The Prevalence and Malignancy Risk of Breast Incidental Uptake Detected by PET/CT with Different Radiopharmaceuticals: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol, Working Group, and Review Question
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Qualitative Synthesis
3.2.1. [18F]FDG PET/CT
3.2.2. Somatostatin Receptor PET/CT (SSA)
3.2.3. [18F]Fluorocholine PET/CT
3.2.4. Overall Study Quality
3.3. Quantitative Synthesis
3.3.1. Prevalence of BIU
| PET Tracer | First Author [Ref.] | BIU-H | DC | % of DC Among BIU-H | DCIS | % of DCIS Among BIU-H | LC | % of LC Among BIU-H | OUM | % of OUM Among BIU-H | MET | % of MET Among BIU-H | FA | % of FA Among BIU-H | OBL | % of OBL Among BIU-H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18F]FDG | Agress et al. [10] | 2 | 1 | 50 | - | - | 1 | 50 | - | - | - | |||||
| Ishimori et al. [11] | 2 | 2 | 100 | - | - | - | - | - | - | |||||||
| Korn et al. [12] | 6 | 5 | 83.3 | - | - | - | - | 1 | 16.7 | - | ||||||
| Wang et al. [13] | 3 | 2 | 66.7 | - | - | - | - | 1 | 33.3 | - | ||||||
| Beatty et al. [14] | 8 | 5 | 62.5 | - | - | - | - | 2 | 25 | 1 | 12.5 | |||||
| Litmanovich et al. [15] | 21 | 7 | 33.3 | - | 2 | 9.5 | - | 3 | 14.3 | 2 | 9.5 | 7 | 33.3 | |||
| Chung et al. [16] | 12 | 6 | 50 | - | - | - | 1 | 8.3 | 1 | 8.3 | 4 | 33.3 | ||||
| Kang et al. [17] | 35 | 14 | 40 | 2 | 5.7 | - | - | 2 | 5.7 | 1 | 2.9 | 16 | 45.7 | |||
| Chae et al. [18] | 60 | 23 | 38.3 | 3 | 5 | 1 | 1.7 | - | 5 | 8.3 | 8 | 13.3 | 20 | 33.3 | ||
| Chopra et al. [19] | 3 | - | - | - | 2 | 66.7 | - | - | 1 | 33.3 | ||||||
| Kim et al. [20] | 23 | 10 | 43.5 | 3 | 13 | - | - | 2 | 8.7 | 2 | 8.7 | 6 | 26.1 | |||
| Dunne et al. [21] | 23 | 8 | 34.8 | 2 | 8.7 | 1 | 4.3 | 4 | 17.4 | 2 | 8.7 | 4 | 17.4 | 2 | 8.7 | |
| Lim et al. [22] | 17 | 5 | 29.4 | 3 | 17.6 | - | 1 | 5.9 | - | 2 | 11.8 | 6 | 35.3 | |||
| Benveniste et al. [23] | 55 | 19 | 34.5 | 1 | 1.8 | 1 | 1.8 | 4 | 7.3 | 12 | 21.8 | - | 18 | 32.7 | ||
| Bertagna et al. [24] | 35 | 17 | 48.6 | 2 | 5.7 | 4 | 11.4 | - | 2 | 5.7 | 9 | 25.7 | 1 | 2.9 | ||
| Minamimoto et al. [25] | 161 | 95 | 59 | 27 | 16.8 | - | 39 | 24.2 | - | - | - | |||||
| Shin et al. [26] | 60 | 21 | 35 | 1 | 1.7 | 1 | 1.7 | 2 | 3.3 | 2 | 3.3 | 10 | 16.7 | 23 | 38.3 | |
| Falomo et al. [27] | 11 | - | - | - | - | - | - | - | ||||||||
| Moletta et al. [28] | 1 | 1 | 100 | - | - | - | - | - | - | |||||||
| Bakhshayeshkaram et al. [29] | 22 | 6 | 27.3 | - | 3 | 13.6 | - | 1 | 4.5 | 4 | 18.2 | 8 | 36.4 | |||
