Formulation and Characterization of Oxiconazole-Loaded Novasomes to Enhance the Treatment of Fungus Infections: Experimentally Induced Candidiasis in Rat
Abstract
1. Introduction
2. Results
2.1. Preparation of Novasomes and Experimental Design
2.2. Characterization of the Oxiconazole-Loaded Novasomes
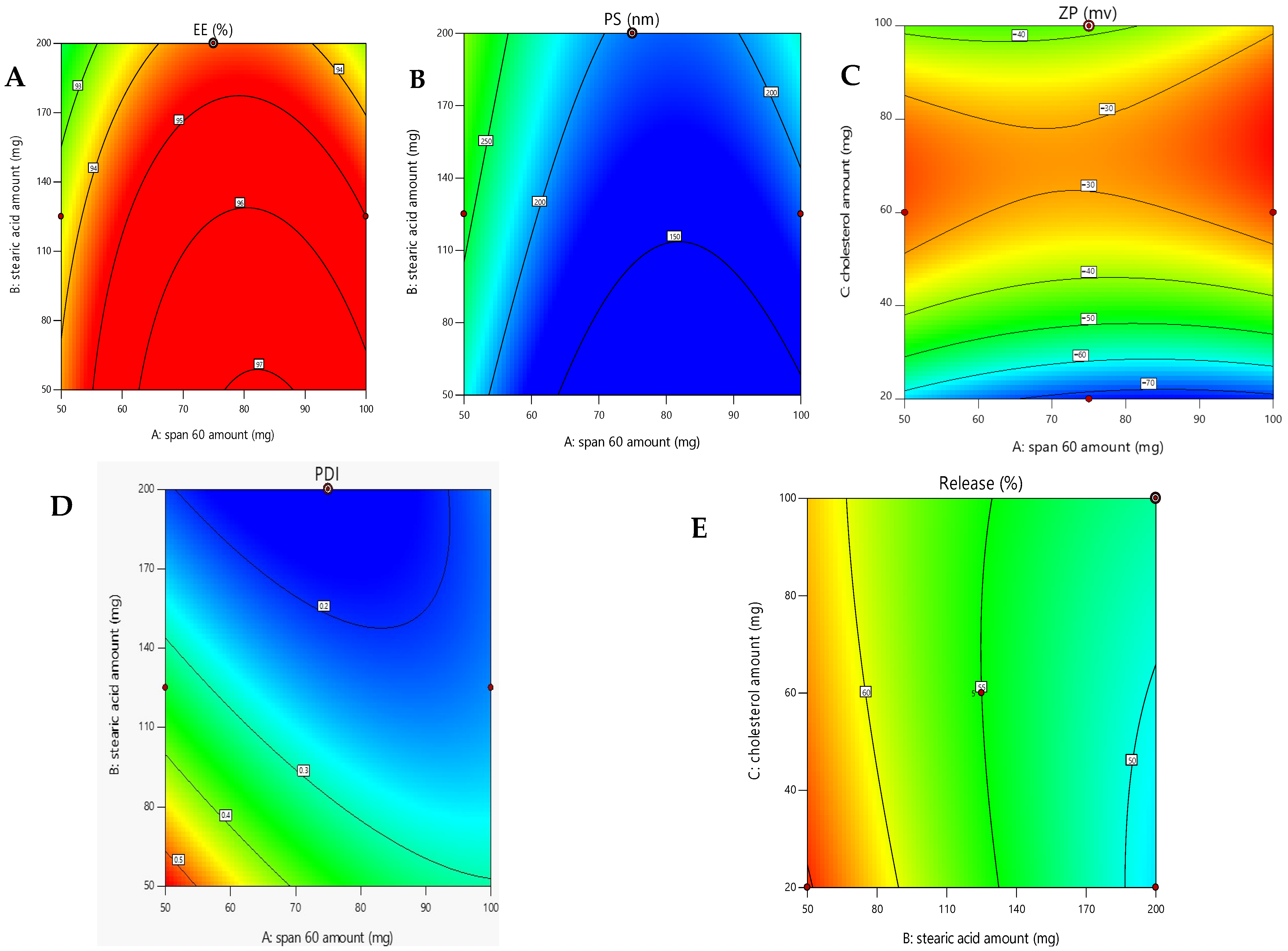
2.2.1. Evaluation of Encapsulation Efficacy (EE%)
2.2.2. Assessment of Formulation Parameters on Vesicle Size
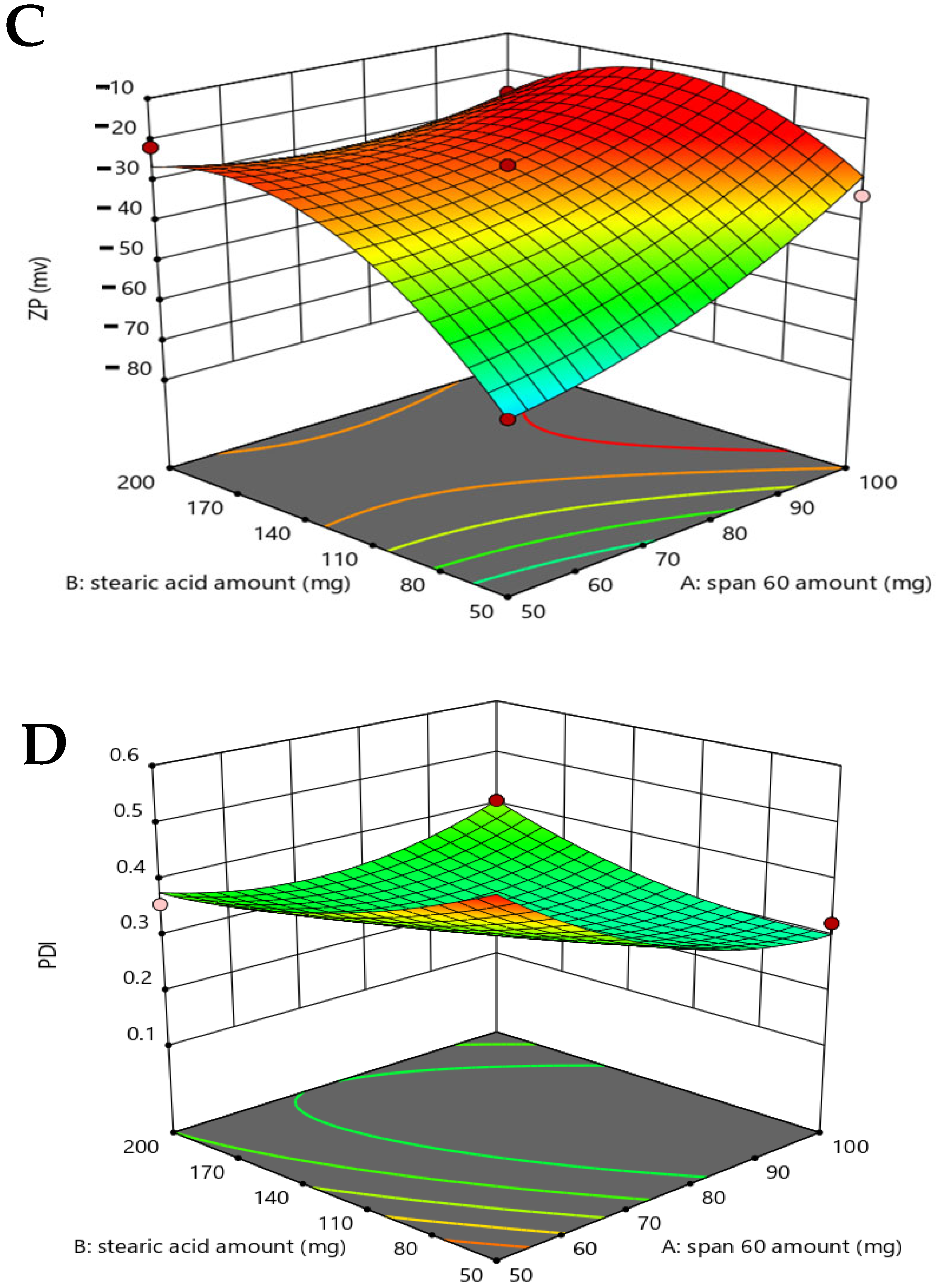
2.2.3. Assessment of Formulation Parameters on Zeta Potential and PDI Analysis
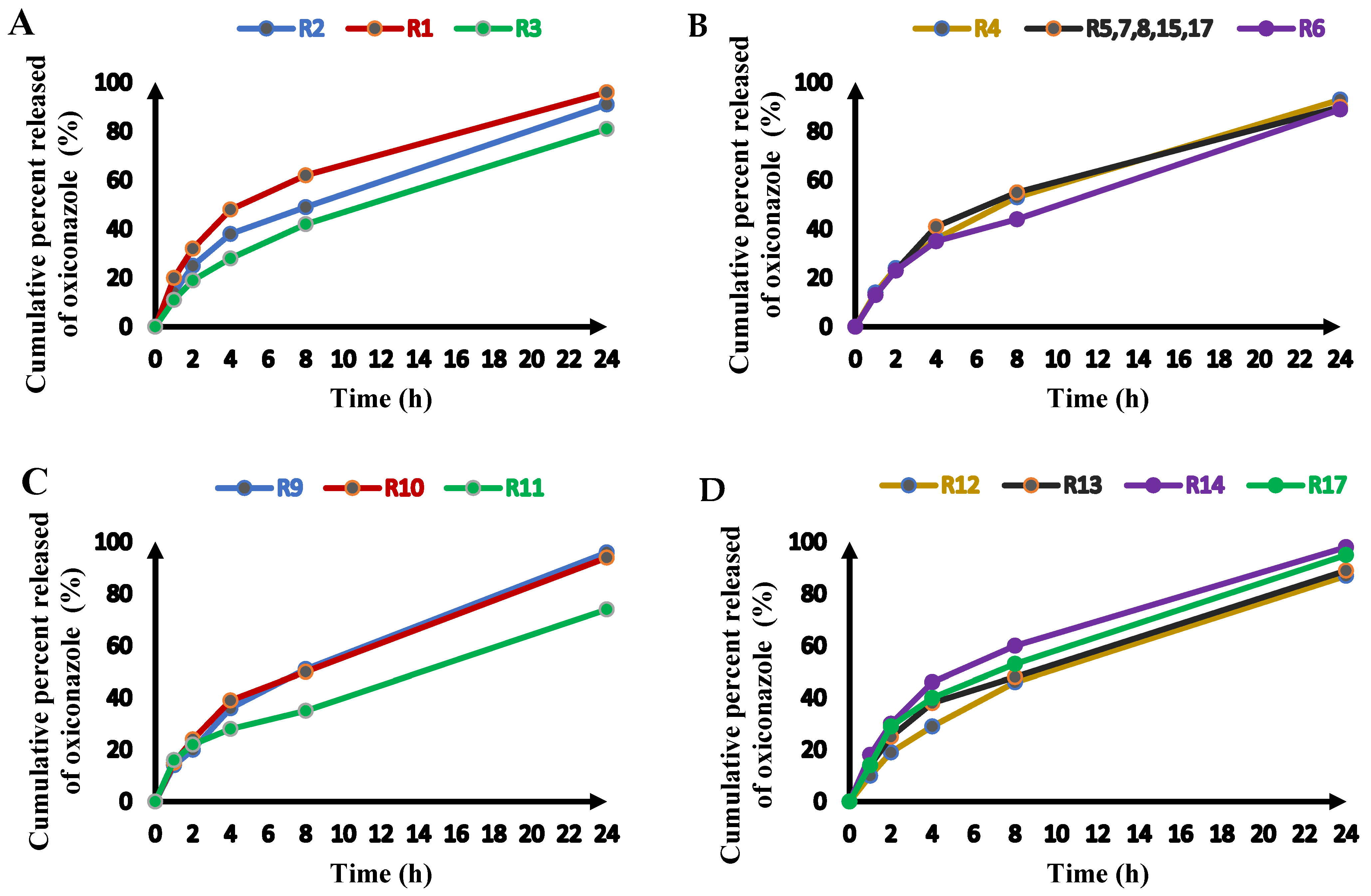
2.2.4. Release of Oxiconazole from Novasomes
2.3. Selection of the Optimized Formula
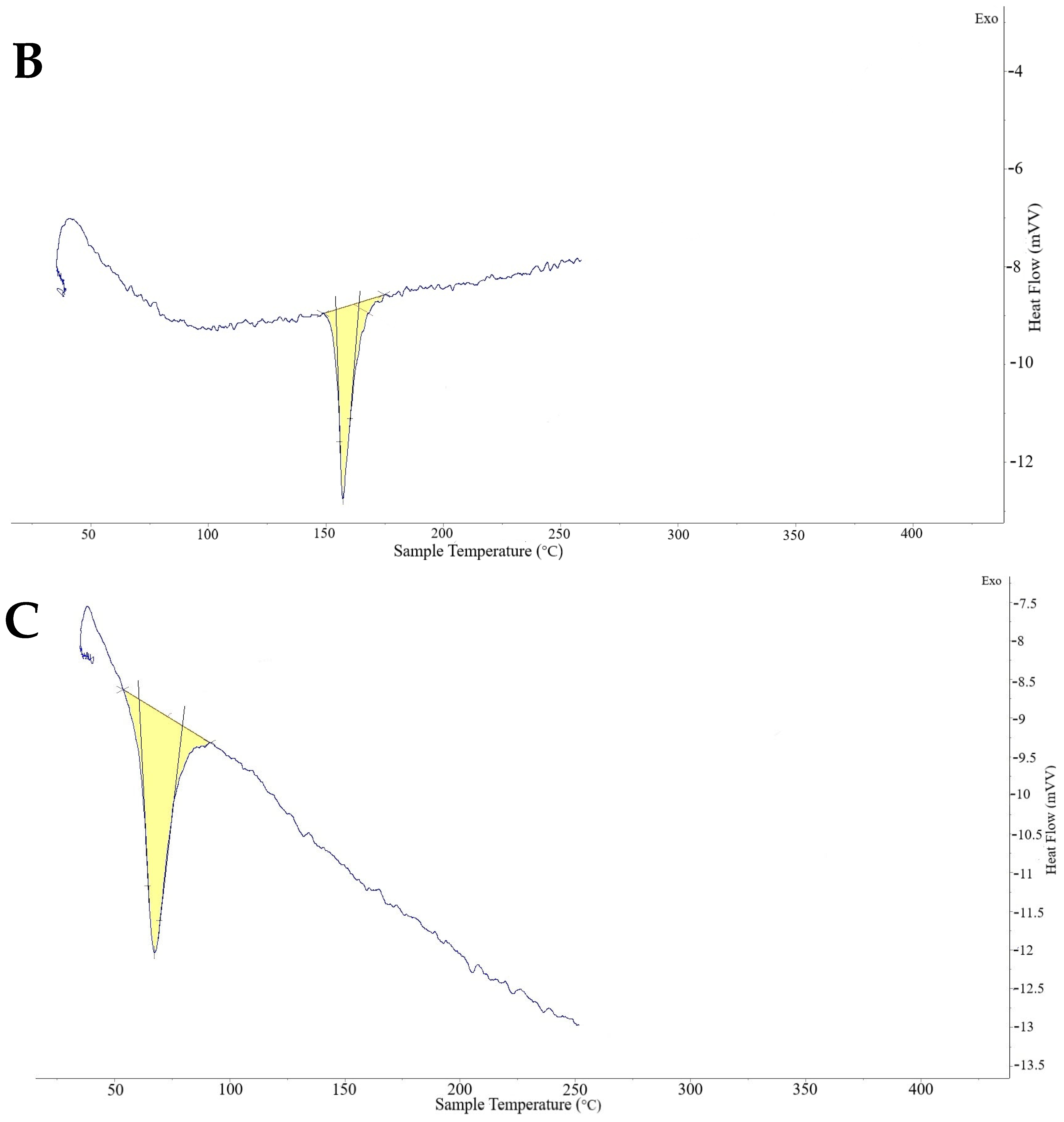
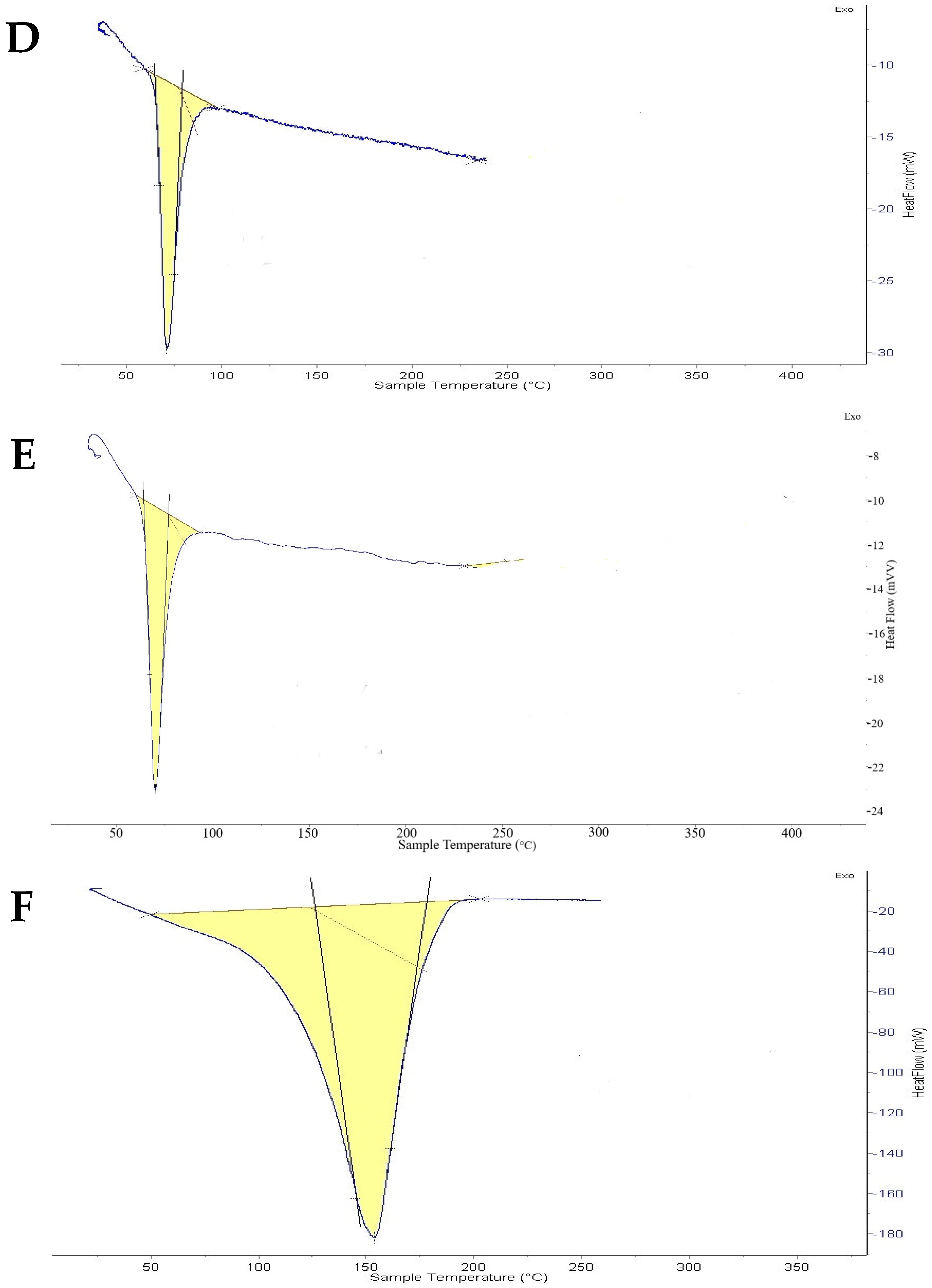
2.4. Thermal Analysis Optimized Oxiconazole Formula
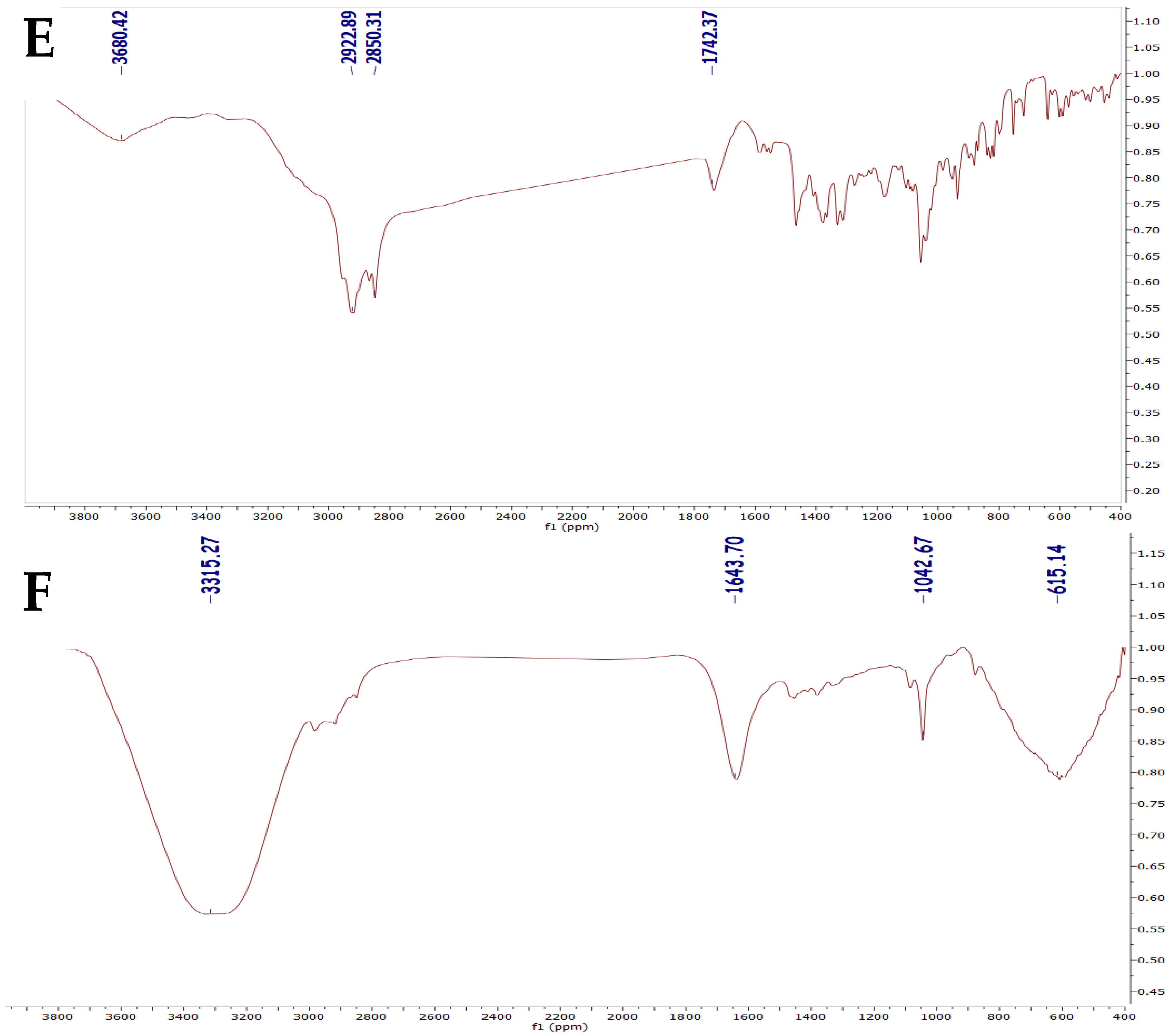
2.5. FTIR Analysis
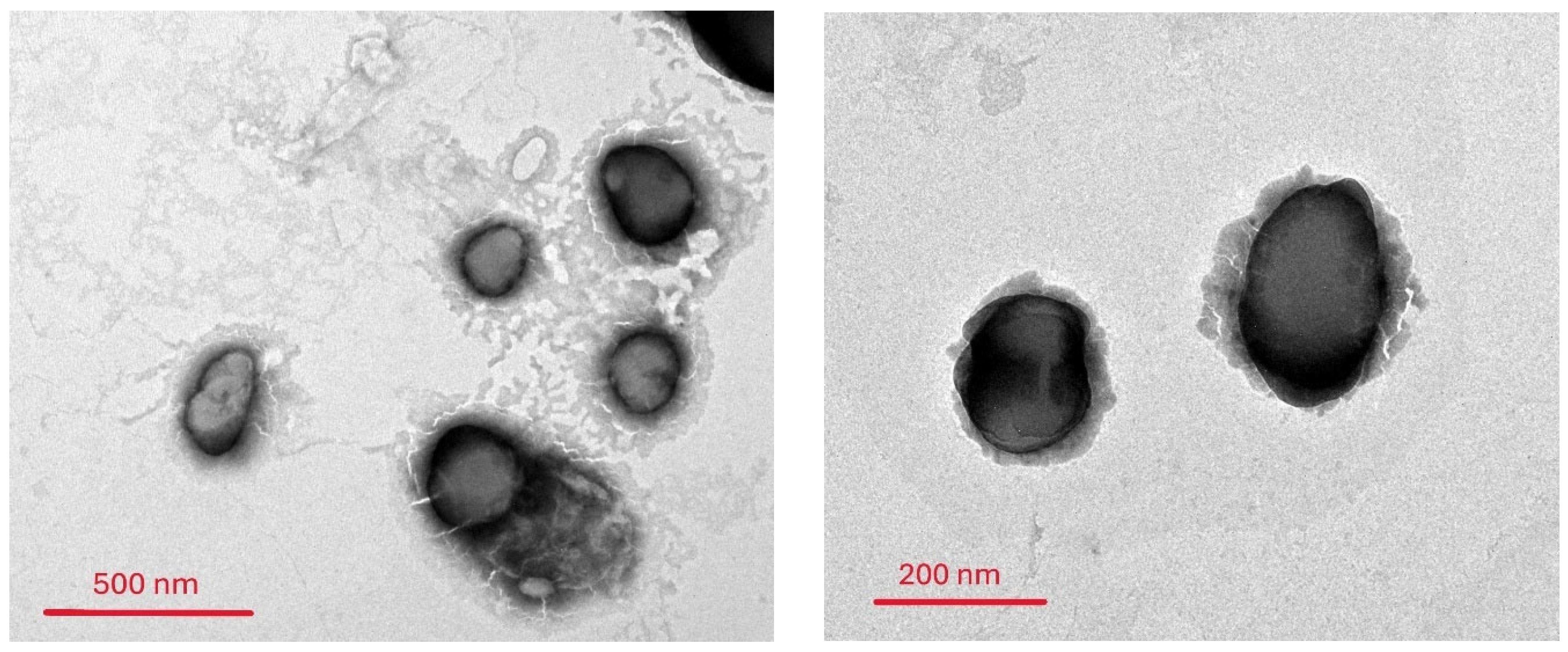
2.6. Morphological Characterization of the Oxiconazole-Loaded Novasomes
2.7. Stability Evaluation for the Optimal Preparation
2.8. Evaluation of Oxiconazole Novasomal Gel
2.9. In Vitro Permeation of Oxiconazole from Prepared Novasomes
2.10. In Vivo Assessment
Histopathology Investigations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design and Optimization:
4.3. Preparation of Oxiconazole-Loaded Novasomes
4.4. Characterization of Novasomes
4.4.1. Encapsulation Efficiency (EE%)
4.4.2. Vesicle Size, Zeta Potential, and Polydispersity Index
4.4.3. In Vitro Drug Release Study
4.5. Optimization of the Novasomal Formulation
4.6. FTIR Spectroscopy
4.7. Differential Scanning Calorimetry (DSC)
4.8. Transmission Electron Microscopy (TEM)
4.9. In Vitro Skin Permeation Study
Skin Integrity (TEER)
4.10. Preparation of Novasomal Gel
4.11. Evaluation of Gel Properties
4.11.1. Homogeneity
4.11.2. Spreadability
4.11.3. pH Measurement
4.11.4. Viscosity
4.12. Stability Study
4.13. In Vivo Antifungal Study
4.13.1. Animals and Ethics
4.13.2. Immunosuppression
4.13.3. Fungal Inoculum Preparation
4.13.4. Infection Procedure
- Negative control
- Positive control (empty gel)
- Oxiconazole gel
- Empty novasomal gel
- Optimized oxiconazole-loaded novasomal gel
4.13.5. Histopathological Examination
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VS | Vesicle Size |
| PS | Particle size |
| PDI | Polydispersity index |
| TLA | Zeta potential |
| EE | Entrapment (Encapsulation) efficiency |
| TEM | Transmission electron microscope |
