Phytotherapeutic Analysis of Chloroform-Based Fractions of Alstonia scholaris and Wrightia tinctoria Extracts Reveals Potent Anti-Psoriatic Activity: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis and Chemical Characterization of the Crude Extracts
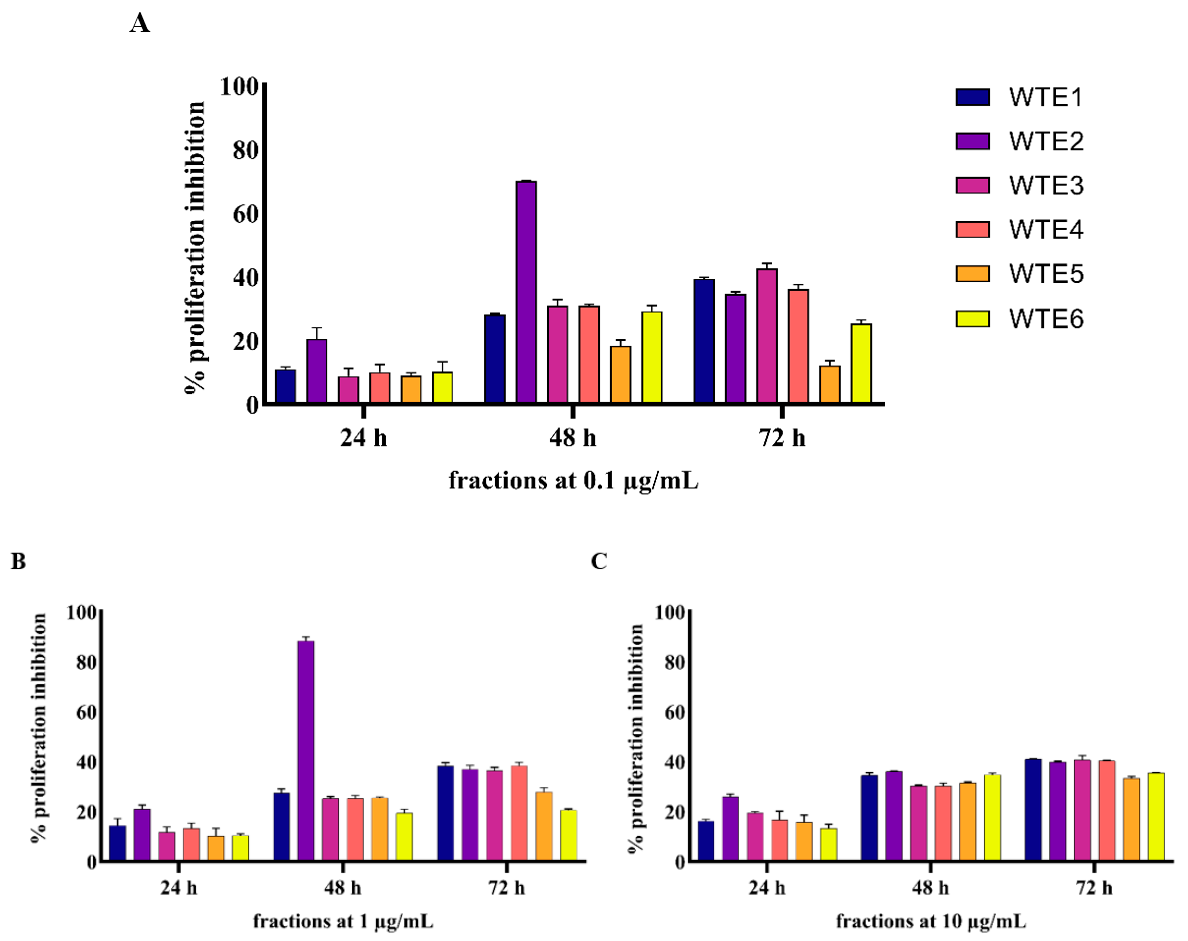
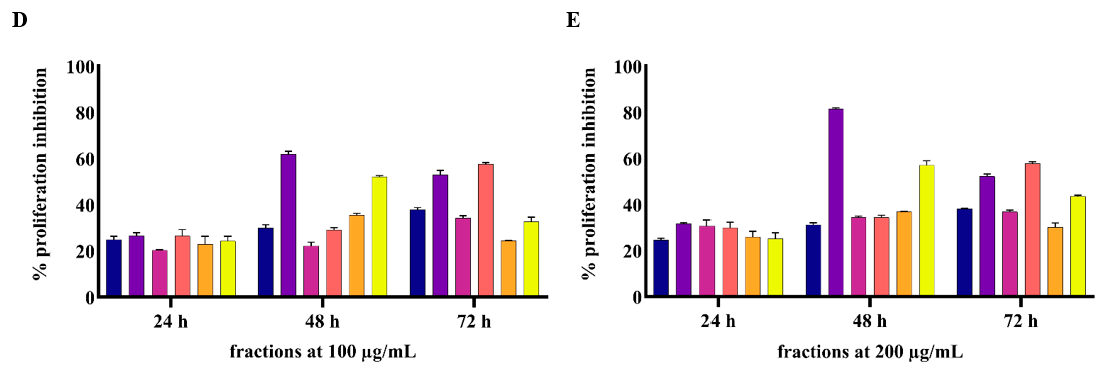
2.2. In Vitro Experiment Results
2.2.1. ASE and WTE Fractions Show Anti-Proliferative Effects on HaCaT Cells
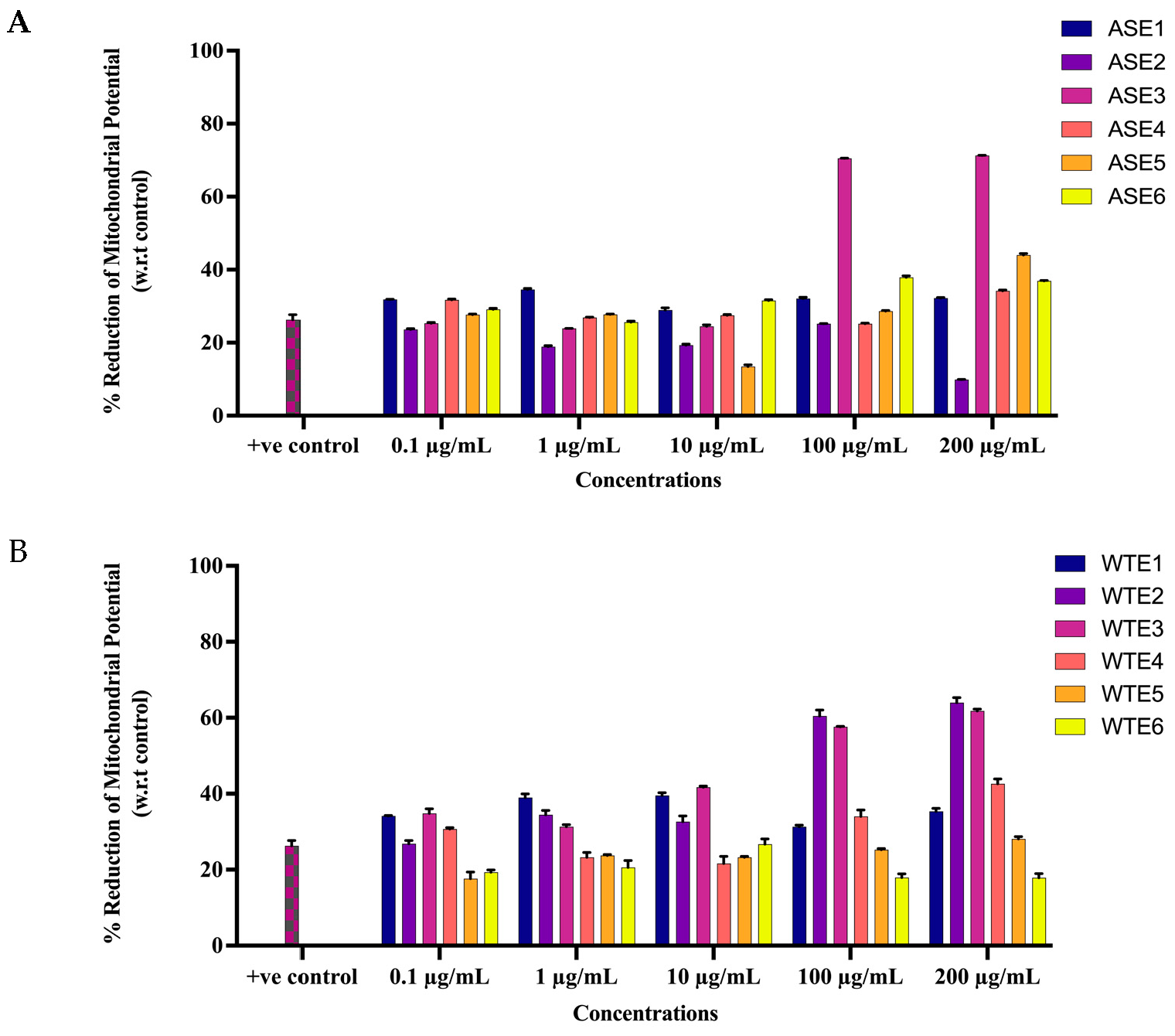
2.2.2. ASE and WTE Fractions Induced Activation of Apoptotic Response
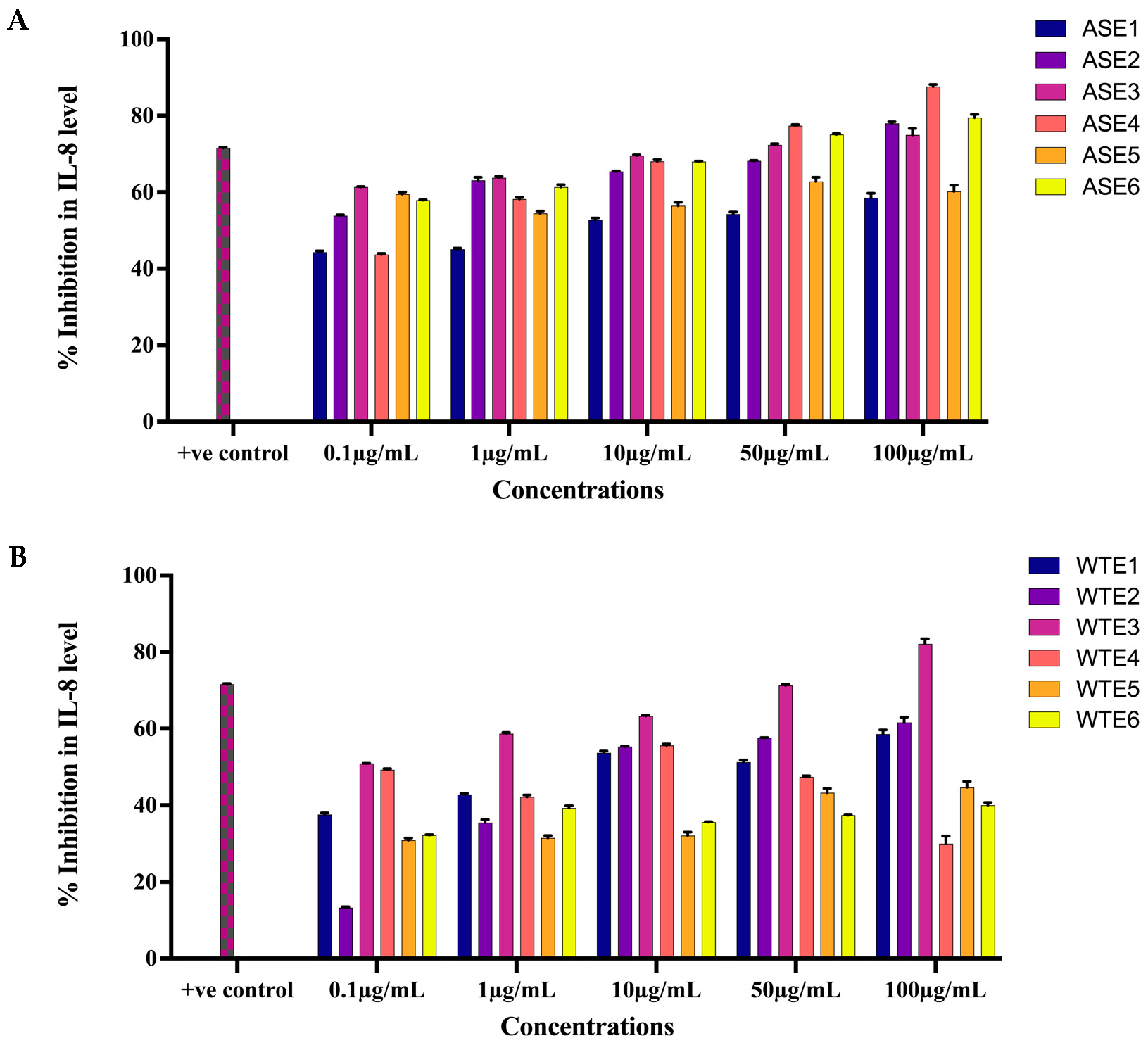
2.2.3. ASE and WTE Fractions Inhibited the Secretion of Inflammatory Mediator IL-8
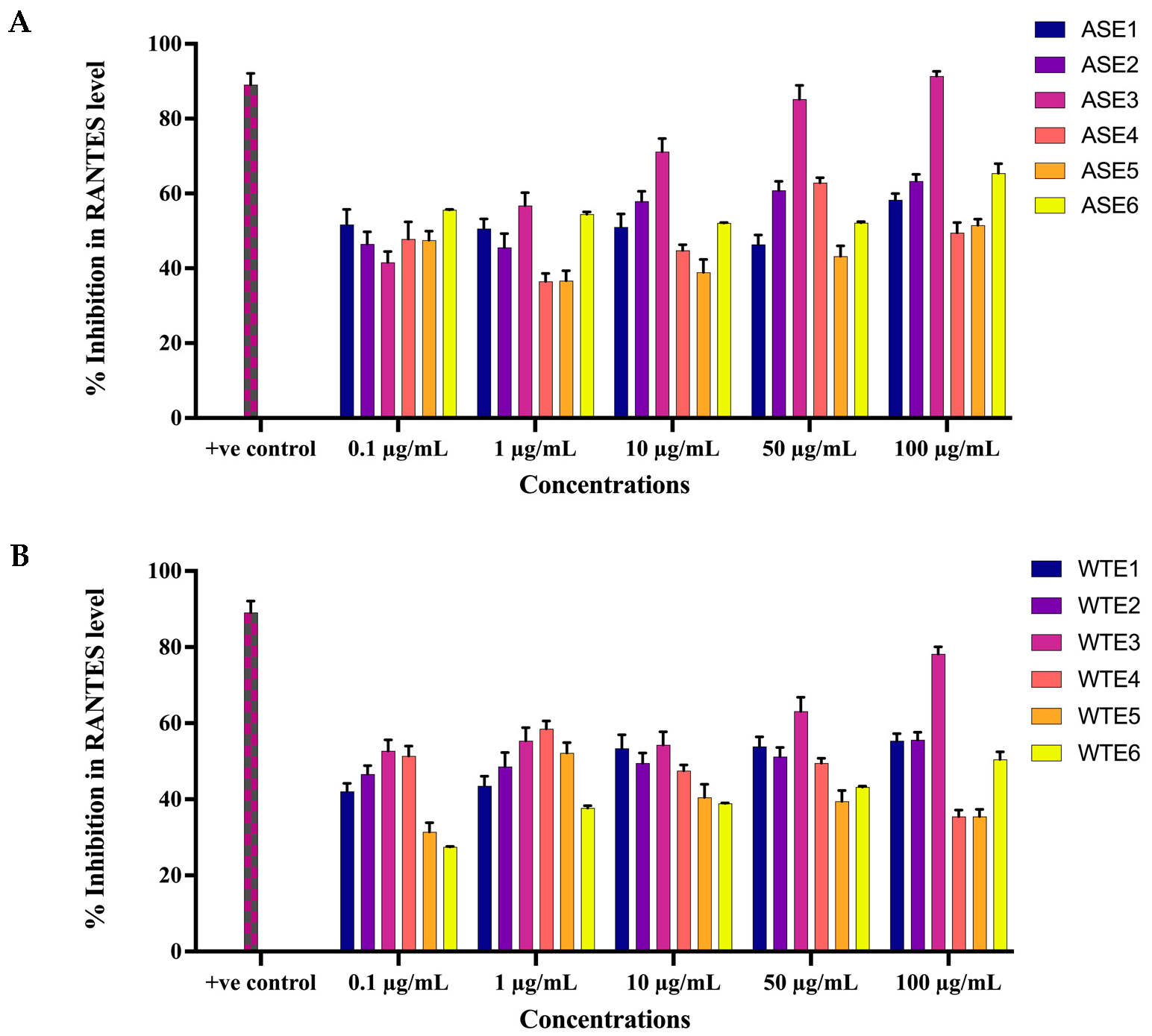
2.2.4. ASE and WTE Fractions Inhibited the Secretion of Inflammatory Mediator RANTES
2.3. In Vivo Experiment Results
2.3.1. Measurement of Body Weight and Skin Edema
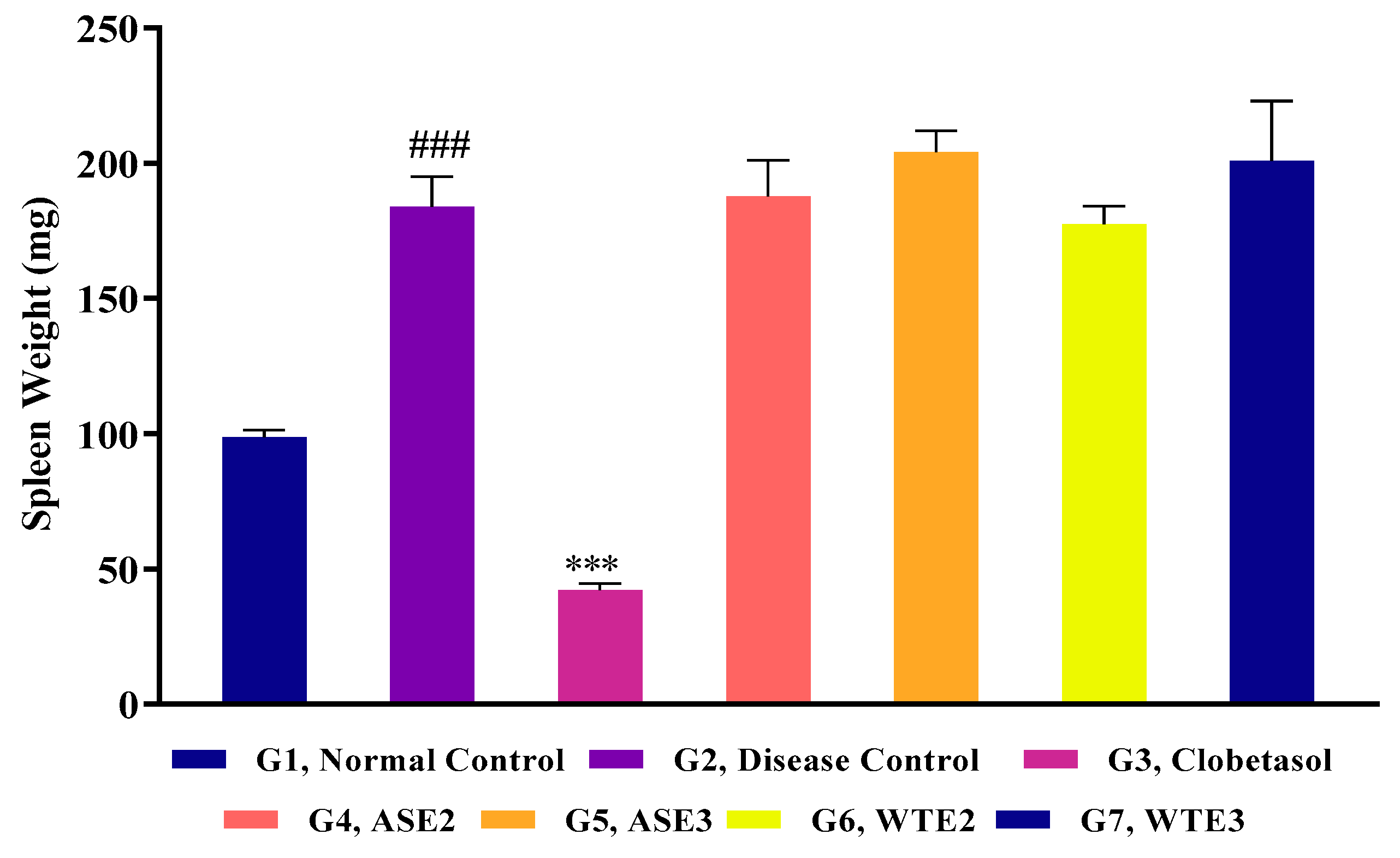
2.3.2. Splenomegaly Assessment
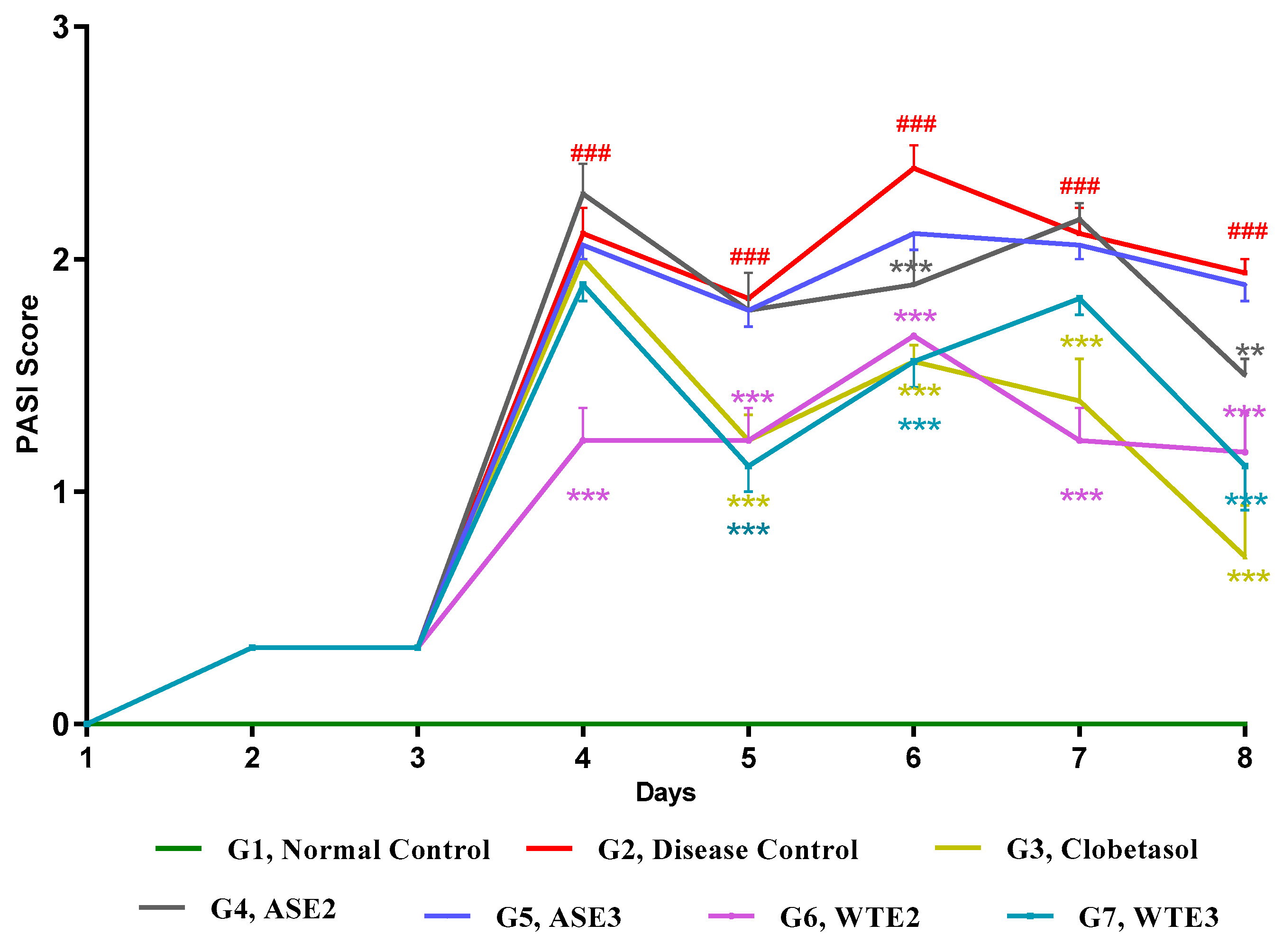
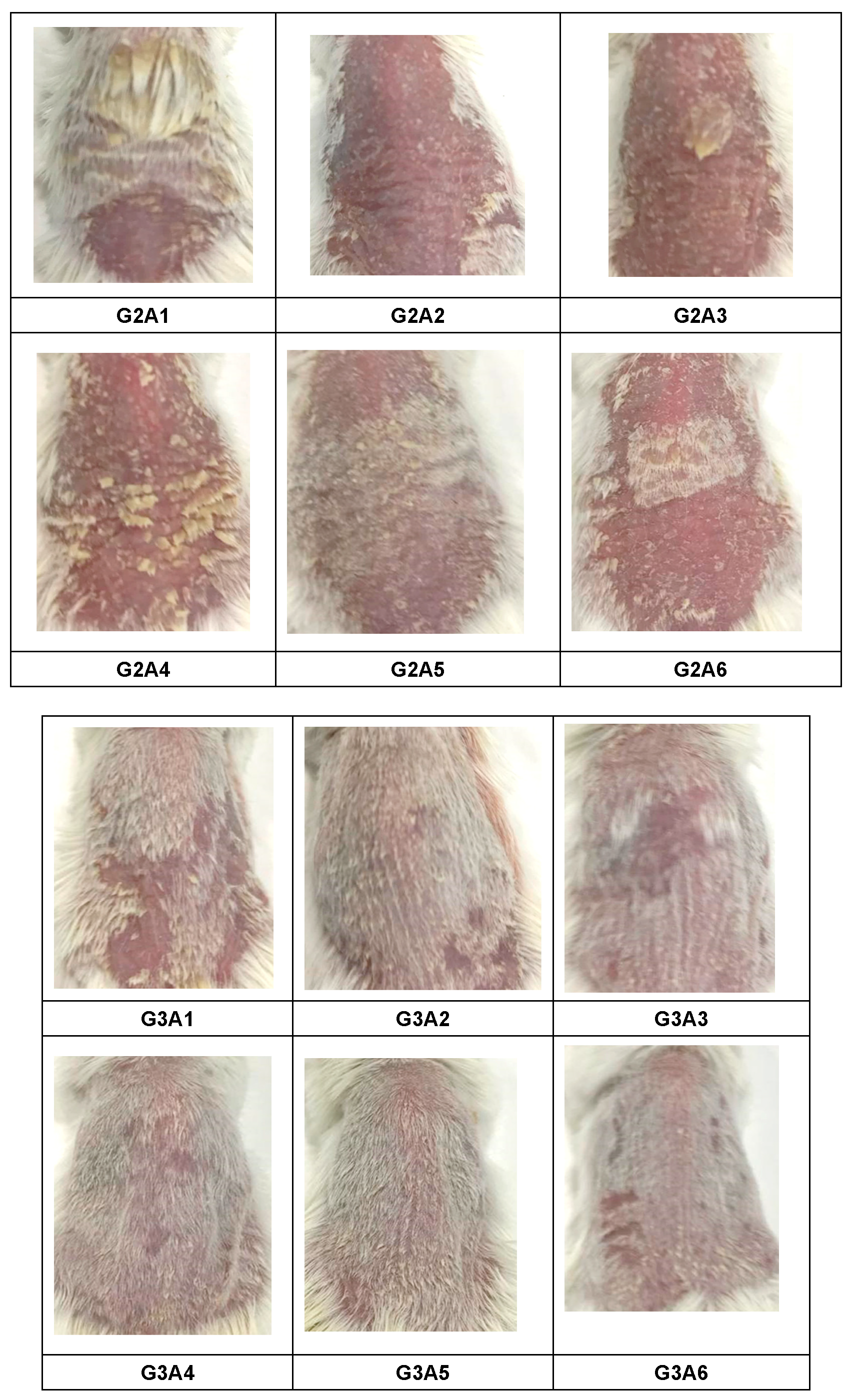
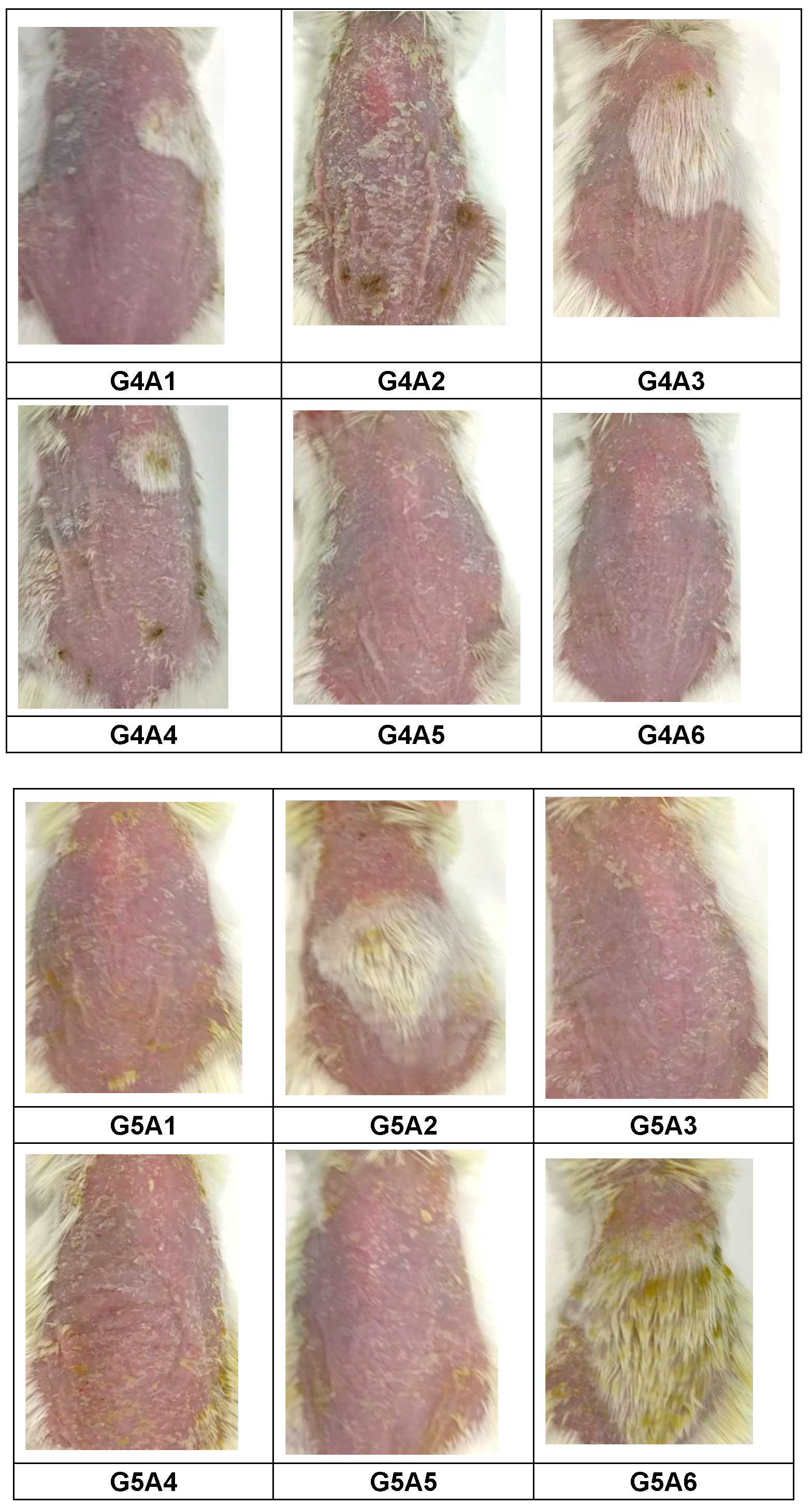
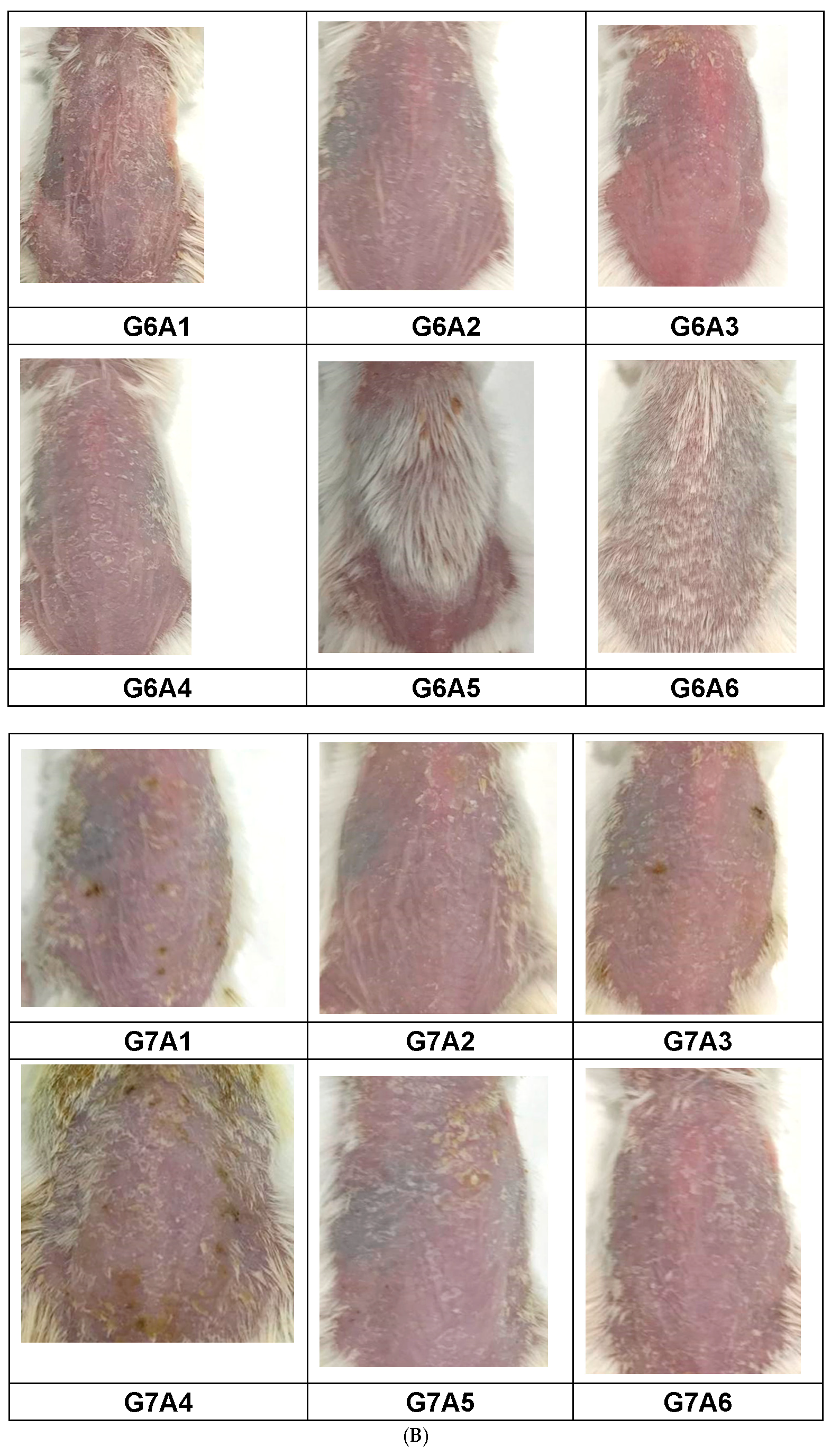
2.3.3. Assessment of PASI Score Post-Treatment
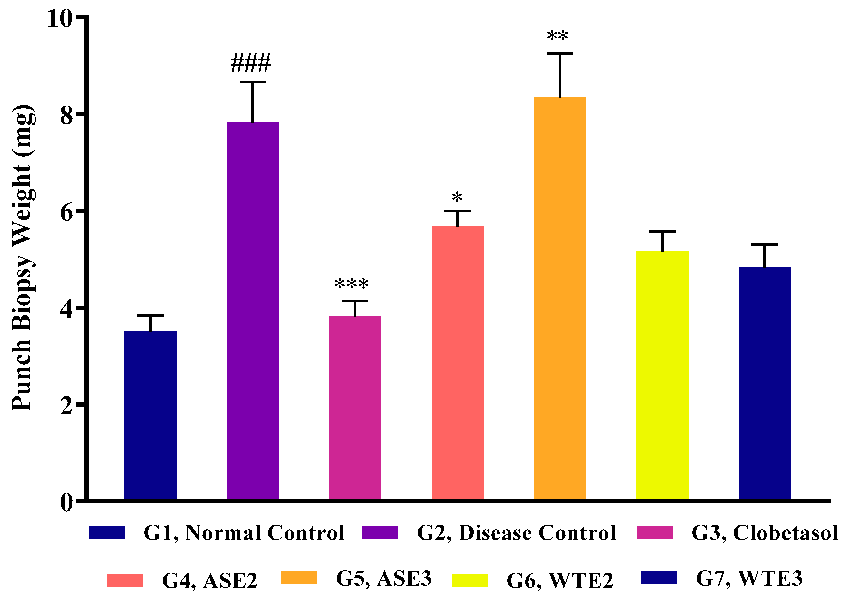
2.3.4. Assessment of Ear Punch Biopsy Weight Post-Treatment
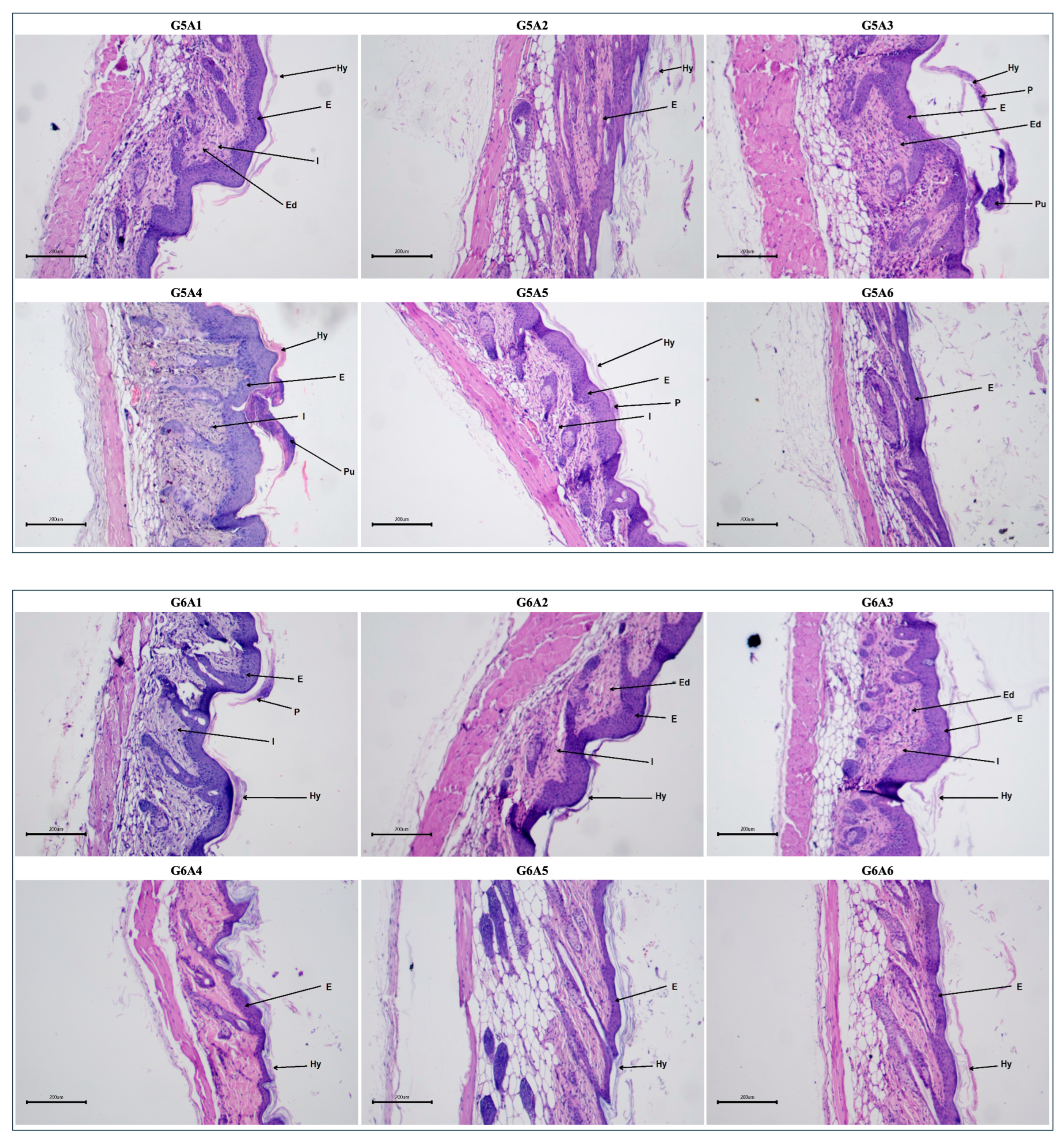
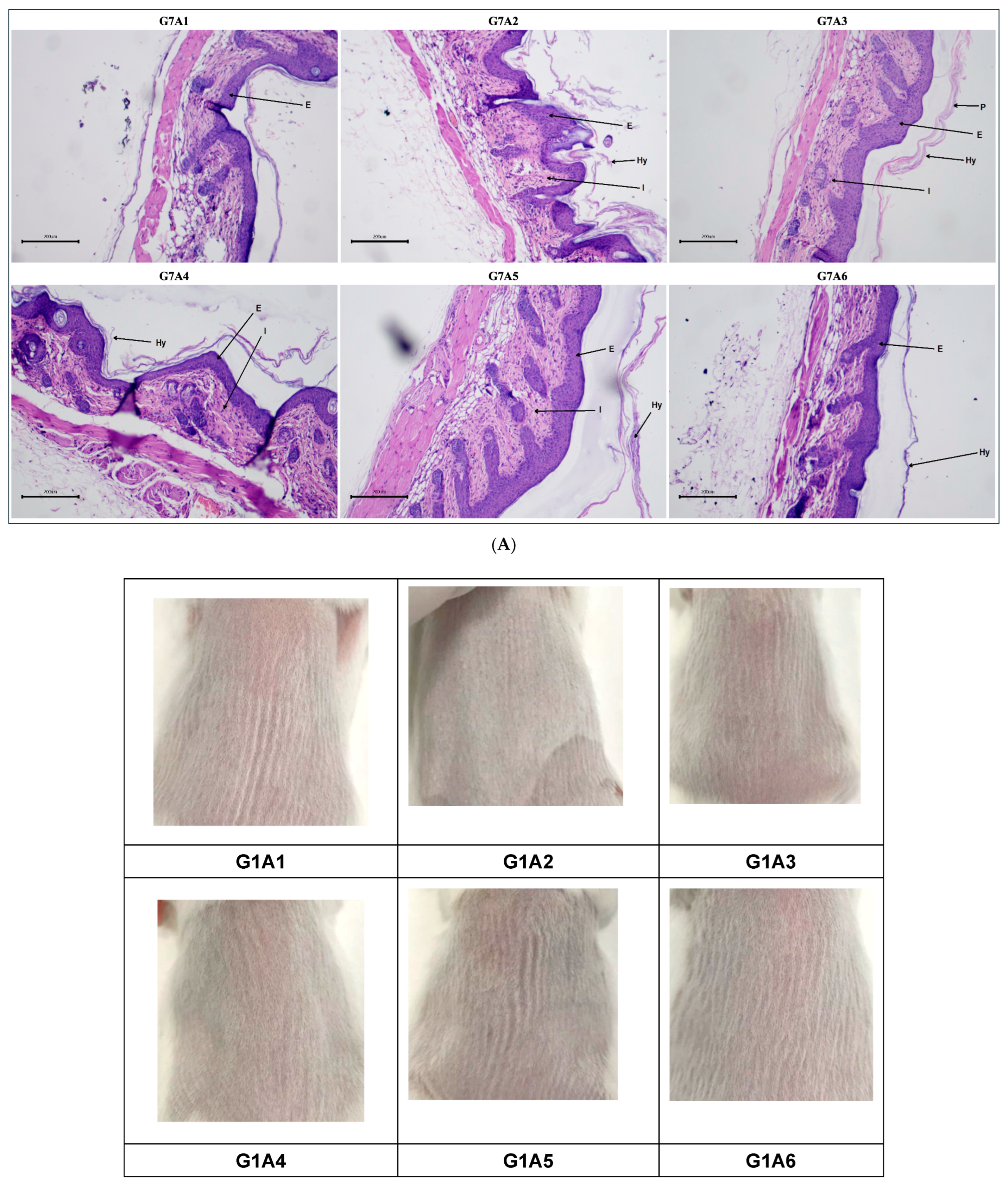
2.3.5. Histopathological Evaluation Post-Treatment
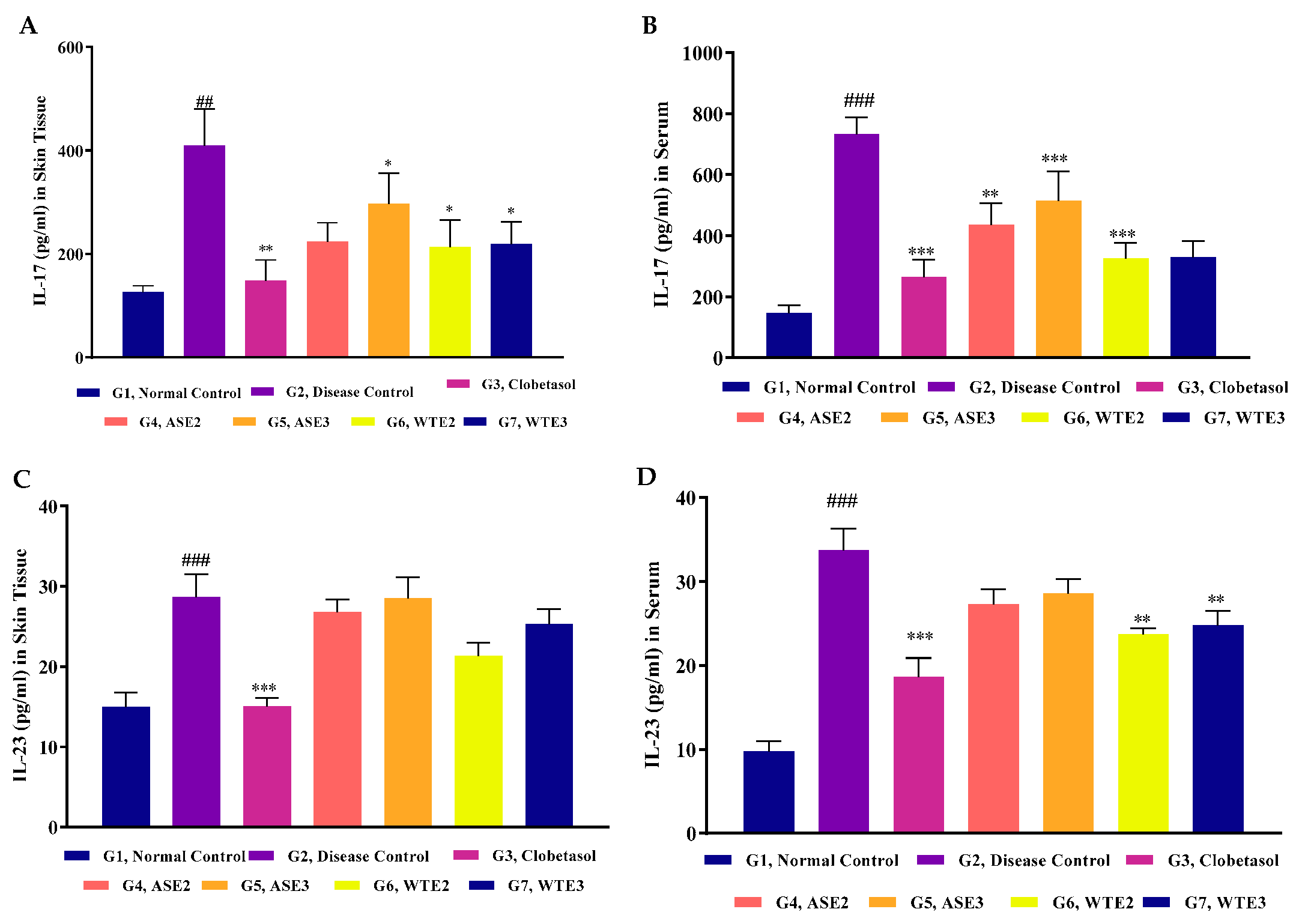
2.3.6. IL-23 and IL-17 Levels in Serum and Skin Homogenate
3. Discussion
4. Material and Methods
4.1. Plant Materials and Preparation of Extracts
4.2. Chemical Characterization of the Crude Extracts
4.3. In Vitro Assessment
4.3.1. Cell Culture
4.3.2. Anti-Proliferative Assay
4.3.3. Mitochondrial Depolarization Assay
4.3.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Inflammatory Markers
4.4. In Vivo Analysis
4.4.1. Mice
4.4.2. Preparation of Test Item Formulation/Hydrogels
4.4.3. Psoriasis Induction, Body Weight, Edema, and Spleen Weight Assessment
4.4.4. Scoring Severity Based on Erythema and Scaling
4.4.5. Histopathological Analysis
4.4.6. Inflammation Detection by ELISA
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASE1 | Fraction Prepared in 100% Chloroform of Ethanolic Extract of Alstonia scholaris |
| ASE2 | Fraction Prepared in 80% Chloroform and 20% Methanol of Ethanolic Extract of Alstonia scholaris |
| ASE3 | Fraction Prepared in 60% Chloroform and 40% Methanol of Ethanolic Extract of Alstonia scholaris |
| ASE4 | Fraction Prepared in 40% Chloroform and 60% Methanol of Ethanolic Extract of Alstonia scholaris |
| ASE5 | Fraction Prepared in 20% Chloroform and 80% Methanol of Ethanolic Extract of Alstonia scholaris |
| ASE6 | Fraction Prepared in 100% Methanol of Ethanolic Extract of Alstonia scholaris |
| CCSEA | Committee for Control and Supervision of Experiments on Animals |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl Sulphoxide |
| E | Ethanolic extract |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| HPLC | High-performance liquid chromatography |
