Drug Treatment Direction Based on the Molecular Mechanism of Breast Cancer Brain Metastasis
Abstract
1. Introduction
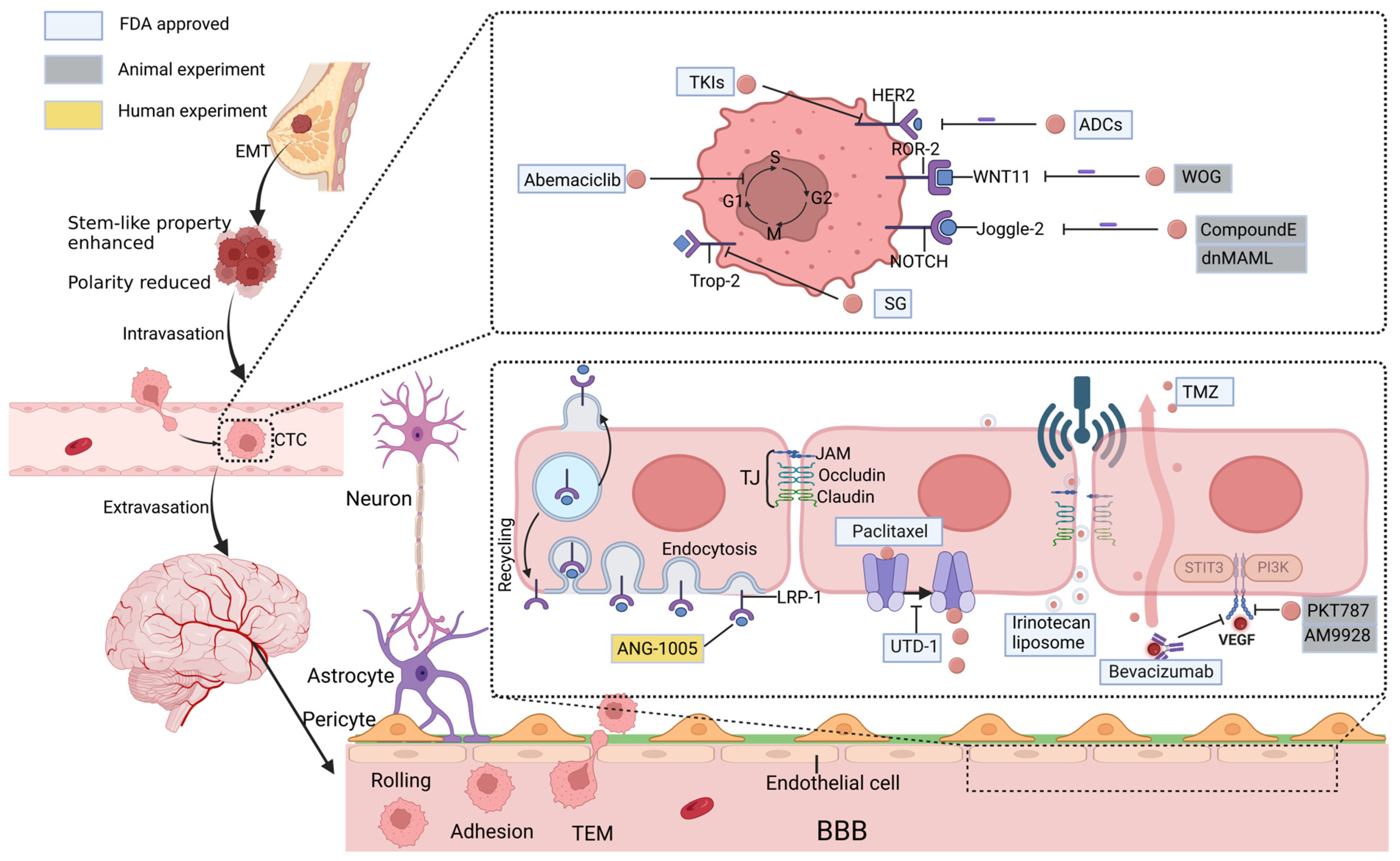
2. Mechanisms of Brain Metastasis
3. Therapeutic Strategies Based on Molecular Mechanisms of BCBM
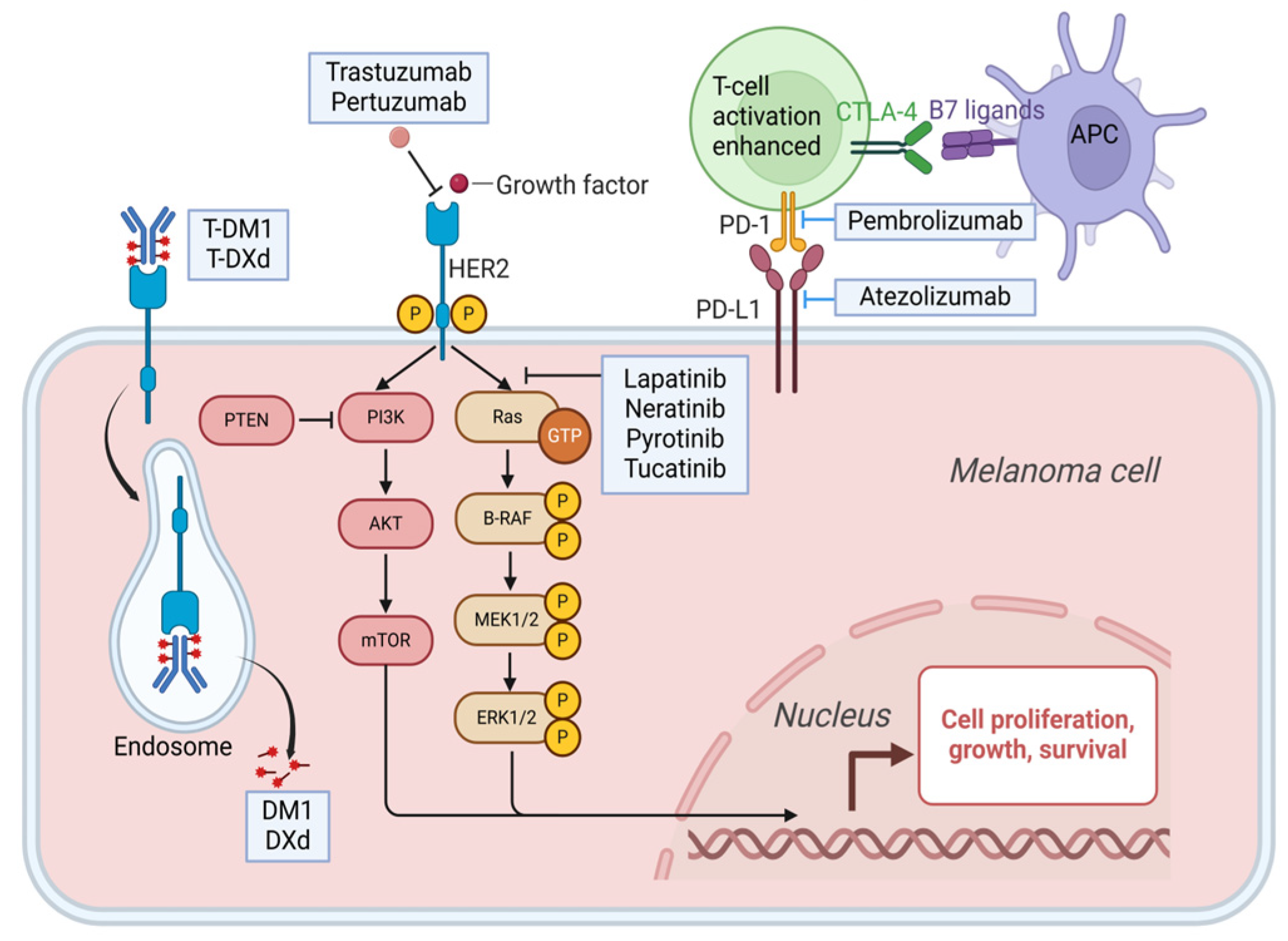
3.1. HER-2 Gene Amplification
3.1.1. Monoclonal Antibody
3.1.1.1. Trastuzumab
3.1.1.2. Pertuzumab
3.1.2. TKI
3.1.2.1. Lapatinib
3.1.2.2. Neratinib
3.1.2.3. Pyrotinib
3.1.2.4. Tucatinib
3.1.3. ADCs
3.1.3.1. T-DM1
3.1.3.2. T-DXd
3.2. Aberrant Signaling Pathway
3.2.1. WNT Signaling Pathway
3.2.2. NOTCH Signaling Pathway
3.2.3. CDK4/6 Pathway
3.2.4. Trophoblast Cell Surface Antigen-2 (Trop-2)
3.2.5. Programmed Cell Death Protein-1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1)
3.2.6. In Summary
4. Therapeutic Strategies Based on BBB Drug Delivery Pathways
4.1. Molecular Pathway
4.1.1. LRP-1
4.1.2. Overcoming the Efflux Pumps
4.1.3. VEGF
4.2. Physicochemical Pathway
4.3. Application of Nanoparticles
4.4. Direct Infiltration
5. Discussion
6. Future Direction
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | antibody-drug conjugate |
| BBB | blood-brain barrier |
| BC | breast cancer |
| BCBM | breast cancer brain metastases |
| BCC | breast cancer cell |
| BCRP | breast cancer resistance protein |
| BM | brain metastases |
| CDK4/6 | cycle protein-dependent kinase 4/6 |
| CNS | central nervous system |
| CRB | clinical benefit rate |
| CSC | cancer stem cell |
| CSF | cerebrospinal fluid |
| CTC | circulating tumor cell |
| cyclin D1 | cell cycle protein D1 |
| DDS | drug delivery system |
| EC | endothelial cell |
| EGF | epidermal growth factor |
| EMT | epithelial mesenchymal transition |
| FUS | Focused Ultrasound |
| ICAM-1 | Intercellular adhesion molecule-1 |
| JAMs | junctional adhesion molecules |
| LITT | laser-induced interstitial thermotherapy |
| LRP-1 | low-density lipoprotein receptor-related protein-1 |
| MBC | metastatic breast cancer |
| MMP | matrix metalloproteinase |
| NBD | nucleotide-binding structural domain |
| NVU | neurovascular unit |
| ORR | objective response rate |
| OS | overall survival |
| PFS | progression-free survival |
| P-gp | P-glycoprotein |
| PTT | photothermal therapy |
| RMT | receptor-mediated transcytosis |
| ROS | reactive oxidative species |
| SG | sacituzumab govitecan |
| TEM | transendothelial migration |
| TJs | tight junctions |
| TKI | tyrosine kinase inhibitor |
| TMZ | Temozolomide |
| TNBC | triple negative breast cancer |
| UTD-1 | Utidelone |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| WBRT | whole-brain radiotherapy |
References
- Riecke, K.; Müller, V.; Neunhöffer, T.; Park-Simon, T.-W.; Weide, R.; Polasik, A.; Schmidt, M.; Puppe, J.; Mundhenke, C.; Lübbe, K.; et al. Long-Term Survival of Breast Cancer Patients with Brain Metastases: Subanalysis of the BMBC Registry. ESMO Open 2023, 8, 101213. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Leone, J.; Vallejo, C.T.; Lin, N.U.; Leone, J.P. Survival Analysis of Patients with Brain Metastases at Initial Breast Cancer Diagnosis over the Last Decade. Breast Cancer Res. Treat. 2024, 205, 579–587. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Custódio-Santos, T.; Videira, M.; Brito, M.A. Brain Metastasization of Breast Cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.P. Epithelial-Mesenchymal Transition in Breast Cancer Progression and Metastasis. Chin. J. Cancer 2011, 30, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Neira, R.; Salazar, E.P. Native Type IV Collagen Induces an Epithelial to Mesenchymal Transition-like Process in Mammary Epithelial Cells MCF10A. Int. J. Biochem. Cell Biol. 2012, 44, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Ota, I.; Li, X.-Y.; Hu, Y.; Weiss, S.J. Induction of a MT1-MMP and MT2-MMP-Dependent Basement Membrane Transmigration Program in Cancer Cells by Snail1. Proc. Natl. Acad. Sci. USA 2009, 106, 20318–20323. [Google Scholar] [CrossRef] [PubMed]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.-Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage–Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef]