| FTIR | Fourier Transform Infra-Red spectroscopy |
| DSC | Differential scanning calorimetry |
| PBS | Phosphate-buffered saline |
| DR | Drug release |
| Jss | Permeation flux value |
| C0 | The preliminary drug concentration |
| HLB | Hydrophilic-lipophilic balance |
| DLS | Dynamic light scattering |
| KP | Permeability coefficient |
| Span 60 | Sorbitan monostearate |
| SC | Stratum corneum |
References
- Da Silva, L.B.B.; Castilho, I.G.; de Souza Silva, F.A.; Ghannoum, M.; Garcia, M.T.; do Carmo, P.H.F. Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update. Microorganisms 2025, 13, 1406. [Google Scholar] [CrossRef]
- Gatina, A.; Trizna, E.; Kolesnikova, A.; Baidamshina, D.; Gorshkova, A.; Drucker, V.; Bogachev, M.; Kayumov, A. The Bovhyaluronidase Azoximer (Longidaza®) Disrupts Candida Albicans and Candida Albicans-Bacterial Mixed Biofilms and Increases the Efficacy of Antifungals. Medicina 2022, 58, 1710. [Google Scholar] [CrossRef]
- Mahmoud, R.A.; Hussein, A.K.; Nasef, G.A.; Mansour, H.F. Oxiconazole Nitrate Solid Lipid Nanoparticles: Formulation, in-Vitro Characterization and Clinical Assessment of an Analogous Loaded Carbopol Gel. Drug Dev. Ind. Pharm. 2020, 46, 706–716. [Google Scholar] [CrossRef]
- Özdemir, Z.; Zenni, Y.N.; Karakurt, A.; Sari, S.; Saraç, S.; Akdağ, M.; Merde, İ.B.; Kart, D.; Venanzoni, R.; Flores, G.A. Potent Antimicrobial Azoles: Synthesis, In Vitro and In Silico Study. Antibiotics 2024, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sghier, K.; Mur, M.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Neubert, R.H.H. Mechanisms of Penetration and Diffusion of Drugs and Cosmetic Actives across the Human Stratum Corneum. Eur. J. Pharm. Biopharm. 2024, 202, 114394. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G.; et al. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules 2022, 27, 2936. [Google Scholar] [CrossRef]
- Richard, C.; Cassel, S.; Blanzat, M. Vesicular Systems for Dermal and Transdermal Drug Delivery. RSC Adv. 2021, 11, 442–451. [Google Scholar] [CrossRef]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of Novasomes as a Vesicular Carrier for Improving the Topical Delivery of Terconazole: In Vitro Characterization, in Vivo Assessment and Exploratory Clinical Experimentation. Int. J. Nanomed. 2021, 16, 119. [Google Scholar] [CrossRef]
- CA, A.A.R.; Krishnan, K.; Sreelekshmi, A.S.; Arjun, K.K.; Nair, S.C. Novasome: A Pioneering Advancement in Vesicular Drug Delivery. Int. J. Appl. Pharm. 2021, 13, 59–64. [Google Scholar]
