Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Technological Characterization of NRG-SLNs
2.1.1. Particle Sizes, Polydispersity Index, and Zeta Potential
2.1.2. In Vitro Drug Release Studies
2.2. Chemical–Physical Characterization of NRG10-SLNs
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Empty SLNs and NRG-SLNs
3.3. Technological Characterization of NRG-SLNs
3.4. In Vitro Drug Release Studies
3.5. UV–Vis Apparatus and Method Validation
3.6. Physical Stability of NRG10-SLNs Formulation
3.7. Physical–Chemical Characterization of NRG10-SLNs
3.8. Antibacterial Activity
3.9. Effect on Biofilm Formation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guedes, B.N.; Krambeck, K.; Durazzo, A.; Lucarini, M.; Santini, A.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Natural Antibiotics against Antimicrobial Resistance: Sources and Bioinspired Delivery Systems. Braz. J. Microbiol. 2024, 55, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological Activities of Naringenin: A Narrative Review Based on in Vitro and in Vivo Studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A Flavanone with Anti-Inflammatory and Anti-Infective Properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Song, H.-S.; Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Park, Y.-L.; Han, Y.-H.; Park, J.Y.; Lee, S.M.; Park, S.L.; et al. Naringenin as an Antibacterial Reagent Controlling of Biofilm Formation and Fatty Acid Metabolism in MRSA. bioRxiv 2020. bioRxiv:2020.03.08.983049. [Google Scholar]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives—Health-Promoting Phytobiotic against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef]
- Wang, L.-H.; Zeng, X.-A.; Wang, M.-S.; Brennan, C.S.; Gong, D. Modification of Membrane Properties and Fatty Acids Biosynthesis-Related Genes in Escherichia Coli and Staphylococcus Aureus: Implications for the Antibacterial Mechanism of Naringenin. Biochim. Biophys. Acta Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of Bacterial Cell–Cell Signalling, Biofilm Formation and Type III Secretion System by Citrus Flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of Naringenin on the Biofilm Formation of Streptococcus Mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Dong, J.; Wei, J.; Wang, Y.; Dai, X.; Wang, X.; Luo, M.; Tan, W.; Deng, X.; et al. Inhibition of α-Toxin Production by Subinhibitory Concentrations of Naringenin Controls Staphylococcus Aureus Pneumonia. Fitoterapia 2013, 86, 92–99. [Google Scholar] [CrossRef] [PubMed]
- The Therapeutic Effects of Naringenin on Bronchial Pneumonia in Children—Yao—2021—Pharmacology Research & Perspectives—Wiley Online Library. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1002/prp2.825 (accessed on 22 October 2024).
- Wen, J.; Liu, B.; Yuan, E.; Ma, Y.; Zhu, Y. Preparation and Physicochemical Properties of the Complex of Naringenin with Hydroxypropyl-β-Cyclodextrin. Molecules 2010, 15, 4401–4407. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Pedotti, S.; Pistarà, V.; Cannavà, C.; Carbone, C.; Cilurzo, F.; Corsaro, A.; Puglisi, G.; Ventura, C.A. Synthesis and Physico-Chemical Characterization of a β-Cyclodextrin Conjugate for Sustained Release of Acyclovir. Carbohydr. Polym. 2015, 131, 159–167. [Google Scholar] [CrossRef]
- De Gaetano, F.; Pastorello, M.; Pistarà, V.; Rescifina, A.; Margani, F.; Barbera, V.; Ventura, C.A.; Marino, A. Rutin/Sulfobutylether-β-Cyclodextrin as a Promising Therapeutic Formulation for Ocular Infection. Pharmaceutics 2024, 16, 233. [Google Scholar] [CrossRef]
- De Gaetano, F.; Margani, F.; Barbera, V.; D’Angelo, V.; Germanò, M.P.; Pistarà, V.; Ventura, C.A. Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2209. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistarà, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef]