| IAEC | Institutional Animal Ethics Committee |
| IMQ | Imiquimod |
| IL-8 | Interleukin-8 |
| IL-23 | Interleukin-23 |
| IL-17 | Interleukin-17 |
| JC-1 | 5,5,6,6′-tetrachloro- 1,1′,3,3′-tetraethylbenzimi-dazoylcarbocyanine iodide |
| MMP | Mitochondrial membrane potential |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| PASI | Psoriatic area severity index |
| PBS | Phosphate-buffered saline |
| Qd | Quaque die (daily) |
| RANTES | Regulated upon activation, normal T Cell expressed and presumably secreted |
| SEM | Standard error of the mean |
| TNF-α | Tumor necrosis factor alpha |
| WTE1 | Fraction Prepared in 100% Chloroform of Ethanolic Extract of Wrightia tinctoria |
| WTE2 | Fraction Prepared in 80% Chloroform and 20% Methanol of Ethanolic Extract of Wrightia tinctoria |
| WTE3 | Fraction Prepared in 60% Chloroform and 40% Methanol of Ethanolic Extract of Wrightia tinctoria |
| WTE4 | Fraction Prepared in 40% Chloroform and 60% Methanol of Ethanolic Extract of Wrightia tinctoria |
| WTE5 | Fraction Prepared in 20% Chloroform and 80% Methanol of Ethanolic Extract of Wrightia tinctoria |
| WTE6 | Fraction Prepared in 100% Methanol of Ethanolic Extract of Wrightia tinctoria |
| µg | Microgram |
| µM | Micromolar |
| µL | Microliter |
| mg | Milligram |
| mL | Milliliter |
| mM | Millimolar |
| nm | Nanometer |
| mg/kg | milligram/kilogram |
| mm | millimeter |
| cm | Centimeter |
| °C | Degree Centigrade |
| % | Percentage |
References
- Gupta, R.; Debbaneh, M.G.; Liao, W. Genetic Epidemiology of Psoriasis. Curr. Dermatol. Rep. 2014, 3, 61–78. [Google Scholar] [CrossRef]
- Tiwari, V.; Brent, L.H. Psoriatic Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Das, R.P.; Jain, A.K.; Ramesh, V. Current Concepts in the Pathogenesis of Psoriasis. Indian J. Dermatol. 2009, 54, 7. [Google Scholar] [CrossRef] [PubMed]
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis—Current Concepts in Pathogenesis. Clin. Rev. Allergy. Immunol. 2024, 66, 164. [Google Scholar] [CrossRef]
- Henry, D.A. Side-Effects of Non-Steroidal Anti-Inflammatory Drugs. Baillieres Clin. Rheumatol. 1988, 2, 425–454. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Bird, H.; Giannoudis, P.V. Nonsteroidal Anti-Inflammatory Drugs: Prostaglandins, Indications, and Side Effects. Int. J. Interferon 2011, 3, 19–27. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-Inflammatory and Side Effects of Cyclooxygenase Inhibitors. Pharmacol. Rep. 2007, 59, 247. [Google Scholar]
- Yilmaz, O.; Pinto, J.P.; Torres, T. New and Emerging Oral Therapies for Psoriasis. Drugs Context 2024, 13, 2024-5-6. [Google Scholar] [CrossRef] [PubMed]
- Portarapillo, A.; Potestio, L.; Tommasino, N.; Lauletta, G.; Feo, F.; Salsano, A.; Fabbrocini, G.; Caso, F.; Megna, M. Novel Pharmacotherapies and Breakthroughs in Psoriasis Treatment: 2024 and beyond. Expert Opin. Pharmacother. 2024, 25, 1187–1198. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, S.A.; An, S.S.; Na, Y.J.; Park, N.H.; Kim, H.S.; Lee, C.W.; Kim, H.K.; Kim, E.K.; Jang, Y.P.; et al. Alstonia Scholaris R. Br. Significantly Inhibits Retinoid-Induced Skin Irritation In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 190370. [Google Scholar] [CrossRef]