- Mustafa, M.A.; Fahad, M.; Mughal, M.; Rasheed, N.; Alqahtani, S.S.; Iqbal, M.Z. Development and Evaluation of Nystatin-Loaded Novasomal Gel for the Treatment of Candida Albicans Infection: In Vitro Microbiological and Skin Compatibility Study. Gels 2025, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, S.; Shah, S.; Shah, P.A.; Irfan, M.; Saadullah, M.; Abbas, G.; Hanif, M.; Rasul, A.; Ahmad, N.; Mahmood, A. Development and Pharmacokinetic Evaluation of Novasomes for the Trans-Nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate. Pharmaceutics 2023, 15, 2259. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal Delivery of Fluvastatin Sodium via Tailored Spanlastic Nanovesicles: Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats through Suppressing P38 MAPK Signaling Pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Ragaie, M.H.; El Hassab, M.A.; El-Haggar, R.; Eldehna, W.M.; Al-Rashood, S.T.; Mosallam, S. Fenticonazole Nitrate Loaded Trans-Novasomes for Effective Management of Tinea Corporis: Design Characterization, in Silico Study, and Exploratory Clinical Appraisal. Drug Deliv. 2022, 29, 1100–1111. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; El-Korany, S.M.; Sayed, S. Corneal Targeted Fenticonazole Nitrate-Loaded Novasomes for the Management of Ocular Candidiasis: Preparation, in Vitro Characterization, Ex Vivo and in Vivo Assessments. Drug Deliv. 2022, 29, 2428–2441. [Google Scholar] [CrossRef]
- Elkomy, M.H.; El Menshawe, S.F.; Kharshoum, R.M.; Abdeltwab, A.M.; Hussein, R.R.S.; Hamad, D.S.; Alsalahat, I.; Aboud, H.M. Innovative Pulmonary Targeting of Terbutaline Sulfate-Laded Novasomes for Non-Invasive Tackling of Asthma: Statistical Optimization and Comparative in Vitro/in Vivo Evaluation. Drug Deliv. 2022, 29, 2058–2071. [Google Scholar] [CrossRef]
- Goyal, G.; Garg, T.; Malik, B.; Chauhan, G.; Rath, G.; Goyal, A.K. Development and Characterization of Niosomal Gel for Topical Delivery of Benzoyl Peroxide. Drug Deliv. 2015, 22, 1027–1042. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; AbouGhaly, M.H.H. Design and Optimization of Topical Methotrexate Loaded Niosomes for Enhanced Management of Psoriasis: Application of Box–Behnken Design, in-Vitro Evaluation and in-Vivo Skin Deposition Study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and Green Fabrication of a Superhydrophobic Surface by One-Step Immersion for Continuous Oil/Water Separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef]
