Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2
Abstract
1. Introduction
2. Results
2.1. DP2 Promotes Mineralization in MC3T3-E1 Cells
2.2. DP2 Enhances Bone Regeneration in a Rat Calvarial Defect
- -
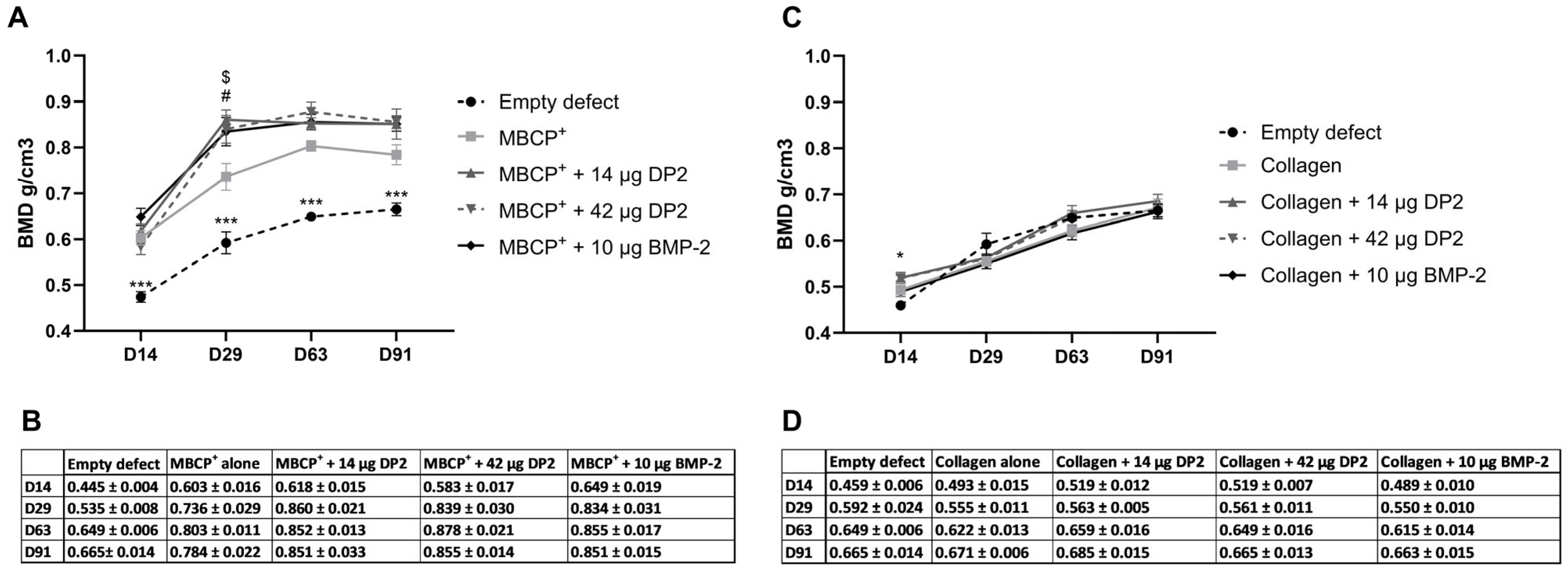
- Quantitative analysis of bone mineral density (BMD)
- -
- Quantitative analysis of bone volume fraction
- -
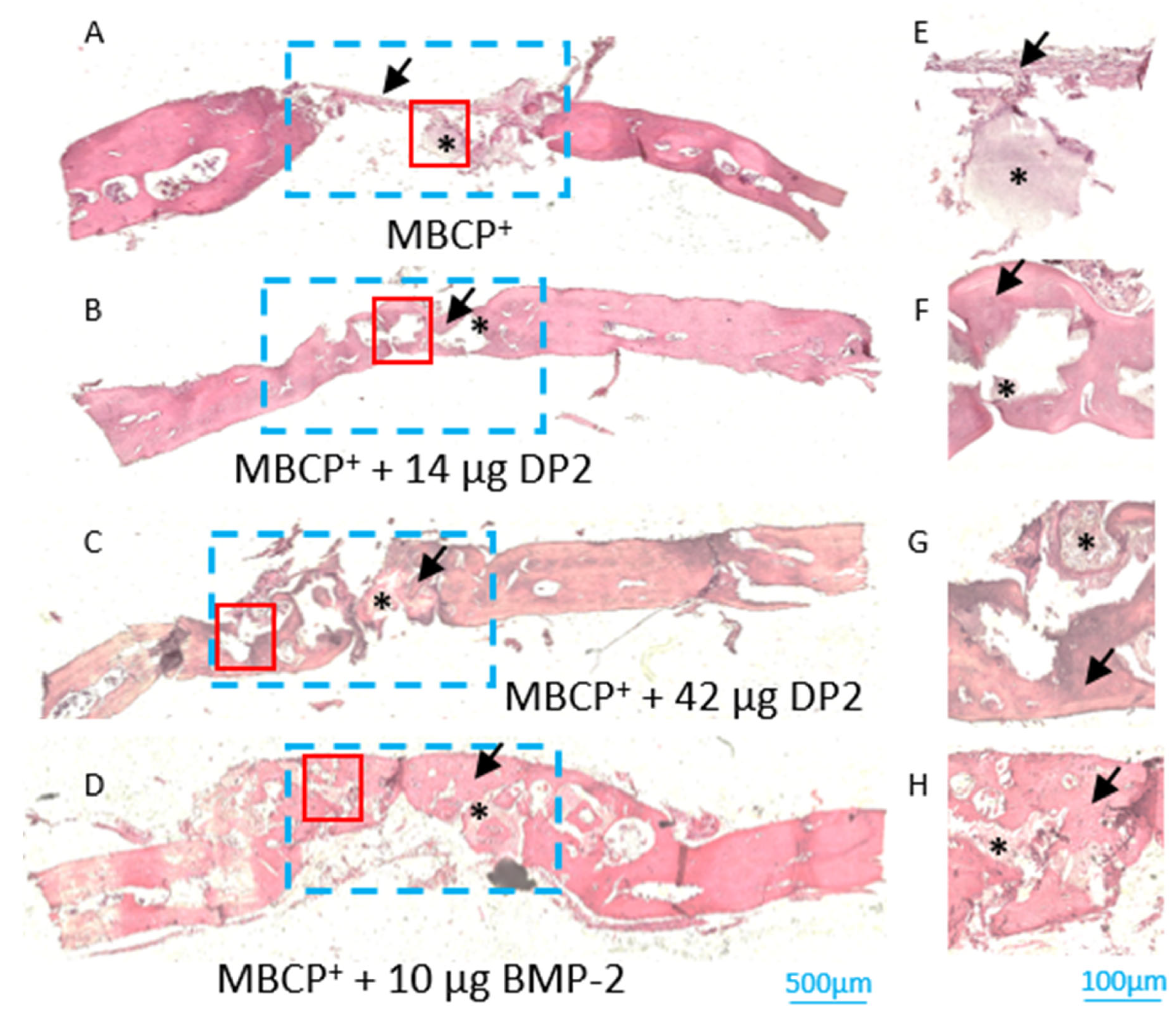
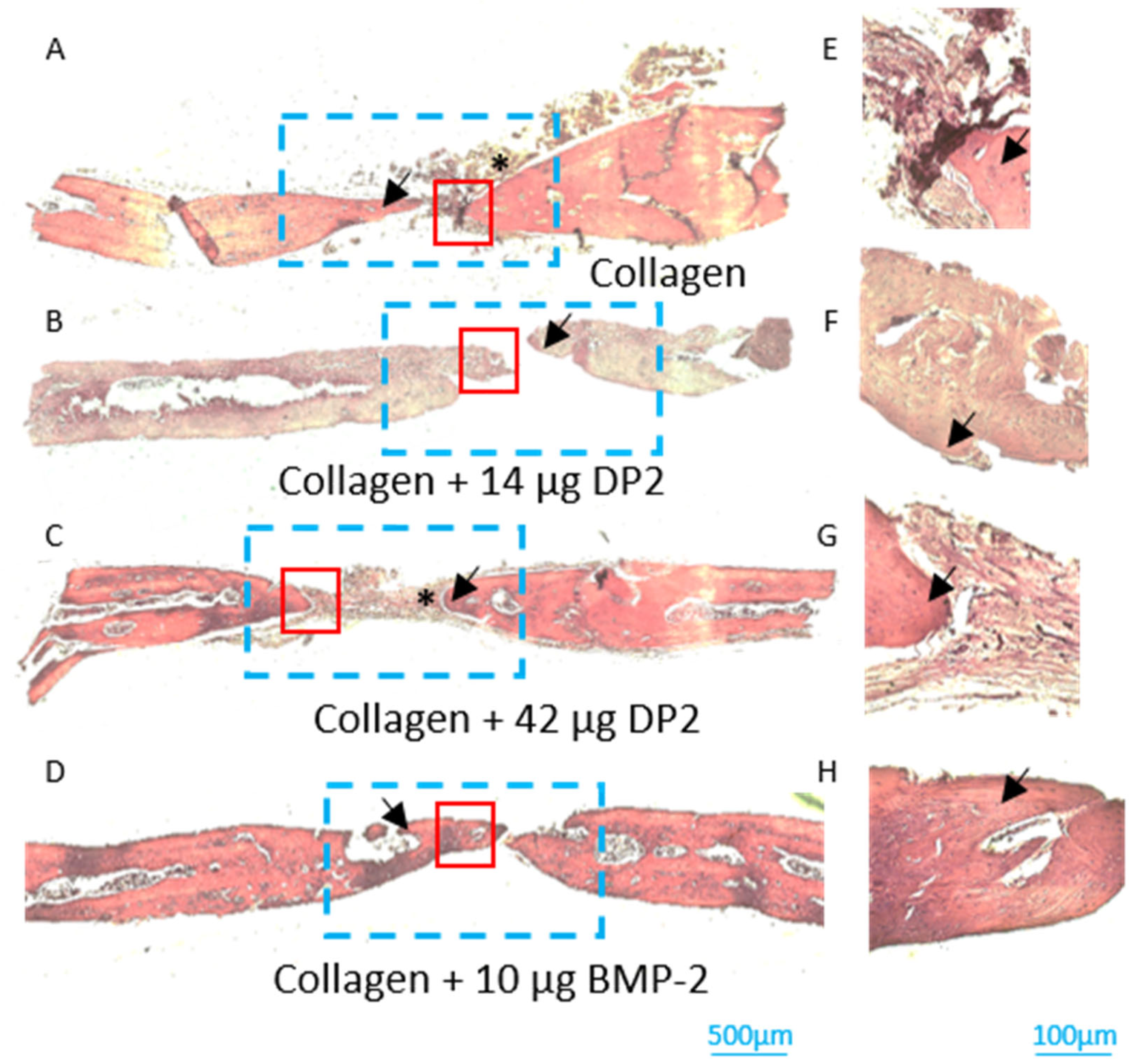
- Histological analyses of newly formed bone
3. Discussion
4. Materials and Methods
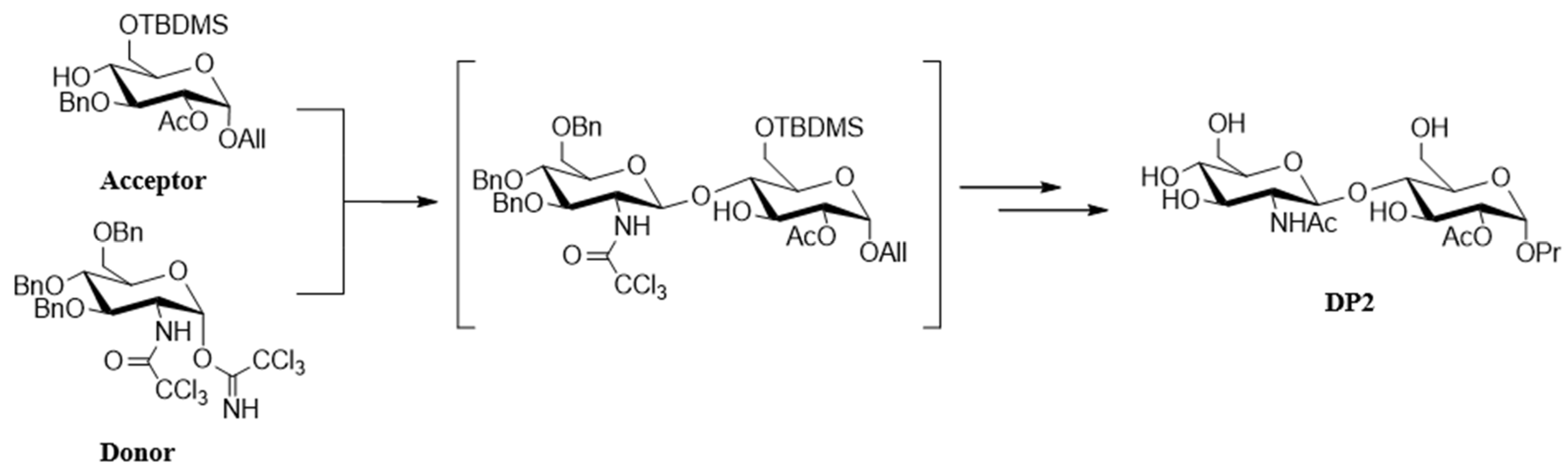
4.1. Chemical Synthesis of DP2
4.2. Cell Culture
4.3. Calcium Quantification Assay
4.4. Animals and Surgical Procedures
4.5. MicroCT Analysis
4.6. Tissue Preparation and Histological Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; De Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Ehrnthaller, C. Long Bone Uninfected Non-Union: Grafting Techniques. EFORT Open Rev. 2024, 9, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Setiawati, R.; Rahardjo, P. Bone Development and Growth. In Osteogenesis and Bone Regeneration; Yang, H., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 1; ISBN 978-1-78985-768-9. [Google Scholar]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Fellah, B.H.; Miramond, T.; Durand, M. Osteoconduction, Osteogenicity, Osteoinduction, What Are the Fundamental Properties for a Smart Bone Substitutes. IRBM 2013, 34, 346–348. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Cahill, K.S.; McCormick, P.C.; Levi, A.D. A Comprehensive Assessment of the Risk of Bone Morphogenetic Protein Use in Spinal Fusion Surgery and Postoperative Cancer Diagnosis. J. Neurosurg. Spine SPI 2015, 23, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, S.; Bennet, S.J.; Arora, M. Bone Morphogenetic Protein-7: Review of Signalling and Efficacy in Fracture Healing. J. Orthop. Transl. 