Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells
Abstract
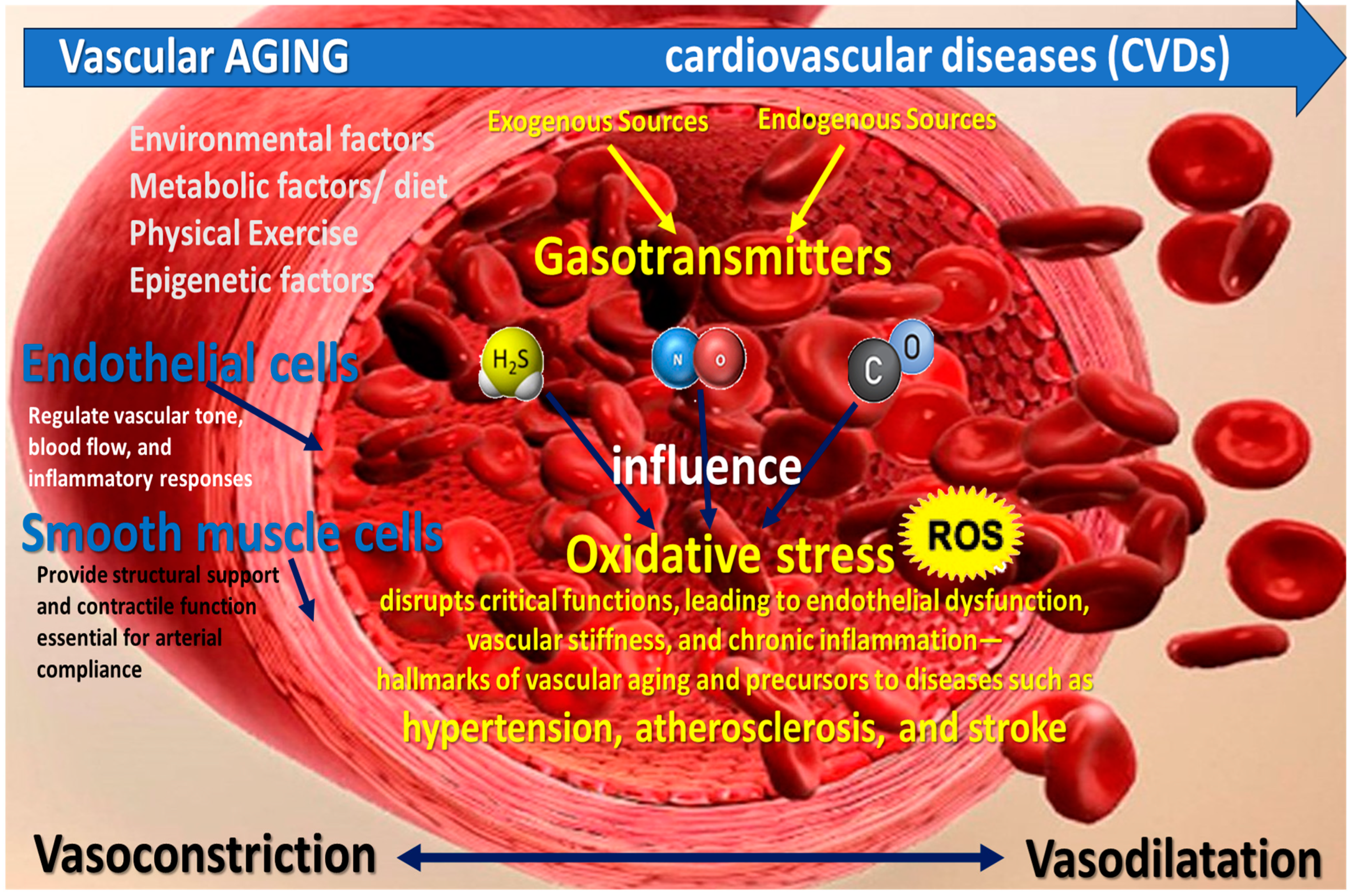
1. Introduction
2. Pathophysiology of Oxidative Stress in Vascular Aging
3. Cellular Senescence in Vascular Smooth Muscle Cells and Its Role in Vascular Stiffness
4. Gasotransmitters Biology and Mechanisms
5. Therapeutic Potential of Gasotransmitters in Vascular Aging
6. Emerging Therapeutic Strategies
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The Implications of Aging on Vascular Health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Cheng, D.C.Y.; Climie, R.E.; Shu, M.; Grieve, S.M.; Kozor, R.; Figtree, G.A. Vascular aging and cardiovascular disease: Pathophysiology and measurement in the coronary arteries. Front. Cardiovasc. Med. 2023, 10, 1206156. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related impairment of vascular structure and functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Bayraktutan, U. Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, J. Deciphering Oxidative Stress in Cardiovascular Disease Progression: A Blueprint for Mechanistic Understanding and Therapeutic Innovation. Antioxidants 2024, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, D.-J.; Jiang, Y.-J.; Tong, J.; Fu, H.; Wu, Y.-H.; Shen, F.-M. Vascular smooth muscle cell senescence and age-related diseases: State of the art. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; Pontes, L.V.d.S.; Júnior, J.F.d.S.; Gonçalves, T.A.F.; Dantas, S.H.; Feitosa, M.S.d.A.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, R.; Aoki, I.; Zhelev, Z.; Higashi, T. Cellular redox imbalance on the crossroad between mitochondrial dysfunction, senescence, and proliferation. Redox Biol. 2022, 53, 102337. [Google Scholar] [CrossRef]
- Čater, M.; Križančić Bombek, L. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.N.; Liu, H.; Wang, Y.; Wang, Y.; Hoogewijs, D.; Yan, L.-J. Redox Imbalance and Mitochondrial Abnormalities in Kidney Disease—Volume II. Biomolecules 2024, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.M.; Hu, Y.Y.; Yang, T.; Wu, N.; Wang, X.N. Reactive Oxygen Species and Oxidative Stress in Vascular-Related Diseases. Oxid. Med. Cell Longev. 2022, 2022, 7906091. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Cheng, W.; Du, Y.; Zhang, Q.; Wang, X.; He, C.; He, J.; Jing, F.; Ren, H.; Guo, M.; Tian, J.; et al. Age-related changes in the risk of high blood pressure. Front. Cardiovasc. Med. 2022, 9, 939103. [Google Scholar] [CrossRef]
- Thoma, A.; Akter-Miah, T.; Reade, R.L.; Lightfoot, A.P. Targeting reactive oxygen species (ROS) to combat the age-related loss of muscle mass and function. Biogerontology 2020, 21, 475–484. [Google Scholar] [CrossRef]
- Munteanu, C. Hydrogen Sulfide and Oxygen Homeostasis in Atherosclerosis: A Systematic Review from Molecular Biology to Therapeutic Perspectives. Int. J. Mol. Sci. 2023, 24, 8376. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Kinoshita, H.; Kawashima, S.; Kawahito, S. Human vascular smooth muscle function and oxidative stress induced by NADPH oxidase with the clinical implications. Cells 2021, 10, 1947. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Călin, M.A.; Manea, D.; Popescu, C.; Iliescu, M.; Ionescu, E.V.; Stanciu, L.; Minea, M.; Oprea, C.; Oprea, D.; et al. Current data regarding homeostasis of tissues oxygenation in pathophysiological and therapeutic circumstances. Balneo PRM Res. J. 2023, 14, 1. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative Stress and Vascular Smooth Muscle Cell Growth: A Mechanistic Linkage by Cyclophilin A. Antioxidants Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Avolio, A.P. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovasc. Res. 2018, 114, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Winner, G.J. Gasotransmitters: A review. Int. J. Basic. Clin. Pharmacol. 2023, 12, 607–615. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, S.; Cao, Y.; Kong, G.; Jiang, F.; Li, Y.; Wang, Q.; Tang, M.; Zhang, Q.; Wang, Q.; et al. Gasotransmitters: Potential Therapeutic Molecules of Fibrotic Diseases. Oxidative Med. Cell Longev. 2021, 2021, 3206982. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.; Florio Pla, A.; Moccia, F.; Tanzi, F.; Munaron, L. Old and New Gasotransmitters in the Cardiovascular System: Focus on the Role of Nitric Oxide and Hydrogen Sulfide in Endothelial Cells and Cardiomyocytes. Curr. Pharm. Biotechnol. 2011, 12, 1406–1415. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, J. New approach of modern pharmacology: From gasotransmitters to traditional mineral drugs. Med. Gas. Res. 2024, 15, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Loscalzo, J.; Brigham, J.L. Vascular-nitric-oxide: Formation-and-function. J. Blood Med. 2010, 1, 147–162. [Google Scholar]
