Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review
Abstract
1. Introduction
2. Seaweed in the Food Chain
3. Nutritional Food Value of Seaweed
3.1. Bioactive Compounds from Seaweed
3.1.1. Pigments
3.1.2. Proteins
3.1.3. Carbohydrates
- Structural polysaccharides
- Storage polysaccharides
3.1.4. Lipids
3.1.5. Vitamins
3.1.6. Minerals
4. Seaweed as an Immune Booster
5. Seaweed as a Sustainable Source for Humans
6. The Biotechnological Advancements in Seaweed-Based Food Supplements
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization, United Nations |
| PUFA | Polyunsaturated fatty acids |
| ASFIS | Aquatic and fisheries information system |
| VEGF | Vascular endothelial growth factor |
| DHA | Docosahexaenoic |
| SOFIA | The State of World Fisheries and Aquaculture |
| FOSHU | Foods for specified health uses |
References
- Burgess, J.G. New and emerging analytical techniques for marine biotechnology. Curr. Opin. Biotechnol. 2012, 23, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, N.; Kim, K. Aquaculture monitoring and control systems for seaweed and fish farming. World J. Agric. Res. 2014, 2, 176–182. [Google Scholar]
- Winberg, P.C.; Fitton, H.J.; Stringer, D.; Karpiniec, S.S.; Gardiner, V.-A. Controlling seaweed biology, physiology and metabolic traits in production for commercially relevant bioactives in glycobiology. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 221–252. [Google Scholar]
- Nicholls, R.J.; Woodroffe, C.; Burkett, V. Coastline degradation as an indicator of global change. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2016; pp. 309–324. [Google Scholar]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Stankus, A.; Zhou, X. Seaweed Revolution: Where Is the Next Milestone? FAO Aquaculture Newsletter; FAO: Rome, Italy, 2021; pp. 13–16. [Google Scholar]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2021. [Google Scholar]
- Mouritsen, O.G.; Mouritsen, J.D. Seaweeds: Edible, Available, and Sustainable; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Rengasamy, K.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Advances in algal drug research with emphasis on enzyme inhibitors. Biotechnol. Adv. 2014, 32, 1364–1381. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide-and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-obesity effects of macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Pirian, K.; Moein, S.; Sohrabipour, J.; Rabiei, R.; Blomster, J. Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. J. Appl. Phycol. 2017, 29, 3151–3159. [Google Scholar] [CrossRef]
- Moffitt, C.M.; Cajas-Cano, L. Blue growth: The 2014 FAO state of world fisheries and aquaculture. Fisheries 2014, 39, 552–553. [Google Scholar] [CrossRef]
- Bhaskar, R. Junk food: Impact on health. J. Drug Deliv. Ther. 2012, 2, 67–73. [Google Scholar] [CrossRef]
- Currie, J.; DellaVigna, S.; Moretti, E.; Pathania, V. The effect of fast food restaurants on obesity. Res. Agric. Appl. Econ. 2009, 1–51. [Google Scholar] [CrossRef]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2020, 62, 1592–1607. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Periyasamy, C.; Kumar, K.S.; Rao, A.S.; Anantharaman, P. Seaweeds: Distribution, production and uses. In Bioprospecting of Algae; Society for Plant Research: Uttar Pradesh, Italy, 2018; pp. 59–78. [Google Scholar]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 247–287. [Google Scholar]
- Kumar, K.S.; Kumari, S.; Singh, K.; Kushwaha, P. Influence of seasonal variation on chemical composition and nutritional profiles of macro-and microalgae. In Recent Advances in Micro and Macroalgal Processing Food and Health Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 14–71. [Google Scholar]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Singh, K.; Kushwaha, P.; Kumar, K.S. Evaluation of nutritional and functional properties of economically important seaweeds. J. Drug Res. Ayurvedic Sci. 2022, 7, 260–275. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Caminiti, J.; Edmundson, S.; Gao, S.; Wick, M.; Huesemann, M. Seaweed proteins are nutritionally valuable components in the human diet. Am. J. Clin. Nutr. 2022, 116, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Tharani, P.; Rao, K.B. A comprehensive review on microbial diversity and anticancer compounds derived from seaweed endophytes: A pharmacokinetic and pharmacodynamic approach. Arch. Microbiol. 2024, 206, 403. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G.; Wildgoose, P.B. The effects of aqueous seaweed extract and kinetin on potato yields. J. Sci. Food Agric. 1977, 28, 121–125. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Fleurence, J. Seaweeds as food. In Seaweed Health Disease Prevention; Academic Press: New York, NY, USA, 2016; pp. 149–167. [Google Scholar]
- Arun, D.; Gayathri, P.; Chandran, M.; Yuvaraj, D. Studies on effect of seaweed extracts on crop plants and microbes. Int. J. ChemTech Res. 2014, 6, 4235–4240. [Google Scholar]
- Chojnacka, K.; Kim, S.K. Introduction of marine algae extracts. In Marine Algae Extracts Processes, Products, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–14. [Google Scholar]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Souza, B.W.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.; Ferreira, A.C.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Ismail, A.; Hong, T.S. Antioxidant activity of selected commercial seaweeds. Malays. J. Nutr. 2002, 8, 167–177. [Google Scholar]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.M.; Brownlee, I.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Farghl, A.A.; Al-Hasawi, Z.M.; El-Sheekh, M.M. Assessment of antioxidant capacity and phytochemical composition of brown and red seaweeds sampled off red sea coast. Appl. Sci. 2021, 11, 11079. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with antioxidant activity from marine macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Susanto, E.; Fahmi, A.S.; Agustini, T.W.; Rosyadi, S.; Wardani, A.D. Effects of different heat processing on fucoxanthin, antioxidant activity and colour of Indonesian brown seaweeds. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012063. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Dong, M.; Zhu, P.; Cai, Y. The anticancer effects and mechanisms of fucoxanthin combined with other drugs. J. Cancer Res. Clin. Oncol. 2019, 145, 293–301. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.F.; Bolton, J.J.; Lategan, C.A.; Smith, P.J.; Beukes, D.R. Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z. Für. Naturforschung C 2008, 63, 848–852. [Google Scholar] [CrossRef]
- Guo, L.; Dang, M.; Song, Q.; Zhang, W.; Li, B. Protective effect of fucoxanthin on ovariectomy-induced osteoporosis in rats. Pharmacogn. Mag. 2020, 16, 242–249. [Google Scholar]
- Rajauria, G. In-Vitro antioxidant properties of lipophilic antioxidant compounds from 3 brown seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro de Carvalho, M. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Othman, R.; Amin, N.; Sani, M.; Fadzillah, N.A.; Jamaludin, M. Carotenoid and chlorophyll profiles in five species of Malaysian seaweed as potential halal active pharmaceutical ingredient (API). Int. J. Adv. Sci. Eng. Inf. Technol 2018, 8, 1610–1616. [Google Scholar]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Vijay, K.; Ambedkar, R.; Ranga Rao, A.; Ravishankar, G.A. Biological activities and health benefits of seaweed carotenoids with special reference to fucoxanthin. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 539–558. [Google Scholar]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Ulagesan, S.; Nam, T.-J.; Choi, Y.-H. Extraction and purification of R-phycoerythrin alpha subunit from the marine red algae Pyropia yezoensis and its biological activities. Molecules 2021, 26, 6479. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Dharani, G.; Paramasivam, A. Evaluation of antimicrobial, antioxidant and cytotoxicity potential of R-phycoerythrin extracted from Gracilaria corticata seaweed. Curr. Res. Green Sustain. Chem. 2023, 6, 100352. [Google Scholar] [CrossRef]