| Andersen et al. [30] | 40 | 26 | 65 | - | 1 | 2.5 | 3 | 7.5 | 4 | 10 | 3 | 7.5 | 2 | 5 | ||
| Panareo et al. [31] | 22 | 9 | 40.9 | - | - | 3 | 13.6 | 4 | 18.2 | 6 | 27.3 | 1 | 4.5 | |||
| Wakfie-Corieh et al. [32] | 23 | - | - | 1 | 4.3 | 8 | 34.8 | - | - | 14 | 60.9 | |||||
| Menon et al. [33] | 26 | 10 | 38.5 | 2 | 7.7 | 1 | 3.8 | 3 | 11.5 | 2 | 7.7 | 4 | 15.4 | 4 | 15.4 | |
| Kayadibi et al. [34] | 63 | 10 | 15.9 | - | 2 | 3.2 | 3 | 4.8 | 5 | 7.9 | 21 | 33.3 | 22 | 34.9 | ||
| Pooled values (95%CI) | 42.2% (34–50.5) | 7.1% (3.4–11.8) | 3.5% (1.7–5.8) | 11.1% (4.8–19) | 8.7% (6–11.6) | 14.8% (10.3–19.9) | 25.3% (17.3–34) | |||||||||
| Radiolabeled Somatostatin analogues | Elgeti et al. [35] | 4 | 2 | 50 | - | - | - | 2 | 50 | - | - | |||||
| Kuyumcu et al. [36] | - | - | - | - | - | - | - | - | ||||||||
| Cavicchioli et al. [37] | 1 | - | - | - | - | 1 | 100 | - | - | |||||||
| [18F]F-choline | Broos et al. [38] | 7 | 3 | 42.9 | 1 | 14.3 | 2 | 28.6 | 1 | 14.3 | - | - | - |
3.3.2. Risk of Malignancy
- −
- For [18F]FDG PET/CT, the pooled malignancy risk was 60.9% (95% CI 52.5–69.0; I2 = 59.5%) (Figure 4);
- −
- For SSA PET/CT, the malignancy risk was 100% (95% CI 66.8–100; I2 = 0%), acknowledging very small numbers;
- −
- For [18F]fluorocholine PET/CT, the malignancy risk was 100% (7/7 malignant lesions in a single study).
- −
- For [18F]FDG PET/CT, 33.5% (95% CI 25.2–42.3; I2 = 87.9%) (Figure 5);
- −
- For SSA PET/CT, 86.4% (95% CI 72.0–100; I2 = 50.7%);
- −
- For [18F]fluorocholine PET/CT, 70% (single study).

3.3.3. Histopathology
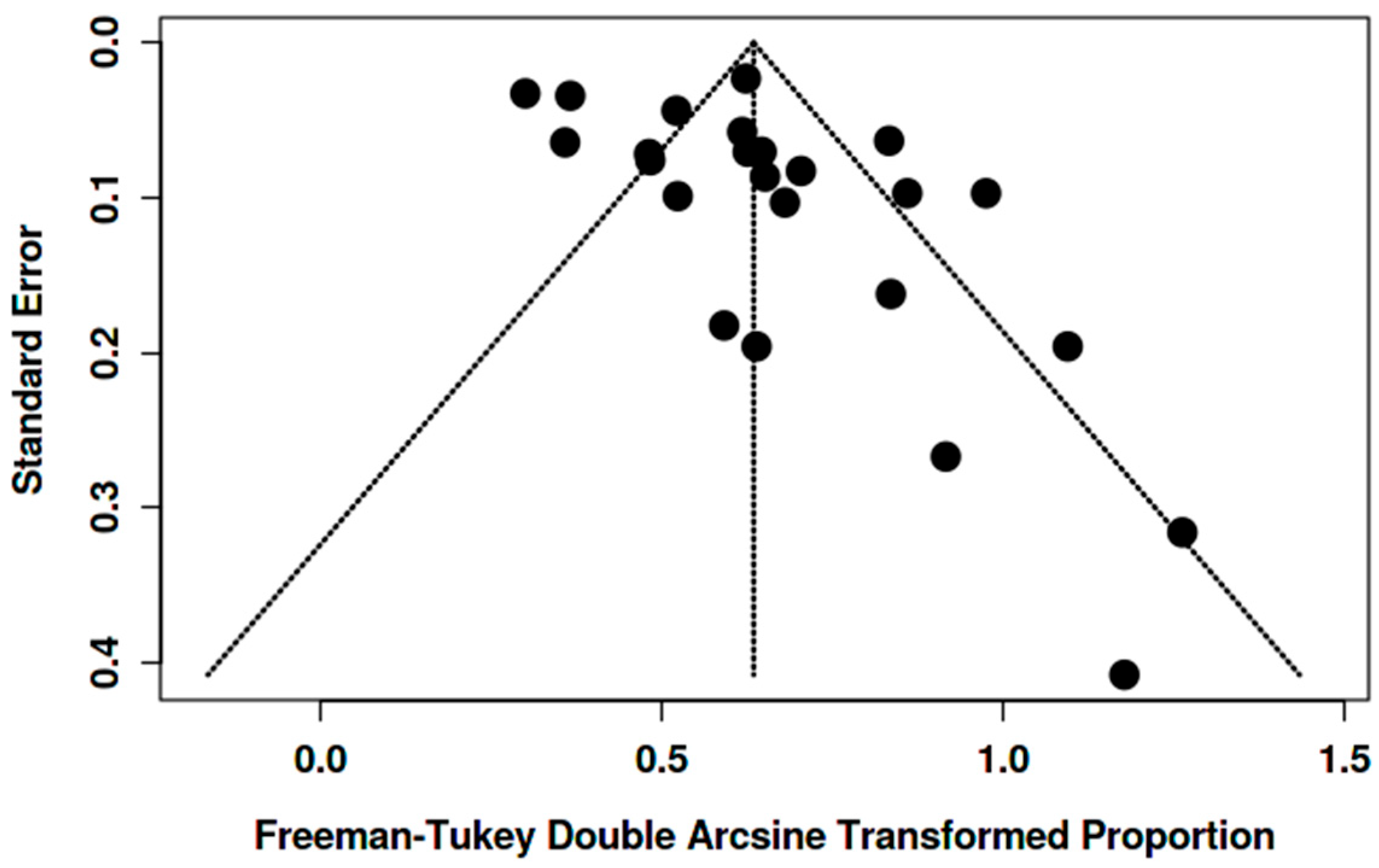
3.3.4. Heterogeneity, Sensitivity Analyses, and Potential Biases
4. Discussion
4.1. Literature Data
4.1.1. Selection Bias in the Outlier Study
4.1.2. What to Do with an Incidental Focus
4.2. Limitations and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Sullivan, J.W.; Muntinga, T.; Grigg, S.; Ioannidis, J.P.A. Prevalence and outcomes of incidental imaging findings: Umbrella review. BMJ 2018, 361, k2387. [Google Scholar] [CrossRef]
- Iacovitti, C.M.; Albano, D.; Rizzo, A.; Piccardo, A.; Cuzzocrea, M.; Paone, G.; Trimboli, P.; Treglia, G. Meta-Analysis on the Prevalence and Significance of Incidental Findings in the Thyroid Gland Using Other PET Radiopharmaceuticals Beyond [18F]FDG. Pharmaceuticals 2025, 18, 723. [Google Scholar] [CrossRef]