- Bachschmid, M.M.; Schildknecht, S.; Matsui, R.; Zee, R.; Haeussler, D.; Cohen, R.A.; Pimental, D.; van der Loo, B. Vascular aging: Chronic oxidative stress and impairment of redox signaling—Consequences for vascular homeostasis and disease. Ann. Med. 2013, 45, 17–36. [Google Scholar] [CrossRef]
- Kaplish, D.; Vagha, J.D.; Meshram, R.J.; Lohiya, S. A Comprehensive Review of Inhaled Nitric Oxide Therapy: Current Trends, Challenges, and Future Directions. Cureus 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.-I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Munteanu, D.; Hoteteu, M.; Iliescu, M.G.; Ionescu, E.V.; Stanciu, L.; Oprea, D.; Minea, M.; Oprea, C.; et al. Biological Evaluation of Balneotherapeutic Mud and Sulfurous Mineral Waters: Insights from In Vivo and In Vitro Studies. Balneo PRM Res. J. 2024, 15, 702. [Google Scholar] [CrossRef]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Liu, D.; Mao, Q.; Bauer, N.; Wang, B. Carbon Monoxide as a Potential Therapeutic Agent: A Molecular Analysis of Its Safety Profiles. J. Med. Chem. 2024, 67, 9789–9815. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y. Mechanisms and therapeutic targets of carbon monoxide poisoning: A focus on reactive oxygen species. Chem. Biol. Interact. 2024, 403, 111223. [Google Scholar] [CrossRef]
- Opoku-Damoah, Y.; Zhang, R.; Ta, H.T.; Xu, Z.P. Therapeutic gas-releasing nanomedicines with controlled release: Advances and perspectives. Exploration 2022, 2, 20210181. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.-S. Hydrogen sulfide from a NaHS source attenuates dextran sulfate sodium (DSS)-induced inflammation via inhibiting nuclear factor-κB. J. Zhejiang Univ. Sci. B 2016, 17, 209–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Davies, L.R.; Martin, S.M.; Coddington, W.J.; Miller, F.J.; Buettner, G.R.; Kerber, R.E. The nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP) increases free radical generation and degrades left ventricular function after myocardial ischemia—Reperfusion. Resuscitation 2003, 59, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kourti, M.; Jiang, W.G.; Cai, J. Aspects of Carbon Monoxide in Form of CO-Releasing Molecules Used in Cancer Treatment: More Light on the Way. Oxid. Med. Cell Longev. 2017, 2017, 9326454. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fang, Y.; Huang, X.; Qiao, R.; Quinn, J.F.; Davis, T.P. Engineering macromolecular nanocarriers for local delivery of gaseous signaling molecules. Adv. Drug Deliv. Rev. 2021, 179, 114005. [Google Scholar] [CrossRef]
- Yang, G.; Sener, A.; Ji, Y.; Pei, Y.; Pluth, M.D. Gasotransmitters in Biology and Medicine: Molecular Mechanisms and Drug Targets. Oxid. Med. Cell Longev. 2016, 2016, 4627308. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, E.; Comità, S.; Pagliaro, P.; Penna, C.; Mancardi, D. Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet? Int. J. Mol. Sci. 2023, 24, 12480. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- Nowak, K.L.; Rossman, M.J.; Chonchol, M.; Seals, D.R. Strategies for achieving healthy vascular aging. Hypertension 2018, 71, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Innovative Therapeutic Strategies for Cardiovascular Disease. EXCLI J. 2023, 22, 690–715. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin De Chantemèle, E.J.; Fulton, D.J.R.; Fukai, T. Interplay between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal 2021, 34, 1319–1354. [Google Scholar] [CrossRef]
