Diabetic Ketoacidosis and the Use of New Hypoglycemic Groups: Real-World Evidence Utilizing the Food and Drug Administration Adverse Event Reporting System
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Institutional Review Board Statement
4.2. Study Design and Setting
4.3. Data Collection and Management
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawami, K.; Tanaka, A.; Node, K. Updated evidence on cardiovascular and renal effects of GLP-1 receptor agonists and combination therapy with SGLT2 inhibitors and finerenone: A narrative review and perspectives. Cardiovasc. Diabetol. 2024, 23, 410. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S158–S178. [Google Scholar] [CrossRef]
- Ahren, B. DPP-4 inhibitors. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, I.; Goblirsch, M.J.; Tuan, W.J.; Beckham, F. Transitioning from insulin to dipeptidyl-peptidase 4 (DPP-4) inhibitors for type 2 diabetes. Geriatr. Nurs. 2022, 46, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gourdy, P.; Darmon, P.; Dievart, F.; Halimi, J.M.; Guerci, B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc. Diabetol. 2023, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Lix, L.M.; Fralick, M.; Dell’Aniello, S.; Shah, B.R.; Ronksley, P.E.; Tremblay, E.; Hu, N.; Alessi-Severini, S.; Fisher, A.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann. Intern. Med. 2020, 173, 417–425. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Avogaro, A. SGLT2 inhibitors and diabetic ketoacidosis: Data from the FDA Adverse Event Reporting System. Diabetologia 2017, 60, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bansal, O.; Garg, R.; Hoskote, A. Euglycemic Ketoacidosis From Semaglutide in a Patient Without Diabetes. JCEM Case Rep. 2024, 2, luae156. [Google Scholar] [CrossRef]
- Taylor, S.I.; Blau, J.E.; Rother, K.I. SGLT2 Inhibitors May Predispose to Ketoacidosis. J. Clin. Endocrinol. Metab. 2015, 100, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; David, J.A.; Udoyen, A.O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.; Ibhiedu, J.O.; Ibhiedu, A.O.; Nwosu, P.U.; et al. Comprehensive review of diabetic ketoacidosis: An update. Ann. Med. Surg. 2023, 85, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Evans, K. Diabetic ketoacidosis: Update on management. Clin. Med. 2019, 19, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Calimag, A.P.P.; Chlebek, S.; Lerma, E.V.; Chaiban, J.T. Diabetic ketoacidosis. Dis. Mon. 2023, 69, 101418. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS), July–September 2023. 2023. Available online: https://www.fda.gov/drugs/fdas-adverse-event-reporting-system-faers/july-september-2023-potential-signals-serious-risksnew-safety-information-identified-fda-adverse#:~:text=The%20%E2%80%9CWarnings%20and%20Precautions%E2%80%9D%20section,prolonged%20diabetic%20ketoacidosis%20and%20glucosuria (accessed on 4 December 2024).
- Mistry, S.; Eschler, D.C. Euglycemic Diabetic Ketoacidosis Caused by SGLT2 Inhibitors and a Ketogenic Diet: A Case Series and Review of Literature. AACE Clin. Case Rep. 2021, 7, 17–19. [Google Scholar] [CrossRef]
- Morace, C.; Lorello, G.; Bellone, F.; Quartarone, C.; Ruggeri, D.; Giandalia, A.; Mandraffino, G.; Minutoli, L.; Squadrito, G.; Russo, G.T.; et al. Ketoacidosis and SGLT2 Inhibitors: A Narrative Review. Metabolites 2024, 14, 264. [Google Scholar] [CrossRef]
- Chou, Y.M.; Seak, C.J.; Goh, Z.N.L.; Seak, J.C.; Seak, C.K.; Lin, C.C. Euglycemic diabetic ketoacidosis caused by dapagliflozin: A case report. Medicine 2018, 97, e11056. [Google Scholar] [CrossRef]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.F. SGLT2 Inhibitor-associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin. Ther. 2016, 38, 2654–2664.e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Li, S.; Wang, Y.; Qin, X.; Deng, K.; Liu, Y.; Zou, K.; Sun, X. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Alduraibi, R.K.; Alrebdi, Y.M.; Altowayan, Y.F. Euglycemic diabetic ketoacidosis after the initiation of dulaglutide in patient with type 2 diabetes. Medicine 2023, 102, e34027. [Google Scholar] [CrossRef] [PubMed]
- Medicines and Healthcare Products Regulatory Agency (2019). GLP-1 Receptor Agonists: Reports of Diabetic Ketoacidosis When Concomitant Insulin Was Rapidly Reduced or Discontinued. 2019. Available online: https://www.gov.uk/drug-safety-update/glp-1-receptor-agonists-reports-of-diabetic-ketoacidosis-when-concomitant-insulin-was-rapidly-reduced-or-discontinued (accessed on 3 December 2024).