- Al-Maghrabi, P.M.; Khafagy, E.-S.; Ghorab, M.M.; Gad, S. Influence of Formulation Variables on Miconazole Nitrate–Loaded Lipid Based Nanocarrier for Topical Delivery. Colloids Surf. B Biointerfaces 2020, 193, 111046. [Google Scholar] [CrossRef]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, Optimization and Characterization of a Transfersomal Gel Using Miconazole Nitrate for the Treatment of Candida Skin Infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Tawfik, M.A.; Mohamed, M.I.; Tadros, M.I.; El-Helaly, S.N. Low-Frequency Sonophoresis as an Active Approach to Potentiate the Transdermal Delivery of Agomelatine-Loaded Novasomes: Design, Optimization, and Pharmacokinetic Profiling in Rabbits. AAPS PharmSciTech 2021, 22, 261. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Zafar, A.; Bin Jumah, M.N.; Alshehri, S. Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to in Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. [Google Scholar] [CrossRef]
- Singh, A.; Yadagiri, G.; Parvez, S.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Formulation, Characterization and in Vitro Anti-Leishmanial Evaluation of Amphotericin B Loaded Solid Lipid Nanoparticles Coated with Vitamin B12-Stearic Acid Conjugate. Mater. Sci. Eng. C 2020, 117, 111279. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A.K.; Hazari, P.P.; Aqil, M. Optimization by Design of Etoposide Loaded Solid Lipid Nanoparticles for Ocular Delivery: Characterization, Pharmacokinetic and Deposition Study. Mater. Sci. Eng. C 2019, 100, 959–970. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In Vitro Characterization, Ex Vivo Permeability, Microbiological Assessment and in Vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; Fahmy, R.H. Diazepam-Loaded Solid Lipid Nanoparticles: Design and Characterization. AAPS PharmSciTech 2009, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and Evaluation of Cubosomes Drug Delivery System for Treatment of Glaucoma: Ex-Vivo Permeation and in-Vivo Pharmacodynamic Study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Nano-Transfersomal Ciprofloxacin Loaded Vesicles for Non-Invasive Trans-Tympanic Ototopical Delivery: In-Vitro Optimization, Ex-Vivo Permeation Studies, and in-Vivo Assessment. Int. J. Pharm. 2014, 472, 304–314. [Google Scholar] [CrossRef]