2016, 4, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, X.; Liu, X.; Mou, X.; Guo, Y.; Yang, L.; Chen, Y.; Zhou, Y.; Shi, Z.; Yang, Z.; et al. Advances in the Application of Bone Morphogenetic Proteins and Their Derived Peptides in Bone Defect Repair. Compos. Part B Eng. 2023, 262, 110805. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A Critical Review of Recombinant Human Bone Morphogenetic Protein-2 Trials in Spinal Surgery: Emerging Safety Concerns and Lessons Learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Fu, R.; Selph, S.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and Harms of Recombinant Human Bone Morphogenetic Protein-2 in Spine Fusion. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the Use of Bone Morphogenetic Protein 2 (BMP-2) in Spine Surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Nguyen, V.; Meyers, C.A.; Yan, N.; Agarwal, S.; Levi, B.; James, A.W. BMP-2-Induced Bone Formation and Neural Inflammation. J. Orthop. 2017, 14, 252–256. [Google Scholar] [CrossRef]
- Suzuki, M.F.; Oliveira, J.E.; Damiani, R.; Lima, E.R.; Amaral, K.C.; Santos, A.M.d.S.; Magalhães, G.S.; Faverani, L.P.; Pereira, L.A.V.D.; Silva, F.M.; et al. Human Bone Morphogenetic Protein-2 (HBMP-2) Characterization by Physical–Chemical, Immunological and Biological Assays. AMB Express 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Büchner, D.; Shoushrah, S.H.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of Biomimetic Hydroxyapatite Coatings on Osteoimmunomodulation. Biomater. Adv. 2022, 134, 112640. [Google Scholar] [CrossRef] [PubMed]
- Dussouy, C.; Bultel, L.; Saguez, J.; Cherqui, A.; Khelifa, M.; Grand, E.; Giordanengo, P.; Kovensky, J. Strong Aphicidal Activity of GlcNAc(Β1→4)Glc Disaccharides: Synthesis, Physiological Effects, and Chitinase Inhibition. Chem.-Eur. J. 2012, 18, 10021–10028. [Google Scholar] [CrossRef]