- Fralick, M.; Schneeweiss, S.; Patorno, E. Risk of Diabetic Ketoacidosis after Initiation of an SGLT2 Inhibitor. N. Engl. J. Med. 2017, 376, 2300–2302. [Google Scholar] [CrossRef]
- Dawwas, G.K.; Flory, J.H.; Hennessy, S.; Leonard, C.E.; Lewis, J.D. Comparative Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Dipeptidyl Peptidase 4 Inhibitors and Sulfonylureas on the Risk of Diabetic Ketoacidosis. Diabetes Care 2022, 45, 919–927. [Google Scholar] [CrossRef]
- Alsuhaibani, D.S.; Edrees, H.H.; Alshammari, T.M. The use and safety risk of repurposed drugs for COVID-19 patients: Lessons learned utilizing the Food and Drug Administration’s Adverse Event Reporting System. Saudi Pharm. J. 2023, 31, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Altebainawi, A.F.; Alfaraj, L.A.; Alharbi, A.A.; Alkhuraisi, F.F.; Alshammari, T.M. Association between proton pump inhibitors and rhabdomyolysis risk: A post-marketing surveillance using FDA adverse event reporting system (FAERS) database. Ther. Adv. Drug Saf. 2023, 14, 20420986231154075. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Suzuki, A.; Wang, D. Statistical methods for exploring spontaneous adverse event reporting databases for drug-host factor interactions. BMC Med. Res. Methodol. 2023, 23, 71. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The Reporting of a Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Saf. 2024, 47, 575–584. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024, 47, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Hauben, M.; Aronson, J.K. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009, 32, 99–110. [Google Scholar] [CrossRef]