- Sarma, N. Evidence and Suggested Therapeutic Approach in Psoriasis of Difficult-to-Treat Areas: Palmoplantar Psoriasis, Nail Psoriasis, Scalp Psoriasis, and Intertriginous Psoriasis. Indian J. Dermatol. 2017, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Albaghdadi, A. Current and Under Development Treatment Modalities of Psoriasis: A Review. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Maddi, S. Molecular Interaction Analysis of Phytochemicals as the Prospective Potential Multi-Target Inhibitors Related to Psoriasis and Other Autoimmune Disorders. J. Drug Des. Med. Chem. 2024, 10, 16–30. [Google Scholar] [CrossRef]
- Ojha, M.; Manocha, N.; Madaan, A.; Gupta, N.; Khurana, S.; Chaudhary, A.; Kumar, V.; Karthikeyan, G.; Toor, D. Anti-psoriatic potential of medicinal plants, Alstonia scholaris, Wrightia tinctoria, and Solanum xanthocarpum, using human HaCaT keratinocytes by multi-parametric analysis. J. Ethnopharmacol. 2024, 334, 118596. [Google Scholar] [CrossRef] [PubMed]
- Biswasroy, P.; Pradhan, D.; Sahu, D.K.; Rai, V.; Halder, J.; Rajwar, T.K.; Bhola, R.K.; Kar, B.; Ghosh, G.; Rath, G. Phytochemical investigation, structural elucidation, in silico study and anti-psoriatic activity of potent bioactive from Betula utilis. J. Biomol. Struct. Dyn. 2022, 41, 8093–8108. [Google Scholar] [CrossRef]
- Gaikwad, R.; Rathod, S.; Shinde, A. In-silico Study of Phytoconstituents from Tribulus terrestris as potential Anti-psoriatic agent. Asian J. Pharm. Res. 2022, 12, 267–274. [Google Scholar] [CrossRef]
- Khan, S.K.; Sawant, N.D.; Mehendale, P.C.; Desai, N.D. Topical Delivery of Extractive Phytoconstituents of Soyabean Seeds for Management of Psoriasis. Indian Drugs 2022, 59, 91–94. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Tripathy, C.S.; Abdulabbas, H.S.; Al-Mawlah, Y.H.; Payrol, J.A.; Asadollah, A.; Abdolmalliki, A.; Khan, A. Psoariasis Vrs Alstonia scholaris: Computational Validation as Functional Food. Asian J. Med. Biomed. 2024, 8, 73–85. [Google Scholar] [CrossRef]
- Akbar, S. Wrightia tinctoria R. Br. (Apocynaceae). In Handbook of 200 Medicinal Plants; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Chan, T.C.; Hawkes, J.E.; Krueger, J.G. Interleukin 23 in the Skin: Role in Psoriasis Pathogenesis and Selective Interleukin 23 Blockade as Treatment. Ther. Adv. Chronic. Dis. 2018, 9, 111. [Google Scholar] [CrossRef]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef] [PubMed]
- Chanprapai, P.; Kubo, I.; Chavasiri, W. Anti-Rice Pathogenic Microbial Activity of Persicaria sp. Extracts. Sci. Technol. Asia 2018, 23, 32–41. [Google Scholar] [CrossRef]