- Bolós, V.; Mira, E.; Martínez-Poveda, B.; Luxán, G.; Cañamero, M.; Martínez-A, C.; Mañes, S.; De La Pompa, J.L. Notch Activation Stimulates Migration of Breast Cancer Cells and Promotes Tumor Growth. Breast Cancer Res. 2013, 15, R54. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood–Brain Barrier in Health and Disease. Acta Neuropathol 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Lorger, M.; Lee, H.; Forsyth, J.S.; Felding-Habermann, B. Comparison of in Vitro and in Vivo Approaches to Studying Brain Colonization by Breast Cancer Cells. J. Neuro-Oncol. 2011, 104, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Lorger, M.; Felding-Habermann, B. Capturing Changes in the Brain Microenvironment during Initial Steps of Breast Cancer Brain Metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of Tumour- and Stroma-Supplied Proteolytic Networks Reveals a Brain-Metastasis-Promoting Role for Cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. The Blood–Tumour Barrier in Cancer Biology and Therapy. Nat. Reviews. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central Nervous System Pericytes in Health and Disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Beeghly, G.F.; Amofa, K.Y.; Fischbach, C.; Kumar, S. Regulation of Tumor Invasion by the Physical Microenvironment: Lessons from Breast and Brain Cancer. Annu. Rev. Biomed. Eng. 2022, 24, 29–59. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, P.; Kruh, G.D.; Vicini, P.; Wang, X.; Gallo, J.M. Predicting Human Tumor Drug Concentrations from a Preclinical Pharmacokinetic Model of Temozolomide Brain Disposition. Clin. Cancer Res. 2007, 13, 4271–4279. [Google Scholar] [CrossRef]
- Cao, K.I.; Lebas, N.; Gerber, S.; Levy, C.; Le Scodan, R.; Bourgier, C.; Pierga, J.-Y.; Gobillion, A.; Savignoni, A.; Kirova, Y.M. Phase II Randomized Study of Whole-Brain Radiation Therapy with or without Concurrent Temozolomide for Brain Metastases from Breast Cancer. Ann. Oncol. 2015, 26, 89–94. [Google Scholar] [CrossRef]
- Gaedcke, J.; Traub, F.; Milde, S.; Wilkens, L.; Stan, A.; Ostertag, H.; Christgen, M.; von Wasielewski, R.; Kreipe, H.H. Predominance of the Basal Type and HER-2/Neu Type in Brain Metastasis from Breast Cancer. Mod. Pathol. 2007, 20, 864–870. [Google Scholar] [CrossRef]
- Tomasik, B.; Bieńkowski, M.; Górska, Z.; Gutowska, K.; Kumięga, P.; Jassem, J.; Duchnowska, R. Molecular Aspects of Brain Metastases in Breast Cancer. Cancer Treat. Rev. 2023, 114, 102521. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New Insights into Molecular Mechanisms of Breast Cancer Metastasis. NPJ Precis. Onc. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and Downstream Pathways Are Involved in Colonization of Brain Metastases from Breast Cancer. Breast Cancer Res. 2010, 12, R46. [Google Scholar] [CrossRef]
- Raghav, K.P.; Moasser, M.M. Molecular Pathways and Mechanisms of HER2 in Cancer Therapy. Clin. Cancer Res. 2022, 29, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB Signaling from the Structure of the ErbB2-Pertuzumab Complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.; Villagrasa, P.; Pascual, T.; Oliveira, M.; Pernas, S.; Paré, L.; Escrivá-de-Romaní, S.; Manso, L.; Adamo, B.; Martínez, E.; et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020, 26, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet. Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Kimura, H.; Nishio, K.; Yamanaka, T.; Ito, Y.; Fukuoka, J.; Tsurutani, J.; Shigeoka, Y.; Uehara, M.; Sato, K.; et al. WJOG6110B (ELTOP): Randomized Phase II Trial Comparing Trastuzumab plus Capecitabine (HX) and Lapatinib plus Capecitabine (LX) in HER2-Positive Metastatic Breast Cancer Patients Previously Treated with Trastuzumab and Taxanes. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef]
- Yan, M.; Ouyang, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z.; Orlandi, A.; et al. Pyrotinib plus Capecitabine for Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases (PERMEATE): A Multicentre, Single-Arm, Two-Cohort, Phase 2 Trial. Lancet Oncol. 2022, 23, 353–361. [Google Scholar] [CrossRef]
- Curigliano, G.; Mueller, V.; Borges, V.; Hamilton, E.; Hurvitz, S.; Loi, S.; Murthy, R.; Okines, A.; Paplomata, E.; Cameron, D.; et al. Tucatinib versus Placebo Added to Trastuzumab and Capecitabine for Patients with Pretreated HER2+ Metastatic Breast Cancer with and without Brain Metastases (HER2CLIMB): Final Overall Survival Analysis. Ann. Oncol. 2021, 33, 321–329. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab Deruxtecan in HER2-Positive Breast Cancer with Brain Metastases: A Single-Arm, Phase 2 Trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results from DESTINY-Breast03, a Randomised, Open-Label, Phase 3 Trial. Lancet 2022, 401, 105–117. [Google Scholar] [CrossRef]