- Ballout, N.; Boullier, A.; Darwiche, W.; Ait-Mohand, K.; Trécherel, E.; Gallégo, T.; Gomila, C.; Yaker, L.; Gennero, I.; Kovensky, J.; et al. DP2, a Carbohydrate Derivative, Enhances In Vitro Osteoblast Mineralisation. Pharmaceuticals 2023, 16, 1512. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Ma, X.; Zhong, Y.; Tang, H.; Mai, S. The Mechanism of Biomineralization: Progress in Mineralization from Intracellular Generation to Extracellular Deposition. Jpn Dent. Sci. Rev. 2023, 59, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ghayor, C.; Guicheux, J.; Magne, D.; Quillard, S.; Kakita, A.; Ono, Y.; Miura, Y.; Oiso, Y.; Itoh, M.; et al. Enhanced Expression of the Inorganic Phosphate Transporter Pit-1 Is Involved in BMP-2–Induced Matrix Mineralization in Osteoblast-Like Cells. J. Bone Miner. Res. 2006, 21, 674–683. [Google Scholar] [CrossRef]
- Zamurovic, N.; Cappellen, D.; Rohner, D.; Susa, M. Coordinated Activation of Notch, Wnt, and Transforming Growth Factor-β Signaling Pathways in Bone Morphogenic Protein 2-Induced Osteogenesis: Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity *. J. Biol. Chem. 2004, 279, 37704–37715. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Abd El Raouf, M.; Saulacic, N.; Kobayashi, E.; Zhang, Y.; Schaller, B.; Miron, R.J. Superior Bone-Inducing Potential of RhBMP9 Compared to RhBMP2. J. Biomed. Mater. Res. Part A 2018, 106, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Notodihardjo, F.Z.; Kakudo, N.; Kushida, S.; Suzuki, K.; Kusumoto, K. Bone Regeneration with BMP-2 and Hydroxyapatite in Critical-Size Calvarial Defects in Rats. J. Cranio-Maxillofac. Surg. 2012, 40, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson, J.C., III; Buxton, A.N.; Wikesjö, U.M.E. Effect of RhBMP-2 Dose on Bone Formation/Maturation in a Rat Critical-Size Calvarial Defect Model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef]