- Juweid, M.E.; Al-Qasem, S.F.; Khuri, F.R.; Gallamini, A.; Lohmann, P.; Ziellenbach, H.J.; Mottaghy, F.M. Beyond fluorodeoxyglucose: Molecular imaging of cancer in precision medicine. CA Cancer J. Clin. 2025, 75, 226–242. [Google Scholar] [CrossRef]
- Lin, M.; Coll, R.P.; Cohen, A.S.; Georgiou, D.K.; Manning, H.C. PET Oncological Radiopharmaceuticals: Current Status and Perspectives. Molecules 2022, 27, 6790. [Google Scholar] [CrossRef]
- Bertagna, F.; Treglia, G.; Orlando, E.; Dognini, L.; Giovanella, L.; Sadeghi, R.; Giubbini, R. Prevalence and clinical significance of incidental F18-FDG breast uptake: A systematic review and meta-analysis. Jpn. J. Radiol. 2014, 32, 59–68. [Google Scholar] [CrossRef]
- Aarstad, E.M.; Nordhaug, P.; Naghavi-Behzad, M.; Larsen, L.B.; Gerke, O.; Hildebrandt, M.G. Prevalence of focal incidental breast uptake on FDG-PET/CT and risk of malignancy: A systematic review and meta-analysis. Eur. J. Hybrid Imaging 2019, 3, 16. [Google Scholar] [CrossRef]
- Iacovitti, C.M.; Muoio, B.; Albano, D.; Rizzo, A.; Cuzzocrea, M.; Paone, G.; Treglia, G. The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1204. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseerf, L.; Tetzlaff, J.M.; Akli, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Agress, H., Jr.; Cooper, B.Z. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: Histopathologic comparison. Radiology 2004, 230, 417–422. [Google Scholar] [CrossRef]
- Ishimori, T.; Patel, P.V.; Wahl, R.L. Detection of unexpected additional primary malignancies with PET/CT. J. Nucl. Med. 2005, 46, 752–757. [Google Scholar] [PubMed]
- Korn, R.L.; Yost, A.M.; May, C.C.; Kovalsky, E.R.; Orth, K.M.; Layton, T.A.; Drumm, D. Unexpected focal hypermetabolic activity in the breast: Significance in patients undergoing 18F-FDG PET/CT. AJR Am. J. Roentgenol. 2006, 187, 81–85. [Google Scholar] [CrossRef]
- Wang, G.; Lau, E.W.; Shakher, R.; Rischin, D.; Ware, R.E.; Hong, E.; Binns, D.S.; Hogg, A.; Drummond, E.; Hicks, R.J. How do oncologists deal with incidental abnormalities on whole-body fluorine-18 fluorodeoxyglucose PET/CT? Cancer 2007, 109, 117–124. [Google Scholar] [CrossRef]
- Beatty, J.S.; Williams, H.T.; Gucwa, A.L.; Hughes, M.P.; Vasudeva, V.S.; Aldridge, B.A.; Fields, D.M.; David, G.S.; Lind, D.S.; Kruse, E.J.; et al. The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am. J. Surg. 2009, 198, 495–499. [Google Scholar] [CrossRef]
- Litmanovich, D.; Gourevich, K.; Israel, O.; Gallimidi, Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: Incidence and clinical significance. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1558–1564. [Google Scholar] [CrossRef]