- Park, Y.H.; Park, M.J.; Ji, S.H.; Yi, S.Y.; Lim, D.H.; Nam, D.H.; Lee, J.-I.; Park, W.; Choi, D.H.; Huh, S.J.; et al. Trastuzumab Treatment Improves Brain Metastasis Outcomes through Control and Durable Prolongation of Systemic Extracranial Disease in HER2-Overexpressing Breast Cancer Patients. Br. J. Cancer 2009, 100, 894. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.-H.; Quah, C.; Lee, L.F.; Cortés, J. Incidence of Central Nervous System Metastases in Patients with HER2-Positive Metastatic Breast Cancer Treated with Pertuzumab, Trastuzumab, and Docetaxel: Results from the Randomized Phase III Study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef]
- Lin, N.U.; Diéras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.-J.; Roché, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter Phase II Study of Lapatinib in Patients with Brain Metastases from HER2-Positive Breast Cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.-Y.; Gonçalves, A.; et al. Lapatinib plus Capecitabine in Patients with Previously Untreated Brain Metastases from HER2-Positive Metastatic Breast Cancer (LANDSCAPE): A Single-Group Phase 2 Study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Meng, J.; Mei, X.; Mo, M.; Xiao, Q.; Han, X.; Zhang, L.; Shi, W.; Chen, X.; Ma, J.; et al. Brain Radiotherapy With Pyrotinib and Capecitabine in Patients With ERBB2-Positive Advanced Breast Cancer and Brain Metastases: A Nonrandomized Phase 2 Trial. JAMA Oncol. 2024, 10, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610. [Google Scholar] [CrossRef] [PubMed]
- Epaillard, N.; Bassil, J.; Pistilli, B. Current Indications and Future Perspectives for Antibody-Drug Conjugates in Brain Metastases of Breast Cancer. Cancer Treat Rev. 2023, 119, 102597. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab Emtansine (T-DM1) versus Lapatinib plus Capecitabine in Patients with HER2-Positive Metastatic Breast Cancer and Central Nervous System Metastases: A Retrospective, Exploratory Analysis in EMILIA. Ann. Oncol. 2014, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzàlez Farré, X.; You, B.; Saura, C.; et al. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov. 2022, 12, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; Jeon, H.-M.; Kim, S.; Kim, M.H.; Lee, Y.-J.; Lee, M.S.; Kim, H.; Joo, K.M.; Lee, D.-S.; Price, J.E.; et al. Activation of Notch Signaling in a Xenograft Model of Brain Metastasis. Clin. Cancer Res. 2008, 14, 4059–4066. [Google Scholar] [CrossRef]
- Klemm, F.; Bleckmann, A.; Siam, L.; Chuang, H.N.; Rietkötter, E.; Behme, D.; Schulz, M.; Schaffrinski, M.; Schindler, S.; Trümper, L.; et al. β-Catenin-Independent WNT Signaling in Basal-like Breast Cancer and Brain Metastasis. Carcinogenesis 2011, 32, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Farnie, G.; Clarke, R.B. Mammary Stem Cells and Breast Cancer—Role of Notch Signalling. Stem. Cell Rev. 2007, 3, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Jackson, K.W.; McNicholas, E.; Kawamura, M.J.; Abdallah, W.M.; Wicha, M.S. Role of Notch Signaling in Cell-Fate Determination of Human Mammary Stem/Progenitor Cells. Breast Cancer Res. 2004, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roarty, K.; Pfefferle, A.D.; Creighton, C.J.; Perou, C.M.; Rosen, J.M. Ror2-Mediated Alternative Wnt Signaling Regulates Cell Fate and Adhesion during Mammary Tumor Progression. Oncogene 2017, 36, 5958–5968. [Google Scholar] [CrossRef]
- Menck, K.; Heinrichs, S.; Wlochowitz, D.; Sitte, M.; Noeding, H.; Janshoff, A.; Treiber, H.; Ruhwedel, T.; Schatlo, B.; Von Der Brelie, C.; et al. WNT11/ROR2 Signaling Is Associated with Tumor Invasion and Poor Survival in Breast Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.-H.; Huang, S.; Fu, Z.; Tomita, Y.; Britton, W.R.; Cho, S.S.; Chen, C.T.; Sun, Y.; Ma, J.; et al. Wnt Signaling Activates MFSD2A to Suppress Vascular Endothelial Transcytosis and Maintain Blood-Retinal Barrier. Sci. Adv. 2020, 6, eaba7457. [Google Scholar] [CrossRef]
- Martin, M.; Vermeiren, S.; Bostaille, N.; Eubelen, M.; Spitzer, D.; Vermeersch, M.; Profaci, C.P.; Pozuelo, E.; Toussay, X.; Raman-Nair, J.; et al. Engineered Wnt Ligands Enable Blood-Brain Barrier Repair in Neurological Disorders. Science 2022, 375, eabm4459. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a Is Critical for the Formation and Function of the Blood–Brain Barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Ju, X.; Miao, T.; Chen, H.; Ni, J.; Han, L. Overcoming Mfsd2a-Mediated Low Transcytosis to Boost Nanoparticle Delivery to Brain for Chemotherapy of Brain Metastases. Adv. Healthc. Mater. 2021, 10, 2001997. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jung, Y.; Shin, D.-H.; Lee, J.-Y.; Oh, M.-Y.; Kim, H.-J.; Jang, K.S.; Jeon, S.J.; Son, K.H.; Kong, G. Anticancer Effects of Wogonin in Both Estrogen Receptor-positive and -negative Human Breast Cancer Cell Lines in Vitro and in Nude Mice Xenografts. Int. J. Cancer 2007, 122, 816–822. [Google Scholar] [CrossRef]