- Karuppannan, S.; Sivakumar, M.; Govindasamy, B.; Chinnaraj, S.; Maluventhan, V.; Arumugam, M. Reliable quality of R-phycoerythrin derived from Portieria hornemannii for effective antioxidant, antibacterial, and anticancer activity. Biomed. Eng. Adv. 2024, 7, 100116. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Ismail, M.M.; El-Fakharany, E.M.; Hegazy, G.E. Purification and fractionation of phycobiliproteins from Arthrospira platensis and Corallina officinalis with evaluating their biological activities. Sci. Rep. 2023, 13, 14270. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; García-Pérez, P.; García-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2023, 22, 1509–1540. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, Y.H.; Nam, T.J. Identification and antioxidant activity of synthetic peptides from phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red seaweed pigments from a biotechnological perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In Vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Amin, A.; Othman, R.; Bakar, A.E.A. Carotenoid and chlorophyll profiles in five species of malaysian seaweed. In Proceedings of the in Products and Services 2018 (i-CHIPS 2018), Hat Yai, Thailand, 13–14 July 2018. [Google Scholar]
- Tabakaeva, O.; Tabakaev, A. Carotenoid profile and antiradical properties of brown seaweed Sargassum miyabei extracts. Chem. Nat. Compd. 2019, 55, 364–366. [Google Scholar] [CrossRef]
- Parida, S.; Jena, M.; Behera, A.K.; Mandal, A.K.; Nayak, R.; Patra, S. A novel phytocolorant, neoxanthin, as a potent chemopreventive: Current progress and future prospects. Curr. Med. Chem. 2024, 31, 5149–5164. [Google Scholar] [CrossRef]
- Frassini, R.; da Silva, Y.P.; Moura, S.; Villela, L.Z.; Martins, A.P.; Colepicolo, P.; Fujii, M.T.; Yokoya, N.S.; de Pereira, C.M.P.; Pereira, V.R.Z.B. Chemical characterization and cytotoxic activity of antarctic macroalgae extracts against colorectal cancer. Adv. Biol. Chem. 2019, 9, 167–177. [Google Scholar] [CrossRef]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Šubarić, D.; Roje, M.; Babić, J.; Jerković, I.; Jokić, S. Recent advances on macroalgal pigments and their biological activities (2016–2021). Algal Res. 2022, 65, 102748. [Google Scholar] [CrossRef]
- El-Din, N.G.S.; Abd El Hafez, M.S.; Abd El-Wahab, M.G.; Ibrahim, H.A. Biological activities of derived pigments and polyphenols from newly recorded alga; Phyllymenia gibbesii. Sci. Rep. 2024, 14, 21284. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid profiling of a red seaweed Pyropia yezoensis: Insights into biosynthetic pathways in the order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. In Proceedings of the Eleventh International Seaweed Symposium, Qingdao, China, 19–25 June 1983; Springer: Berlin/Heidelberg, Germany, 1984; pp. 513–516. [Google Scholar]
- Abdel-fattah, A.F.; Sary, H.H. Glycoproteins from Ulva lactuca. Phytochemistry 1987, 26, 1447–1448. [Google Scholar] [CrossRef]
- Augier, H.; Santimone, M. Composition en azote total, en protéines et en acides aminés protéiques de fertilisant foliaire «Goémar», à base d’algues marines. Bot. Mar. 1978, 21, 337–342. [Google Scholar] [CrossRef]
- Wiencke, C.; Amsler, C.D. Seaweeds and their communities in polar regions. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–291. [Google Scholar]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.G.P.B.; Quek, S.Y. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Gengatharan, A. Alternative protein sources as functional food ingredients. In Future Proteins; Elsevier: Amsterdam, The Netherlands, 2023; pp. 359–390. [Google Scholar]
- Astorga-España, M.S.; Rodríguez-Galdón, B.; Rodríguez-Rodríguez, E.M.; Díaz-Romero, C. Amino acid content in seaweeds from the Magellan Straits (Chile). J. Food Compos. Anal. 2016, 53, 77–84. [Google Scholar] [CrossRef]
- Milinovic, J.; Campos, B.; Mata, P.; Diniz, M.; Noronha, J.P. Umami free amino acids in edible green, red, and brown seaweeds from the Portuguese seashore. J. Appl. Phycol. 2020, 32, 3331–3339. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Zhou, A.Y.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Changes in total nitrogen and amino acid composition of New Zealand Undaria pinnatifida with growth, location and plant parts. Food Chem. 2015, 186, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, C.; Agrasar, A.M.T.; Mallo, F.; Rúa, M.L.; Fucinos, C. Red seaweed proteins: Valuable marine-origin compounds with encouraging applications. Algal Res. 2023, 75, 103262. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; Sanchez de Medina, F.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Seaweed proteins, peptides, and amino acids. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 125–140. [Google Scholar]
- Vásquez, P.; Cian, R.E.; Drago, S.R. Marine Bioactive Peptides (Fishes, Algae, Cephalopods, Molluscs, and Crustaceans). In Handbook of Food Bioactive Ingredients: Properties and Applications; Springer: Cham, Switzerland, 2023; pp. 1–30. [Google Scholar]
- Kraiem, M.; Ben Hamouda, S.; Eleroui, M.; Ajala, M.; Feki, A.; Dghim, A.; Boujhoud, Z.; Bouhamed, M.; Badraoui, R.; Pujo, J.M. Anti-Inflammatory and immunomodulatory properties of a crude polysaccharide derived from green seaweed Halimeda tuna: Computational and experimental evidences. Mar. Drugs 2024, 22, 85. [Google Scholar] [CrossRef]
- Lozano, I.; Wacyk, J.M.; Carrasco, J.; Cortez-San Martín, M.A. Red macroalgae Pyropia columbina and Gracilaria chilensis: Sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J. Appl. Phycol. 2016, 28, 1343–1351. [Google Scholar] [CrossRef]
- Dumay, J.; Morancais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins: Valuable proteinic pigments in red seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 321–343. [Google Scholar]
- Vissers, A.M. Leaf Phenolics and Seaweed Tannins: Analysis, Enzymatic Oxidation and Non-Covalent Protein Binding; Wageningen University and Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Akköz, C.; Arslan, D.; Ünver, A.; Özcan, M.; Yilmaz, B. Chemical composition, total phenolic and mineral contents of Enteromorpha intestinalis (L.) Kütz. and Cladophora glomerata (L.) Kütz. seaweeds. J. Food Biochem. 2011, 35, 513–523. [Google Scholar] [CrossRef]
- Manivannan, K.; Thirumaran, G.; Karthikai Devi, G.; Anantharaman, P.; Balasubramanian, T. Proximate composition of different group of seaweeds from Vedalai Coastal waters (Gulf of Mannar): Southeast Coast of India. Middle-East J. Sci. Res. 2009, 4, 72–77. [Google Scholar]
- Ramos, M.V.; Monteiro, A.C.O.; Moreira, R.A.; Carvalho, A.D.F.A.F.U. Amino acid composition of some Brazilian seaweed species. J. Food Biochem. 2000, 24, 33–39. [Google Scholar] [CrossRef]
- Manivannan, K.; Thirumaran, G.; Devi, G.K.; Hemalatha, A.; Anantharaman, P. Biochemical composition of seaweeds from Mandapam coastal regions along Southeast Coast of India. Am.-Eurasian J. Bot. 2008, 1, 32–37. [Google Scholar]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C. Nutritional evaluation of some subtropical red and green seaweeds: Part I—Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Shanmugam, A.; Palpandi, C. Biochemical Composition and Fatty Acid Profile of the Green Alga Ulva reticulata; Academic Journals Inc.: Berlin, Germany, 2008. [Google Scholar]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Mancini Filho, J.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.M.; Ali, F.S.; Desouky, M.G.; Ashour, M.; El-Shafei, A.; Maaty, M.M.; Sharawy, Z.Z. Novel comprehensive molecular and ecological study introducing coastal mud shrimp (Solenocera crassicornis) recorded at the Gulf of suez, Egypt. J. Mar. Sci. Eng. 2021, 9, 9. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural features and rheological properties of a sulfated Xylogalactan-rich fraction isolated from Tunisian red seaweed Jania adhaerens. Appl. Sci. 2020, 10, 1655. [Google Scholar] [CrossRef]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook; IntechOpen: London, UK, 2012. [Google Scholar]
- Sedlmeyer, F. Xylan as by-product of biorefineries: Characteristics and potential use for food applications. Food Hydrocoll. 2011, 25, 1891–1898. [Google Scholar] [CrossRef]
- Matulewicz, M.C.; Ciancia, M.; Noseda, M.D.; Cerezo, A.S. Carrageenan systems from tetrasporic and cystocarpic stages of Gigartina skottsbergii. Phytochemistry 1989, 28, 2937–2941. [Google Scholar] [CrossRef]
- De Sf-Tischer, P.C.; Talarico, L.B.; Noseda, M.D.; Guimarães, S.M.P.B.; Damonte, E.B.; Duarte, M.E.R. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar] [CrossRef]