- De Gaetano, F.; Mannino, D.; Celesti, C.; Bulzomí, M.; Iraci, N.; Vincenzo Giofrè, S.; Esposito, E.; Paterniti, I.; Anna Ventura, C. Randomly Methylated β-Cyclodextrin Improves Water—Solubility, Cellular Protection and Mucosa Permeability of Idebenone. Int. J. Pharm. 2024, 665, 124718. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Leggio, L.; Celesti, C.; Genovese, F.; Falcone, M.; Giofrè, S.V.; Iraci, N.; Ventura, C.A. Study of Host-Guest Interaction and In Vitro Neuroprotective Potential of Cinnamic Acid/Randomly Methylated β-Cyclodextrin Inclusion Complex. Int. J. Mol. Sci. 2024, 25, 12778. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, S.; Raneri, D.; Ficarra, R.; Calabrò, M.L.; Stancanelli, R.; Ficarra, P. Improvement in Solubility and Dissolution Rate of Flavonoids by Complexation with β-Cyclodextrin. J. Pharm. Biomed. Anal. 2004, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, S.; Calabrò, M.L.; Raneri, D.; Ficarra, P.; Ficarra, R. Combined Effect of pH and Polysorbates with Cyclodextrins on Solubilization of Naringenin. J. Pharm. Biomed. Anal. 2004, 36, 327–333. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of Naringenin Bioavailability by Complexation with Hydroxypropoyl-β-Cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda Redondo, M.; Martínez, G.; Merinero de Los Santos, M.; Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- De Gaetano, F.; Scala, A.; Celesti, C.; Lambertsen Larsen, K.; Genovese, F.; Bongiorno, C.; Leggio, L.; Iraci, N.; Mazzaglia, A.; Ventura, C.A. Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study. Molecules 2023, 28, 3023. [Google Scholar] [CrossRef]
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206. [Google Scholar] [CrossRef]
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Mancuso, A.; Fresta, M.; Torella, D.; De Gaetano, F.; Ventura, C.A.; Paolino, D. Topical Unsaturated Fatty Acid Vesicles Improve Antioxidant Activity of Ammonium Glycyrrhizinate. Pharmaceutics 2021, 13, 548. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Hensel, A.; Goycoolea, F.M. Chitosan/Cyclodextrin Surface-Adsorbed Naringenin-Loaded Nanocapsules Enhance Bacterial Quorum Quenching and Anti-Biofilm Activities. Colloids Surf. B Biointerfaces 2022, 211, 112281. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Sajadimajd, S.; Hoseinzadeh, L.; Bahrami, G.; Arkan, E.; Moradi, S.; Abdi, F.; Farzaei, M.H. Neuroprotective Effect of Naringenin-Loaded Solid Lipid Nanoparticles against Streptozocin-Induced Neurotoxicity through Autophagy Blockage. J. Food Biochem. 2022, 46, e14408. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Asfour, H.Z. Neuroprotective and Antioxidant Effect of Naringenin-Loaded Nanoparticles for Nose-to-Brain Delivery. Brain Sci. 2019, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery—A Review of the State of the Art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Venuti, V.; Crupi, V.; Majolino, D.; Paladini, G.; Acri, G.; Testagrossa, B.; Irrera, A.; Paolino, D.; et al. Rutin-Loaded Solid Lipid Nanoparticles: Characterization and In Vitro Evaluation. Molecules 2021, 26, 1039. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- De Gaetano, F.; Celesti, C.; Paladini, G.; Venuti, V.; Cristiano, M.C.; Paolino, D.; Iannazzo, D.; Strano, V.; Gueli, A.M.; Tommasini, S.; et al. Solid Lipid Nanoparticles Containing Morin: Preparation, Characterization, and Ex Vivo Permeation Studies. Pharmaceutics 2023, 15, 1605. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid Nanoparticles: Different Preparation Techniques, Characterization, Hurdles, and Strategies for the Production of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Oral Drug Delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Pour, P.M.; Nouri, Z.; Ghasemi, D.; Sajadimajd, S.; Farzaei, M.H. Cytotoxic Impact of Naringenin-Loaded Solid Lipid Nanoparticles on RIN5F Pancreatic β Cells via Autophagy Blockage. Recent Patents Drug Deliv. Formul. 2024, 18, 304–314. [Google Scholar] [CrossRef]
- Mani, M.; Balasubramanian, S.; Manikandan, K.R.; Kulandaivel, B. Neuroprotective Potential of Naringenin-Loaded Solid-Lipid Nanoparticles against Rotenone-Induced Parkinson’s Disease Model. J. Appl. Pharm. Sci. 2021, 11, 19–28. [Google Scholar] [CrossRef]
- Munir, A.; Muhammad, F.; Zaheer, Y.; Ali, M.; Iqbal, M.; Rehman, M.; Munir, M.U.; Akhtar, B.; Webster, T.; Sharif, A.; et al. Synthesis of Naringenin Loaded Lipid Based Nanocarriers and Their In-Vivo Therapeutic Potential in a Rheumatoid Arthritis Model. J. Drug Deliv. Sci. Technol. 2021, 66, 102854. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-Loaded Solid Lipid Nanoparticles: Preparation, Controlled Delivery, Cellular Uptake, and Pulmonary Pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef]
- Souto, E.B.; Mehnert, W.; Müller, R.H. Polymorphic Behaviour of Compritol®888 ATO as Bulk Lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef]