- Tong, Y.; An, P.; Tang, P.; Mu, R.; Zeng, Y.; Sun, H.; Zhao, M.; Lv, Z.; Wang, P.; Han, W.; et al. Suppressing Wnt Signaling of the Blood-tumor Barrier to Intensify Drug Delivery and Inhibit Lipogenesis of Brain Metastases. Acta Pharm. Sin. B 2024, 14, 2716–2731. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; van Es, J.H.; van den Born, M.; Clevers, H. Porcupine Inhibitor Suppresses Paracrine Wnt-Driven Growth of Rnf43;Znrf3-Mutant Neoplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 7548–7550. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Moon, J.; Dodge, M.E.; Pan, X.; Zhang, L.; Hanson, J.M.; Tuladhar, R.; Ma, Z.; Shi, H.; Williams, N.S.; et al. The Development of Highly Potent Inhibitors for Porcupine. J. Med. Chem. 2013, 56, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-Driven Cancer through the Inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic Heterogeneity of Breast Cancer: Molecular Mechanism and Potential Therapeutic Targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ma, B.; Sun, W.; Zhang, N.; Liu, Y.; Jia, L.; Liu, C. JAG1 Is Associated with the Prognosis and Metastasis in Breast Cancer. Sci. Rep. 2022, 12, 21986. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Kobayashi, A.; Okuda, H.; Watabe, M.; Pai, S.K.; Pandey, P.R.; Hirota, S.; Wilber, A.; Mo, Y.-Y.; Moore, B.E.; et al. Reactive Astrocytes Promote the Metastatic Growth of Breast Cancer Stem-like Cells by Activating Notch Signalling in Brain. EMBO Mol. Med. 2013, 5, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Raub, T.J.; Wishart, G.N.; Kulanthaivel, P.; Staton, B.A.; Ajamie, R.T.; Sawada, G.A.; Gelbert, L.M.; Shannon, H.E.; Sanchez-Martinez, C.; De Dios, A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. 2015, 43, 1360–1371. [Google Scholar] [CrossRef]
- Yu, Q.; Sicinska, E.; Geng, Y.; Ahnström, M.; Zagozdzon, A.; Kong, Y.; Gardner, H.; Kiyokawa, H.; Harris, L.N.; Stål, O.; et al. Requirement for CDK4 Kinase Function in Breast Cancer. Cancer Cell 2006, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chong, Q.-Y.; Kok, Z.-H.; Bui, N.-L.-C.; Xiang, X.; Wong, A.L.-A.; Yong, W.-P.; Sethi, G.; Lobie, P.E.; Wang, L.; Goh, B.-C. A Unique CDK4/6 Inhibitor: Current and Future Therapeutic Strategies of Abemaciclib. Pharmacol. Res. 2020, 156, 104686. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Cantanelli, P.; Guerra, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 Quantitatively Stimulates Human Cancer Growth. Oncogene 2013, 32, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and Its Overexpression in Cancers: Regulation and Clinical/Therapeutic Implications. Genes Cancer 2015, 6, 84. [Google Scholar] [CrossRef]
- Ambrogi, F.; Fornili, M.; Boracchi, P.; Trerotola, M.; Relli, V.; Simeone, P.; La Sorda, R.; Lattanzio, R.; Querzoli, P.; Pedriali, M.; et al. Trop-2 Is a Determinant of Breast Cancer Survival. PLoS ONE 2014, 9, e96993. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Huang, J.-F.; Qiu, J.-R.; Zhang, H.-L.; Tang, X.-J.; Li, H.; Wang, C.-J.; Wang, Z.-C.; Feng, Z.-Q.; Zhu, J. Significantly Upregulated TACSTD2 and Cyclin D1 Correlate with Poor Prognosis of Invasive Ductal Breast Cancer. Exp. Mol. Pathol. 2013, 94, 73–78. [Google Scholar] [CrossRef]
- Wu, M.; Liu, L.; Chan, C. Identification of Novel Targets for Breast Cancer by Exploring Gene Switches on a Genome Scale. BMC Genom. 2011, 12, 547. [Google Scholar] [CrossRef]
- Ning, S.; Liang, N.; Liu, B.; Chen, X.; Pang, Q.; Xin, T. TROP2 Expression and Its Correlation with Tumor Proliferation and Angiogenesis in Human Gliomas. Neurol. Sci. 2013, 34, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Balinda, H.U.; Kelly, W.J.; Kaklamani, V.G.; Lathrop, K.I.; Canola, M.M.; Ghamasaee, P.; Sareddy, G.R.; Michalek, J.; Gilbert, A.R.; Surapaneni, P.; et al. Sacituzumab Govitecan in Patients with Breast Cancer Brain Metastases and Recurrent Glioblastoma: A Phase 0 Window-of-Opportunity Trial. Nat. Commun. 2024, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.-K.; Tafuri, A.; Berg-Brown, N.N.; Shahinian, A.; Plyte, S.; Duncan, G.S.; Okada, H.; Wakeham, A.; Odermatt, B.; Ohashi, P.S.; et al. The Inducible Costimulator Plays the Major Costimulatory Role in Humoral Immune Responses in the Absence of CD28. J. Immunol. 