- Jeong, B.-C.; Choi, H.; Hur, S.-W.; Kim, J.-W.; Oh, S.-H.; Kim, H.-S.; Song, S.-C.; Lee, K.-B.; Park, K.-B.; Koh, J.-T. Repair of Cranial Bone Defects Using RhBMP2 and Submicron Particle of Biphasic Calcium Phosphate Ceramics with Through-Hole. Biomed. Res. Int. 2015, 2015, 926291. [Google Scholar] [CrossRef]
- Brown, J.P.; Adachi, J.D.; Schemitsch, E.; Tarride, J.-E.; Brown, V.; Bell, A.; Reiner, M.; Oliveira, T.; Motsepe-Ditshego, P.; Burke, N.; et al. Mortality in Older Adults Following a Fragility Fracture: Real-World Retrospective Matched-Cohort Study in Ontario. BMC Musculoskelet. Disord. 2021, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Haleem, S.; Choudri, M.J.; Kainth, G.S.; Parker, M.J. Mortality Following Hip Fracture: Trends and Geographical Variations over the Last SIXTY Years. Injury 2023, 54, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Payen, M.; Mainard, N.; Accadbled, F.; Sales de Gauzy, J.; Abid, A. Surgical Treatment of Congenital Pseudarthrosis of the Clavicle: A Series of 10 Cases. Orthop. Traumatol. Surg. Res. 2022, 110, 103518. [Google Scholar] [CrossRef]
- Zargarbashi, R.; Bagherpour, A.; Panjavi, B.; Bagherpour Zarchi, M. Is Using a Ring External Fixator in the Treatment of Congenital Pseudarthrosis of the Tibia Associated With Better Results or Using a Locking Plate? J. Pediatr. Orthop. 2024, 44, e419–e425. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef]
- Durham, E.L.; Howie, R.N.; Hall, S.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; LaRue, A.C.; Muise-Helmericks, R.; et al. Optimizing Bone Wound Healing Using BMP2 with Absorbable Collagen Sponge and Talymed Nanofiber Scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef]