- Zarkovic, N. Roles and functions of ROS and RNS in cellular physiology and pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Chen, J.H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Naish, E.; Wood, A.J.; Stewart, A.P.; Routledge, M.; Morris, A.C.; Chilvers, E.R.; Lodge, K.M. The formation and function of the neutrophil phagosome. Immunol. Rev. 2023, 314, 158–180. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular Redox Homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; Alsalamat, H.A.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Themed Section: Spotlight on Small Molecules in Cardiovascular Diseases. Br. J. Pharmacol. 2018, 175, 1279. [Google Scholar] [CrossRef]
- Obradovic, M.; Essack, M.; Zafirovic, S.; Sudar-Milovanovic, E.; Bajic, V.P.; Van Neste, C.; Trpkovic, A.; Stanimirovic, J.; Bajic, V.B.; Isenovic, E.R. Redox control of vascular biology. BioFactors 2020, 46, 246–262. [Google Scholar] [CrossRef]
- Zweier, J.L.; Ilangovan, G. Regulation of nitric oxide metabolism and vascular tone by cytoglobin. Antioxid. Redox Signal 2020, 32, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. Medcomm 2024, 5, e651. [Google Scholar] [CrossRef]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: Intertwined roads to neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 360203. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, A.; Madej, M.; Kasprzyk, A.; Doroszko, A. Role of the eNOS Uncoupling and the Nitric Oxide Metabolic Pathway in the Pathogenesis of Autoimmune Rheumatic Diseases. Oxid. Med. Cell Longev. 2020, 2020, 1417981. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, U.; Kaur, U.; Chakrabarti, S.S.; Sharma, P.; Agrawal, B.K.; Saso, L. Oxidative Stress, Neuroinflammation, and NADPH Oxidase: Implications in the Pathogenesis and Treatment of Alzheimer’s Disease. Oxid. Med. Cell Longev. 2021, 2021, 7086512. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Meijles, D.N.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef]
- Kwon, O.S.; Noh, S.G.; Park, S.H.; Andtbacka, R.H.I.; Hyngstrom, J.R.; Richardson, R.S. Ageing and endothelium-mediated vascular dysfunction: The role of the NADPH oxidases. J. Physiol. 2023, 601, 451–467. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Lee, J.E.; Yenari, M.A. NOX inhibitors—A promising avenue for ischemic stroke. Exp. Neurobiol. 2017, 26, 195–205. [Google Scholar] [CrossRef]
- Nazari, B.; Jaquet, V.; Krause, K.H. NOX family NADPH oxidases in mammals: Evolutionary conservation and isoform-defining sequences. Redox Biol. 2023, 66, 102851. [Google Scholar] [CrossRef]
- Burtenshaw, D.; Hakimjavadi, R.; Redmond, E.M.; Cahill, P.A. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants 2017, 6, 90. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef] [PubMed]
- Sacks, B.; Onal, H.; Martorana, R.; Sehgal, A.; Harvey, A.; Wastella, C.; Ahmad, H.; Ross, E.; Pjetergjoka, A.; Prasad, S.; et al. Mitochondrial targeted antioxidants, mitoquinone and SKQ1, not vitamin C, mitigate doxorubicin-induced damage in H9c2 myoblast: Pretreatment vs. co-treatment. BMC Pharmacol. Toxicol. 2021, 22, 49. [Google Scholar] [CrossRef]
- Pasini, A.M.F.; Cominacini, L. Effect of Antioxidant Therapy on Oxidative Stress In Vivo. Antioxidants 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Victor, V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antioxid. Redox Signal 2015, 22, 686–729. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska-Gęsiak, S.; Płóciniczak, A.; Wilemska-Kucharzewska, K.; Kokot, T.; Muc-Wierzgoń, M.; Wysocka, E. The relationship between plasma lipids, oxidant– antioxidant status, and glycated proteins in individuals at risk for atherosclerosis. Clin. Interv. Aging 2019, 14, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.S.; Apple, C.G.; Kannan, K.B.; Funk, Z.M.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Chronic stress induces persistent low-grade inflammation. Am. J. Surg. 2019, 218, 677–683. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Redman, L.M.; Heilbronn, L.K.; Smith, J.V.; Martin, C.K.; Rood, J.C.; Greenway, F.L.; Williamson, D.A.; Smith, S.R.; Ravussin, E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis 2009, 203, 206–213. [Google Scholar] [CrossRef]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2005, 103, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Redman, L.M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, C.-H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.; Silva, A.N.; de Oliveira, M.R. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid. Med. Cell Longev. 2012, 2012, 756132. [Google Scholar] [CrossRef] [PubMed]