- Goldman, A.; Fishman, B.; Twig, G.; Raschi, E.; Cukierman-Yaffe, T.; Moshkovits, Y.; Pomerantz, A.; Ben-Zvi, I.; Dankner, R.; Maor, E. The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (>/=75 years): A retrospective, pharmacovigilance study. Cardiovasc. Diabetol. 2023, 22, 16. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication. FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections (2015). 2015. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious (accessed on 12 November 2024).
- FDA Drug Safety Communication: FDA Strengthens Kidney Warnings for Diabetes Medicines Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR) (2016). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin (accessed on 12 November 2024).
- Erondu, N.; Desai, M.; Ways, K.; Meininger, G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care 2015, 38, 1680–1686. [Google Scholar] [CrossRef]
- Thiruvenkatarajan, V.; Meyer, E.J.; Nanjappa, N.; Van Wijk, R.M.; Jesudason, D. Perioperative diabetic ketoacidosis associated with sodium-glucose co-transporter-2 inhibitors: A systematic review. Br. J. Anaesth. 2019, 123, 27–36. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Master, V.; Gurell, M.N. Clinical Impact of SGLT2 Inhibitors and Euglycemic Diabetic Ketoacidosis: Insights From The Faers Database. Chest 2023, 164, A1750. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, X.; Guo, X.; Liu, D.; Xu, J.; Hu, F.; Zhai, Y.; Gao, Y.; Xu, X.; Dong, Z.; et al. Safety of SGLT2 Inhibitors: A Pharmacovigilance Study from 2013 to 2021 Based on FAERS. Front. Pharmacol. 2021, 12, 766125. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Lam, K.; Zhao, W.; Yang, S.; Li, Y.; Mo, J.; Gao, S.; Liang, D.; Qiu, K.; Huang, M.; et al. SGLT-2 inhibitors and euglycemic diabetic ketoacidosis/diabetic ketoacidosis in FAERS: A pharmacovigilance assessment. Acta Diabetol. 2023, 60, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Zhang, S.; Wu, F.; Liu, D.; Wu, Q.; Zheng, H.; Fan, P.; Su, N. Risk of diabetic ketoacidosis of SGLT2 inhibitors in patients with type 2 diabetes: A systematic review and network meta-analysis of randomized controlled trials. Front. Pharmacol. 2023, 14, 1145587. [Google Scholar] [CrossRef]
- Seidu, S.; Kunutsor, S.K.; Ajjan, R.A.; Choudhary, P. Efficacy and Safety of Continuous Glucose Monitoring and Intermittently Scanned Continuous Glucose Monitoring in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis of Interventional Evidence. Diabetes Care 2024, 47, 169–179. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists. In StatPearls; Disclosure: Ryan Costello Declares No Relevant Financial Relationships with Ineligible Companies; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Monami, M.; Nreu, B.; Zannoni, S.; Lualdi, C.; Mannucci, E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: A meta-analysis of randomised controlled trials. Diabetes Res. Clin. Pract. 2017, 130, 53–60. [Google Scholar] [CrossRef]
- Weber, C.; Kocher, S.; Neeser, K.; Joshi, S.R. Prevention of diabetic ketoacidosis and self-monitoring of ketone bodies: An overview. Curr. Med. Res. Opin. 2009, 25, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Ferhatbegovic, L.; Mrsic, D.; Macic-Dzankovic, A. The benefits of GLP1 receptors in cardiovascular diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef] [PubMed]