2004, 172, 5917–5923. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective Effects of PD-1 on Akt and Ras Pathways Regulate Molecular Components of the Cell Cycle and Inhibit T Cell Proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schwartz, J.-C.D.; Guo, X.; Bhatia, S.; Cao, E.; Chen, L.; Zhang, Z.-Y.; Edidin, M.A.; Nathenson, S.G.; Almo, S.C. Structural and Functional Analysis of the Costimulatory Receptor Programmed Death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, K.A.; Jeon, H.; Zang, X. The PD-1/PD-L1 (B7-H1) Pathway in Chronic Infection-Induced Cytotoxic T Lymphocyte Exhaustion. BioMed Res. Int. 2011, 2011, 451694. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-Infiltrating Lymphocytes Are Correlated with Higher Expression Levels of PD-1 and PD-L1 in Early Breast Cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortés, J.; Dent, R.; Pusztai, L.; McArthur, H.L.; Kuemmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. LBA8_PR—KEYNOTE-522: Phase III Study of Pembrolizumab (Pembro) + Chemotherapy (Chemo) vs Placebo (Pbo) + Chemo as Neoadjuvant Treatment, Followed by Pembro vs Pbo as Adjuvant Treatment for Early Triple-Negative Breast Cancer (TNBC). Ann. Oncol. 2019, 30, v853–v854. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus Chemotherapy as Neoadjuvant Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: Results from the Phase 1b Open-Label, Multicohort KEYNOTE-173 Study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Oba, T.; Kajihara, R.; Abrams, S.I.; Ito, F. Local, Multimodal Intralesional Therapy Renders Distant Brain Metastases Susceptible to PD-L1 Blockade in a Preclinical Model of Triple-Negative Breast Cancer. Sci. Rep. 2021, 11, 21992. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Lin, N.U.; Overmoyer, B.; Wen, P.Y.; Nayak, L.; Cohen, J.V.; Dietrich, J.; et al. Pembrolizumab in Brain Metastases of Diverse Histologies: Phase 2 Trial Results. Nat. Med. 2023, 29, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Kumthekar, P.U.; Jin, Q.; Binboga Kurt, B.; Ren, S.; Li, T.; Leone, J.P.; Mittendorf, E.A.; Pereslete, A.M.; Sharp, L.; et al. A Phase II Study of Atezolizumab, Pertuzumab, and High-Dose Trastuzumab for Central Nervous System Metastases in Patients with HER2-Positive Breast Cancer. Clin. Cancer Res. 2024, 30, 4856–4865. [Google Scholar] [CrossRef]
- Winger, R.C.; Koblinski, J.E.; Kanda, T.; Ransohoff, R.M.; Muller, W.A. Rapid Remodeling of Tight Junctions during Paracellular Diapedesis in a Human Model of the Blood–Brain Barrier. J. Immunol. 2014, 193, 2427–2437. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for Delivering Therapeutics across the Blood–Brain Barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential Expression of Receptors Mediating Receptor-Mediated Transcytosis (RMT) in Brain Microvessels, Brain Parenchyma and Peripheral Tissues of the Mouse and the Human. Fluids Barriers CNS 2020, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Tang, S.-C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Zan, B.; Ulmschneider, J.P.; Wimley, W.C.; Lu, T.K.; Ulmschneider, M.B.; Zhou, L. Development of Membrane-active Peptide Therapeutics in Oncology. J. Pept. Sci. 2023, 29, e3482. [Google Scholar] [CrossRef] [PubMed]
- Uceda-Castro, R.; Margarido, A.S.; Song, J.-Y.; de Gooijer, M.C.; Messal, H.A.; Chambers, C.R.; Nobis, M.; Çitirikkaya, C.H.; Hahn, K.; Seinstra, D.; et al. BCRP Drives Intrinsic Chemoresistance in Chemotherapy-Naïve Breast Cancer Brain Metastasis. Sci. Adv. 2023, 9, eabp9530. [Google Scholar] [CrossRef] [PubMed]
- De Gooijer, M.C.; De Vries, N.A.; Buckle, T.; Buil, L.C.M.; Beijnen, J.H.; Boogerd, W.; Van Tellingen, O. Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2. Neoplasia 2018, 20, 710–720. [Google Scholar] [CrossRef]
- Bailey-Dell, K.J.; Hassel, B.; Doyle, L.A.; Ross, D.D. Promoter Characterization and Genomic Organization of the Human Breast Cancer Resistance Protein (ATP-Binding Cassette Transporter G2) Gene1. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2001, 1520, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.-H.; Shen, T.; Chen, X.; Sodani, K.; Hopper-Borge, E.; Tiwari, A.K.; Lee, J.W.K.K.; Fu, L.-W.; Chen, Z.-S. Lapatinib and Erlotinib Are Potent Reversal Agents for MRP7 (ABCC10)-Mediated Multidrug Resistance. Biochem. Pharmacol. 2010, 79, 154–161. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. BIOCHEMICAL, CELLULAR, AND PHARMACOLOGICAL ASPECTS OF THE MULTIDRUG TRANSPORTER1. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Kwan Law, B.Y.; Wai Wong, V.K.; Bik Chan, D.S.; Fai Mok, S.W.; Ying Gao, J.J.; Yan Ho, R.K.; Liang, X.; Li, J.H.; Lee, M.T.; et al. HM30181A, a Potent P-Glycoprotein Inhibitor, Potentiates the Absorption and in Vivo Antitumor Efficacy of Paclitaxel in an Orthotopic Brain Tumor Model. Cancer Biol. Med. 2020, 17, 986–1001. [Google Scholar] [CrossRef]