- Broskey, N.T.; Marlatt, K.L.; Most, J.; Erickson, M.L.; Irving, B.A.; Redman, L.M. The Panacea of Human Aging: Calorie Restriction Versus Exercise. Exerc. Sport. Sci. Rev. 2019, 47, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Yazid, M.D.; Teoh, S.H.; Balan, S.S.; Shariff, H.; Kumar, J.; Bahari, H.; Embong, H. Dietary Polyphenols as a Protection against Cognitive Decline: Evidence from Animal Experiments; Mechanisms and Limitations. Antioxidants 2023, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A review. Int. J. Mol. Sci. 2020, 21, 140. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Lazaridis, A.; Dogan, S.; Fraenkel, E.; Tuna, B.G.; Mozos, I.; Vukicevic, M.; Yalcin, O.; Gopcevic, K. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8672. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Faragher, R.G.A. Cellular senescence: From growth arrest to immunogenic conversion. Age 2015, 37, 27. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The role of vascular smooth muscle cells in arterial remodeling: Focus on calcification-related processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [PubMed]
- Elmarasi, M.; Elmakaty, I.; Elsayed, B.; Elsayed, A.; Al Zein, J.; Boudaka, A.; Eid, A.H. Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell Physiol. 2024, 239, e31200. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Seawright, J.W.; Sreenivasappa, H.; Gibbs, H.C.; Padgham, S.; Shin, S.Y.; Chaponnier, C.; Yeh, A.T.; Trzeciakowski, J.P.; Woodman, C.R.; Trache, A. Vascular Smooth Muscle Contractile Function Declines with Age in Skeletal Muscle Feed Arteries. Front. Physiol. 2018, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Marcozzi, S.; Beltrami, A.P.; Malavolta, M. Molecular Mechanisms to Target Cellular Senescence in Aging and Disease. Cells 2022, 11, 3732. [Google Scholar] [CrossRef] [PubMed]
- Shreeya, T.; Ansari, M.S.; Kumar, P.; Saifi, M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Senescence: A DNA damage response and its role in aging and Neurodegenerative Diseases. Front. Aging 2024, 4, 1292053. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Feng, J.; Wang, J. Underlying Mechanisms and Treatment of Cellular Senescence-Induced Biological Barrier Interruption and Related Diseases. Aging Dis. 2024, 15, 612–639. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef]
- Stephens, K.E.; Miaskowski, C.A.; Levine, J.D.; Pullinger, C.R.; Aouizerat, B.E. Epigenetic Regulation and Measurement of Epigenetic Changes. Biol. Res. Nurs. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Dogaru, B.G.; Munteanu, C. The Role of Hydrogen Sulfide (H2S) in Epigenetic Regulation of Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12555. [Google Scholar] [CrossRef]
- Nyhan, D.; Steppan, J.; Barodka, V.; Berkowitz, D.E. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract. 2011, 2011, 263585. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular Stiffness in Aging and Disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef] [PubMed]
- Kajuluri, L.P.; Singh, K.; Morgan, K.G. Vascular aging, the vascular cytoskeleton and aortic stiffness. Explor. Med. 2021, 2, 186–197. [Google Scholar] [CrossRef]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular endothelial cells: Heterogeneity and targeting approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef]
- Charbonier, F.W.; Zamani, M.; Huang, N.F. Endothelial Cell Mechanotransduction in the Dynamic Vascular Environment. Adv. Biosyst. 2019, 3, e1800252. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflugers Arch. 2010, 459, 807–816. [Google Scholar] [CrossRef]
- Sandoo, A.; Veldhuijzen Van Zanten, J.J.C.S.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302. [Google Scholar] [CrossRef] [PubMed]
- Faraonio, R. Oxidative Stress and Cell Senescence Process. Antioxidants 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Ramprasath, T.; Zou, M.H. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid. Redox Signal 2021, 34, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H459–H476. [Google Scholar] [CrossRef]