- Venuti, I.; Ceruso, M.; D’angelo, C.; Casillo, A.; Pepe, T. Antimicrobial Activity Evaluation of Pure Compounds Obtained from Pseudoalteromonas Haloplanktis against Listeria Monocytogenes: Preliminary Results. Ital. J. Food Saf. 2022, 11, 10320. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive Steroids Bearing Oxirane Ring. Biomedicines 2023, 11, 2237. [Google Scholar] [CrossRef]
- Kyoui, D.; Saito, Y.; Takahashi, A.; Tanaka, G.; Yoshida, R.; Maegaki, Y.; Kawarai, T.; Ogihara, H.; Suzuki, C. Antibacterial Activity of Hexanol Vapor In Vitro and on the Surface of Vegetables. Foods 2023, 12, 3097. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In Vitro Antimicrobial Activities and Mechanism of 1-Octen-3-Ol against Food-Related Bacteria and Pathogenic Fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, J.; Jia, Y.; Yang, X.; Yan, X.; Wu, L. Design, Synthesis, and Biological Evaluation of Quinolinedione-Linked Sulfonylpiperazine Derivatives as NQO1-Directed Antitumor Agents. Bioorg. Chem. 2023, 132, 106385. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Bȩbenek, E.; Chrobak, E.; Boryczka, S. 5,8-Quinolinedione Scaffold as a Promising Moiety of Bioactive Agents. Molecules 2019, 24, 4115. [Google Scholar] [CrossRef] [PubMed]
- Dank, C.; Ielo, L. Recent Advances in the Accessibility, Synthetic Utility, and Biological Applications of Aziridines. Org. Biomol. Chem. 2023, 21, 4553–4573. [Google Scholar] [CrossRef]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine Alkaloids as Potential Therapeutic Agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Lorenz, I.-P.; Sadowska, B.; Mucha, P. Metal Ion Complexes with Pyrazoles, Aziridines and Diaziridines—Synthesis and Biological Activity. Curr. Med. Chem. 2019, 26, 648–663. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Terent’ev, A.O.; Levitsky, D.O. Aziridine Alkaloids: Origin, Chemistry and Activity. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 977–1006. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, O.; Nawaz, F.; Alam, M.J.; Alam, P. Current Status of Pyrazole and Its Biological Activities. J. Pharm. Bioallied. Sci. 2016, 8, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Ayman Salkini, M.; Ibnouf Ahmed, E.O.; Yusufoglu, H.S. Evaluation of the Composition and in Vitro Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Cilantro (Coriandrum Sativum L. Leaves) Cultivated in Saudi Arabia (Al-Kharj). Saudi J. Biol. Sci. 2021, 28, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Jordaan, M.A.; Ebenezer, O. Biological Activities of Tetrahydroisoquinolines Derivatives. J. Org. Pharm. Chem. 2023, 21, 20–38. [Google Scholar] [CrossRef]
- Bagheri, G.; Mirzaei, M.; Mehrabi, R.; Sharifi-Rad, J. Cytotoxic and Antioxidant Activities of Alstonia scholaris, Alstonia venenata and Moringa oleifera Plants From India. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 31129. [Google Scholar] [CrossRef]
- Mahadevan, N.; Moorthy, K.; Perumal, P.; Raju, S.V. Pharmacognosy of leaves of Wrightia tinctoria R. Br. Anc. Sci. Life 1998, 18, 78–83. [Google Scholar] [PubMed]