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A Multifunctional Lipid Excipient in Drug Delivery Systems and Nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In Vitro Neuroprotective Effects of Naringenin Nanoemulsion against β-Amyloid Toxicity through the Regulation of Amyloidogenesis and Tau Phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Sahu, A.; Sahu, G.K.; Dash, D.; Mishra, S.P.; Mishra, K.; Kashyap, P.; Jain, V. Assessment of in Vitro Naringenin Release from Solid Lipid Nanoparticles and Kinetic Model Profiling: Applied Ultraviolet-Visible Spectrophotometer. Indian Drugs 2017, 54, 46–57. [Google Scholar] [CrossRef]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin Loaded Multifunctional Nanoparticles to Enhance the Chemotherapeutic Efficacy in Hepatic Fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Wani, F.A.; Behera, K.; Patel, R. Amphiphilic Micelles as Superior Nanocarriers in Drug Delivery: From Current Preclinical Surveys to Structural Frameworks. ChemistrySelect 2022, 7, e202201928. [Google Scholar] [CrossRef]
- Yıldırım, M.; Acet, Ö.; Yetkin, D.; Acet, B.Ö.; Karakoc, V.; Odabası, M. Anti-Cancer Activity of Naringenin Loaded Smart Polymeric Nanoparticles in Breast Cancer. J. Drug Deliv. Sci. Technol. 2022, 74, 103552. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Release from Lipid Dosage Forms. Int. J. Pharm. 2011, 418, 42–53. [Google Scholar] [CrossRef]
- Modeling and Comparison of Dissolution Profiles—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11297896/ (accessed on 8 March 2024).
- Parhi, R.; Suresh, P. Production of Solid Lipid Nanoparticles-Drug Loading and Release Mechanism. J. Chem. Pharm. Res. 2010, 2, 211–227. [Google Scholar]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Celiz, G.; Suarez, S.A.; Arias, A.; Molina, J.; Brondino, C.D.; Doctorovich, F. Synthesis, Structural Elucidation and Antiradical Activity of a Copper (II) Naringenin Complex. Biometals 2019, 32, 595–610. [Google Scholar] [CrossRef]
- Unsalan, O.; Gulluoglu, M. FT-Raman and FT-IR Spectral and Quantum Chemical Studies on Some Flavonoid Derivatives: Baicalein and Naringenin. J. Raman Spectrosc. 2009, 40, 562–570. [Google Scholar] [CrossRef]
- Wang, L.-H.; Wang, M.-S.; Zeng, X.-A.; Xu, X.-M.; Brennan, C.S. Membrane and Genomic DNA Dual-Targeting of Citrus Flavonoid Naringenin against Staphylococcus Aureus. Integr. Biol. 2017, 9, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) Based Microemulsions: Preclinical Drug Delivery, Toxicity and Antimicrobial Applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on Growth and Biofilm Formation in Laboratory Media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef] [PubMed]
- Menberu, M.A.; Hayes, A.J.; Liu, S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Tween 80 and Its Derivative Oleic Acid Promote the Growth of Corynebacterium Accolens and Inhibit Staphylococcus Aureus Clinical Isolates. Int. Forum Allergy Rhinol. 2021, 11, 810–813. [Google Scholar] [CrossRef]
- Wen, Q.-H.; Wang, R.; Zhao, S.-Q.; Chen, B.-R.; Zeng, X.-A. Inhibition of Biofilm Formation of Foodborne Staphylococcus Aureus by the Citrus Flavonoid Naringenin. Foods 2021, 10, 2614. [Google Scholar] [CrossRef]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory Effects of Polysorbate 80 on MRSA Biofilm Formed on Different Substrates Including Dermal Tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B Solid Lipid Nanoparticles: Design and Development. Colloids Surf. B Biointerfaces 2016, 139, 17–24. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Jha, D.K.; Shah, D.S.; Talele, S.R.; Amin, P.D. Correlation of Two Validated Methods for the Quantification of Naringenin in Its Solid Dispersion: HPLC and UV Spectrophotometric Methods. SN Appl. Sci. 2020, 2, 698. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In Vitro Dissolution Testing Strategies for Nanoparticulate Drug Delivery Systems: Recent Developments and Challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chen, Y.; Xie, H. In Vitro Dissolution Considerations Associated with Nano Drug Delivery Systems (NDDS). Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1732. [Google Scholar] [CrossRef] [PubMed]
- M100Ed34|Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 27 March 2024).