- Stottlemyer, B.A.; McDermott, M.C.; Minogue, M.R.; Gray, M.P.; Boyce, R.D.; Kane-Gill, S.L. Assessing adverse drug reaction reports for antidiabetic medications approved by the food and drug administration between 2012 and 2017: A pharmacovigilance study. Ther. Adv. Drug Saf. 2023, 14, 20420986231181334. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services, Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Quarterly Data Extract Files. 2024. Available online: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html (accessed on 4 October 2024).
- Alenzi, K.A.; Alsuhaibani, D.; Batarfi, B.; Alshammari, T.M. Pancreatitis with use of new diabetic medications: A real-world data study using the post-marketing FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 2024, 15, 1364110. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, T.M.; Ata, S.I.; Mahmoud, M.A.; Alhawassi, T.M.; Aljadhey, H.S. Signals of bleeding among direct-acting oral anticoagulant users compared to those among warfarin users: Analyses of the post-marketing FDA Adverse Event Reporting System (FAERS) database, 2010–2015. Ther. Clin. Risk Manag. 2018, 14, 803–809. [Google Scholar] [CrossRef]
- Khaleel, M.A.; Khan, A.H.; Ghadzi, S.M.S.; Adnan, A.S.; Abdallah, Q.M. A Standardized Dataset of a Spontaneous Adverse Event Reporting System. Healthcare 2022, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.D.; Walley, R.J.; Colopy, M.W.; Hilzinger, T.S.; Verdru, P. Pooling Different Safety Data Sources: Impact of Combining Solicited and Spontaneous Reports on Signal Detection In Pharmacovigilance. Drug Saf. 2019, 42, 1191–1198. [Google Scholar] [CrossRef]