- Lüneburg, N.; Harbaum, L.; Hennigs, J.K. The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases. Biomed. Res. Int. 2014, 2014, 501612. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G.; Szurszewski, J.H. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology 2014, 147, 303–313. [Google Scholar] [CrossRef]
- Bryan, N.S.; Lefer, D.J. Update on gaseous signaling molecules nitric oxide and hydrogen sulfide: Strategies to capture their functional activity for human therapeutics. Mol. Pharmacol. 2019, 96, 109–114. [Google Scholar] [CrossRef]
- Agarwal, S.R.; Clancy, C.E.; Harvey, R.D. Mechanisms Restricting Diffusion of Intracellular cAMP. Sci. Rep. 2016, 6, 19577. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wang, Y.; Wu, L.; Yang, G. Gasotransmitter signaling in energy homeostasis and metabolic disorders. Free Radic. Res. 2021, 55, 83–105. [Google Scholar] [CrossRef]
- Gantner, B.N.; LaFond, K.M.; Bonini, M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020, 34, 101550. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, U.; Brzozowski, T.; Magierowski, M. Synergisms, Discrepancies and Interactions between Hydrogen Sulfide and Carbon Monoxide in the Gastrointestinal and Digestive System Physiology, Pathophysiology and Pharmacology. Biomolecules 2020, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon monoxide poisoning: Pathogenesis, management, and future directions of therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal 2009, 2, re2. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Tain, Y.-L. Gasotransmitters for the therapeutic prevention of hypertension and kidney disease. Int. J. Mol. Sci. 2021, 22, 7808. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Shared signaling pathways among gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon monoxide and nitric oxide as examples of the youngest class of transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef]
- Gorini, F.; Del Turco, S.; Sabatino, L.; Gaggini, M.; Vassalle, C. H2S as a bridge linking inflammation, oxidative stress and endothelial biology: A possible defense in the fight against SARS-CoV-2 infection? Biomedicines 2021, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Rodkin, S.; Nwosu, C.; Sannikov, A.; Tyurin, A.; Chulkov, V.S.; Raevskaya, M.; Ermakov, A.; Kirichenko, E.; Gasanov, M. The Role of Gasotransmitter-Dependent Signaling Mechanisms in Apoptotic Cell Death in Cardiovascular, Rheumatic, Kidney, and Neurodegenerative Diseases and Mental Disorders. Int. J. Mol. Sci. 2023, 24, 6014. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in nrf2- and sirtuin-dependent maintenance of cellular Redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Iordan, D.A.; Hoteteu, M.; Popescu, C.; Postoiu, R.; Onu, I.; Onose, G. Mechanistic Intimate Insights into the Role of Hydrogen Sulfide in Alzheimer’s Disease: A Recent Systematic Review. Int. J. Mol. Sci. 2023, 24, 15481. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Myers, I.; Angelova, P.R. Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death. Antioxidants 2024, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Gasomediators (·NO, CO, and H2S) and their role in hemostasis and thrombosis. Clin. Chim. Acta 2015, 445, 115–121. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2014, 172, 1587–1606. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, S.E.; Borland, G.; Carter, R.N.; Morton, N.M.; Selman, C. Hydrogen sulfide in ageing, longevity and disease. Biochem. J. 2021, 478, 3485–3504. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, M.S.; Snyder, S.H. Hydrogen sulfide: A gasotransmitter of clinical relevance. J. Mol. Med. 2012, 90, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Suschek, C.V.; Feibel, D.; von Kohout, M.; Opländer, C. Enhancement of Nitric Oxide Bioavailability by Modulation of Cutaneous Nitric Oxide Stores. Biomedicines 2022, 10, 2124. [Google Scholar] [CrossRef]
- Pyrgidis, N.; Mykoniatis, I.; Haidich, A.-B.; Tirta, M.; Talimtzi, P.; Kalyvianakis, D.; Ouranidis, A.; Hatzichristou, D. The Effect of Phosphodiesterase-type 5 Inhibitors on Erectile Function: An Overview of Systematic Reviews. Front. Pharmacol. 2021, 12, 735708. [Google Scholar] [CrossRef]