- Chung, A.; Schoder, H.; Sampson, M.; Morrow, M.; Port, E. Incidental breast lesions identified by 18F-fluorodeoxyglucose-positron emission tomography. Ann. Surg. Oncol. 2010, 17, 2119–2125. [Google Scholar] [CrossRef]
- Kang, B.J.; Lee, J.H.; Yoo, I.eR.; Kim, S.H.; Choi, J.J.; Jeong, S.H.; Yim, H.W. Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am. J. Roentgenol. 2011, 197, 341–347. [Google Scholar] [CrossRef]
- Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Kim, H.J.; Oh, H.Y.; Koh, Y.H.; Moon, D.H. Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: Clinical significance and differential diagnosis. Acta Radiol. 2012, 53, 530–535. [Google Scholar] [CrossRef]
- Chopra, A.; Ford, A.; De Noronha, R.; Matthews, S. Incidental findings on positron emission tomography/CT scans performed in the investigation of lung cancer. Br. J. Radiol. 2012, 85, e229–e237. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, N.; Chang, J.M.; Yun, B.L.; Bae, M.S.; Kang, K.W.; Moon, W.K. Mammography and ultrasonography evaluation of unexpected focal 18F-FDG uptakes in breast on PET/CT. Acta Radiol. 2012, 53, 249–254. [Google Scholar] [CrossRef]
- Dunne, R.M.; O’Mahony, D.; Wilson, G.; McDermott, R.; O’Keeffe, S.A. The role of the breast radiologist in evaluation of breast incidentalomas detected on 18-fludeoxyglucose positron emission tomography/CT. Br. J. Radiol. 2013, 86, 20130034. [Google Scholar] [CrossRef]
- Lim, S.; Lee, E.H.; Park, J.M.; Chang, Y.W.; Kim, H.H.; Jeong, S.H. Role of combined BI-RADS assessment using mammography and sonography for evaluation of incidental hypermetabolic lesions in the breast on 18F-FDG PET-CT. Acta Radiol. 2013, 54, 1117–1124. [Google Scholar] [CrossRef]
- Benveniste, A.P.; Marom, E.M.; Benveniste, M.F.; Mawlawi, O.; Fox, P.S.; Yang, W. Incidental primary breast cancer detected on PET-CT. Breast Cancer Res. Treat. 2015, 151, 261–268. [Google Scholar] [CrossRef]
- Bertagna, F.; Evangelista, L.; Piccardo, A.; Bertoli, M.; Bosio, G.; Giubbini, R.; Orlando, E.; Treglia, G. Multicentric study on 18F-FDG-PET/CT breast incidental uptake in patients studied for non-breast malignant purposes. Rev. Esp. Med. Nucl. Imagen Mol. 2015, 34, 24–29. [Google Scholar] [CrossRef]
- Minamimoto, R.; Senda, M.; Jinnouchi, S.; Terauchi, T.; Yoshida, T.; Inoue, T. Detection of breast cancer in an FDG-PET cancer screening program: Results of a nationwide Japanese survey. Clin. Breast Cancer 2015, 15, e139–e146. [Google Scholar] [CrossRef]