- Rugo, H.S.; Umanzor, G.A.; Barrios, F.J.; Vasallo, R.H.; Chivalan, M.A.; Bejarano, S.; Ramírez, J.R.; Fein, L.; Kowalyszyn, R.D.; Kramer, E.D.; et al. Open-Label, Randomized, Multicenter, Phase III Study Comparing Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer. J. Clin. Oncol. 2023, 41, 65–74. [Google Scholar] [CrossRef]
- Christie, E.L.; Pattnaik, S.; Beach, J.; Copeland, A.; Rashoo, N.; Fereday, S.; Hendley, J.; Alsop, K.; Brady, S.L.; Lamb, G.; et al. Multiple ABCB1 Transcriptional Fusions in Drug Resistant High-Grade Serous Ovarian and Breast Cancer. Nat. Commun. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Lv, H.; Liu, X.; Cuizhi, G.; Wang, S.; Song, Y.; Liu, Z.; Niu, L.; Zhang, M.; Feng, Y. Utidelone plus Bevacizumab for the Treatment of HER2-Negative Breast Cancer Brain Metastases (U-BOMB): A Multicenter, Single-Arm Phase II Study. JCO 2024, 42, 1081. [Google Scholar] [CrossRef]
- Xu, B.; Sun, T.; Zhang, Q.; Zhang, P.; Yuan, Z.; Jiang, Z.; Wang, X.; Cui, S.; Teng, Y.; Hu, X.-C.; et al. Efficacy of Utidelone plus Capecitabine versus Capecitabine for Heavily Pretreated, Anthracycline- and Taxane-Refractory Metastatic Breast Cancer: Final Analysis of Overall Survival in a Phase III Randomised Controlled Trial. Ann. Oncol. 2021, 32, 218–228. [Google Scholar] [CrossRef]
- Kim, L.S.; Huang, S.; Lu, W.; Chelouche Lev, D.; Price, J.E. Vascular Endothelial Growth Factor Expression Promotes the Growth of Breast Cancer Brain Metastases in Nude Mice. Clin. Exp. Metastasis 2004, 21, 107–118. [Google Scholar] [CrossRef]
- Lee, T.-H.; Avraham, H.K.; Jiang, S.; Avraham, S. Vascular Endothelial Growth Factor Modulates the Transendothelial Migration of MDA-MB-231 Breast Cancer Cells through Regulation of Brain Microvascular Endothelial Cell Permeability*. J. Biol. Chem. 2003, 278, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Bhattacharya, S.; Bowden, C.; Miller, K.; Comis, R.L. Independent Review of E2100: A Phase III Trial of Bevacizumab Plus Paclitaxel Versus Paclitaxel in Women With Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.W.; Chan, A.; Dirix, L.Y.; Cortés, J.; Pivot, X.; Tomczak, P.; Delozier, T.; Sohn, J.H.; Provencher, L.; Puglisi, F.; et al. Phase III Study of Bevacizumab Plus Docetaxel Compared With Placebo Plus Docetaxel for the First-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. JCO 2010, 28, 3239–3247. [Google Scholar] [CrossRef]
- Robert, N.J.; Diéras, V.; Glaspy, J.; Brufsky, A.M.; Bondarenko, I.; Lipatov, O.N.; Perez, E.A.; Yardley, D.A.; Chan, S.Y.T.; Zhou, X.; et al. RIBBON-1: Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Chemotherapy With or Without Bevacizumab for First-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative, Locally Recurrent or Metastatic Breast Cancer. JCO 2011, 29, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Leone, J.P.; Emblem, K.E.; Weitz, M.; Gelman, R.S.; Schneider, B.P.; Freedman, R.A.; Younger, J.; Pinho, M.C.; Sorensen, A.G.; Gerstner, E.R.; et al. Phase II Trial of Carboplatin and Bevacizumab in Patients with Breast Cancer Brain Metastases. Breast Cancer Res. 2020, 22, 131. [Google Scholar] [CrossRef]
- Chen, T.W.-W.; Dai, M.-S.; Tseng, L.-M.; Chen, S.-C.; Chao, T.-Y.; Chao, T.-C.; Chang, Y.-C.; Chiu, C.-F.; Liu, C.-T.; Lin, C.-H.; et al. Whole-Brain Radiotherapy Alone vs Preceded by Bevacizumab, Etoposide, and Cisplatin for Untreated Brain Metastases From Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2024, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, S.M.; Sayyad, M.; Puchalapalli, M.; Sullivan, M.; Singh, J.; Ratchford, B.; Abrams, J.; Jahromi, M.; Hu, B.; Idowu, M.; et al. Abstract 3058: Syndecan-1 Mediates Breast Cancer Metastasis to the Brain through IL-8 and PECAM-1 Signaling. Cancer Res. 2017, 77, 3058. [Google Scholar] [CrossRef]
- Benchama, O.; Tyukhtenko, S.; Malamas, M.S.; Williams, M.K.; Makriyannis, A.; Avraham, H.K. Inhibition of Triple Negative Breast Cancer-Associated Inflammation, Tumor Growth and Brain Colonization by Targeting Monoacylglycerol Lipase. Sci. Rep. 2022, 12, 5328. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.; Rommel, F.; Kondakci, M.; Gorol, M.; Willers, R.; Bilzer, T. Locoregional Opening of the Rodent Blood–Brain Barrier for Paclitaxel Using Nd:YAG Laser-Induced Thermo Therapy: A New Concept of Adjuvant Glioma Therapy? Lasers Surg. Med. 2003, 33, 75–80. [Google Scholar] [CrossRef]