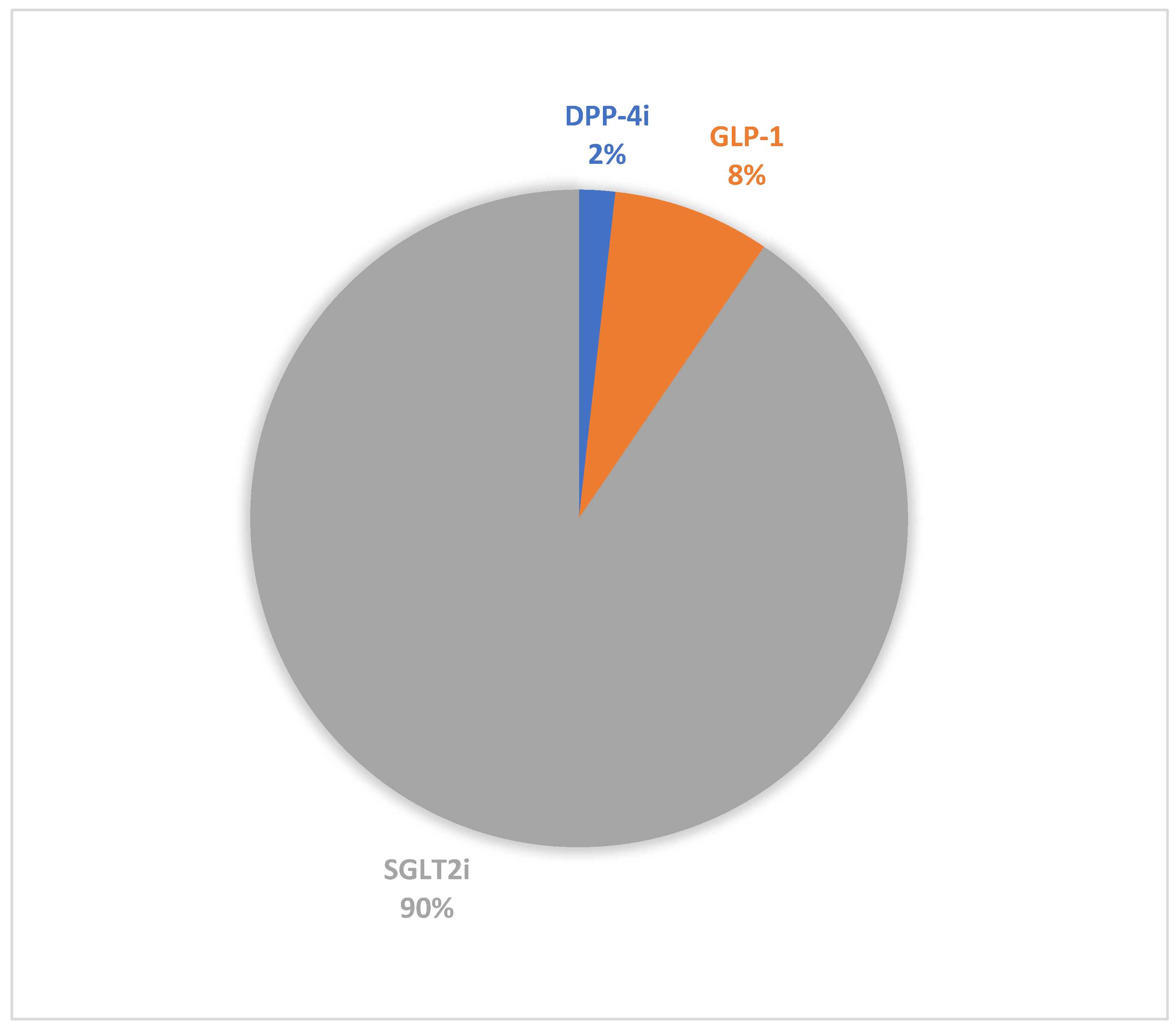
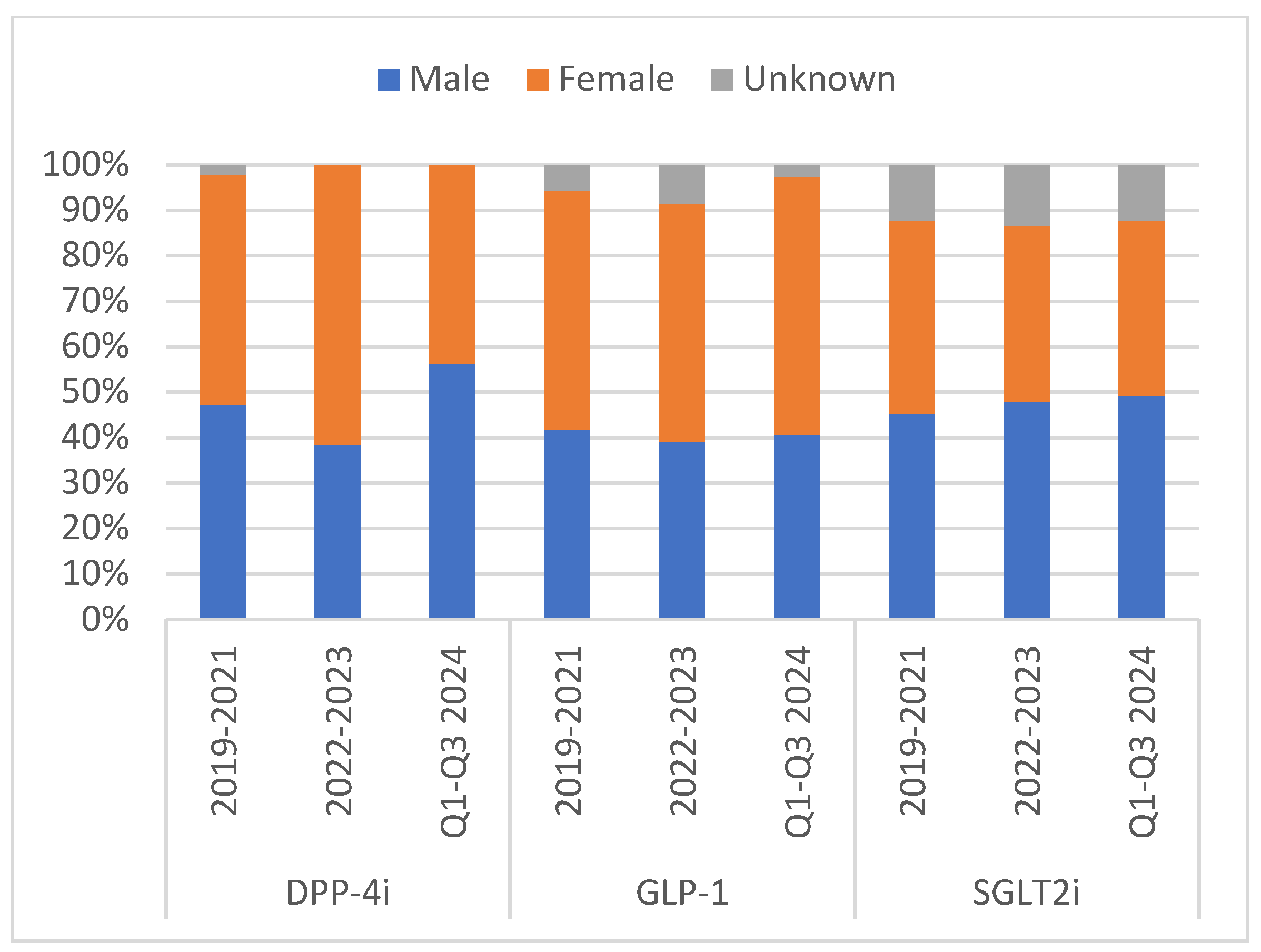
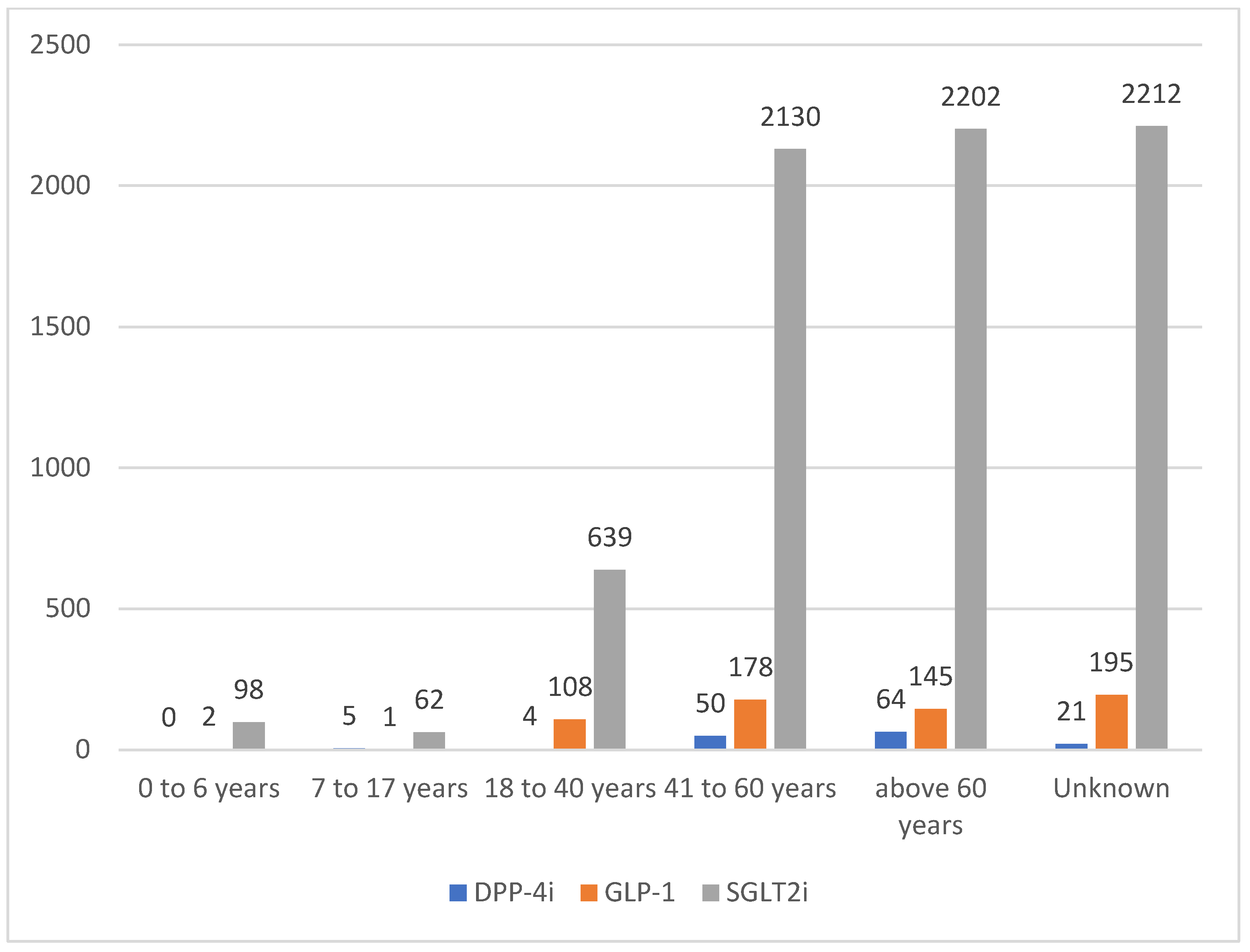
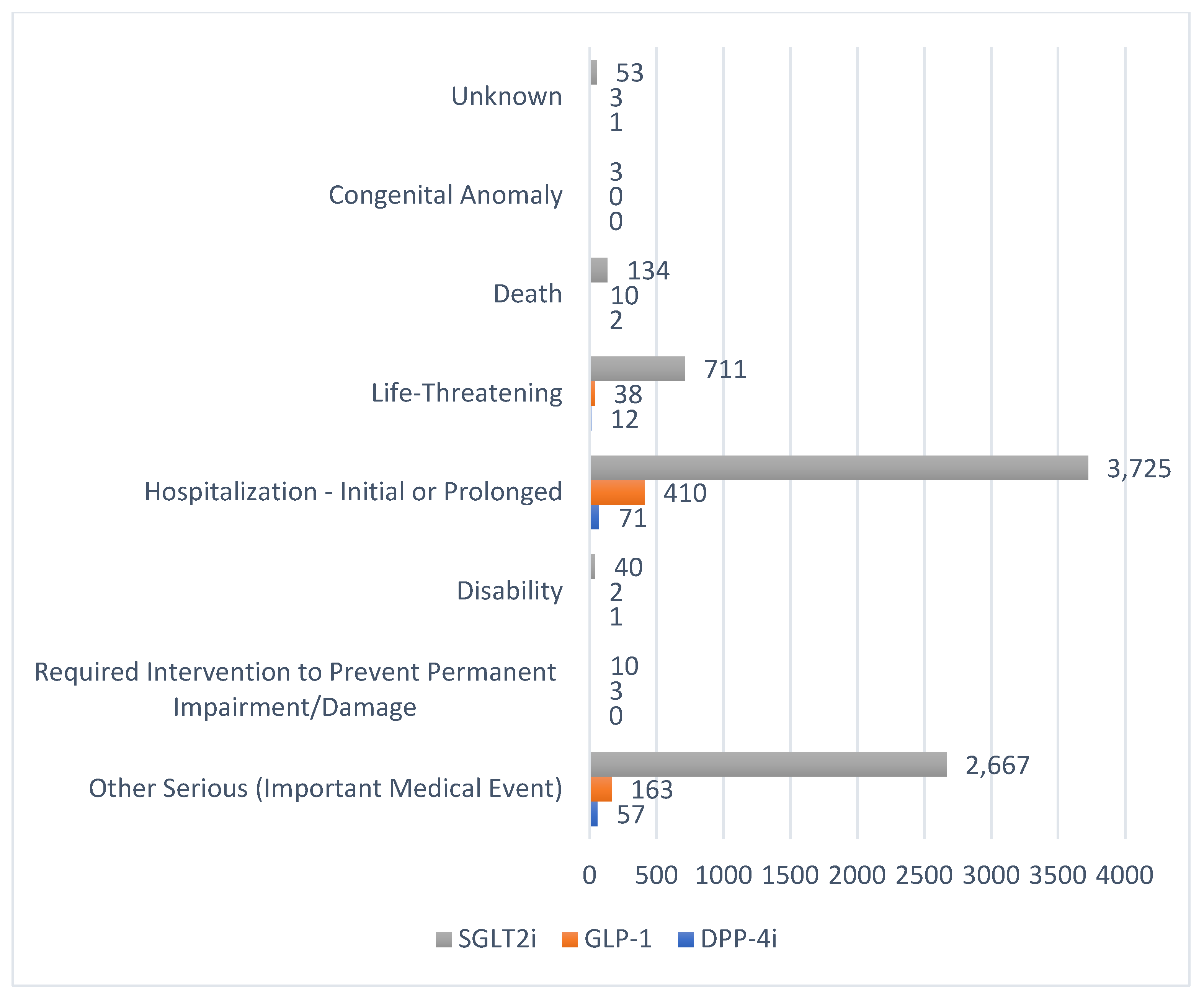
| Medication Class | DPP-4i * | GLP-1 ** | SGLT2i § | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | 2019–2021 | 2022–2023 | Q1–Q3 2024 | 2019–2021 | 2022–2023 | Q1–Q3 2024 | 2019–2021 | 2022–2023 | Q1–Q3 2024 | |||||||||
| Characteristics | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) |
| Patient’s Age (years) | ||||||||||||||||||
| 0–6 | - | - | - | - | - | - | 1 | 0.36 | - | - | 1 | 0.85 | 58 | 1.18 | 32 | 1.75 | 8 | 1.35 |
| 7–17 | 4 | 4.49 | 1 | 2.56 | - | - | - | - | - | - | 1 | 0.85 | 35 | 0.71 | 23 | 1.26 | 4 | 0.68 |
| 18–40 | 2 | 2.25 | 1 | 2.56 | 1 | 6.25 | 47 | 16.9 | 38 | 16.3 | 23 | 19.5 | 506 | 10.3 | 115 | 6.29 | 18 | 3.05 |
| 41–60 | 32 | 36 | 14 | 35.9 | 4 | 25 | 77 | 27.7 | 67 | 28.8 | 34 | 28.8 | 1548 | 31.4 | 432 | 23.7 | 150 | 25.4 |
| >60 | 36 | 40.5 | 20 | 51.3 | 8 | 50 | 61 | 21.9 | 58 | 24.9 | 26 | 22 | 1354 | 27.5 | 620 | 33.9 | 228 | 38.6 |
| Unknown | 15 | 16.9 | 3 | 7.69 | 3 | 18.8 | 92 | 33.1 | 70 | 30 | 33 | 28 | 1424 | 28.9 | 605 | 33.1 | 183 | 31 |
| Reporter’s Occupation | ||||||||||||||||||
| Physician | 46 | 51.7 | 26 | 66.7 | 8 | 50 | 115 | 41.4 | 83 | 35.6 | 42 | 35.6 | 2567 | 52.1 | 1080 | 59.1 | 327 | 55.3 |
| Pharmacist | 4 | 4.49 | 2 | 5.13 | 5 | 31.3 | 18 | 6.47 | 21 | 9.01 | 11 | 9.32 | 651 | 13.2 | 271 | 14.8 | 89 | 15.1 |
| Other Health Professional | 16 | 18 | 9 | 23.1 | 3 | 18.8 | 43 | 15.5 | 52 | 22.3 | 25 | 21.2 | 969 | 19.7 | 339 | 18.6 | 112 | 19 |
| Lawyer | 2 | 2.25 | - | - | - | - | - | - | - | - | - | - | 1 | 0.02 | 1 | 0.05 | - | - |