- Shin, K.M.; Kim, H.J.; Jung, S.J.; Lim, H.S.; Lee, S.W.; Cho, S.H.; Jang, Y.J.; Lee, H.J.; Kim, G.C.; Jung, J.H.; et al. Incidental Breast Lesions Identified by (18)F-FDG PET/CT: Which Clinical Variables Differentiate between Benign and Malignant Breast Lesions? J. Breast Cancer 2015, 18, 73–79. [Google Scholar] [CrossRef]
- Falomo, E.; Strigel, R.M.; Bruce, R.; Munoz Del Rio, A.; Adejumo, C.; Kelcz, F. Incidence and outcomes of incidental breast lesions detected on cross-sectional imaging examinations. Breast J. 2018, 24, 743–748. [Google Scholar] [CrossRef]
- Moletta, L.; Bissoli, S.; Fantin, A.; Passuello, N.; Valmasoni, M.; Sperti, C. PET/CT incidental detection of second tumor in patients investigated for pancreatic neoplasms. BMC Cancer 2018, 18, 531. [Google Scholar] [CrossRef]
- Bakhshayeshkaram, M.; Salehi, Y.; Abbasi, M.; Hashemi Beni, R.; Seifi, S.; Hassanzad, M.; Jamaati, H.R.; Aghahosseini, F. A preliminary study to propose a diagnostic algorithm for PET/CT-detected incidental breast lesions: Application of BI-RADS lexicon for US in combination with SUVmax. Eur. Radiol. 2019, 29, 5507–5516. [Google Scholar] [CrossRef]
- Andersen, J.D.; Zacho, H.D.; Petersen, L.J. The frequency and malignancy rate of incidental focal breast lesions identified by 18F-fluorodeoxyglucose positron emission tomography. Nucl. Med. Commun. 2021, 42, 93–100. [Google Scholar] [CrossRef]
- Panareo, S.; Urso, L.; Nieri, A.; Caracciolo, M.; Valpiani, G.; Torricelli, P.; Frassoldati, A.; Cittanti, C.; Rollo, M.; Bartolomei, M. Clinical-Diagnostic Relevance of Breast “Incidentaloma” Detected During 18F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography: Correlation with Radiological Imaging and Histopathology. Indian. J. Nucl. Med. 2021, 36, 385–390. [Google Scholar] [CrossRef]
- Wakfie-Corieh, C.G.; Rodríguez Rey, C.; Ortega Candil, A.; Ferrando-Castagnetto, F.; Valhondo-Rama, R.; Ruiz Tolón, M.; Pascual Martin, A.; Carreras Delgado, J.L. Clinical relevance of incidental focal breast uptake on fluorine-18 fluorodeoxyglucose PET/computed tomography studies: An experience in a high-load center of Spain. Nucl. Med. Commun. 2021, 42, 678–684. [Google Scholar] [CrossRef]
- Menon, P.; Bourke, A. Breast incidentalomas on 18-Fluorodeoxyglucose positron emission tomography-computed tomography performed for a non-mammary cause: Significance and outcomes. J. Med. Imaging Radiat. Oncol. 2023, 67, 357–364. [Google Scholar] [CrossRef]