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Bonferroni, C.E. Teoria Statistica Delle Classi e Calcolo Delle Probabilità; Seeber: Castelbello-Ciardes, Italy, 1936. [Google Scholar]
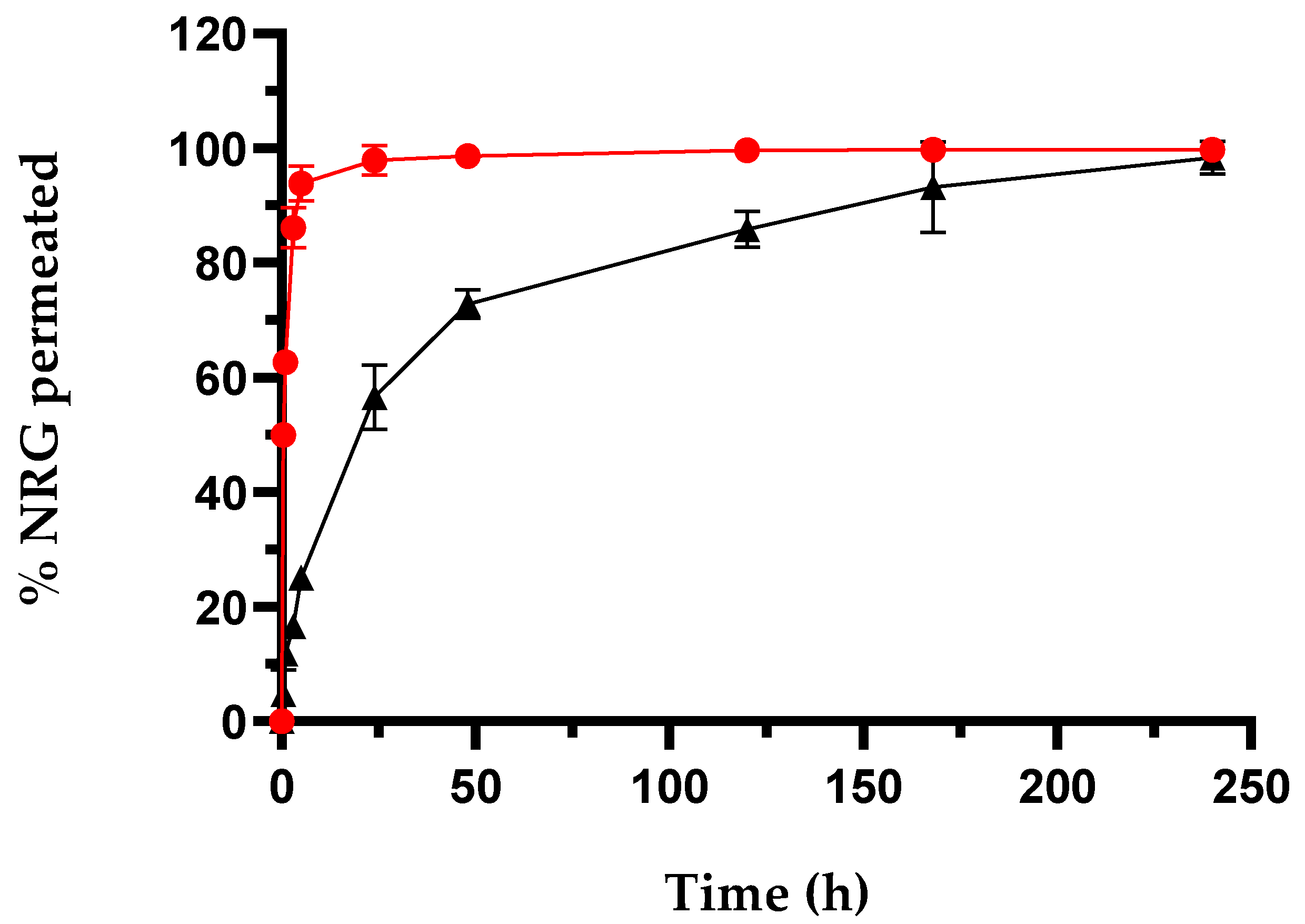
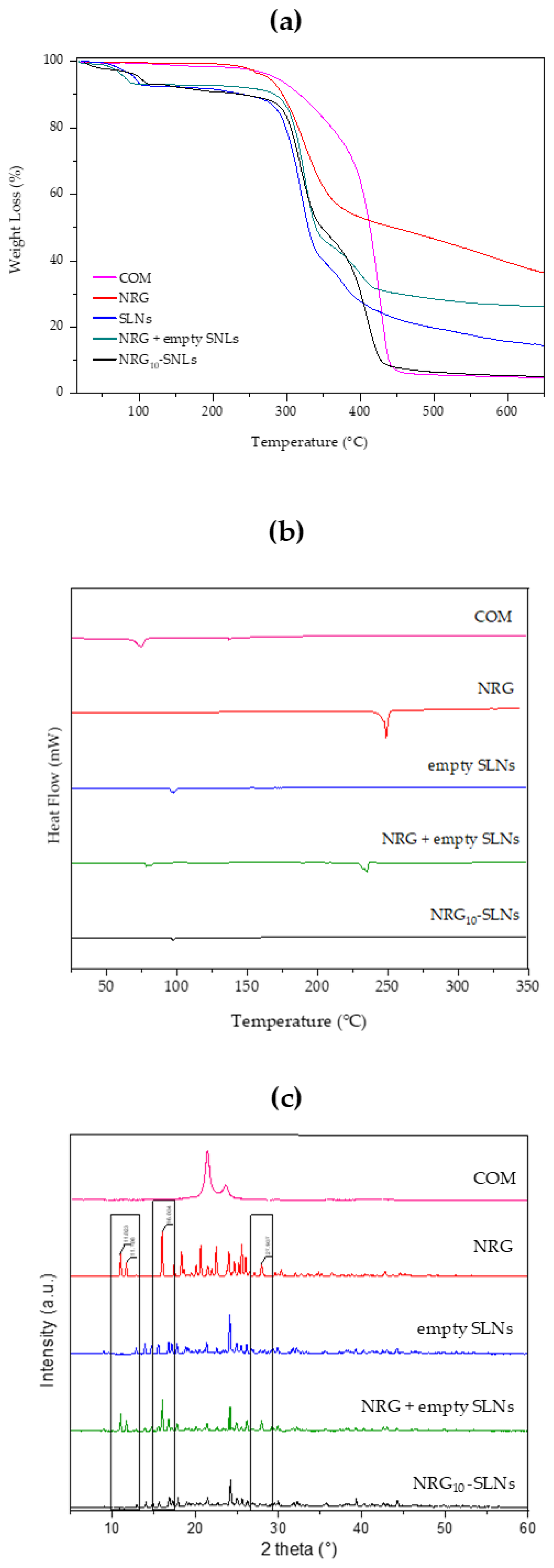
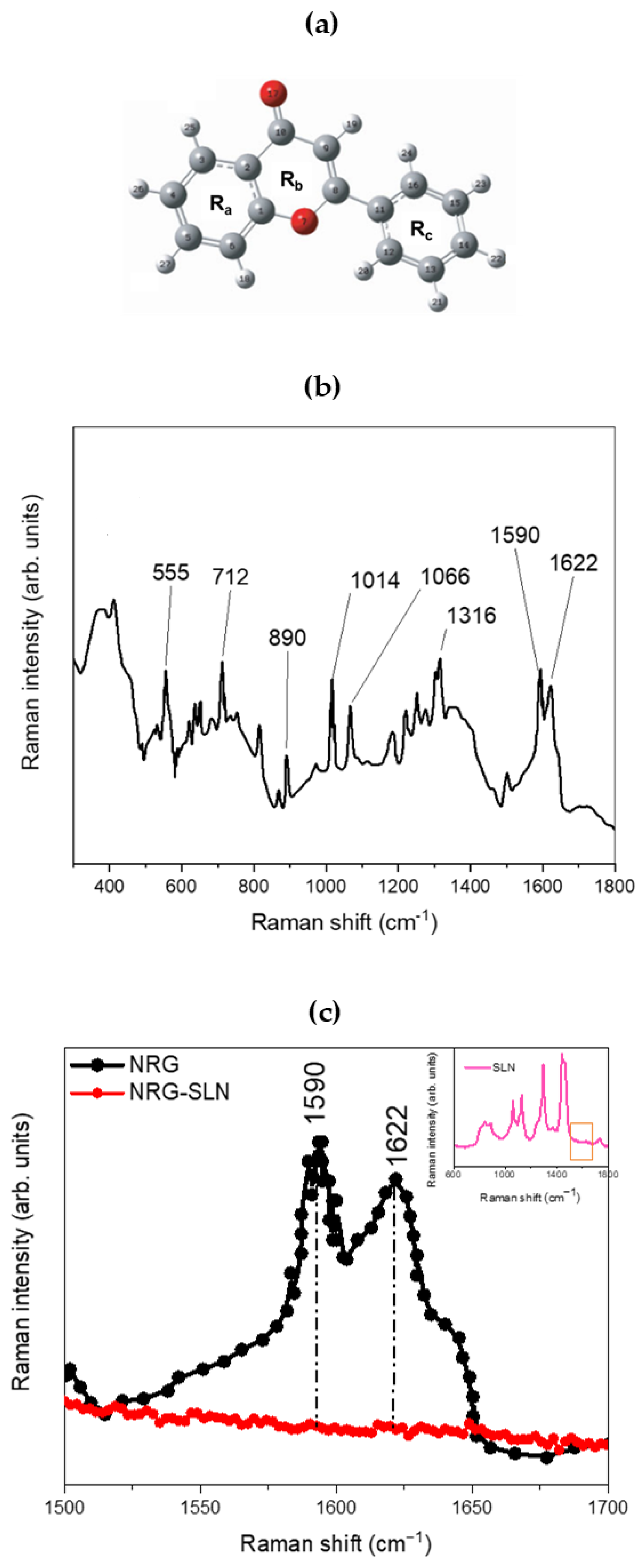



| Sample | Yield % ± S.D. | E.E. % ± S.D. | D.C. % ± S.D. |
|---|---|---|---|
| Empty SLNs | 48.1 ± 0.5 | --- | --- |
| NRG1-SLNs | 52.3 ± 0.4 | 89.2 ± 2.5 | 0.37 ± 0.05 |
| NRG2-SLNs | 54.8 ± 3.3 | 92.5 ± 0.3 | 0.73 ± 0.06 |
| NRG3-SLNs | 58.1 ± 7.8 | 95.6 ± 1.1 | 1.07 ± 0.16 |
| NRG4-SLNs | 46.8 ± 2.2 | 93.8 ± 2.0 | 1.72 ± 2.40 |
| NRG5-SLNs | 54.0 ± 3.1 | 94.6 ± 0.5 | 1.88 ± 3.25 |
| NRG6-SLNs | 52.5 ± 6.2 | 94.4 ± 2.1 | 2.65 ± 1.58 |
| NRG7-SLNs | 48.8 ± 4.2 | 92.5 ± 4.0 | 2.80 ± 0.80 |
| NRG8-SLNs | 48.6 ± 6.6 | 92.7 ± 0.5 | 3.26 ± 2.13 |
| NRG9-SLNs | 56.2 ± 5.2 | 94.2 ± 4.8 | 3.20 ± 1.20 |
| NRG10-SLNs | 51.5 ± 4.1 | 97.9 ± 0.7 | 4.11 ± 0.20 |