- Srivastava, R. A Review on Phytochemical, Pharmacological, and Pharmacognostical Profile of Wrightia tinctoria: Adulterant of Kurchi. Pharmacogn. Rev. 2014, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1β and IL-6 production in mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Dogra, S.; Mahajan, R. Systemic Methotrexate Therapy for Psoriasis: Past, Present, and Future. Clin. Exp. Dermatol. 2016, 41, 820–826. [Google Scholar] [CrossRef]
- Guillaume, P.; Rupp, T.; Froget, G.; Goineau, S. Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model. Int. J. Mol. Sci. 2024, 25, 9254. [Google Scholar] [CrossRef]
- Chen, C.; Wu, N.; Duan, Q.; Yang, H.; Wang, X.; Yang, P.; Zhang, M.; Liu, J.; Liu, Z.; Shao, Y.; et al. C10orf99 Contributes to the Development of Psoriasis by Promoting the Proliferation of Keratinocytes. Sci. Rep. 2018, 8, 8590. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Thongrakard, V.; Tencomnao, T. Anti-Inflammatory and Anti-Proliferative Effects of Plant Extracts on Human Keratinocytes. J. Ethnopharmacol. 2016, 189, 161–171. [Google Scholar]
- Duan, L.; Chen, J.; Zhang, H.; Yang, H.; Zhang, D. Role of Keratinocyte Proliferation in Psoriasis Pathogenesis. Exp. Dermatol. 2020, 29, 891–898. [Google Scholar]
- Huang, X.; Li, X.; Mahavadi, P. Mitochondrial Dysfunction and Apoptosis Induction by Natural Compounds in Keratinocytes. Mol. Cell. Biochem. 2015, 409, 93–104. [Google Scholar]
- Lee, J.H.; Park, C.; Jeong, J.S.; Kim, G.Y.; Choi, Y.H. Caspase Activation and Apoptosis in Keratinocytes by Plant-Derived Compounds. Int. J. Mol. Med. 2016, 37, 1303–1310. [Google Scholar]
- Moriyama, H.; Asai, H.; Fukumoto, Y. Contact Inhibition and Cell Cycle Regulation in Confluent Keratinocyte Cultures. J. Dermatol. Sci. 2014, 74, 45–53. [Google Scholar]
- Balaji, S.; Watson, C.L.; Ranjan, A.; King, A. Metabolic Waste Accumulation and Its Impact on Cell Viability in Prolonged Cultures. J. Cell Biol. 2017, 216, 543–556. [Google Scholar]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. Curr. Drug Targets 2011, 12, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. Hormesis Provides a Generalized Quantitative Estimate of Biological Plasticity. J. Cell Sci. 2011, 124, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, J.; Liu, S. Oxidative Stress and Mitochondrial Dysfunction in Psoriasis. Redox Biol. 2017, 13, 308–314. [Google Scholar]
- Wang, X.; Zhang, Y.; Sun, G. Apoptosis Induction in Keratinocytes by Plant Extracts. Phytomedicine 2020, 68, 153–160. [Google Scholar]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Miśkiewicz, J.; Strzelec, K.; Wcisło-Dziadecka, D.; Strzałka-Mrozik, B. Apoptosis in Autoimmunological Diseases, with Particular Consideration of Molecular Aspects of Psoriasis. Med. Sci. Monit. 2020, 26, e922035. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-Induced Apoptosis in Neuronal Cells Is Mediated by Fas/FasL-Mediated Mitochondrial Apoptotic Signaling Pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef]