- Kayadibi, Y.; Karagoz, S.H.; Kurt, S.A.; Kargin, O.A.; Guneren, C.; Sahin, O.E.; Hamid, R.; Yilmaz, M.H. Diagnostic Characteristics and Clinical Relevance of Incidental Hypermetabolic Breast Lesions Detected on 18F-FDG PET-CT: A Retrospective Evaluation. Acad. Radiol. 2025, 32, 1806–1815. [Google Scholar] [CrossRef]
- Elgeti, F.; Amthauer, H.; Denecke, T.; Steffen, I.; Heuck, F.; Stelter, L.; Ruf, J. Incidental detection of breast cancer by 68Ga-DOTATOC-PET/CT in women suffering from neuroendocrine tumours. Nuklearmedizin. 2008, 47, 261–265. [Google Scholar] [PubMed]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of (68)Ga-DOTATATE: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Bitencourt, A.G.V.; Lima, E.N.P. 68Ga-DOTATATE PET/CT versus 111In-octreotide scintigraphy in patients with neuroendocrine tumors: A prospective study. Radiol. Bras. 2022, 55, 13–18. [Google Scholar] [CrossRef]
- Broos, W.A.M.; Knol, R.J.J.; Zant, F.M.V.; Schaper, N.C.; Wondergem, M. Incidental Findings on 18 F-Fluorocholine PET/CT for Parathyroid Imaging. World J. Nucl. Med. 2022, 21, 192–199. [Google Scholar] [CrossRef]
- Avril, N.; Menzel, M.; Dose, J.; Schelling, M.; Weber, W.; Jänicke, F.; Nathrath, W.; Schwaiger, M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. J. Nucl. Med. 2001, 42, 9–16. [Google Scholar] [PubMed]
- Buck, A.; Schirrmeister, H.; Kühn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1317–1323. [Google Scholar] [CrossRef]
- Crippa, F.; Seregni, E.; Agresti, R.; Chiesa, C.; Pascali, C.; Bogni, A.; Decise, D.; De Sanctis, V.; Greco, M.; Daidone, M.G.; et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: A preliminary observation. Eur. J. Nucl. Med. 1998, 25, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hemminki, K. Second primary neoplasms among 53,159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: A search for common mechanisms. Br. J. Cancer 2001, 85, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Muto, T.; Oya, M.; Ota, H.; Azekura, K.; Yamaguchi, T. Multiple primary cancer: An experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int. J. Clin. Oncol. 2003, 8, 162–167. [Google Scholar] [CrossRef] [PubMed]