| Sample | DH (nm) ± S.D. | PDI (%) ± S.D. | ζ (mV) ± S.D. | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Empty SLNs | 54.4 ± 2.5 | 50.2 ± 4.2 | 0.12 ± 0.01 | 0.15 ± 0.02 | −30.5 ± 0.6 | −29.3 ± 1.4 |
| NRG10-SLNs | 58.8 ± 0.3 | 56.8 ± 6.2 | 0.15 ± 0.00 | 0.15 ± 0.00 | −30.3 ± 0.1 | −30.5 ± 2.6 |
| Day | 4° C | 25° C in the Daylight | 25 ° C in the Dark | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DH (nm) ± S.D. | PDI (%) ± S.D. | ζ (mV) ± S.D. | DH (nm) ± S.D. | PDI (%) ± S.D. | ζ (mV) ± S.D. | DH (nm) ± S.D. | PDI (%) ± S.D. | ζ (mV) ± S.D. | |
| 1 | 58.8 ± 0.3 | 0.15 ± 0.00 | −30.3 ± 0.1 | 58.8 ± 0.3 | 0.15 ± 0.00 | −30.3 ± 0.1 | 58.8 ± 1.3 | 0.15 ± 0.01 | −30.3 ± 0.1 |
| 7 | 68.6 ± 3.1 | 0.15 ± 0.01 | −28.1 ± 0.6 | 58.4 ± 3.6 | 0.13 ± 0.00 | −31.2 ± 0.8 | 61.4 ± 0.6 | 0.14 ± 0.02 | −29.2 ± 0.8 |
| 15 | 78.4 ± 1.6 | 0.12 ± 0.01 | −27.8 ± 0.2 | 64.6 ± 0.5 | 0.12 ± 0.01 | −29.9 ± 1.0 | 64.4 ± 6.4 | 0.14 ± 0.01 | −28.6 ± 1.0 |
