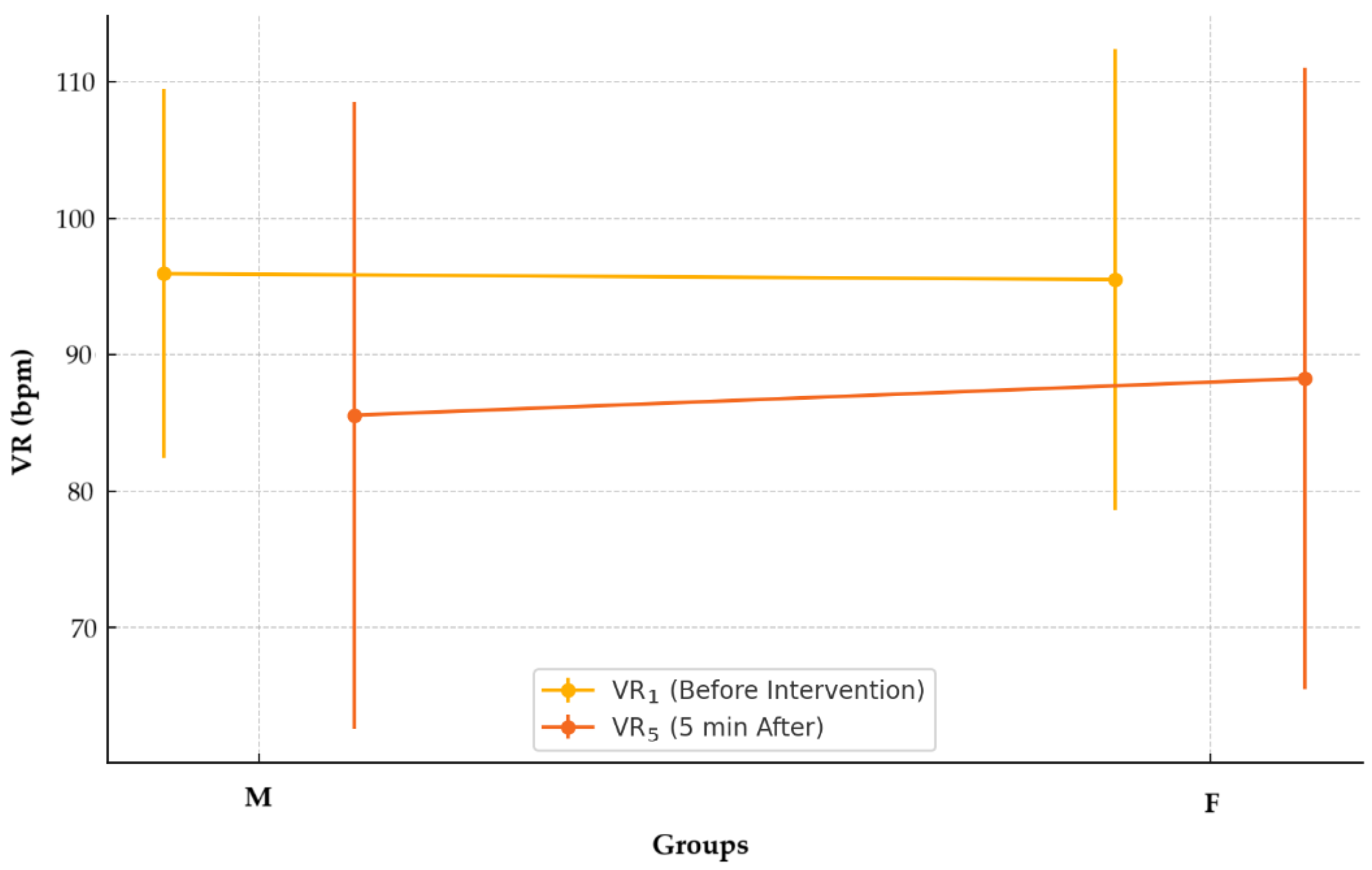
 —mean; °—outliers.