| PET Tracer | First Author [Ref.] | Year | Country/ Study Type | No. of Scans (Both Genders) | Patients with BIU (Both Genders) | Prevalence of BIU on Scans (Both Genders) | No. of Scans (Female) | Patients with BIU (Female) | Prevalence of BIU on Scans (Female) | BIU-H | MBIU | Percentage of MBIU Among BIU-H | Overall Risk of Malignancy of BIU (Female) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18F]FDG | Agress et al. [10] | 2004 | USA/R | 1750 | 2 | 0.1% | NR | 2 | NC | 2 | 2 | 100% | 100% |
| Ishimori et al. [11] | 2005 | USA/R | 1912 | 7 | 0.4% | 988 | 7 | 0.7% | 2 | 2 | 100% | 28.6% | |
| Korn et al. [12] | 2006 | USA/R | 1098 | 6 | 0.5% | 533 | 6 | 1.1% | 6 | 5 | 83.3% | 83.3% | |
| Wang et al. [13] | 2007 | Australia/R | 1727 | 3 | 0.2% | 747 | 3 | 0.4% | 3 | 2 | 66.7% | 66.7% | |
| Beatty et al. [14] | 2009 | USA/P | NR | NR | NC | 1500 | 9 | 0.6% | 8 | 5 | 62.5% | 55.6% | |
| Litmanovich et al. [15] | 2009 | USA/R | NR | NR | NC | 4038 | 33 | 0.8% | 21 | 12 | 57.1% | 36.4% | |
| Chung et al. [16] | 2010 | USA/R | 45,000 | 60 | 0.1% | NR | 60 | NC | 12 | 7 | 58.3% | 11.7% | |
| Kang et al. [17] | 2011 | Korea/R | NR | NR | NC | 13,897 | 50 | 0.4% | 35 | 18 | 51.4% | 36% | |
| Chae et al. [18] | 2012 | Korea/R | 32,988 | 131 | 0.4% | NR | 130 | NC | 60 | 32 | 53.3% | 24.6% | |
| Chopra et al. [19] | 2012 | UK/R | 818 | 6 | 0.7% | NR | 6 | NC | 3 | 2 | 66.7% | 33.3% | |
| Kim et al. [20] | 2012 | Korea/R | 5214 | 27 | 0.5% | NR | 26 | NC | 23 | 15 | 65.2% | 57.7% | |
| Dunne et al. [21] | 2013 | Ireland/R | 6050 | 50 | 0.8% | 2719 | 50 | 1.8% | 23 | 17 | 73.9% | 34% | |
| Lim et al. [22] | 2013 | Korea/R | NR | NR | NC | 7594 | 43 | 0.6% | 17 | 9 | 52.9% | 20.9% | |
| Benveniste et al. [23] | 2015 | USA/R | 1951 | 438 | 22.5% | 1866 | 438 | 23.5% | 55 | 37 | 67.3% | 8.4% | |
| Bertagna et al. [24] | 2015 | Italy/R | 42,927 | 79 | 0.2% | NR | 75 | NC | 35 | 25 | 71.4% | 33.3% | |
| Minamimoto et al. [25] | 2015 | Japan/R | NR | NR | NC | 62,054 | 473 | 0.8% | NR | 161 | NC | 34% | |
| Shin et al. [26] | 2015 | Korea/R | 55,762 | 214 | 0.4% | 21,224 | 214 | 1% | 60 | 27 | 45% | 12.6% | |
| Falomo et al. [27] | 2018 | USA/R | 8687 | 25 | 0.3% | NR | 25 | NC | 11 | 6 | 54.5% | 24% | |
| Moletta et al. [28] | 2018 | Italy/R | 399 | 1 | 0.3% | NR | 1 | NC | 1 | 1 | 100% | 100% | |
| Bakhshayeshkaram et al. [29] | 2019 | Iran/R | 5029 | 51 | 1% | NR | 48 | NC | 22 | 10 | 45.5% | 20.8% | |
| Andersen et al. [30] | 2021 | Denmark/R | 19,551 | 66 | 0.3% | NR | 62 | NC | 40 | 34 | 85% | 54.8% | |
| Panareo et al. [31] | 2021 | Italy/R | 3675 | 43 | 1.2% | NR | 36 | NC | 22 | 15 | 68.2% | 41.7% | |