- Anand, J.; Sathuvan, M.; Babu, G.V.; Sakthivel, M.; Palani, P.; Nagaraj, S. Bioactive potential and composition analysis of sulfated polysaccharide from Acanthophora spicifera (Vahl) Borgeson. Int. J. Biol. Macromol. 2018, 111, 1238–1244. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R. In Vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Lins, K.O.; Bezerra, D.P.; Alves, A.P.N.; Alencar, N.M.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.A.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; da Silva Jr, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Albuquerque, P.B.; Barros Jr, W.; Santos, G.R.; Correia, M.T.; Mourão, P.A.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Characterization and rheological study of the galactomannan extracted from seeds of Cassia grandis. Carbohydr. Polym. 2014, 104, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In Vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular λ-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Barabanova, A.; Bogdanovich, R.; Khomenko, V.; Solov’eva, T.; Yermak, I. In Vitro antioxidant properties of red algal polysaccharides. Biomed. Prev. Nutr. 2011, 1, 161–167. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Mohsin, S.; Kurup, G.M. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Biomed. Prev. Nutr. 2011, 1, 294–301. [Google Scholar] [CrossRef]
- Diogo, J.V.; Novo, S.G.; González, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015, 98, 142–144. [Google Scholar] [CrossRef]
- Ghosh, T.; Pujol, C.A.; Damonte, E.B.; Sinha, S.; Ray, B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: Structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009, 19, 235–242. [Google Scholar] [CrossRef]
- Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Xylans from Scinaia hatei: Structural features, sulfation and anti-HSV activity. Int. J. Biol. Macromol. 2010, 46, 173–178. [Google Scholar] [CrossRef]
- Cai, L.; Zheng, J.; Liu, L.; Chen, X.; Wang, H. Unlocking the potential of β-1, 3-xylooligosaccharides from Caulerpa lentillifera: Structural characterization, antioxidative and anti-osteoarthritis applications. Chem. Biol. Technol. Agric. 2024, 11, 54. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.C.P.; Nascimento, F.G.; Oliveira, S.R.; Lima, G.C.; Chagas, F.D.S.; Sombra, V.G.; Feitosa, J.P.; Soriano, E.M.; Souza, M.H.; Zocolo, G.J. Protective effect against gastric mucosa injury of a sulfated agaran from Acanthophora spicifera. Carbohydr. Polym. 2021, 261, 117829. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Kan, J.; Hu, Z.; Liu, Y.; Du, H.; Pang, G.-C.; Cheong, K.-L. Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules 2018, 23, 1354. [Google Scholar] [CrossRef] [PubMed]
- Takano, R.; Iwane-Sakata, H.; Hayashi, K.; Hara, S.; Hirase, S. Concurrence of agaroid and carrageenan chains in funoran from the red seaweed Gloiopeltis furcata Post. et Ruprecht (Cryptonemiales, Rhodophyta). Carbohydr. Polym. 1998, 35, 81–87. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Errea, M.I.; Matulewicz, M.C.; Damonte, E.B. Antiviral activity of natural sulphated galactans on herpes virus multiplication in cell culture. Planta Medica 1997, 63, 429–432. [Google Scholar] [CrossRef]
- Duarte, M.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.A.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, X.; Liao, W.; Fang, J.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, l-fuco-d-manno-1, 6-α-d-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohydr. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Bourgougnon, N.; Roussakis, C.; Kornprobst, J.-M.; Lahaye, M. Effects in vitro of sulfated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales) on a non-small-cell bronchopulmonary carcinoma line (NSCLC-N6). Cancer Lett. 1994, 85, 87–92. [Google Scholar] [CrossRef]
- Zúñiga, E.A.; Matsuhiro, B.; Mejías, E. Preparation of a low-molecular weight fraction by free radical depolymerization of the sulfated galactan from Schizymenia binderi (Gigartinales, Rhodophyta) and its anticoagulant activity. Carbohydr. Polym. 2006, 66, 208–215. [Google Scholar] [CrossRef]
- Sen Sr, A.K.; Das, A.; Banerji, N.; Siddhanta, A.; Mody, K.; Ramavat, B.; Chauhan, V.; Vedasiromoni, J.; Ganguly, D. A new sulfated polysaccharide with potent blood anti-coagulant activity from the red seaweed Grateloupia indica. Int. J. Biol. Macromol. 1994, 16, 279–280. [Google Scholar] [CrossRef]
- Pujol, C.; Estevez, J.; Carlucci, M.; Ciancia, M.; Cerezo, A.; Damonte, E. Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir. Chem. Chemother. 2002, 13, 83–89. [Google Scholar] [CrossRef]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar] [CrossRef]
- Pereira, M.G.; Benevides, N.M.; Melo, M.R.; Valente, A.P.; Melo, F.R.; Mourão, P.A. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005, 340, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Noda, H. Health benefits and nutritional properties of nori. J. Appl. Phycol. 1993, 5, 255–258. [Google Scholar] [CrossRef]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Usui, T.; Hosokawa, S.; Mizuno, T.; Suzuki, T.; Meguro, H. Investigation of the heterogeneity of heterogalactan from the fruit bodies of Fomitopsis pinicola, by employing concanavalin A-Sepharose affinity chromatography. J. Biochem. 1981, 89, 1029–1037. [Google Scholar] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Usui, T.; Asari, K.; Mizuno, T. Isolation of highly purified “fucoidan” from Eisenia bicyclis and its anticoagulant and antitumor activities. Agric. Biol. Chem. 1980, 44, 1965–1966. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Duc Thinh, P.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef] [PubMed]
- Tsumuraya, Y.; Hashimoto, Y.; Yamamoto, S.; Shibuya, N. Structure of L-arabino-D-galactan-containing glycoproteins from radish leaves. Carbohydr. Res. 1984, 134, 215–228. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Taran, I.V.; Usoltseva, R.V.; Zvyagintsev, N.V.; Zueva, A.O.; Rubtsov, N.K.; Lembikova, D.E.; Nedashkovskaya, O.I.; Kusaykin, M.I.; Isaeva, M.P. The discovery of the fucoidan-active Endo-1→ 4-α-L-Fucanase of the GH168 family, which produces fucoidan derivatives with regular sulfation and anticoagulant activity. Int. J. Mol. Sci. 2023, 25, 218. [Google Scholar] [CrossRef]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.-B.; Sankawa, U. An antivirally active sulfated polysaccharide from Sargassum horneri (Turner) C. Agardh. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Karmakar, P.; Ghosh, T.; Sinha, S.; Saha, S.; Mandal, P.; Ghosal, P.K.; Ray, B. Polysaccharides from the brown seaweed Padina tetrastromatica: Characterization of a sulfated fucan. Carbohydr. Polym. 2009, 78, 416–421. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2, 4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Alekseyenko, T.; Zhanayeva, S.Y.; Venediktova, A.; Zvyagintseva, T.; Kuznetsova, T.; Besednova, N.; Korolenko, T. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, Y.-C. Extraction and characterization of fucoidan from six brown macroalgae. J. Mar. Sci. Technol. 2016, 24, 26. [Google Scholar]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.; Córdoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomón, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.; Rajendran, M.; Pasanban, B.; Rengasamy, R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine 2010, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon Okamuranus tokida in U937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Shibata, H.; Kimura-Takagi, I.; Nagaoka, M.; Hashimoto, S.; Aiyama, R.; Iha, M.; Ueyama, S.; Yokokura, T. Properties of fucoidan from Cladosiphon okamuranus tokida in gastric mucosal protection. Biofactors 2000, 11, 235–245. [Google Scholar] [CrossRef]