—mean; °—outliers.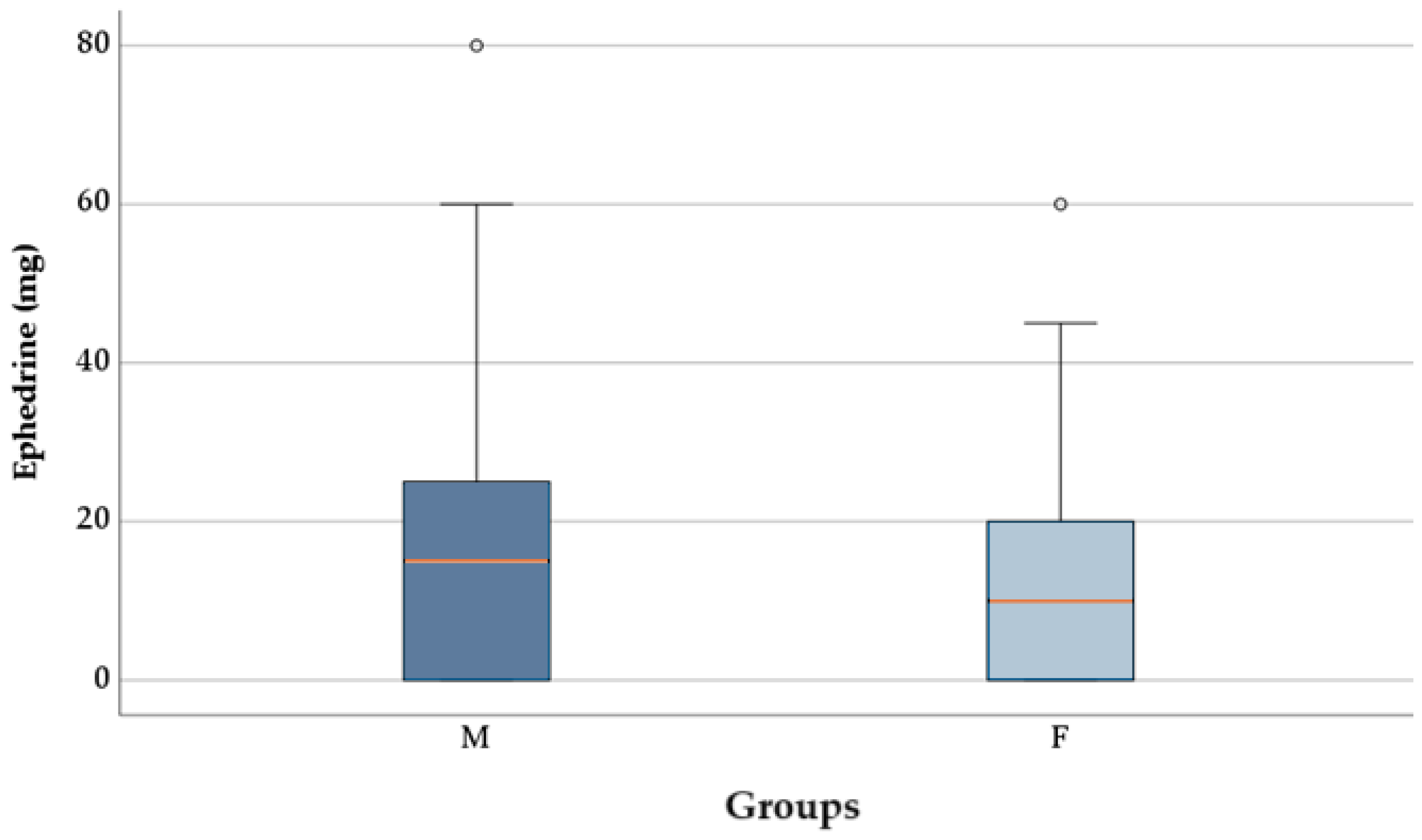
| Parameter | Group F (n = 80) | Group M (n = 90) | p-Value | SMD |
|---|---|---|---|---|
| DD | ||||
| Age, years, M ± SD (min, max) | 31.61 ± 4.37 (23.50) | 32.17 ± 4.38 (22.44) | 0.434 * | 0.1 |
| Urban residence, n (%) | 55 (68.8) | 62 (68.9) | 0.984 ** | 0.0 |
| Smoker, n (%) | 13 (14.44) | 15 18.75) | 0.450 ** | 0.0 |
| Clinical and laboratory data | ||||
| Weight, kg, M ± SD (min, max) | 77 ± 12.83 (50, 110) | 77.84 ± 15.05 (53, 131) | 0.805 * | 0.0 |
| Height, cm, M ± SD (min, max) | 164.35 ± 5.86 (150, 180) | 165.21 ± 6.31 (150, 178) | 0.945 * | 0.0 |
| BMI, M ± SD (min, max) | 28.25 ± 3.55 (19.20, 39.06) | 28.82 ± 5.29 (19.47, 44.37) | 0.384 * | 0.0 |
| Number of pregnancies, n (%) | ||||
| 0 | 42 (52.5) | 50 (55.6) | 0.471 *** | 0.0 |
| 1 | 28 (35.0) | 32 (35.6) | 0.0 | |
| 2 | 7 (8.8) | 3 (3.3) | 0.1 | |
| 3 | 3 (3.8) | 5 (5.6) | 0.0 | |
| Number of births, n (%) | ||||
| 0 | 51 (63.8) | 55 (61.1) | 0.402 *** | 0.0 |
| 1 | 25 (31.2) | 30 (33.3) | 0.0 | |
| 2 | 4 (5.0) | 3 (3.3) | 0.0 | |
| 3 | 0 (0.0) | 2 (2.2) | 0.0 | |
| PCS, n (%) | 26 (32.5) | 27 (30.0) | 0.725 ** | 0.0 |
| Contractions present, n (%) | 21 (26.2) | 16 (17.8) | 0.181 ** | 0.1 |
| Previous analgesia, n (%) | 4 (5.0) | 1 (1.1) | 0.189 **** | 0.1 |
| BP1S, mmHg, M ± SD | 132.09 ± 14.85 | 130.42 ± 14.51 | 0.475 ** | 0.1 |
| BP1D, mmHg, M ± SD | 80.50 ± 9.09 | 80.97 ± 8.62 | 0.446 ** | 0.0 |
| MAP1, mmHg, M ± SD | 97.70 ± 9.72 | 97.45 ± 9.63 | 0.989 ** | 0.0 |
| VR1, bpm, M ± SD | 95.51 ± 16.89 | 95.94 ± 13.54 | 0.854 ° | 0.0 |