| 30 | 89.5 ± 9.3 | 0.14 ± 0.09 | −24.5 ± 0.4 | 65.3 ± 3.7 | 0.15 ± 0.02 | −28.9 ± 2.2 | 68.5 ± 2.8 | 0.15 ± 0.01 | −27.9 ± 2.9 |
| Sample | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | K0 (d−1) | R2 | K1 (d−1) | R2 | KH (d−1/2) | R2 | n | |
| NRG10-SLNs | 0.8009 | 0.5665 | 0.9939 | 0.0275 | 0.9535 | 8.0534 | 0.72 | 0.5764 |
| Strains | NRG10-SLNs | NRG | Empty SLNs | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| mg/mL | ||||||
| S. aureus ATCC 6538 | 0.5 | >1 | 0.5 | >1 | 1 | >1 |
| S. aureus (MRSA)ATCC 43300 | 0.25 | >1 | 0.5 | >1 | 0.5 | >1 |
| E. coli ATCC 10536 | 1 | >1 | 1 | >1 | >1 | >1 |
| P. aeruginosa ATCC 9027 | >1 | >1 | >1 | >1 | >1 | >1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gaetano, F.; Caridi, F.; Totaro, N.; Celesti, C.; Venuti, V.; Ginestra, G.; Nostro, A.; Tommasini, S.; Ventura, C.A.; Stancanelli, R. Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity. Pharmaceuticals 2025, 18, 232. https://doi.org/10.3390/ph18020232
De Gaetano F, Caridi F, Totaro N, Celesti C, Venuti V, Ginestra G, Nostro A, Tommasini S, Ventura CA, Stancanelli R. Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity. Pharmaceuticals. 2025; 18(2):232. https://doi.org/10.3390/ph18020232
Chicago/Turabian StyleDe Gaetano, Federica, Francesco Caridi, Noemi Totaro, Consuelo Celesti, Valentina Venuti, Giovanna Ginestra, Antonia Nostro, Silvana Tommasini, Cinzia Anna Ventura, and Rosanna Stancanelli. 2025. "Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity" Pharmaceuticals 18, no. 2: 232. https://doi.org/10.3390/ph18020232
APA StyleDe Gaetano, F., Caridi, F., Totaro, N., Celesti, C., Venuti, V., Ginestra, G., Nostro, A., Tommasini, S., Ventura, C. A., & Stancanelli, R. (2025). Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity. Pharmaceuticals, 18(2), 232. https://doi.org/10.3390/ph18020232