- Doh-Ura, K.; Kuge, T.; Uomoto, M.; Nishizawa, K.; Kawasaki, Y.; Iha, M. Prophylactic effect of dietary seaweed fucoidan against enteral prion infection. Antimicrob. Agents Chemother. 2007, 51, 2274–2277. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Somova, L.M.; Plekhova, N.G. Fucoidan extracted from Fucus evanescens prevents endotoxin-induced damage in a mouse model of endotoxemia. Mar. Drugs 2014, 12, 886–898. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Murakami, K.; Nishimura, I.; Nakano, T.; Obata, A. A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria. Int. J. Mol. Med. 2012, 29, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kyung, J.; Kim, D.; Park, D.; Yang, Y.-H.; Choi, E.-K.; Lee, S.-P.; Kim, T.-S.; Lee, Y.-B.; Kim, Y.-B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012, 28, 91–97. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lim, J.-D.; Sohn, E.-H.; Choi, Y.-S.; Han, E.-T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Medica 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- O’Shea, C.; O’Doherty, J.; Callanan, J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef]
- Kuda, T.; Kosaka, M.; Hirano, S.; Kawahara, M.; Sato, M.; Kaneshima, T.; Nishizawa, M.; Takahashi, H.; Kimura, B. Effect of sodium-alginate and laminaran on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Carbohydr. Polym. 2015, 125, 113–119. [Google Scholar] [CrossRef]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.-H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin effects, a β-(1, 3)-glucan, on skin cell inflammation and oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Kim, I.-H.; Kim, J.; Nam, T.-J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Springer: Singapore, 2018; pp. 27–66. [Google Scholar]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Fundueanu, G.; Esposito, E.; Mihai, D.; Carpov, A.; Desbrieres, J.; Rinaudo, M.; Nastruzzi, C. Preparation and characterization of Ca-alginate microspheres by a new emulsification method. Int. J. Pharm. 1998, 170, 11–21. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kong, H.; Mooney, D. Polysaccharide-based hydrogels in tissue engineering. In Polysacharides, Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 817–837. [Google Scholar]
- Qin, Y. The characterization of alginate wound dressings with different fiber and textile structures. J. Appl. Polym. Sci. 2006, 100, 2516–2520. [Google Scholar] [CrossRef]
- Wang, T.; Gu, Q.; Zhao, J.; Mei, J.; Shao, M.; Pan, Y.; Zhang, J.; Wu, H.; Zhang, Z.; Liu, F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6636. [Google Scholar]
- Khaire, K.C.; Maibam, P.D.; Thakur, A.; Goyal, A. Biomedical and pharmaceutical applications of xylan and its derivatives. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 447–465. [Google Scholar]
- Bahú, J.O.; de Andrade, L.R.M.; de Melo Barbosa, R.; Crivellin, S.; da Silva, A.P.; Souza, S.D.; Cárdenas Concha, V.O.; Severino, P.; Souto, E.B. Plant polysaccharides in engineered pharmaceutical gels. Bioengineering 2022, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Garg, S. Xylanase: Applications in biofuel production. Curr. Metabolomics 2016, 4, 23–37. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kuthiala, T.; Singh, G.; Rarotra, S.; Kaur, A.; Arya, S.K.; Kumar, P. Current status of xylanase for biofuel production: A review on classification and characterization. Biomass Convers. Biorefinery 2021, 13, 8773–8791. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Aydar, A.Y. An overview of plant-based food alternatives (PBFAs): Classification, textural and sensory characteristics. In Plant-Based Foods: Ingredients, Technology and Health Aspects; Springer: Cham, Switzerland, 2023; pp. 1–17. [Google Scholar]
- Armisen, R.; Galatas, F. Production, properties and uses of agar. In Production and Utilization of Products from Commercial Seaweeds; FAO Fish. Tech. Pap.; FAO: Rome, Italy, 1987; Volume 288, pp. 1–57. [Google Scholar]
- Lomartire, S.; Gonçalves, A.M. Algal phycocolloids: Bioactivities and pharmaceutical applications. Mar. Drugs 2023, 21, 384. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y. Production of seaweed-derived food hydrocolloids. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–69. [Google Scholar]
- Mori, M.; Parmar, E.; Taral, P. Seaweeds processing and its application in food and industries. In Innovations in Agriculture, Environment and Health Research for Ecological Restoration; Society of Biological Sciences and Rural Development: Uttar Pradesh, Italy, 2019; p. 82. [Google Scholar]
- Davis, L. Basic Methods in Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chew, K.W.; Juan, J.C.; Phang, S.M.; Ling, T.C.; Show, P.L. An overview on the development of conventional and alternative extractive methods for the purification of agarose from seaweed. Sep. Sci. Technol. 2018, 53, 467–480. [Google Scholar] [CrossRef]
- Tyeb, S.; Kumar, N.; Kumar, A.; Verma, V. Flexible agar-sericin hydrogel film dressing for chronic wounds. Carbohydr. Polym. 2018, 200, 572–582. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Bhatnagar, A. Wound dressings from algal polymers. In Marine Algae Extracts: Processes, Products, and Applications; Wiley: Hoboken, NJ, USA, 2015; pp. 523–556. [Google Scholar]
- Shoaib, M.H.; Sikandar, M.; Ahmed, F.R.; Ali, F.R.; Qazi, F.; Yousuf, R.I.; Irshad, A.; Jabeen, S.; Ahmed, K. Applications of polysaccharides in controlled release drug delivery system. In Polysaccharides: Properties and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 607–656. [Google Scholar]
- Almukainzi, M.; El-Masry, T.A.; Selim, H.; Saleh, A.; El-Sheekh, M.; Makhlof, M.E.; El-Bouseary, M.M. New insight on the cytoprotective/antioxidant pathway keap1/Nrf2/HO-1 modulation by Ulva intestinalis extract and its selenium nanoparticles in rats with carrageenan-induced paw edema. Mar. Drugs 2023, 21, 459. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Kennedy, J.F. β–glucan-induced disease resistance in plants: A review. Int. J. Biol. Macromol. 2023, 25, 127043. [Google Scholar] [CrossRef]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Gopal, R.K.; Damodharan, R.; Arumugam, A.; Sampath Kumar, M.; Senthilkumar, N.; Anbalagan, M. In Vitro anticancer potential of laminarin and fucoidan from Brown seaweeds. Sci. Rep. 2023, 13, 14452. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef] [PubMed]
- Morparia, S.; Suvarna, V. Recent advancements in applications of alginates in drug delivery, tissue engineering, and biomedical field. Nat. Prod. J. 2024, 14, 83–100. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-based biomaterials in tissue engineering and regenerative medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- Abbasi, P.; Alemzadeh, I.; Vossoughi, M. Characterizing an injectable alginate hydrogel as a co-encapsulating system for beta cells and curcumin in type 1 diabetes therapy. Can. J. Chem. Eng. 2024, 102, 3358–3371. [Google Scholar] [CrossRef]
- Mišurcová, L.; Ambrožová, J.; Samek, D. Seaweed lipids as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 339–355. [Google Scholar]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Jebali, A.; Karray, F.; Hassairi, I.; Abdelkafi, S.; Ayadi, H.; Sayadi, S.; Dhouib, A. Characterization of Amphora sp., a newly isolated diatom wild strain, potentially usable for biodiesel production. Bioprocess Biosyst. Eng. 2015, 38, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of lipid extracts and complex lipids from seaweeds: Current knowledge and future prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, Y.-S.; Jeon, Y.-J. Seaweeds and their natural products for preventing cardiovascular associated dysfunction. Mar. Drugs 2021, 19, 507. [Google Scholar] [CrossRef]
- Eilander, A.; Harika, R.K.; Zock, P.L. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur. J. Lipid Sci. Technol. 2015, 117, 1370–1377. [Google Scholar] [CrossRef]
- Schmid, M.; Kraft, L.G.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Shin, D.; Lee, S.; Huang, Y.-H.; Lim, H.-W.; Lee, Y.; Jang, K.; Cho, Y.; Park, S.J.; Kim, D.D.; Lim, C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Yoon, S.-J.; Choi, J.-S.; Park, N.G.; Lee, H.-H.; Cho, J.-Y.; Hong, Y.-K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.-Y.; Sun, Z.-W.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.-H. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jung, S.H.; Lee, S.H.; Shin, K.H. Effects of the extracts from the marine algae Pelvetia siliquosa on hyperlipidemia in rats. Korean J. Pharmacogn. 2004, 35, 143–146. [Google Scholar]
- Huynh, A.; Maktabi, B.; Reddy, C.M.; O’Neil, G.W.; Chandler, M.; Baki, G. Evaluation of alkenones, a renewably sourced, plant-derived wax as a structuring agent for lipsticks. Int. J. Cosmet. Sci. 2020, 42, 146–155. [Google Scholar] [CrossRef]