- Shang, X.; Wang, P.; Liu, Y.; Zhang, Z.; Xue, Y. Mechanism of Low-Frequency Ultrasound in Opening Blood-Tumor Barrier by Tight Junction. J. Mol. Neurosci. 2011, 43, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem Blood Brain Barrier Disruption Using Focused Ultrasound: A Demonstration of Feasibility and Enhanced Doxorubicin Delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Zhang, Y.-Z.; Vykhodtseva, N.; McDannold, N. Ultrasound-Mediated Blood-brain/Blood-Tumor Barrier Disruption Improves Outcomes with Trastuzumab in a Breast Cancer Brain Metastasis Model. J. Control. Release 2012, 163, 277–284. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging Blood–Brain-Barrier-Crossing Nanotechnology for Brain Cancer Theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Ansari, M.A.; Thiruvengadam, M.; Farooqui, Z.; Rajakumar, G.; Sajid Jamal, Q.M.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Chung, I.-M.; Al-Suhaimi, E.A. Nanotechnology, in Silico and Endocrine-Based Strategy for Delivering Paclitaxel and miRNA: Prospects for the Therapeutic Management of Breast Cancer. Semin. Cancer Biol. 2021, 69, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in Cancer Nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- He, C.; Cai, P.; Li, J.; Zhang, T.; Lin, L.; Abbasi, A.Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Blood-Brain Barrier-Penetrating Amphiphilic Polymer Nanoparticles Deliver Docetaxel for the Treatment of Brain Metastases of Triple Negative Breast Cancer. J. Control. Release 2017, 246, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.S.; Griffith, J.I.; Adkins, C.E.; Shah, N.; Sechrest, E.; Dolan, E.L.; Terrell-Hall, T.B.; Hendriks, B.S.; Lee, H.; Lockman, P.R. Liposomal Irinotecan Accumulates in Metastatic Lesions, Crosses the Blood-Tumor Barrier (BTB), and Prolongs Survival in an Experimental Model of Brain Metastases of Triple Negative Breast Cancer. Pharm. Res. 2018, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, J.C.; Munster, P.; Northfelt, D.W.; Han, H.S.; Ma, C.; Maxwell, F.; Wang, T.; Belanger, B.; Zhang, B.; Moore, Y.; et al. Phase I Study of Liposomal Irinotecan in Patients with Metastatic Breast Cancer: Findings from the Expansion Phase. Breast Cancer Res. Treat. 2021, 185, 759–771. [Google Scholar] [CrossRef]
- Zhang, T.; Lip, H.; He, C.; Cai, P.; Wang, Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Multitargeted Nanoparticles Deliver Synergistic Drugs across the Blood–Brain Barrier to Brain Metastases of Triple Negative Breast Cancer Cells and Tumor-Associated Macrophages. Adv. Healthc. Mater. 2019, 8, 1900543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, F.; Li, K.; Zhang, H.; Yin, Y.; Yu, Y.; Lu, G.; Zhang, S.; Wei, Y.; Xu, K.; et al. Gold Nanoparticle-Directed Autophagy Intervention for Antitumor Immunotherapy via Inhibiting Tumor-Associated Macrophage M2 Polarization. Acta Pharm. Sin. B 2022, 12, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting Tumor-Associated Macrophages to Synergize Tumor Immunotherapy. Signal Transduct Target Ther. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic Significance of Tumor-Associated Macrophages in Breast Cancer: A Meta-Analysis of the Literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron Oxide Nanoparticles Inhibit Tumour Growth by Inducing Pro-Inflammatory Macrophage Polarization in Tumour Tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Tong, F.; Hu, H.; Xu, Y.; Zhou, Y.; Xie, R.; Lei, T.; Du, Y.; Yang, W.; He, S.; Huang, Y.; et al. Hollow Copper Sulfide Nanoparticles Carrying ISRIB for the Sensitized Photothermal Therapy of Breast Cancer and Brain Metastases through Inhibiting Stress Granule Formation and Reprogramming Tumor-associated Macrophages. Acta Pharm. Sin. B 2023, 13, 3471–3488. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Zhao, S.-K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, e2004853. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Bigham, A.; Taheriazam, A.; Saghari, Y.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Karimi-Maleh, H.; Nazarzadeh Zare, E.; et al. (Nano)Platforms in Breast Cancer Therapy: Drug/Gene Delivery, Advanced Nanocarriers and Immunotherapy. Med. Res. Rev. 2023, 43, 2115–2176. [Google Scholar] [CrossRef]