- Kutwin, M.; Woźniacka, A. Interleukins 20 and 8–Less Widely Known Cytokines in Psoriasis. Adv. Dermatol. Allergol./Postȩpy Dermatol. I Alergol. 2023, 40, 194. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Ogino, Y.; Sato, H.; Ohta, T.; Komoriya, K.; Nishioka, K.; Katayama, I. RANTES Expression in Psoriatic Skin, and Regulation of RANTES and IL-8 Production in Cultured Epidermal Keratinocytes by Active Vitamin D3 (Tacalcitol). Br. J. Dermatol. 1998, 138, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aderka, D.; Goldenberg, D. Clobetasol and Its Effects on Weight Gain in Inflammatory Disease Management. Clin. Pharmacol. Ther. 2018, 103, 988–998. [Google Scholar]
- Stern, S.D.; Mahalingam, S.; Kim, J. Body Weight Loss and Systemic Inflammation in the Psoriasis Model. J. Immunol. 2017, 198, 1234–1244. [Google Scholar]
- Mucsi, I.; Gábor, I.; Farkas, S. Mechanisms of Weight Loss in Chronic Inflammatory Diseases: The Role of Cachexia. Inflamm. Bowel Dis. 2014, 20, 123–136. [Google Scholar]
- Qadir, A.; Gupta, D.K.; Mir Najib Ullah, S.N.; Aqil, M.; Jahan, S.; Khan, N. Pinpoint and Stewardship of Psoriasis by Using Phytoconstituent-Based Novel Formulation. Curr. Bioact. Compd. 2023, 19, 12–30. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis Pathogenesis and the Development of Novel Targeted Immune Therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Weigert, C.; Röcken, M. Immunopathogenesis and Role of T Cells in Psoriasis. Clin. Dermatol. 2007, 25, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Prinz, J.C.; Gottlieb, A.B.; Kingo, K.; Vanaclocha, F.; You, R.; Burge, D.J. Secukinumab Administration by Pre-Filled Syringe Maintains Significant Efficacy in Moderate to Severe Plaque Psoriasis: Results from the CLEAR Study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1513–1522. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, J.; Dou, W.; Hu, J.-H. Curcumin Inhibits Proliferation of Interleukin-22-Treated HaCaT Cells. Int. J. Clin. Exp. Med. 2015, 8, 9580–9584. [Google Scholar]
- Brooks, M.M.; Neelam, S.; Fudala, R.; Gryczynski, I.; Cammarata, P.R. Lenticular Mitoprotection. Part A: Monitoring Mitochondrial Depolarization with JC-1 and Artifactual Fluorescence by the Glycogen Synthase Kinase-3β Inhibitor, SB216763. Mol. Vis. 2013, 19, 1406. [Google Scholar]
- Achoui, M.; Appleton, D.; Abdulla, M.A.; Awang, K.; Mohd, M.A.; Mustafa, M.R. In Vitro and In Vivo Anti-Inflammatory Activity of 17-O-Acetylacuminolide through the Inhibition of Cytokines, NF-ΚB Translocation and IKKβ Activity. PLoS ONE 2010, 5, e15105. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, H.; Din, F.U.; Malik, M.; Mukhtiar, M.; Choi, H.G.; Ur-Rehman, T.; Khan, G.M. A Cutback in Imiquimod Cutaneous Toxicity; Comparative Cutaneous Toxicity Analysis of Imiquimod Nanotransethosomal Gel with 5% Marketed Cream on the BALB/c Mice. Sci. Rep. 2022, 12, 14244. [Google Scholar] [CrossRef] [PubMed]
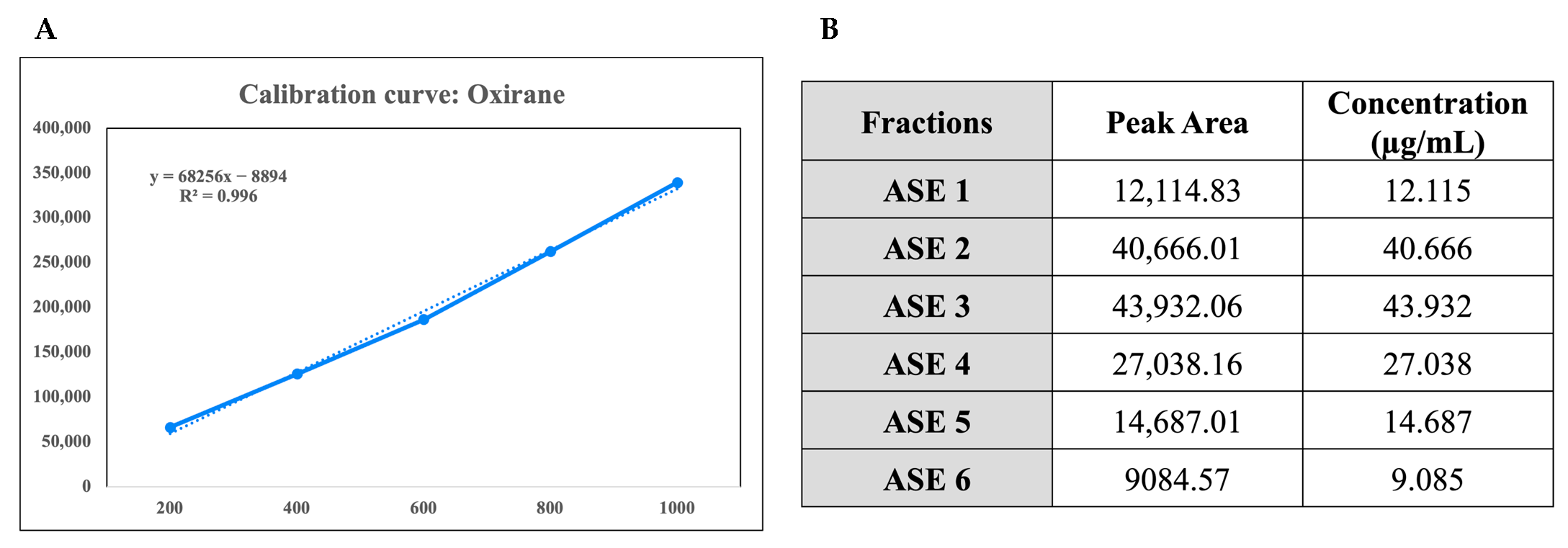
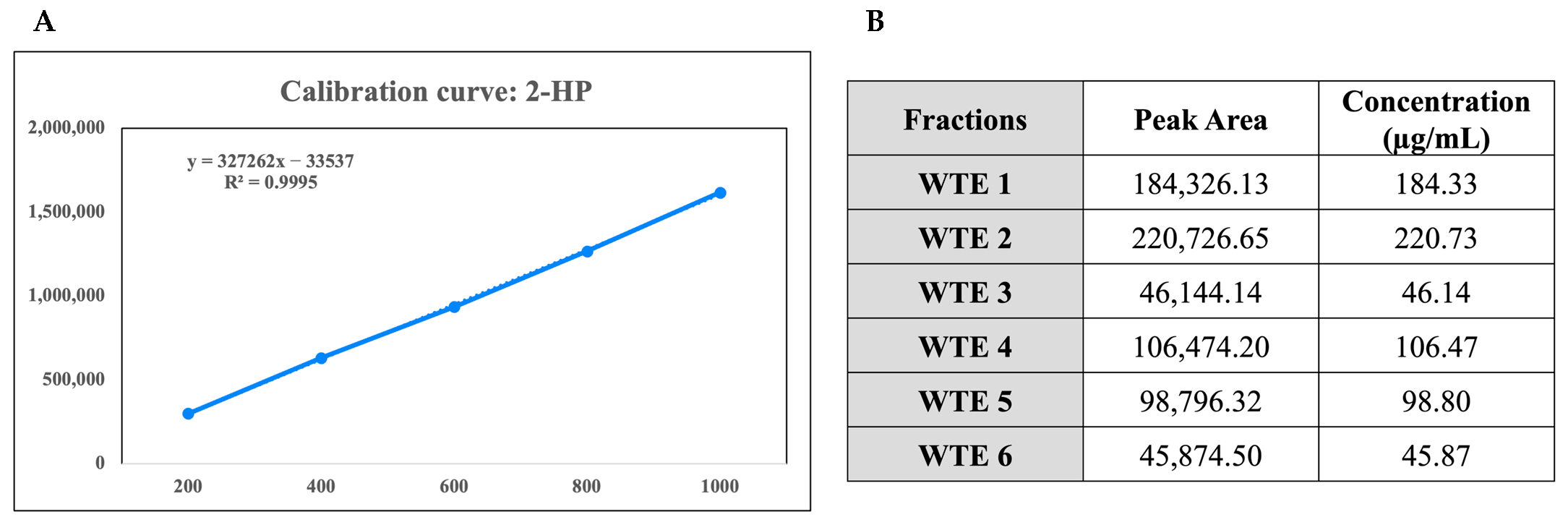




| RT | Compound Name | Molecular Formula | Molecular Weight |
|---|---|---|---|
| 12.43 | Oxirane | C12H24O | 184.182715 |