- Lee, H.J.; Dang, H.T.; Kang, G.J.; Yang, E.J.; Park, S.S.; Yoon, W.J.; Jung, J.H.; Kang, H.K.; Yoo, E.S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-κB and STAT1 activity in lipopolysaccharide-stimulated Raw 264.7 cells. Arch. Pharmacal Res. 2009, 32, 453–462. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Vyssotski, M.; Lagutin, K.; MacKenzie, A.; Mitchell, K.; Scott, D. Phospholipids of New Zealand edible brown algae. Lipids 2017, 52, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata (Kütz.) Okamura. Glycobiology 2006, 16, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Akbari, V.; Abedi, M.; Yegdaneh, A. Bioassay-guided isolation of glycolipids from the seaweed Gracilaria corticata. Res. Pharm. Sci. 2020, 15, 473–480. [Google Scholar] [PubMed]
- Aumeerun, S.; Soulange-Govinden, J.; Driver, M.F.; Rao, A.R.; Ravishankar, G.A.; Neetoo, H. 19 Macroalgae and Microalgae. In Handbook of Algal Technologies and Phytochemicals: Two Volume Set, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–219. [Google Scholar]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Kumar, C.S.; Ganesan, P.; Suresh, P.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds-a review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Noriega-Fernández, E.; Sone, I.; Astráin-Redín, L.; Prabhu, L.; Sivertsvik, M.; Álvarez, I.; Cebrián, G. Innovative ultrasound-assisted approaches towards reduction of heavy metals and iodine in macroalgal biomass. Foods 2021, 10, 649. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Ravishankar, C. Seaweeds as a Source of Micro and Macro Nutrients; ICAR-Central Institute of Fisheries Technology: Cochin, India, 2018. [Google Scholar]
- Inam, A.; Oncu-Oner, T.; Deniz, I. Algae in Biomedicine. Cell Biol. Transl. Med. 2024, 22, 147–163. [Google Scholar]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; IntechOpen: London, UK, 2013. [Google Scholar]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-Y.; Liu, S.-L.; Chiang, Y.-R. Biosynthesis of ascorbic acid as a glucose-induced photoprotective process in the extremophilic red alga Galdieria partita. Front. Microbiol. 2020, 10, 3005. [Google Scholar] [CrossRef]
- Ivanov, B. Role of ascorbic acid in photosynthesis. Biochemistry 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- Antunes, W.M.; Luna, A.S.; Henriques, C.A.; da Costa, A.C.A. An evaluation of copper biosorption by a brown seaweed under optimized conditions. Electron. J. Biotechnol. 2003, 6, 174–184. [Google Scholar]
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Lee, K.W.; Cho, M.S.; Shin, D.; Song, W.O. Changes in iodine status among US adults, 2001–2012. Int. J. Food Sci. Nutr. 2016, 67, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144. [Google Scholar]
- Sapkota, M.; Knoell, D.L. Essential role of zinc and zinc transporters in myeloid cell function and host defense against infection. J. Immunol. Res. 2018, 2018, 4315140. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Chauhan, O.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Milner, J. Molecular targets for bioactive food components. J. Nutr. 2004, 134, 2492S–2498S. [Google Scholar] [CrossRef]
- Mohanty, S.; Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jena, M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020, 10, 1–8. [Google Scholar]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Horie, Y.; Sugase, K.; Horie, K. Physiological differences of soluble and insoluble dietary fibre fractions of brown algae and mushrooms in pepsin activity in vitro and protein digestibility. Asia Pac. J. Clin. Nutr. 1995, 4, 251–255. [Google Scholar]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.F.; Siqueira Jr, J.M.; Maraschin, M.; Ferreira, A.G.; Gagliardi, A.R.; Ribeiro-do-Valle, R.M. A polysaccharide isolated from the brown seaweed Sargassum stenophyllum exerts antivasculogenic effects evidenced by modified morphogenesis. Microvasc. Res. 2008, 75, 34–44. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Enomoto, A.; Todoh, H.; Ametani, A.; Kaminogawa, S. Activation of murine macrophages by polysaccharide fractions from marine algae (Porphyra yezoensis). Biosci. Biotechnol. Biochem. 1993, 57, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Ametani, A.; Kaminogawa, S. In vivo macrophage-stimulation activity of the enzyme-degraded water-soluble polysaccharide fraction from a marine alga (Gracilaria verrucosa). Biosci. Biotechnol. Biochem. 1996, 60, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Vasconcelos, J.B.; De Vasconcelos, E.R.; Urrea-Victoria, V.; Bezerra, P.S.; Reis, T.N.; Cocentino, A.L.; Navarro, D.M.; Chow, F.; Areces, A.J.; Fujii, M.T. Antioxidant activity of three seaweeds from tropical reefs of Brazil: Potential sources for bioprospecting. J. Appl. Phycol. 2019, 31, 835–846. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.G.; Kang, M.-C.; Sanjeewa, K.A.; Oh, J.Y.; Jeon, Y.-J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.-S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Sun, X.; Sun, S.; Xu, N. Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2585–2596. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Fradinho, P.; Flórez-Fernández, N.; Sousa, I.; Raymundo, A.; Domínguez, H.; Torres, M. Environmentally friendly processing of Laminaria ochroleuca for soft food applications with bioactive properties. J. Appl. Phycol. 2020, 32, 1455–1465. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of I rish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishida, M.; Nishi, K.; Oda, H.; Toyohara, H.; Sugahara, T. Studies on anti-allergic activity of Sargassum horneri extract. J. Funct. Foods 2014, 10, 154–160. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, Á.; Pereira, D.M.; Andrade, P.B. Edible seaweeds’ phlorotannins in allergy: A natural multi-target approach. Food Chem. 2018, 265, 233–241. [Google Scholar] [CrossRef]
- Na, H.J.; Moon, P.D.; Ko, S.G.; Lee, H.J.; Jung, H.A.; Hong, S.H.; Seo, Y.; Oh, J.M.; Lee, B.H.; Choi, B.W. Sargassum hemiphyllum inhibits atopic allergic reaction via the regulation of inflammatory mediators. J. Pharmacol. Sci. 2005, 97, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Na, H.J.; Moon, P.D.; Lee, H.J.; Kim, H.R.; Chae, H.J.; Shin, T.; Seo, Y.; Hong, S.H.; Kim, H.M. Regulatory effect of atopic allergic reaction by Carpopeltis affinis. J. Ethnopharmacol. 2005, 101, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kimiya, T.; Ohtani, K.; Satoh, S.; Abe, Y.; Ogita, Y.; Kawakita, H.; Hamada, H.; Konishi, Y.; Kubota, S.; Tominaga, A. Inhibitory effects of edible marine algae extracts on degranulation of RBL-2H3 cells and mouse eosinophils. Fish. Sci. 2008, 74, 1157–1165. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Kakinuma, M.; Amano, H. Anti-allergic effects of the brown alga Eisenia arborea on Brown Norway rats. Fish. Sci. 2008, 74, 180–186. [Google Scholar] [CrossRef]
- Chin, Y.X.; Lim, P.E.; Maggs, C.A.; Phang, S.M.; Sharifuddin, Y.; Green, B.D. Anti-diabetic potential of selected Malaysian seaweeds. J. Appl. Phycol. 2015, 27, 2137–2148. [Google Scholar] [CrossRef]
- Yang, C.F.; Lai, S.S.; Chen, Y.H.; Liu, D.; Liu, B.; Ai, C.; Wan, X.Z.; Gao, L.Y.; Chen, X.H.; Zhao, C. Anti-diabetic effect of oligosaccharides from seaweed Sargassum confusum via JNK-IRS1/PI3K signalling pathways and regulation of gut microbiota. Food Chem. Toxicol. 2019, 131, 110562. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Bhattamisra, S.K.; Panigrahy, R.C.; Parida, S.K. Evaluation of the antioxidant, hypoglycaemic and anti-diabetic activities of some seaweed collected from the east coast of India. Biomed. Pharmacol. J. 2016, 9, 365–375. [Google Scholar] [CrossRef]
- Jia, R.B.; Wu, J.; Li, Z.R.; Ou, Z.R.; Lin, L.; Sun, B.; Zhao, M. Structural characterization of polysaccharides from three seaweed species and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 1040–1049. [Google Scholar] [CrossRef]
- Sanger, G.; Rarung, L.; Damongilala, L.; Kaseger, B.; Montolalu, L. Phytochemical constituents and antidiabetic activity of edible marine red seaweed (Halymenia durvilae). IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012069. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Chen, M.; Jia, R.; Liu, B.; Zhao, C. The antidiabetic activity of brown seaweed Sargassum confusum polysaccharide hydrolysates in insulin resistance HepG2 cells in vitro. Res. J. Biotechnol 2017, 12, 1–9. [Google Scholar]