- Zolghadri, S.; Asad, A.G.; Farzi, F.; Ghajarzadeh, F.; Habibi, Z.; Rahban, M.; Zolghadri, S.; Stanek, A. Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent in Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity. Pharmaceuticals 2023, 16, 1680. [Google Scholar] [CrossRef]
- Zubairu, Y.; Negi, L.M.; Iqbal, Z.; Talegaonkar, S. Design and Development of Novel Bioadhesive Niosomal Formulation for the Transcorneal Delivery of Anti-Infective Agent: In-Vitro and Ex-Vivo Investigations. Asian J. Pharm. Sci. 2015, 10, 322–330. [Google Scholar] [CrossRef]
- Mahmoud, M.O.; Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Johnston, T.P. Transdermal Delivery of Atorvastatin Calcium from Novel Nanovesicular Systems Using Polyethylene Glycol Fatty Acid Esters: Ameliorated Effect without Liver Toxicity in Poloxamer 407-Induced Hyperlipidemic Rats. J. Control. Release 2017, 254, 10–22. [Google Scholar] [CrossRef]
- Ali, A.A.; Hassan, A.H.; Eissa, E.M.; Aboud, H.M. Response Surface Optimization of Ultra-Elastic Nanovesicles Loaded with Deflazacort Tailored for Transdermal Delivery: Accentuated Bioavailability and Anti-Inflammatory Efficacy. Int. J. Nanomed. 2021, 16, 591–607. [Google Scholar] [CrossRef]
- Huang, R.; Song, H.; Wang, X.; Shen, H.; Li, S.; Guan, X. Fatty Acids-Modified Liposomes for Encapsulation of Bioactive Peptides: Fabrication, Characterization, Storage Stability and in Vitro Release. Food Chem. 2024, 440, 138139. [Google Scholar] [CrossRef] [PubMed]
- Killen, B.U.; Corrigan, O.I. Factors Influencing Drug Release from Stearic Acid Based Compacts. Int. J. Pharm. 2001, 228, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.; Sade, A.; Severcan, F.; Keskin, D.; Tezcaner, A.; Banerjee, S. Celecoxib-Loaded Liposomes: Effect of Cholesterol on Encapsulation and in Vitro Release Characteristics. Biosci. Rep. 2010, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an Ocular Delivery System for Acetazolamide: In Vitro and in Vivo Studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and Appraisal of Self-Assembled Valsartan-Loaded Amalgamated Pluronic F127/Tween 80 Polymeric Micelles: Boosted Cardioprotection via Regulation of Mhrt/Nrf2 and Trx1 Pathways in Cisplatin-Induced Cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abdel Hakim, L.F.; Abdelrahim, M.E. Edge Activators and a Polycationic Polymer Enhance the Formulation of Porous Voriconazole Nanoagglomerate for the Use as a Dry Powder Inhaler. J. Liposome Res. 2016, 26, 324–335. [Google Scholar] [CrossRef]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Corneal Targeted Sertaconazole Nitrate Loaded Cubosomes: Preparation, Statistical Optimization, in Vitro Characterization, Ex Vivo Permeation and in Vivo Studies. Int. J. Pharm. 