| Consumer | 19 | 21.4 | 2 | 5.13 | - | - | 97 | 34.9 | 77 | 33.1 | 40 | 33.9 | 410 | 8.32 | 126 | 6.9 | 60 | 10.2 |
| Unknown | 2 | 2.25 | - | - | - | - | 5 | 1.8 | - | - | - | - | 327 | 6.64 | 10 | 0.55 | 3 | 0.51 |
| Reporter’s Country | ||||||||||||||||||
| United States | 30 | 33.7 | 3 | 7.69 | 4 | 25 | 140 | 50.4 | 74 | 31.8 | 38 | 32.2 | 1106 | 22.5 | 674 | 36.9 | 255 | 43.2 |
| Outside the United States | 57 | 64 | 36 | 92.3 | 12 | 75 | 137 | 49.3 | 159 | 68.2 | 80 | 67.8 | 3814 | 77.4 | 1153 | 63.1 | 336 | 56.9 |
| Unknown | 2 | 2.25 | - | - | - | - | 1 | 0.36 | - | - | - | - | 5 | 0.1 | - | - | - | - |
| Total DKA Reports | 89 | 39 | 16 | 278 | 233 | 118 | 4925 | 1827 | 591 | |||||||||
| Medication Class | Duration | DKA Reports | Reported ADEs | ROR | PRR | EBGM | IC |
|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | ||||||
| DPP-4i | 2019–2021 | 89 | 7333 | 6.81 | 6.74 | 6.48 | 2.69 |
| (5.52–8.40) | (5.46–8.31) | ||||||
| 2022–2023 | 39 | 3373 | 8.57 | 8.48 | 7.69 | 2.94 | |
| (6.24–11.76) | (6.17–11.64) | ||||||
| Q1–Q3 2024 | 16 | 925 | 11.02 | 10.85 | 8.3 | 3.05 | |
| (6.71–18.10) | (6.60–17.82) | ||||||
| GLP-1 | 2019–2021 | 278 | 54,286 | 2.88 | 2.87 | 2.81 | 1.5 |
| (2.56–3.25) | (2.55–3.24) | ||||||
| 2022–2023 | 233 | 38,192 | 4.64 | 4.4 | 4.41 | 2.14 | |
| (4.06–5.29) | (3.63–5.34) | ||||||
| Q1–Q3 2024 | 118 | 19,563 | 3.95 | 3.93 | 3.71 | 1.9 | |
| (3.27–4.76) | (3.26–4.74) | ||||||
| SGLT2i | 2019–2021 | 4925 | 22,348 | 314.86 | 245.69 | 120 | 6.9 |
| (301.76–328.53) | (235.47–256.36) | ||||||
| 2022–2023 | 1827 | 18,641 | 126.96 | 114.61 | 70 | 6.13 | |
| (119.54–138.48) | (107.92–121.73) | ||||||