- Zhao, C.; Yang, C.; Chen, M.; Lv, X.; Liu, B.; Yi, L.; Cornara, L.; Wei, M.C.; Yang, Y.C.; Tundis, R. Regulatory efficacy of brown seaweed Lessonia nigrescens extract on the gene expression profile and intestinal microflora in type 2 diabetic mice. Mol. Nutr. Food Res. 2018, 62, 1700730. [Google Scholar] [CrossRef]
- Manggau, M.; Hamzah, M.; Mamada, S.; Nurdin, W.; Zaenuddin, E. Anti-coagulant activities of brown seaweed Sargassum cristaefolium extract. J. Phys. Conf. Ser. 2019, 1341, 072006. [Google Scholar] [CrossRef]
- da Silva Chagas, F.D.; Lima, G.C.; Dos Santos, V.I.N.; Costa, L.E.C.; de Sousa, W.M.; Sombra, V.G.; de Araújo, D.F.; Barros, F.C.N.; Marinho-Soriano, E.; de Andrade Feitosa, J.P. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Liu, S.; Yu, H.; Li, R.; Wang, X.; Qin, Y.; Li, P. Preparation of low molecular weight Sargassum fusiforme polysaccharide and its anticoagulant activity. J. Oceanol. Limnol. 2018, 36, 882–891. [Google Scholar] [CrossRef]
- de Carvalho, M.M.; de Freitas, R.A.; Ducatti, D.R.; Ferreira, L.G.; Gonçalves, A.G.; Colodi, F.G.; Mazepa, E.; Aranha, E.M.; Noseda, M.D.; Duarte, M.E.R. Modification of ulvans via periodate-chlorite oxidation: Chemical characterization and anticoagulant activity. Carbohydr. Polym. 2018, 197, 631–640. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Athukorala, Y.; Jeon, Y.-J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In Vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana JV Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by high-performance liquid chromatography (HPLC) with quadrupole-time-of-flight mass spectroscopy (qTOF-MS) and antiviral activities of phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; de Souza Barros, C.; de Oliveira, M.C.; Rabelo, V.W.-H.; Azevedo, R.C.; Teixeira, V.L.; Ferreira, D.F.; de Palmer Paixão, I.C.N. In Vitro studies on the inhibition of replication of Zika and Chikungunya viruses by dolastane isolated from seaweed Canistrocarpus cervicornis. Sci. Rep. 2020, 10, 8263. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; de Souza Barros, C.; Nogueira, C.C.R.; Amorim, L.d.S.C.; de Mendonça Campos, R.; Ratcliffe, N.A.; Teixeira, V.L.; Ferreira, D.F.; de Palmer Paixao, I.C.N. Antiviral effect of the seaweed Osmundaria obtusiloba against the Zika virus. J. Med. Plants Res. 2018, 12, 387–395. [Google Scholar]
- Palanisamy, S.K.; Arumugam, V.; Rajendran, S.; Ramadoss, A.; Nachimuthu, S.; Peter, D.M.; Sundaresan, U. Chemical diversity and anti-proliferative activity of marine algae. Nat. Prod. Res. 2019, 33, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.G.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernández-Kelly, L.C.; Ortega, A. Brown seaweed Egregia menziesii’s cytotoxic activity against brain cancer cell lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Gonçalves-Fernández, C.; Sineiro, J.; Moreira, R.; Gualillo, O. Extraction and characterization of phlorotannin-enriched fractions from the Atlantic seaweed Bifurcaria bifurcata and evaluation of their cytotoxic activity in murine cell line. J. Appl. Phycol. 2019, 31, 2573–2583. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.; Chitra, J. In Vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In Vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- de Sousa Pinheiro, T.; Nascimento Santos, M.d.S.; Will Castro, L.S.E.; Paiva, A.A.d.O.; Alves, L.G.; Cruz, A.K.M.; Nobre, L.T.D.; Alves, M.G.d.C.; Leite, E.L. A fucan of a brown seaweed and its antitumoral property on HT-29 and immunomodulatory activity in murine RAW 264. 7 macrophage cell line. J. Appl. Phycol. 2017, 29, 2061–2075. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1, 3/1, 6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef]
- Cho, M.; Lee, D.-J.; Kim, J.-K.; You, S. Molecular characterization and immunomodulatory activity of sulfated fucans from Agarum cribrosum. Carbohydr. Polym. 2014, 113, 507–514. [Google Scholar] [CrossRef]
- Ahn, G.; Hwang, I.; Park, E.; Kim, J.; Jeon, Y.-J.; Lee, J.; Park, J.W.; Jee, Y. Immunomodulatory effects of an enzymatic extract from Ecklonia cava on murine splenocytes. Mar. Biotechnol. 2008, 10, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Kudus, K.; Grasian, I.; Madasamy, S.; John, A. Immunomodulatory effect of alginic acid from brown seaweed Sargassum wightii on disease resistance in Penaeus monodon. J. Clean WAS 2017, 1, 26–29. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K.; Makkar, F. Pharmacological activities of brown seaweed Sargassum wightii (Family Sargassaceae) using different in vitro models. Int. J. Food Prop. 2017, 20, 931–945. [Google Scholar] [CrossRef]
- Ramirez-Higuera, A.; Quevedo-Corona, L.; Paniagua-Castro, N.; Chamorro-Ceballos, G.; Milliar-Garcia, A.; Jaramillo-Flores, M.E. Antioxidant enzymes gene expression and antihypertensive effects of seaweeds Ulva linza and Lessonia trabeculata in rats fed a high-fat and high-sucrose diet. J. Appl. Phycol. 2014, 26, 597–605. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K. Pharmacological potential of sulfated polygalactopyranosyl-fucopyranan from the brown seaweed Sargassum wightii. J. Appl. Phycol. 2018, 30, 1971–1988. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Rhatigan, P. The Irish Seaweed Kitchen; Booklink: Co Down, Ireland, 2009. [Google Scholar]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Cofrades, S.; Serdaroğlu, M.; Jiménez-Colmenero, F. Design of healthier foods and beverages containing whole algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 609–633. [Google Scholar]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef]
- González, A.; Paz, S.; Rubio, C.; Gutiérrez, Á.J.; Hardisson, A. Human exposure to iodine from the consumption of edible seaweeds. Biol. Trace Elem. Res. 2020, 197, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Yuan, X.; Kobayashi, S.; Sasaki, S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS ONE 2017, 12, e0173722. [Google Scholar] [CrossRef]
- Taylor, V.F.; Li, Z.; Sayarath, V.; Palys, T.J.; Morse, K.R.; Scholz-Bright, R.A.; Karagas, M.R. Distinct arsenic metabolites following seaweed consumption in humans. Sci. Rep. 2017, 7, 3920. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A.; Tewari, A.; Joshi, H.V.; Viswanath, S.; Ramesh, H.P.; Rao, S.V. Evaluation of nutritional quality and food safety of seaweeds of India. J. Food Saf. 1993, 13, 77–90. [Google Scholar] [CrossRef]
- Moreira, A.S.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Benedi, J.; Sánchez-Muniz, F.J. Wakame and Nori in restructured meats included in cholesterol-enriched diets affect the antioxidant enzyme gene expressions and activities in Wistar rats. Plant Foods Hum. Nutr. 2010, 65, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Halldorsdottir, S.M.; Sveinsdottir, H.; Gudmundsdottir, A.; Thorkelsson, G.; Kristinsson, H.G. High quality fish protein hydrolysates prepared from by-product material with Fucus vesiculosus extract. J. Funct. Foods 2014, 9, 10–17. [Google Scholar] [CrossRef]
- Egodavitharana, D.I.; Manikkrama, S.; Bambaranda, B.V.A.S.M.; Mudannayake, D.C. Utilization of Sargassum crassifolium seaweed powder as a functional ingredient in wheat noodles. J. Appl. Phycol. 2024, 36, 2903–2915. [Google Scholar] [CrossRef]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive exploration of marine algae diversity, bioactive compounds, health benefits, regulatory issues, and food and drug applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Aware, C.; Nalavade, V.; Jadhav, R.; Bhatia, S.K.; Yang, Y.-H.; Jadhav, J.; Gurav, R. Algae as food and nutraceuticals. In Algal Biorefineries and the Circular Bioeconomy; CRC Press: Boca Raton, FL, USA, 2022; pp. 295–337. [Google Scholar]
- Athukorala, Y.; Lee, K.W.; Park, E.J.; Heo, M.S.; Yeo, I.K.; Lee, Y.D.; Jeon, Y.J. Reduction of lipid peroxidation and H2O2-mediated DNA damage by a red alga (Grateloupia filicina) methanolic extract. J. Sci. Food Agric. 2005, 85, 2341–2348. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Lee, K.-W.; Kim, S.-H.; Ha, J.-H.; Park, G.-T.; Jeon, Y.-J. Lipid peroxidation inhibitory effects of Hizikia fusiformis methanolic extract on fish oil and linoleic acid. Food Sci. Technol. Int. 2004, 10, 65–72. [Google Scholar] [CrossRef]