- Stokovic, N.; Ivanjko, N.; Maticic, D.; Luyten, F.P.; Vukicevic, S. Bone Morphogenetic Proteins, Carriers, and Animal Models in the Development of Novel Bone Regenerative Therapies. Materials 2021, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation in Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef]
- Van Griensven, M. Preclinical Testing of Drug Delivery Systems to Bone. Adv. Drug Deliv. Rev. 2015, 94, 151–164. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell Therapy Induced Regeneration of Severely Atrophied Mandibular Bone in a Clinical Trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and Safety of Treating Non-Unions in Tibia, Femur and Humerus with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells Associated with Biphasic Calcium Phosphate Biomaterials in a Multicentric, Non-Comparative Trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Boraiah, S.; Paul, O.; Hawkes, D.; Wickham, M.; Lorich, D.G. Complications of Recombinant Human BMP-2 for Treating Complex Tibial Plateau Fractures: A Preliminary Report. Clin. Orthop. Relat. Res. 2009, 467, 3257–3262. [Google Scholar] [CrossRef]
- Brower, R.S.; Vickroy, N.M. A Case of Psoas Ossification From the Use of BMP-2 for Posterolateral Fusion at L4–L5. Spine 2008, 33, E653–E655. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.; Rampersaud, Y.R. Heterotopic Bone Formation With the Use of RhBMP2 in Posterior Minimal Access Interbody Fusion: A CT Analysis. Spine 2007, 32, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sun, W.; Gao, F.; Cheng, L.; Li, Z. Heterotopic Ossification Related to the Use of Recombinant Human BMP-2 in Osteonecrosis of Femoral Head. Medicine 2017, 96, e7413. [Google Scholar] [CrossRef]