| Tetradecanal | C14H28O | 212.214016 | |
| Pentadecanal | C15H30O | 226.229666 | |
| Vinyl lauryl ether | C14H28O | 212.214016 | |
| Dodecanal | C12H24O | 184.182715 | |
| Undecane | C10H16N2O | 180.126264 | |
| Hexadecanal | C16H32O | 240.245316 | |
| Pentadecanal | C15H30O | 226.229666 | |
| Hexadecanal | C16H32O | 240.245316 | |
| 1,2-Epoxyundecane | C11H22O | 170.167066 | |
| 13.48 | 1-Ethoxypentan-3-ol | C7H16O2 | 132.115029 |
| Hexanol | C7H16O | 116.1201153 | |
| N-Acetyl-d-threo-O-methylthreonine | C7H13NO4 | 175.084458 | |
| 2-Butene | C8H16O2 | 144.115029 | |
| Silane | C8H20Si | 144.133428 | |
| 2-Acetylamino-3-hydroxy-propionic acid | C5H9NO4 | 147.053158 | |
| Oxirane | C6H12O2 | 116.0837297 | |
| Heptane | C7H14O | 114.104465 | |
| Oxirane | C5H10O2 | 102.0680795 | |
| Rhamnitol | C8H14O5 | 190.084124 | |
| 14.94 | Aziridine | C6H14N2 | 114.1156983 |
| Tris(aziridinomethyl)hydrazine | C9H19N5 | 197.164045 | |
| 5-Aziridinopentanol | C7H15NO | 129.115364 | |
| 5-Ethyl-3-nonanol | C11H24O | 172.182715 | |
| Quinolinedione | C25H38N2O3 | 414.288242 | |
| Methyl 5-piperidino-4-ketocaproate | C11H19NO3 | 213.136494 | |
| Oxirane | C11H22O2 | 186.16198 | |
| 1,3-Diethoxy-2-methylenepropane | C8H16O2 | 144.115029 | |
| 2-Butene | C8H16O2 | 144.115029 | |
| 15.11 | 1,3-Dioxane-2-propanol | C8H16O3 | 160.109944 |
| Carboxylic acid | C4H6O3 | 102.031694 | |
| Hexanol | C7H16O | 116.1201153 | |
| Dimethyldiaziridine | C7H17N3 | 143.142247 | |
| Oxirane | C5H10O2 | 102.0680795 | |
| 1-Tetradecanamine | C14H31N | 213.24565 | |
| Dimethyldiaziridine | C7H14N2O | 142.110613 | |
| 4-Oxopentyl formate | C6H10O3 | 130.062994 | |
| Heptanol | C9H20O | 144.151415 | |
| Heptane | C7H14O | 114.104465 |
| RT | Compound Name | Molecular Formula | Molecular Weight |
|---|---|---|---|
| 12.48 | N-[4-Aminobutyl]aziridine | C6H14N2 | 114.11 |
| 1,4,5,8-Tetraazadecalin | C6H14N4 | 142.12 | |
| Methyldicyanophosphine | C3H3N2P | 98 | |
| 5-Aziridinopentanol | C7H15NO | 129.11 | |
| 1,4-Butanediamine, N-(3-aminopropyl) | C7H19N3 | 145.15 | |
| 1,3-Diethoxy-2-methylenepropane | C8H16O2 | 144.11 | |
| Betaine | C5H11NO2 | 117.07 | |
| Tris(aziridinomethyl)hydrazine | C9H19N5 | 197.16 | |
| 4-Methyl-2-hexanol | C7H16O | 116.12 | |
| 3-Ethyl-2-heptanol | C9H20O | 144.15 | |
| 13.44 | 4(1H)-Pyridinone, 2,3-dihydro-1-methyl | C6H9NO | 111.06 |
| Pyrazol-4-amine | C5H9N3 | 111.07 | |
| 2(1H)-Pyridinone, 5-hydroxy | C5H5NO2 | 111.03 | |
| 2(1H)-Pyridinone, 3-hydroxy | C5H5NO2 | 111.03 | |
| N-Methylmaleimide | C5H5NO2 | 111.03 | |
| Tropinone, 6β-methoxy-, (+) | C9H15NO2 | 169.11 | |
| Quinuclidine | C7H13N | 111.1 | |
| 14.86 | (E)-Tetradec-2-enal | C14H26O | 210.19 |
| Vinyl lauryl ether | C14H28O | 212.21 | |
| 6-Dodecanol acetate | C14H28O2 | 228.2 | |
| 13-Methyltetradecanal | C15H30O | 226.22 | |
| Hexadecane, 1-(ethenyloxy) | C18H36O | 268.27 | |
| 3,7,11-Trimethyldodecylacetate | C17H34O2 | 270.25 | |
| (E)-Hexadec-2-enal | C16H30O | 238.22 | |
| 2-Tridecenal, (E) | C13H24O | 196.18 | |