- Buedenbender, L.; Astone, F.A.; Tasdemir, D. Bioactive molecular networking for mapping the antimicrobial constituents of the baltic brown alga Fucus vesiculosus. Mar. Drugs 2020, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Sandsdalen, E.; Haug, T.; Stensvåg, K.; Styrvold, O.B. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J. Microbiol. Biotechnol. 2003, 19, 777–782. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- Khalil, H.A.; Saurabh, C.K.; Tye, Y.; Lai, T.; Easa, A.; Rosamah, E.; Fazita, M.; Syakir, M.; Adnan, A.; Fizree, H. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Gu, C.-H.; Wang, J.-J.; Yu, Y.; Sun, H.; Shuai, N.; Wei, B. Biodegradable multilayer barrier films based on alginate/polyethyleneimine and biaxially oriented poly (lactic acid). Carbohydr. Polym. 2013, 92, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.T.L.; Thien, V.Y.; Misson, M.; Chin, G.J.W.L.; Hussin, S.N.I.S.; Chong, H.L.H.; Yusof, N.A.; Ma, N.L.; Rodrigues, K.F. Seaweed: A bioindustrial game-changer for the green revolution. Biomass Bioenergy 2024, 183, 107122. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Seaweed: A sustainable solution for greening drug manufacturing in the pursuit of sustainable healthcare. Explor. Drug Sci. 2024, 2, 50–84. [Google Scholar] [CrossRef]
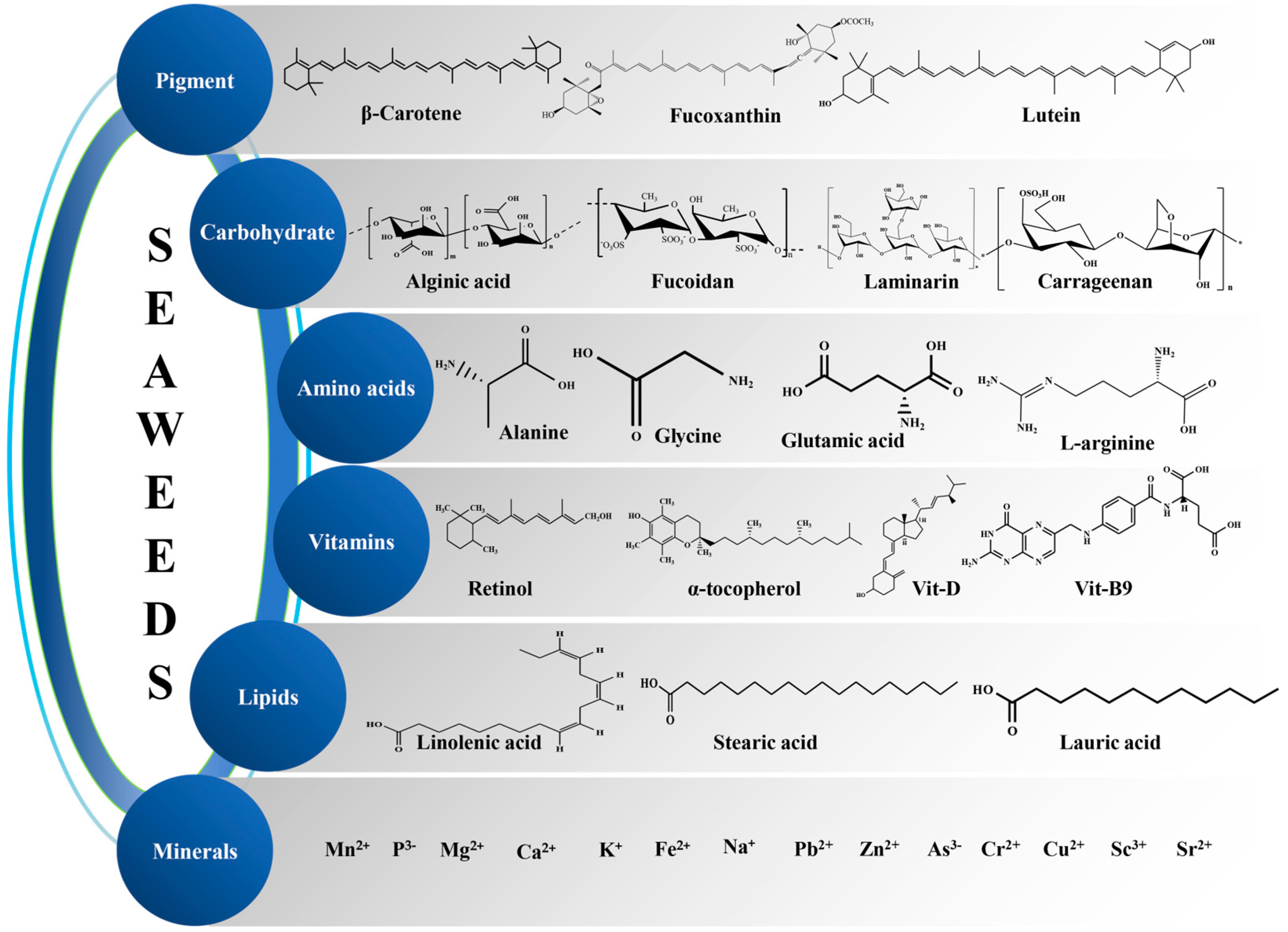
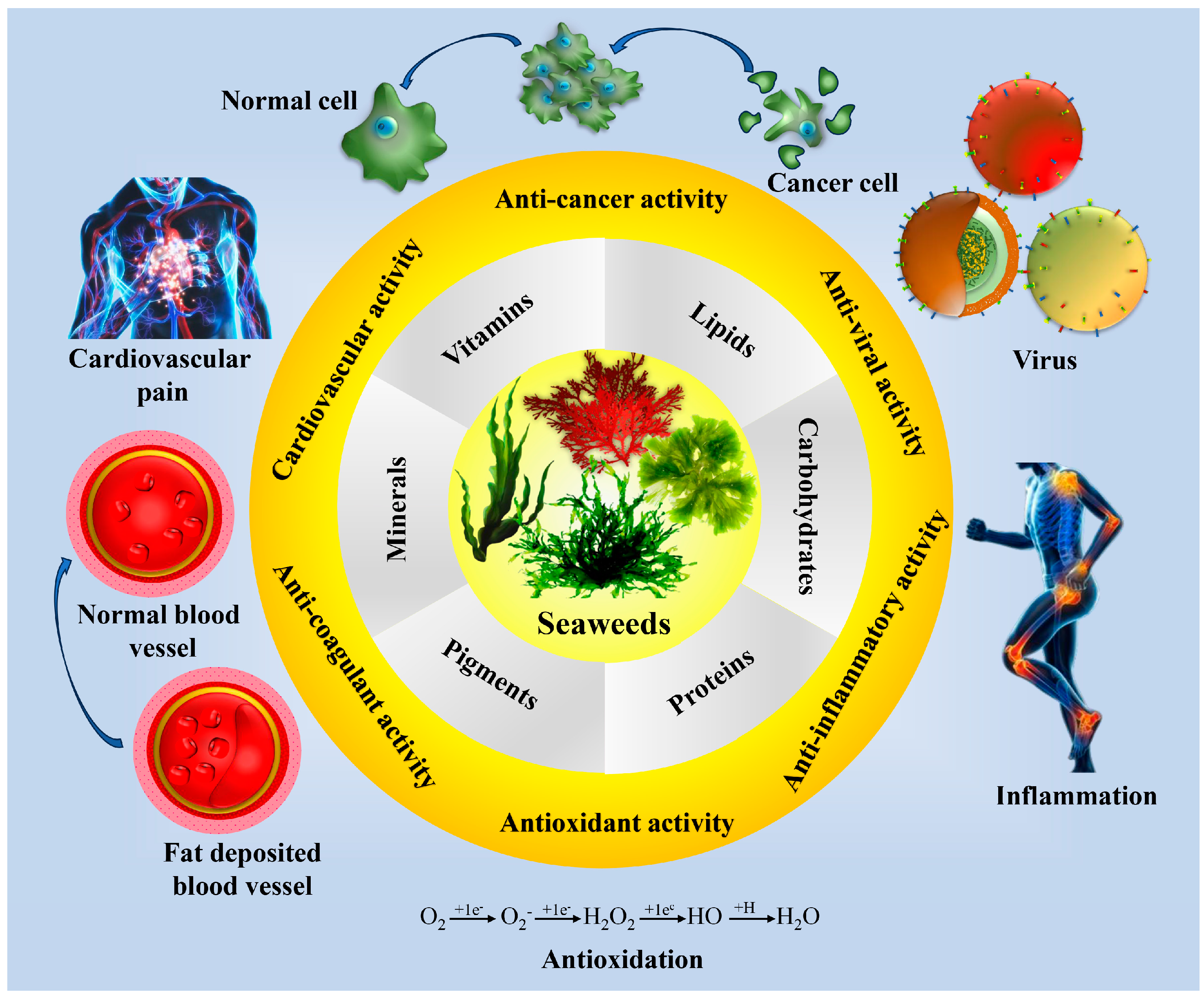
| Country | Seaweed Productions (2019) | |
|---|---|---|
| Wet Weight (In Tons) | Total Percentage of World Production | |
| China | 20,296,592 | 56.75 |
| Indonesia | 9,962,900 | 27.86 |
| South Korea | 1,821,475 | 5.09 |
| Philippines | 1,500,326 | 4.20 |
| North Korea | 553,300 | 1.71 |
| Chile | 426,605 | 1.19 |
| Japan | 412,300 | 1.15 |
| Malaysia | 188,110 | 0.53 |
| Norway | 163,197 | 0.46 |
| Tanzania | 106,069 | 0.30 |
| Vietnam | 20,300 | 0.06 |
| Russia | 19,544 | 0.05 |
| Solomon Islands | 5600 | 0.02 |
| India | 5700 | 0.02 |
| Madagascar | 9665 | 0.03 |
| Papua New Guinea | 4300 | 0.01 |
| Kiribati | 3650 | 0.01 |
| Total global production | 35,762,504 | |
| Pigment | Biological Activities | Algal Source | References |
|---|---|---|---|
| β-Carotene | Antioxidant potential in vivo, antimutagenic, provitamin A | Phaeophyta, Rhodophyta, Chlorophyta | [39,40,41,42,43] |
| Fucoxanthin | Antioxidant food additives, antidiabetic properties, anti-obesity properties, antihypertensive activity and lower stroke risk factors, prevent osteoporosis, anticancer activities, prevent breast cancer, immune-modulatory, antiangiogenic, antimalarial activities, neuroprotective and anti-inflammatory property | Phaeophyta | [44,45,46,47,48,49,50,51] |
| Zeaxanthin | Antioxidant potential in vivo | Rhodophyta, Chlorophyta | [41,52,53] |
| Lutein | Antioxidant potential in vivo, antimutagenic, protects eyes from oxidative stress, prevents heart disease | Rhodophyta, Chlorophyta | [41,54,55] |
| Astaxanthin | Antioxidant, immune-modulatory, antiangiogenic, antimalarial activities, and anti-inflammatory effects, antioxidant, and immune-enhancing properties | Chlorophyta | [56,57,58,59] |
| Phycoerythrin | Antioxidant, anticancer activity | Rhodophyta | [60,61,62] |
| Phycocyanin | Anticancer activity, antidiabetic and anti-inflammatory activity, antimicrobial activity | Rhodophyta | [63,64,65] |
| Pheophorbide a | Antioxidant | Rhodophyta | [65,66] |
| Phycoerythrobilin | Antioxidant | Rhodophyta | [67,68] |
| Siphonaxanthin | Anticancer, antiangiogenic | Chlorophyta | [53,69] |
| Chlorophyll a | Antioxidant, antimutagenic | Phaeophyta, Rhodophyta, Chlorophyta | [55,70,71] |
| Neoxanthin | Antiproliferative activity, anticancer activity, anti-inflammatory activity, anti-obesity properties, antioxidant activity | Chlorophyta | [53,58,72,73] |
| Violaxanthin | Antiproliferative activity, anti-inflammatory activity, antioxidant activity | Chlorophyta | [74,75] |
| Antheraxanthin | Antioxidant activity, anticancer activity | Chlorophyta | [76,77,78] |
| Name of Seaweed | Protein Content (Dry Weight in %) | References |
|---|---|---|
| Chlorophyta | ||
| Caulerpa lentillifera | 9.5–20.4 | [99,100] |
| Cladophora glomerata | 14.1–20.4 | [101,102] |
| Caulerpa sertularioides | 20.0 | [103] |
| Enteromorpha compressa | 12.3 | [102] |
| Enteromorpha flexuosa | 7.9 | [99] |
| Enteromorpha intestinalis | 15.2–16.4 | [101,104] |
| Ulva fasciata | 6.6–8.8 | [99,103] |
| Ulva lactuca | 3.3–27.2 | [104,105,106,107] |
| Ulva reticulata | 13.5–20.0 | [102,108] |
| Phaeophyta | ||
| Dictyota acutiloba | 12 | [99] |
| Dictyota sadvincensis | 6.4 | [99] |
| Laminaria sp. | 7.5 | [109] |
| Padina gymnospora | 11.2–17.1 | [103,104] |
| Padina pavonica | 13.6 | [102] |
| Sargassum echinocarpum | 10.3 | [99] |
| Sargassum obtusifolium | 13 | [99] |
| Sargassum vulgaris | 16.3 | [103] |
| Undarina pinnatifida | 19.8 | [109] |
| Rhodophyta | ||
| Amansia multifida | 25.6 | [103] |
| Bryothamnion seaforthii | 17.3 | [103] |
| Corallina officinalis | 2.3–6.9 | [103,110] |
| Enantiocladia duperreyi | 19.5 | [103] |
| Gelidiella acerosa | 31.1 | [102] |
| Gracilaria birdiae | 7.1 | [111] |
| Gracilaria folifera | 7 | [104] |
| Gracilaria salicornia | 5.6 | [99] |
| Hypnea charoides | 18.4 | [106] |
| Hypnea japonica | 19.1 | [106] |
| Laurencia filiformis | 18.3 | [111] |
| Palmaria palmata | 18.3 | [112] |
| Porphyra sp. | 31.3–44 | [109,110] |
| Solieria filiformis | 21.3 | [103] |
| Vidalia obtusiloba | 18.1 | [103] |
| Polysaccharides | Algal Sources | Properties | References |
|---|---|---|---|
| Carrageenan | Gigartina skottsbergii | Antiviral, inhibits influenza virus | [119] |
| Meristiella gelidium | Antiviral | [120] | |
| Acanthophora spicifera | Antiviral against HSV-1, inhibits virus replication | [121] | |
| Hypnea musciformis | Anticancer | [122] | |
| Champia feldmannii | Antitumor | [123] | |
| Stenogramme interrupta | Antiviral | [124] | |
| E. spinosa | Anticoagulant, antithrombotic | [125] | |
| Chondrus crispus | Antiviral, anticoagulant, antithrombotic | [126] | |
| C. ocellatus | Antitumor | [127] | |
| C. ocellatus | Antitumor | [128] | |
| Gigartinaceae Tichocarpaceae algae | Antioxidant | [129] | |
| Tribonema minus | Anticancer | [130] | |
| Padina tetrastromatic | Anti-inflammation | [131] | |
| G. skottsbergii | Antiviral | [132] | |
| Xylans | Sebdenia polydactyla | Antiviral | [133] |
| Scinaia hatei | Antiviral | [134] | |
| Caulerpa lentillifera | Antioxidative and antitumor | [135] | |
| Spatoglossum schröederi | Antithrombotic; peripheral anti-nociceptive; antiproliferative, anti-adhesive, antioxidant | [136] | |
| Agar | Acanthophora spicifera | Antiviral against HSV-2, inhibits the initial attachment of the virus to the cells | [137] |
| Gelidium amansii | Antioxidant activity | [138] | |
| Gloiopeltis complanata | Antiviral | [139] | |
| Cryptopleura ramosa | Antiviral | [140] | |
| Bostrychia montagnei | Antiviral | [141] | |
| Gracilaria corticata | Antiviral | [142] | |
| Galactans | Callophyllis variegate Agardhiella tenera Schizymenia binderi Cryptonemia crenulata | Antiviral | [143] |
| Laminaria japonica | Anti-lipidemic, antiviral, antitumor, immunomodulator, antioxidant neuroprotective | [144,145] | |
| Sargassum sp. | Antitumor | [146] | |
| Adenocystis utricularis | Antiviral | [147] | |
| Spatoglossum schröederi | Antithrombotic | [148] | |
| Schizymenia dubyi | Antiviral | [149] | |
| S. binderi | Anticoagulant | [150] | |
| Grateloupia indica | Anticoagulant, antithrombotic | [151] | |
| Gymnogongrus torulosus | Antiviral | [152] | |
| Gigartina acicularis | Antioxidant | [148] | |
| Euchema cottonii | Antioxidant | ||
| Aghardiella tenera | Antiviral | [153] | |
| Gelidium crinale | Anticoagulant | [154] | |
| Porphyra sp. | Antitumor, hypotensive, regulates blood cholesterol | [155] | |
| Ulvans | Enteromorpha compressa | Antiviral against HSV, inhibits the adsorption and replication of the virus | [156] |
| Ulva intestinalis | Antiviral against measles virus, reduction in syncytia formation and low cytotoxicity | [157] | |
| U. armoricana | Antiviral against HSV-1 | [158] | |
| U. pertusa | Antioxidant, antiproliferative, hypocholesterolemic | [159] | |
| U. rigida | Immunostimulatory | [160] | |
| U. pertusa | Antioxidant and antihyperlipidemic activity | [130] | |
| U. pertusa | Antioxidant activity | [161] | |
| Ulva sp. | Anti-aging | [162] | |
| Enteromorpha prolifera | Immunomodulator, antioxidant, hypolipidemic | [139] | |
| Ulva sp. | Anti-adhesive, antiproliferative, hepatoprotective | [160] | |