- Wei, C.; Vanhatalo, A.; Black, M.I.; Blackwell, J.R.; Rajaram, R.; Kadach, S.; Jones, A.M. Relationships between nitric oxide biomarkers and physiological outcomes following dietary nitrate supplementation. Nitric Oxide 2024, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, Z.; Chen, Y.; Su, H.; Deng, X.; Liu, X.; Fan, Y. Delivery of nitric oxide in the cardiovascular system: Implications for clinical diagnosis and therapy. Int. J. Mol. Sci. 2021, 22, 12166. [Google Scholar] [CrossRef]
- Levy, R.J. Carbon monoxide pollution and neurodevelopment: A public health concern. Neurotoxicol. Teratol. 2015, 49, 31–40. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, Y.M. Regulation of Endothelial and Vascular Functions by Carbon Monoxide via Crosstalk with Nitric Oxide. Front. Cardiovasc. Med. 2021, 8, 649630. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, C.; Dias-Pedroso, D.; Soares, N.L.; Vieira, H.L.A. CO-mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 2020, 32, 101470. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Barale, C.; Melchionda, E.; Penna, C.; Pagliaro, P. Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide. Int. J. Mol. Sci. 2023, 24, 6107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response. Pharmacol. Rev. 2022, 74, 823–873. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Muhammad, N.; Niazi, K.U.K.; Hu, Y.; Wang, Y.; Wu, Y.; Sun, H.; Liu, R.; Dong, W.; Zhang, W.; et al. CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials 2019, 12, 1643. [Google Scholar] [CrossRef] [PubMed]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and its application in drug delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, Z.; Li, H.; Yang, Y.G.; Zhang, Y.; Sun, T. Nanocarriers for targeted drug delivery in the vascular system: Focus on endothelium. J. Nanobiotechnol. 2024, 22, 620. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Guo, W.; Hu, C.; Wang, Y.; Zhang, W.; Zhang, S.; Peng, J.; Wang, Y.; Wu, J. NO-releasing double-crosslinked responsive hydrogels accelerate the treatment and repair of ischemic stroke. Acta Pharm. Sin. B 2025, in press. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.S.; Alqabbani, A.A.; Aldayel, A.M. Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Motta, I.; Soccio, M.; Guidotti, G.; Lotti, N.; Pasquinelli, G. Hydrogels for Cardio and Vascular Tissue Repair and Regeneration. Gels 2024, 10, 196. [Google Scholar] [CrossRef]
- Estes Bright, L.M.; Wu, Y.; Brisbois, E.J.; Handa, H. Advances in nitric oxide-releasing hydrogels for biomedical applications. Curr. Opin. Colloid. Interface Sci. 2023, 66, 101704. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Matson, J.B. Hydrogels for Gasotransmitter Delivery: Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide. Macromol. Biosci. 2024, 24, e2300138. [Google Scholar] [CrossRef]
- Yuan, S.; Patel, R.P.; Kevil, C.G. Working with nitric oxide and hydrogen sulfide in biological systems. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, 403–415. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Z.; Hua, T.; Ji, G.; Wang, Z.; Liu, P.; Zhang, Y.; Li, S.; Chao, Y.; Qian, M.; et al. Targeted codelivery of nitric oxide and hydrogen sulfide for enhanced antithrombosis efficacy. Bioact. Mater. 2025, 48, 29–42. [Google Scholar] [CrossRef]
- Wang, R. Gasotransmitters: Growing pains and joys. Trends Biochem. Sci. 2014, 39, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoon, Y.R. Understanding the pharmacokinetics of prodrug and metabolite. Transl. Clin. Pharmacol. 2018, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Sato, W.; Kosugi, T.; Yamamoto, T.; Kimura, T.; Taniguchi, S.; Kojima, H.; Maruyama, S.; Imai, E.; Matsuo, S.; et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin. Exp. Nephrol. 2013, 17, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.H.; Rice, S.A. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules 2022, 27, 674. [Google Scholar] [CrossRef]