2018, 553, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.L.B.; Simões, S. Development, Characterization, and Skin Delivery Studies of Related Ultradeformable Vesicles: Transfersomes, Ethosomes, and Transethosomes. Int. J. Nanomedicine 2015, 10, 5837–5851. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.J.; Garg, N.K.; Beg, S.; Singh, B.; Katare, O.P. Nanosized Ethosomes-Based Hydrogel Formulations of Methoxsalen for Enhanced Topical Delivery against Vitiligo: Formulation Optimization, in Vitro Evaluation and Preclinical Assessment. J. Drug Target. 2016, 24, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Dhatt, B.; Kaur, A. Ethosomes-Based Topical Delivery System of Antihistaminic Drug for Treatment of Skin Allergies. J. Microencapsul. 2014, 31, 716–724. [Google Scholar] [CrossRef]
- Mousa, I.A.; Hammady, T.M.; Gad, S.; Zaitone, S.A.; El-Sherbiny, M.; Sayed, O.M. Formulation and Characterization of Metformin-Loaded Ethosomes for Topical Application to Experimentally Induced Skin Cancer in Mice. Pharmaceuticals 2022, 15, 657. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Alam, M.A. Enhanced Anti-Inflammatory Activity of Carbopol Loaded Meloxicam Nanoethosomes Gel. Int J Biol Macromol 2014, 67, 99–104. [Google Scholar] [CrossRef]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of Skin Integrity Tests for Dermal Absorption Studies in Vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- El-Menshawe, S.F.; Sayed, O.M.; Abou-Taleb, H.A.; El Tellawy, N. Skin Permeation Enhancement of Nicotinamide through Using Fluidization and Deformability of Positively Charged Ethosomal Vesicles: A New Approach for Treatment of Atopic Eczema. J. Drug Deliv. Sci. Technol. 2019, 52, 687–701. [Google Scholar] [CrossRef]
- Olesen, U.H.; Clergeaud, G.; Lerche, C.M.; Andresen, T.L.; Haedersdal, M. Topical Delivery of Vismodegib Using Ablative Fractional Laser and Micro-emulsion Formulation in Vitro. Lasers Surg. Med. 2019, 51, 79–87. [Google Scholar] [CrossRef]
- Aboud, H.M.; Hussein, A.K.; Zayan, A.Z.; Makram, T.S.; Sarhan, M.O.; El-Sharawy, D.M. Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice. Pharmaceutics 2022, 14, 875. [Google Scholar] [CrossRef]