| 1-Eicosanol | C20H42O | 298.32 | |
| Tridecanal | C13H26O | 198.19 | |
| 14.99 | (3S,5R,8aR)-3-(Hex-5-en-1-yl)-5-(pent-4-en-1-yl)octahydroindolizine | C19H33N | 275.26 |
| (5R,8aR)-3-(Hex-5-en-1-yl)-5-(pent-4-en-1-yl)octahydroindolizine | C19H33N | 275.26 | |
| 1-Methyl-3-[4-(1-trimethylsilyloxyethylidene)-cyclohexa-3,5-dienylidene]-triazene | C12H19N3OSi | 249.12 | |
| 6-Azaspiro[2.5]octa-4,7-diene-6-carboxylic acid, 2,2-dimethyl-, ethyl ester | C12H17NO2 | 207.12 | |
| Benzoic acid, 3-(diethylamino)-, methyl ester | C12H17NO2 | 207.12 | |
| Piperidine-2,6-dione, 2-oxo-2-[6-oxo-2-(1-pyrrolidinyl)cyclohexenyl]ethyl | C17H22N2O4 | 318.15 | |
| Pyrrolidine, N-(menth-3-en-3-yl) | C14H25N | 207.19 | |
| 3-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)propan-1-amine | C14H22N2O2 | 250.16 | |
| (+)-Salsolidine | C12H17NO2 | 207.12 | |
| 2,9-Dimethyl-4-ethynyl-trans-decahydroquinol-4-ol | C13H21NO | 207.16 |
| Groups | Treatment | Dose, Regimen and Route of Administration | Animals per Group |
|---|---|---|---|
| G1: Normal Control | Vehicle | 250 mg, qdx8, Topical | 6 |
| G2: Psoriasis Control | IMQ + Vehicle | ~63 mg, qdx8, Topical + 250 mg, qdx8, Topical | 6 |
| G3: Reference Item | IMQ + Clobetasol | ~63 mg, qdx8, Topical + 100 mg (0.1%), qdx8, Topical | 6 |
| G4: Test Item Treatment-1 | IMQ + ASE2 | ~63 mg, qdx8, Topical + 250 mg, qdx8, Topical | 6 |
| G5: Test Item Treatment-2 | IMQ + ASE3 | ~63 mg, qdx8, Topical + 250 mg, qdx8, Topical | 6 |
| G6: Test Item Treatment-3 | IMQ + WTE2 | ~63 mg, qdx8, Topical + 250 mg, qdx8, Topical | 6 |
| G7: Test Item Treatment-4 | IMQ + WTE3 | ~63 mg, qdx8, Topical + 250 mg, qdx8, Topical | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojha, M.; Manocha, N.; Kumar, V.; Karthikeyan, G.; Toor, D. Phytotherapeutic Analysis of Chloroform-Based Fractions of Alstonia scholaris and Wrightia tinctoria Extracts Reveals Potent Anti-Psoriatic Activity: An In Vitro and In Vivo Study. Pharmaceuticals 2025, 18, 304. https://doi.org/10.3390/ph18030304
Ojha M, Manocha N, Kumar V, Karthikeyan G, Toor D. Phytotherapeutic Analysis of Chloroform-Based Fractions of Alstonia scholaris and Wrightia tinctoria Extracts Reveals Potent Anti-Psoriatic Activity: An In Vitro and In Vivo Study. Pharmaceuticals. 2025; 18(3):304. https://doi.org/10.3390/ph18030304
Chicago/Turabian StyleOjha, Madhavi, Nilanshu Manocha, Vinod Kumar, Ganeshan Karthikeyan, and Devinder Toor. 2025. "Phytotherapeutic Analysis of Chloroform-Based Fractions of Alstonia scholaris and Wrightia tinctoria Extracts Reveals Potent Anti-Psoriatic Activity: An In Vitro and In Vivo Study" Pharmaceuticals 18, no. 3: 304. https://doi.org/10.3390/ph18030304
APA StyleOjha, M., Manocha, N., Kumar, V., Karthikeyan, G., & Toor, D. (2025). Phytotherapeutic Analysis of Chloroform-Based Fractions of Alstonia scholaris and Wrightia tinctoria Extracts Reveals Potent Anti-Psoriatic Activity: An In Vitro and In Vivo Study. Pharmaceuticals, 18(3), 304. https://doi.org/10.3390/ph18030304