- Lazarus, L.S.; Benninghoff, A.D.; Berreau, L.M. Development of Triggerable, Trackable, and Targetable Carbon Monoxide Releasing Molecules. Acc. Chem. Res. 2020, 53, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Mouodi, S.; Hosseini, S.R.; Ghadimi, R.; Cumming, R.G.; Bijani, A.; Mouodi, M.; Pasha, Y.Z. Lifestyle Interventions to Promote Healthy Nutrition and Physical Activity in Middle-Age (40–60 Years) Adults: A Randomized Controlled Trial in the North of Iran. J. Res. Health Sci. 2019, 19, e00434. [Google Scholar] [PubMed]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Shefa, U.; Yeo, S.G.; Kim, M.-S.; Song, I.O.; Jung, J.; Jeong, N.Y.; Huh, Y. Role of Gasotransmitters in Oxidative Stresses, Neuroinflammation, and Neuronal Repair. Biomed. Res. Int. 2017, 2017, 1689341. [Google Scholar] [CrossRef] [PubMed]
- Van Den Born, J.C.; Hammes, H.P.; Greffrath, W.; Van Goor, H.; Hillebrands, J.L. Gasotransmitters in vascular complications of diabetes. Diabetes 2016, 65, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Kruzliak, P.; Kovacova, G.; Pechanova, O. Therapeutic potential of nitric oxide donors in the prevention and treatment of angiogenesis-inhibitor-induced hypertension. Angiogenesis 2013, 16, 289–295. [Google Scholar] [CrossRef]
- Hendriks, K.D.; Maassen, H.; van Dijk, P.R.; Henning, R.H.; van Goor, H.; Hillebrands, J.L. Gasotransmitters in health and disease: A mitochondria-centered view. Curr. Opin. Pharmacol. 2019, 45, 87–93. [Google Scholar] [CrossRef]
- Panchin, A.Y.; Ogmen, A.; Blagodatski, A.S.; Egorova, A.; Batin, M.; Glinin, T. Targeting multiple hallmarks of mammalian aging with combinations of interventions. Aging 2024, 16, 12073–12100. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Adams, J.C. Gaso-transmitters: Expanding the kinetic universe of cell signaling. Am. J. Physiol. Cell Physiol. 2017, 312, C1–C2. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gaseotransmitters: New frontiers for translational science. Sci. Transl. Med. 2010, 2, 59ps54. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Herrald, A.L.; Ambrogi, E.K.; Mirica, K.A. Electrochemical Detection of Gasotransmitters: Status and Roadmap. ACS Sens. 2024, 9, 1682–1705. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P.; Livanos, G.P. Pharmacology of the “gasotransmitters” NO, CO and H2S: Translational opportunities. Br. J. Pharmacol. 2015, 172, 1395. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Donald, J.A. Nervous control of circulation—The role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009, 111, 244–256. [Google Scholar] [CrossRef] [PubMed]
- McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and oxytocin systems in early life stress and cardiovascular disease. J. Clin. Med. 2021, 10, 3484. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol. 2013, 1, 313–318. [Google Scholar] [CrossRef]
- Wen, Y.-D.; Wang, H.; Kho, S.-H.; Rinkiko, S.; Sheng, X.; Shen, H.-M.; Zhu, Y.-Z. Hydrogen Sulfide Protects HUVECs against Hydrogen Peroxide Induced Mitochondrial Dysfunction and Oxidative Stress. PLoS ONE 2013, 8, e53147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, L.; Wang, W.; Gao, H. Nitric Oxide, Nitric Oxide Formers and Their Physiological Impacts in Bacteria. Int. J. Mol. Sci. 2022, 23, 10778. [Google Scholar] [CrossRef]
- Litvinova, L.; Atochin, D.N.; Fattakhov, N.; Vasilenko, M.; Zatolokin, P.; Kirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef]
- Pichette, J.; Gagnon, J. Implications of Hydrogen Sulfide in Glucose Regulation: How H2S Can Alter Glucose Homeostasis through Metabolic Hormones. Oxid. Med. Cell Longev. 2016, 2016, 3285074. [Google Scholar] [CrossRef]
- Althaus, M.; Clauss, W.G. Gasotransmitters: Novel regulators of ion channels and transporters. Front. Physiol. 2013, 4, 46416. [Google Scholar] [CrossRef]
- Pagliaro, P.; Weber, N.C.; Femminò, S.; Alloatti, G.; Penna, C. Gasotransmitters and noble gases in cardioprotection: Unraveling molecular pathways for future therapeutic strategies. Basic. Res. Cardiol. 2024, 119, 509–544. [Google Scholar] [CrossRef]