| SpO2_1, %, M ± SD | 98.49 ± 0.76 | 98.49 ± 0.70 | 0.934 * | 0.0 |
| Bupivacaine, mg, M ± SD | 9.84 ± 0.75 | 9.96 ± 0.79 | 0.342 ° | 0.1 |
| Parameter | Group F | Group M | p-Value |
|---|---|---|---|
| ΔBPS, mmHg, M ± SD | −36.79 ± 17.64 | −39.84 ± 18.88 | 0.278 * |
| ΔBPD, mmHg, M ± SD | −25.24 ± 13.29 | −27.89 ± 11.73 | 0.168 ** |
| ΔMAP, mmHg, M ± SD | −29.09 ± 13.40 | −31.87 ± 13.08 | 0.172 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisa, R.C.; Negrut, N.; Macovei, I.C.; Moisa, C.C.M.; John, H.T.; Marian, P. The Impact of Fentanyl and Morphine on Maternal Hemodynamics in Spinal Anesthesia for Cesarean Section. Pharmaceuticals 2025, 18, 392. https://doi.org/10.3390/ph18030392
Moisa RC, Negrut N, Macovei IC, Moisa CCM, John HT, Marian P. The Impact of Fentanyl and Morphine on Maternal Hemodynamics in Spinal Anesthesia for Cesarean Section. Pharmaceuticals. 2025; 18(3):392. https://doi.org/10.3390/ph18030392
Chicago/Turabian StyleMoisa, Ramona Celia, Nicoleta Negrut, Iulia Codruta Macovei, Cezar Cristian Mihai Moisa, Harrie Toms John, and Paula Marian. 2025. "The Impact of Fentanyl and Morphine on Maternal Hemodynamics in Spinal Anesthesia for Cesarean Section" Pharmaceuticals 18, no. 3: 392. https://doi.org/10.3390/ph18030392
APA StyleMoisa, R. C., Negrut, N., Macovei, I. C., Moisa, C. C. M., John, H. T., & Marian, P. (2025). The Impact of Fentanyl and Morphine on Maternal Hemodynamics in Spinal Anesthesia for Cesarean Section. Pharmaceuticals, 18(3), 392. https://doi.org/10.3390/ph18030392