| Wakfie-Corieh et al. [32] | 2021 | Spain/R | 10,615 | 26 | 0.2% | NR | 23 | NC | 23 | 9 | 39.1% | 39.1% | |
| Menon et al. [33] | 2023 | Australia/R | 5728 | 27 | 0.5% | NR | 26 | NC | 26 | 18 | 69.2% | 69.2% | |
| Kayadibi et al. [34] | 2025 | Turkey/R | 12,633 | 234 | 1.9% | 4263 | 234 | 5.5% | 63 | 20 | 31.7% | 8.5% | |
| Pooled values (95%CI) | 0.5% (0.3–0.6) | 1% (0.6–1.6) | 60.9% (52.5–69) | 33.5% (25.2–42.3) | |||||||||
| Radiolabeled somatostatin analogues | Elgeti et al. [35] | 2008 | Germany/R | 33 | 4 | 12.1% | 33 | 4 | 12.1% | 4 | 4 | 100% | 66.7% |
| Kuyumcu et al. [36] | 2013 | Turkey/R | 120 | 1 | 0.8% | 63 | 1 | 1.6% | 0 | 0 | 0% | 0% | |
| Cavicchioli et al. [37] | 2021 | Brazil/P | 41 | 1 | 2.4% | 16 | 1 | 6.2% | 1 | 1 | 100% | 100% | |
| Pooled values (95%CI) | 3.4% (0.0–11.5) | 5.2% (0.1–14.7) | 100% (66.8–100) | 86.4% (72–100) | |||||||||
| [18F]F-choline | Broos et al. [38] | 2022 | Netherlands/R | 388 | 10 | 2.6% | 290 | 10 | 3.4% | 7 | 7 | 100% | 70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovitti, C.M.; Marin, A.; Tasevski, S.; Martinello, C.; Cuzzocrea, M.; Paone, G.; Rizzo, A.; Albano, D.; Treglia, G. The Prevalence and Malignancy Risk of Breast Incidental Uptake Detected by PET/CT with Different Radiopharmaceuticals: An Updated Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 1831. https://doi.org/10.3390/ph18121831
Iacovitti CM, Marin A, Tasevski S, Martinello C, Cuzzocrea M, Paone G, Rizzo A, Albano D, Treglia G. The Prevalence and Malignancy Risk of Breast Incidental Uptake Detected by PET/CT with Different Radiopharmaceuticals: An Updated Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(12):1831. https://doi.org/10.3390/ph18121831
Chicago/Turabian StyleIacovitti, Cesare Michele, Andreea Marin, Slavko Tasevski, Chiara Martinello, Marco Cuzzocrea, Gaetano Paone, Alessio Rizzo, Domenico Albano, and Giorgio Treglia. 2025. "The Prevalence and Malignancy Risk of Breast Incidental Uptake Detected by PET/CT with Different Radiopharmaceuticals: An Updated Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 12: 1831. https://doi.org/10.3390/ph18121831
APA StyleIacovitti, C. M., Marin, A., Tasevski, S., Martinello, C., Cuzzocrea, M., Paone, G., Rizzo, A., Albano, D., & Treglia, G. (2025). The Prevalence and Malignancy Risk of Breast Incidental Uptake Detected by PET/CT with Different Radiopharmaceuticals: An Updated Systematic Review and Meta-Analysis. Pharmaceuticals, 18(12), 1831. https://doi.org/10.3390/ph18121831