- Shields, L.B.E.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse Effects Associated With High-Dose Recombinant Human Bone Morphogenetic Protein-2 Use in Anterior Cervical Spine Fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of Protein Dose and Delivery System on BMP-Mediated Bone Regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; Gao, T.; Porter, T.J.; Friess, W.; Wozney, J.M. Delivery Systems for BMPs: Factors Contributing to Protein Retention at an Application Site. J. Bone Jt. Surg. 2001, 83, S128–S135. [Google Scholar] [CrossRef]
- Sarkar, B.C.; Chauhan, U.P.S. A New Method for Determining Micro Quantities of Calcium in Biological Materials. Anal. Biochem. 1967, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Adeeb, S.; Doschak, M.R. Using Micro-CT Derived Bone Microarchitecture to Analyze Bone Stiffness—A Case Study on Osteoporosis Rat Bone. Front. Endocrinol. 2015, 6, 80. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballout, N.; Toumieux, S.; Darwiche, W.; Gomila, C.; Trécherel, E.; Accadbled, F.; Laurencin-Dalicieux, S.; Gennero, I.; Kovensky, J.; Boullier, A.; et al. Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2. Pharmaceuticals 2025, 18, 215. https://doi.org/10.3390/ph18020215
Ballout N, Toumieux S, Darwiche W, Gomila C, Trécherel E, Accadbled F, Laurencin-Dalicieux S, Gennero I, Kovensky J, Boullier A, et al. Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2. Pharmaceuticals. 2025; 18(2):215. https://doi.org/10.3390/ph18020215
Chicago/Turabian StyleBallout, Nissrine, Sylvestre Toumieux, Walaa Darwiche, Cathy Gomila, Eric Trécherel, Franck Accadbled, Sara Laurencin-Dalicieux, Isabelle Gennero, José Kovensky, Agnès Boullier, and et al. 2025. "Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2" Pharmaceuticals 18, no. 2: 215. https://doi.org/10.3390/ph18020215
APA StyleBallout, N., Toumieux, S., Darwiche, W., Gomila, C., Trécherel, E., Accadbled, F., Laurencin-Dalicieux, S., Gennero, I., Kovensky, J., Boullier, A., & Ausseil, J. (2025). Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2. Pharmaceuticals, 18(2), 215. https://doi.org/10.3390/ph18020215