- Cai, H.; Wang, X. Effect of sulfur dioxide on vascular biology. Histol. Histopathol. 2021, 36, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, C.; Du, J.; Jin, H. Endogenous sulfur dioxide: A new member of gasotransmitter family in the cardiovascular system. Oxid. Med. Cell Longev. 2016, 2016, 8961951. [Google Scholar] [CrossRef] [PubMed]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; de Arenas-Arroyo, S.N.; Martínez-Vizcaíno, V. Accuracy of pulse wave velocity predicting cardiovascular and all-cause mortality. A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Wentland, A.L.; Grist, T.M.; Wieben, O. Review of MRI-based measurements of pulse wave velocity: A biomarker of arterial stiffness. Cardiovasc. Diagn. Ther. 2014, 4, 193–206. [Google Scholar] [CrossRef]
- Calin, M.A.; Manea, D.; Parasca, S.V.; Popescu, C.; Ionescu, E.V.; Munteanu, C. Hyperspectral imaging reveals that sapropelic mud therapy may improve local tissue oxygenation in elderly. Int. J. Biometeorol. 2024, 69, 591–604. [Google Scholar] [CrossRef]
- Sander, O.; Magnusson, B.; Ludwig, I.; Jullion, A.; Meille, C.; Lorand, D.; Bornkamp, B.; Hinder, M.; Kovacs, S.J.; Looby, M. A framework to guide dose & regimen strategy for clinical drug development. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1276–1280. [Google Scholar] [CrossRef]

| Gasotransmitter | Therapeutic Applications | Mechanism of Action | Delivery Systems | Clinical Development Status | Comparison with Alternative Therapies |
|---|---|---|---|---|---|
| Nitric Oxide (NO) | Cardiovascular diseases (hypertension, restenosis), wound healing, pulmonary hypertension | Vasodilation, inhibition of platelet aggregation, reduction of inflammation | NO-releasing nanoparticles, drug-eluting stents, hydrogels, NONOates | Phase II–III clinical trials for cardiovascular applications | Antioxidants reduce oxidative stress but do not directly improve endothelial function as NO does; NO has immediate vasodilatory effects |
| Hydrogen Sulfide (H2S) | Myocardial protection, ischemia-reperfusion injury, wound healing, neuroprotection | Antioxidant defense, mitochondrial protection, stimulation of angiogenesis | H2S-loaded nanoparticles, hydrogels, slow-releasing prodrugs (e.g., GYY4137) | Preclinical to early clinical trials | Antioxidants provide general cytoprotection, whereas H2S directly supports mitochondrial bioenergetics and promotes angiogenesis |
| Carbon Monoxide (CO) | Anti-inflammatory therapy, organ transplantation, ischemic stroke protection | Modulation of heme oxygenase pathway, reduction of pro-inflammatory cytokines, cytoprotection | CORMs, CO-releasing nanoparticles, photocontrolled delivery systems | Preclinical trials for inflammation and organ protection | Anti-inflammatory drugs like corticosteroids suppress inflammation but do not offer the cytoprotective effects of CO |
| Combination Therapy (NO + H2S) | Cardiovascular repair, chronic wound healing | Synergistic effects on vasodilation, inflammation reduction, and endothelial protection | Dual gasotransmitter hydrogels, combined nanoparticles | Preclinical studies | Combination antioxidant therapies (e.g., vitamin C and E) have limited synergy compared to the broader cellular effects of gasotransmitters |
| Balneotherapy (Mineral Springs) | Rheumatologic disorders, cardiovascular recovery, skin diseases, chronic inflammation | Natural sources of H2S and CO from sulfurous and carbonated waters, stimulation of antioxidant defense | Inhalation, immersion in mineral-rich baths, topical application of mineral mud | Traditional use; growing scientific evidence from clinical studies | Non-invasive and holistic, balneotherapy provides sustained exposure to low-dose gasotransmitters and minerals, complementing pharmacological approaches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells. Pharmaceuticals 2025, 18, 344. https://doi.org/10.3390/ph18030344
Munteanu C, Galaction AI, Onose G, Turnea M, Rotariu M. Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells. Pharmaceuticals. 2025; 18(3):344. https://doi.org/10.3390/ph18030344
Chicago/Turabian StyleMunteanu, Constantin, Anca Irina Galaction, Gelu Onose, Marius Turnea, and Mariana Rotariu. 2025. "Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells" Pharmaceuticals 18, no. 3: 344. https://doi.org/10.3390/ph18030344
APA StyleMunteanu, C., Galaction, A. I., Onose, G., Turnea, M., & Rotariu, M. (2025). Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells. Pharmaceuticals, 18(3), 344. https://doi.org/10.3390/ph18030344