| Q1–Q3 2024 | 591 | 9258 | 60.48 | 56.68 | 38.46 | 5.26 | |
| (54.78–66.76) | (51.34–62.58) |
| Adverse Drug Reaction of Interest | Other Adverse Drug Reactions | |
|---|---|---|
| Drug of Interest | a | b |
| All Other Drugs in the Database | c | d |
| Metric | Formula | Criteria |
|---|---|---|
| ROR, 95% CI | 95% CI > 1, n ≥ 2 | |
| PRR, 95% CI | PRR ≥ 2, chi-squared ≥ 4, n ≥ 3 | |
| EBGM | EBGM 05 ≥ 2 | |
| IC | Lower limit of 95% CI ≥ 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaibah, H.A.; Banji, O.J.F.; Banji, D.; Alshammari, T.M. Diabetic Ketoacidosis and the Use of New Hypoglycemic Groups: Real-World Evidence Utilizing the Food and Drug Administration Adverse Event Reporting System. Pharmaceuticals 2025, 18, 214. https://doi.org/10.3390/ph18020214
Thaibah HA, Banji OJF, Banji D, Alshammari TM. Diabetic Ketoacidosis and the Use of New Hypoglycemic Groups: Real-World Evidence Utilizing the Food and Drug Administration Adverse Event Reporting System. Pharmaceuticals. 2025; 18(2):214. https://doi.org/10.3390/ph18020214
Chicago/Turabian StyleThaibah, Hilal A., Otilia J. F. Banji, David Banji, and Thamir M. Alshammari. 2025. "Diabetic Ketoacidosis and the Use of New Hypoglycemic Groups: Real-World Evidence Utilizing the Food and Drug Administration Adverse Event Reporting System" Pharmaceuticals 18, no. 2: 214. https://doi.org/10.3390/ph18020214
APA StyleThaibah, H. A., Banji, O. J. F., Banji, D., & Alshammari, T. M. (2025). Diabetic Ketoacidosis and the Use of New Hypoglycemic Groups: Real-World Evidence Utilizing the Food and Drug Administration Adverse Event Reporting System. Pharmaceuticals, 18(2), 214. https://doi.org/10.3390/ph18020214