| U. pertusa | Antioxidant, antiproliferative, hypocholesterolemic | [163] | |
| U. pertusa | Antioxidant, hypotriglyceridemic, decreases LDL- and increases HDL-cholesterol, immunostimulatory | [164] | |
| Fucoidans | Sargassum mcclurei | Antiviral against HIV-1, blocks entry of the virus | [165] |
| Adenocytis utricularis Undaria pinnatifida Stoechospermum marginatum Cystoseira indica | Antiviral against HSV-1, HSV-2, HCMV, VSV, Sindbis virus, and HIV-1 | [166] | |
| Ecklonia cava E. kurome | Antiproliferative, antitumor, anticoagulant, antioxidant, antithrombotic, anti-inflammatory | [167,168] | |
| S. horneri | Antitumor, antiviral | [169] | |
| Fucus sp. | Immunostimulant, antiviral, antitumor, antiproliferative, antiadhesive | [148] | |
| Ascophyllum nodosum | Immunomodulatory, anti-inflammatory, anticoagulant, antithrombotic | [170] | |
| Padina tetrastromatica | Antitumor, antiviral | [171] | |
| A. nodosum Cladosiphon okamuranus Fucus spiralis F. distichus, F. evanescens F. vesiculosus F. serratus L. digitata L. saccharina | Anticoagulant, anti-inflammatory, antiadhesive, antiangiogenic | [170] | |
| F. vesiculosus | Anti-atopic dermatitis | [172] | |
| F. evanescens | Antitumor | [173] | |
| S. fusiforme | Antiangiogenic | [174] | |
| S. fusiforme | Anticancer | [175] | |
| C. okamuranus | Anticancer | [176] | |
| Adenocystis utricularis | Antiretroviral | [177] | |
| A. utricularis | Antiviral | [147] | |
| C. okamuranus | Cardioprotective | [178] | |
| C. okamuranus | Antiproliferative | [179] | |
| C. okamuranus | Gastric protection | [180] | |
| C. okamuranus | Antiprion | [181] | |
| F. evanescens | Anticoagulant | [182] | |
| F. evanescens | Anti-inflammatory | [183] | |
| F. vesiculosus | Anti-obesity | [184] | |
| Undaria piaantifida | Immunostimulatory | [185] | |
| L. japonica | Antioxidant | [186] | |
| L. japonica | Anti-inflammatory | [187] | |
| Lessonia vadosa | Anticoagulant and elicitor | [188] | |
| U. pinnatifida | Antiplasmodial | [189] | |
| U. pinnatifida | Anti-allergy | [190] | |
| U. pinnatifida | Antitumor | [191] | |
| U. pinnatifida | Antitumor | [192] | |
| Laminarins | Laminaria sp. | Anti-inflammatory | [193] |
| Eisenia bicyclis | Antibacterial | [194] | |
| E. bicyclis | Anticancer | [195] | |
| L. digitata | Antioxidant protection | [196] | |
| L. japonica | ROS scavenging potential | [197] | |
| L. digitata | Anticancer | [198] | |
| Alginates | L. hyperborea L. digitata L. japonica A. nodosum Macrocystis pyrifera | Antiviral | [199] |
| Eucheuma cottonii S. polycystum | Antidiabetic | [200] | |
| Laminaria sp. | Drug carriers | [201] | |
| A. nodosum | Scaffolds for ligaments and tissue engineering | [202] | |
| Ecklonia sp. | Regeneration of tissues | [203] | |
| M. pyrifera | Wound healing and dressing | [204] | |
| L. hyperborean | Wound healing | [205] |
| Lipid Type | Algal Sources | Properties | References |
|---|---|---|---|
| Omega-3 fatty acids | Phaeophyta | Ant-inflammation, boosts brain function, supports eye health, improves heart health | [244] |
| Arachidonic acid | Porphyridium cruentum | Improves growth and development of neonates | [245] |
| Fucosterol | Sargassum fusiformis | Anti-aging | [246] |
| Docosahexaenoic acid | Phaeophyta | Cardiovascular health, eye and brain health, development of nervous system | [245] |
| Eicosapentaenoic acid | Porphyridium cruentum | Cognition, heart health, protection against arthrosclerosis, anti-inflammatory potential | [247] |
| Polyunsaturated fatty acid | Undaria pinnatifida | Anti-inflammation | [248] |
| Palmitic acid | Fucus vesiculosus Saccharina latissima Gracilaria sp. Ulva rigida | Enzyme inhibition, antioxidant | [249] |
| Fucosterol | S. fusiformis Pelvetia siliquosa | Anti-inflammatory, antioxidant, increased antioxidative enzymes | [250,251] |
| Essential oil | Laminaria japonica Gracilaria verrucosa | Anti-inflammatory, antioxidant, antibacterial activity | [252,253] |
| Phospholipids | Grateloupia turuturu Ecklonia radiata Hormosira banksii | Antioxidant and anti-inflammatory potential | [254,255] |
| Glycolipids | Chondria armata Gracilaria corticata | Antimicrobial activity | [256,257] |
| Biological Activity | Bioactive Compounds/Extracts | Seaweed Species | Reference |
|---|---|---|---|
| Antioxidant | Eckol | Ecklonia cava sub sp. Stolonifera | [296] |
| Methanolic extract | Osmundaria obtusiloba | [297] | |
| Polysaccharide | Mazzaella canaliculate | [298] | |
| Fucoidan | Sargassum fusiforme | [299] | |
| Histidyl dipeptide | Acanthophora nayadiformis | [300] | |
| Aqueous extract | Hypnea musciformis | [301] | |
| Protein hydrolysates | Gracilariopsis lemaneiformis | [302] | |
| Crude phlorotannin | Ecklonia stolonifera | [303] | |
| Crude phlorotannin | Eisenia bicyclis | [303] | |
| Aqueous extract | Laminaria ochroleuca | [304] | |
| Polyphenol | Ascophyllum nodosum | [305] | |
| Polyphenol | Pelvetia canaliculate | [305] | |
| Polyphenol | Fucus spiralis | [305] | |
| Polyphenol | Ulva intestinalis | [305] | |
| Polyphenol | Saccharina japonica | [306] | |
| Anti-allergic | Chlorophyll c2 | Sargassum horneri | [307] |
| Phlorotannin | Fucus sp. | [308] | |
| Methanolic extract | Sargassum hemiphyllum | [309] | |
| Methanolic extract | Polyopes affinis | [310] | |
| Aqueous extract | Ecklonia cava | [311] | |
| Seaweed powder | Eisenia arborea | [312] | |
| Antidiabetic | Aqueous extract | Halimeda macroloba | [313] |
| Oligosaccharide | Sargassum confusum | [314] | |
| Ethyl acetate extract | Ulva lactuca | [315] | |
| Polysaccharides | Sargassum sp. | [316] | |
| Aqueous extract | Halymenia durvillei | [317] | |
| Polysaccharide hydrolysates | Sargassum confusum | [318] | |
| Ethanolic extract | Lessonia nigrescens | [319] | |
| Anticoagulant | Flavonoid | Sargassum cristaefolium | [320] |
| Sulfated polysaccharide | Gelidiella acerosa | [321] | |
| Polysaccharide | Sargassum fusiforme | [322] | |
| Ulvans | Ulva lactuca | [323] | |
| Sulfated polysaccharide | Ecklonia cava | [324] | |
| Antiviral | Sulfated polysaccharide | Dictyota bartayesiana | [325] |
| Fucoidans | Nizamuddinia zanardinii | [326] | |
| Fucoidans | Fucus distichus subsp. Evanescens | [327] | |
| Phlorotannin | Ecklonia cava | [328] | |
| Crude extract | Canistrocarpus cervicornis | [329] | |
| Crude extract | Osmundaria obtusiloba | [330] | |
| Antiproliferative | Polyphenol | Ulva reticulata | [331] |
| Ethanolic extract | Egregia menziesii | [332] | |
| Fucoidan | Nizamuddinia zanardinii | [333] | |
| Phlorotannin | Bifurcaria bifurcate | [334] | |
| Fucoxanthin | Phaeodactylum tricornutum | [335] | |
| Sulfated polysaccharide | Sargassum cinereum | [336] | |
| Immunomodulator | Sulfated polysaccharides | Ulva prolifera | [337] |
| Fucoidans | Lobophora variegate | [338] | |
| b-1,3/1,6-glucan | Durvillaea antarctica | [339] | |
| Fucoidans | Agarum clathratum | [340] | |
| Enzymatic extract | Ecklonia cava | [341] | |
| Alginic acid | Sargassum wightii | [342] | |
| Antihypertensive | Phlorotannins | Ascophyllum nodosum | [343] |
| Chloroform: methanol extract | Sargassum wightii | [344] | |
| Aqueous extract | Ulva linza | [345] | |
| Protein content | Macrocystis pyrifera | [346] | |
| Fucopyranan | Sargassum wightii | [347] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.K.; Parida, S.; Behera, A.K.; Adhikary, S.P.; Lukatkin, A.A.; Lukatkin, A.S.; Jena, M. Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review. Pharmaceuticals 2025, 18, 367. https://doi.org/10.3390/ph18030367
Mandal AK, Parida S, Behera AK, Adhikary SP, Lukatkin AA, Lukatkin AS, Jena M. Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review. Pharmaceuticals. 2025; 18(3):367. https://doi.org/10.3390/ph18030367
Chicago/Turabian StyleMandal, Amiya Kumar, Sudhamayee Parida, Akshaya Kumar Behera, Siba Prasad Adhikary, Andrey A. Lukatkin, Alexander S. Lukatkin, and Mrutyunjay Jena. 2025. "Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review" Pharmaceuticals 18, no. 3: 367. https://doi.org/10.3390/ph18030367
APA StyleMandal, A. K., Parida, S., Behera, A. K., Adhikary, S. P., Lukatkin, A. A., Lukatkin, A. S., & Jena, M. (2025). Seaweed in the Diet as a Source of Bioactive Metabolites and a Potential Natural Immunity Booster: A Comprehensive Review. Pharmaceuticals, 18(3), 367. https://doi.org/10.3390/ph18030367