- Subhan, M.A.; Muzibur Rahman, M. Recent Development in Metallic Nanoparticles for Breast Cancer Therapy and Diagnosis. Chem. Rec. 2022, 22, e202100331. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, M.E.; Crump, M.; Charpentier, D.; Yelle, L.; Bordeleau, L.; Matthews, S.; Eisenhauer, E. Temozolomide in Metastatic Breast Cancer (MBC): A Phase II Trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann. Oncol. 2006, 17, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Pang, D.; Yang, H.; Li, W.; Wang, S.; Cui, S.; Liao, N.; Wang, Y.; Wang, C.; Chang, Y.-C.; et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e193692. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Wu, Y.; Wang, J.; Ma, F. Efficacy and Safety of Trastuzumab with or Without a Tyrosine Kinase Inhibitor for HER2-Positive Breast Cancer: A Systematic Review and Meta-Analysis. Biochim. Biophys. Acta BBA Rev. Cancer 2023, 1878, 188969. [Google Scholar] [CrossRef]
- Klocker, E.V.; Egle, D.; Bartsch, R.; Rinnerthaler, G.; Gnant, M. Efficacy and Safety of CDK4/6 Inhibitors: A Focus on HR+/HER2- Early Breast Cancer. Drugs 2025, 85, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Su, Z.; Sullivan, R.J.; Blum, S.M.; Qi, Z.; Liu, Y.; Huo, Y.; Fang, Y.; Zhang, L.; et al. Toxicities Associated with Immune Checkpoint Inhibitors: A Systematic Study. Int. J. Surg. 2023, 109, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Loo, P.V.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef] [PubMed]
- Mattos-Arruda, L.D. Liquid Biopsy for HER2-Positive Breast Cancer Brain Metastasis: The Role of the Cerebrospinal Fluid. ESMO Open 2017, 2, e000270. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
| Drug Classification | Drug Name | Research Title | Study Design | Phase | ORR | OS | PFS |
|---|---|---|---|---|---|---|---|
| Monoclonal antibodies | Pertuzumab | PATRICIA [31] | Pertuzumab plus high-dose trastuzumab | Phase II | Clinical benefit rates at 4 months and 6 months were 68% and 51% | --- | --- |
| CLEOPATRA [32] | Pertuzumab, trastuzumab, and docetaxel vs. placebo, trastuzumab, and docetaxel | Phase III | --- | 57.1 months(Pertuzumab) vs. 40.8 months(placebo) | 53.8 months(pertuzumab) vs. 46.6 months(placebo) | ||
| TKI | Lapatinib | ELTOP [33] | Lapatinib plus capecitabine vs. trastuzumab plus capecitabine | Phase II | 41% (lapatinib plus capecitabine) vs. 40% (trastuzumab plus capecitabine) | --- vs. 6.1 months | 7.1 months (lapatinib plus capecitabine) vs. 6.1 months (trastuzumab plus capecitabine) |
| Neratinib | TBCRC 022 [34] | Lapatinib-naïve vs. lapatinib-treated added to neratinib, capecitabine | Phase II | 49% (lapatinib-naïve) vs. 33% (lapatinib-treated) | 13.3 months (lapatinib-naïve) vs. 15.1 months (lapatinib-treated) | 5.5 months (lapatinib-naïve) vs. 3.1 months (lapatinib-treated) | |
| Pyrotinib | PERMEATE [35] | No radiotherapy vs. radiotherapy | Phase II | --- | 36.0 months vs. 31.5 months | 11.3 months (No radiotherapy) vs. 5.6 months (radiotherapy) | |
| Tucatinib | HER2CLIMB [36] | Tucatinib vs. Placebo added to trastuzumab and capecitabine | Phase III | --- | 21.9 months vs. 14.7 months | 7.6 months (tucatinib) vs. 5.4 months (placebo plus trastuzumab and capecitabine) | |
| ADC | T-DM1 | EMILIA [27] | T-DM1 vs. Lapatinib plus capecitabine | Phase III | --- | 30.9 months vs. 25.1 months | 9.6 months (T-DM1) vs. 6.4 months (lapatinib plus capecitabine) |
| T-Dxd | TUXEDO-1 [37] | --- | Phase II | --- | --- | 14 months | |
| DESTINY-Breast03 [38] | T-Dxd vs. T-DM1 | Phase III | --- | --- | 25.1 months (T-Dxd) vs. 7.2 months (T-DM1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shang, H.; Zhang, J.; Jiang, Y.; Li, J.; Xiong, H.; Chao, T. Drug Treatment Direction Based on the Molecular Mechanism of Breast Cancer Brain Metastasis. Pharmaceuticals 2025, 18, 262. https://doi.org/10.3390/ph18020262
Zhang Y, Shang H, Zhang J, Jiang Y, Li J, Xiong H, Chao T. Drug Treatment Direction Based on the Molecular Mechanism of Breast Cancer Brain Metastasis. Pharmaceuticals. 2025; 18(2):262. https://doi.org/10.3390/ph18020262
Chicago/Turabian StyleZhang, Yumin, Haotian Shang, Jiaxuan Zhang, Yizhi Jiang, Jiahao Li, Huihua Xiong, and Tengfei Chao. 2025. "Drug Treatment Direction Based on the Molecular Mechanism of Breast Cancer Brain Metastasis" Pharmaceuticals 18, no. 2: 262. https://doi.org/10.3390/ph18020262
APA StyleZhang, Y., Shang, H., Zhang, J., Jiang, Y., Li, J., Xiong, H., & Chao, T. (2025). Drug Treatment Direction Based on the Molecular Mechanism of Breast Cancer Brain Metastasis. Pharmaceuticals, 18(2), 262. https://doi.org/10.3390/ph18020262