- Amr Gamal, F.; Kharshoum, R.M.; Sayed, O.M.; El-Ela, F.I.A.; Salem, H.F. Control of Basal Cell Carcinoma via Positively Charged Ethosomes of Vismodegib: In Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2020, 56, 101556. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of Permeation Enhancers on the Penetration Mechanism of Transfersomal Gel of Ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sheer, A.; Chauhan, M. Ethosomes as Vesicular Carrier for Enhanced Transdermal Delivery of Ketoconazole-Formulation and Evaluation. IJPI’s J. Pharm. Cosmetol. 2011, 1, 1–14. [Google Scholar]
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A.F. Sertaconazole Nitrate Loaded Nanovesicular Systems for Targeting Skin Fungal Infection: In-Vitro, Ex-Vivo and in-Vivo Evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef]
- Fayyaz, H.A.; El-Massik, M.A.; Bahey-El-Din, M.; Abdel-Bary, A.; Abdallah, O.Y.; Eltaher, H.M. Targeted DPPC/DMPG Surface-Modified Voriconazole Lipid Nanoparticles Control Invasive Pulmonary Aspergillosis in Immunocompromised Population: In-Vitro and in-Vivo Assessment. Int. J. Pharm. 2024, 649, 123663. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Co-Polymer Mixed Micelles Enhanced Transdermal Transport of Lornoxicam: In Vitro Characterization, and in Vivo Assessment of Anti-Inflammatory Effect and Antinociceptive Activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102365. [Google Scholar] [CrossRef]











| Run | Independent Parameters | Dependent Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (mg) | Y1 (%) | Y2 (nm) | Y3 (mV) | Y4 | Y5 (%) | |
| R1 | 50 | 50 | 60 | 94.24 ± 3.80 | 246.2 ± 12.33 | −61.6 ± 2.39 | 0.532 ± 0.02 | 62 ± 1.90 |
| R2 | 75 | 200 | 20 | 90.37 ± 4.80 | 337.8 ± 14.50 | −72.5 ± 4.30 | 0.375 ± 0.02 | 49 ± 2.10 |
| R3 | 75 | 50 | 100 | 88.47 ± 4.60 | 374.4 ± 45.20 | −64.6 ± 1.65 | 0.47 ± 0.08 | 42 ± 2.80 |
| R4 | 50 | 125 | 100 | 93.45 ± 1.30 | 258.6 ± 4.30 | −69.5 ± 1.10 | 0.34 ± 0.03 | 53 ± 2.0 |
| R5 * | 75 | 125 | 60 | 94.51 ± 2.50 | 235.3 ± 4.60 | −26 ± 2.15 | 0.32 ± 0.02 | 55 ± 1.10 |
| R6 | 50 | 200 | 60 | 91.9 ± 6.10 | 327.8 ± 17.80 | −21.7 ± 0.20 | 0.358 ± 0.13 | 44 ± 1.60 |
| R7 * | 75 | 125 | 60 | 94.51 ± 2.50 | 235.3 ± 4.60 | −26 ± 2.15 | 0.32 ± 0.02 | 55 ± 1.10 |
| R8 * | 75 | 125 | 60 | 94.51 ± 2.50 | 235.3 ± 4.60 | −26 ± 2.15 | 0.32 ± 0.02 | 55 ± 1.10 |
| R9 | 75 | 200 | 100 | 94.63 ± 1.60 | 174 ± 1.15 | −46.5 ± 1.61 | 0.184 ± 0.01 | 51 ± 0.50 |
| R10 | 50 | 125 | 20 | 92.8 ± 2.30 | 280.2 ± 62.79 | −44.9 ± 0.90 | 0.383 ± 0.04 | 50 ± 0.80 |
| R11 | 100 | 125 | 20 | 86.4 ± 4.80 | 387.3 ± 49.02 | −73.8 ± 0.87 | 0.49 ± 0.14 | 35 ± 3.20 |
| R12 | 100 | 200 | 60 | 90.1 ± 5.10 | 354 ± 11.80 | −25.8 ± 0.20 | 0.408 ± 0.13 | 46 ± 1.50 |
| R13 | 100 | 125 | 100 | 94.78 ± 1.40 | 209.1 ± 1.33 | −38.2 ± 1.15 | 0.212 ± 0.04 | 48 ± 1.8 |
| R14 | 75 | 50 | 20 | 92.94 ± 3.40 | 286.5 ± 35.96 | −36.4 ± 0.97 | 0.197 ± 0.02 | 60 ± 1.50 |
| R15 * | 75 | 125 | 60 | 94.51 ± 2.50 | 235.3 ± 4.60 | −26 ± 2.15 | 0.32 ± 0.02 | 55 ± 1.10 |
| R16 * | 75 | 125 | 60 | 94.51 ± 2.50 | 235.3 ± 4.60 | −26 ± 2.15 | 0.32 ± 0.02 | 55 ± 1.10 |
| R17 | 100 | 50 | 60 | 93.52 ± 2.10 | 253.2 ± 22.10 | −33.5 ± 2.10 | 0.324 ± 0.04 | 53 ± 1.40 |
| Response | Order | p-Value | F-Value | R2 | Adeq. Precision | Significant Independent Parameters |
|---|---|---|---|---|---|---|
| EE % | Quadratic | <0.0001 | 61.98 | 0.991 | 24.723 | X1, X2, X3 |
| VS | Quadratic | <0.0178 | 7.83 | 0.933 | 9.497 | X2, X3 |
| ZP | Quadratic | <0.0036 | 15.78 | 0.966 | 10.954 | X1, X2, X3 |
| PDI | Quadratic | <0.0016 | 22.42 | 0.976 | 19.944 | X1 |
| DR% 8 h | Quadratic | <0.0001 | 276.89 | 0.998 | 59.256 | X1, X2, X3 |
| Optimized Formula | Fresh Prepared | After 3 Months | After 6 Months |
|---|---|---|---|
| EE % | 94.63 ± 1.6 | 93.3 ± 1.3 | 92.1 ± 2.4 |
| PS (nm) | 174 ± 1.15 | 174 ± 2.1 | 176 ± 2.7 |
| ZP (mv) | −46.5 ± 1.61 | −46 ± 1.1 | −43.5 ± 3.3 |
| PDI | 0.184 ± 0.014 | 0.2 ± 0.02 | 0.173 ± 0.01 |
| DR % 8 h | 51 ± 0.50 | 49 ± 1.5 | 53 ± 1.2 |
| The Permeated Amount of Oxiconazole (µg/cm2) | The Steady-State Flux (g/cm2/h) | The Cumulative Permeation Percentage (%) | The Permeability Coefficient (cm/h) | |
|---|---|---|---|---|
| Optimal Gel Formula | 1820 ± 35 | 3.508 | 94 ± 1.75 | 1.75 × 10−3 |
| Oxiconazole Gel | 1440 ± 22 | 2.759 | 72 ± 1.1 | 1.38 × 10−3 |
| Group | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| Epidermal thickness (µm) (mean ± SD) | 22.3 ± 2.1 | 77 ± 8.3 | 41.3 ± 2.9 | 64 ± 6.8 | 21.7 ± 1.2 |
| Ulceration | Not Detected | Detected | Not Detected | Detected | Not Detected |
| Skin inflammatory infiltrate and edema | Not Detected | Detected | Not Detected | Detected | Not Detected |
| Dermal fibroblastic proliferation and collagen arrangement | No fibroblastic proliferation and uniform dermal collagen | There is fibroblastic proliferation and fibrosis | No fibroblastic proliferation and uniform dermal collagen | There is fibroblastic proliferation and fibrosis | No fibroblastic proliferation and uniform dermal collagen |
| Factor | Level | ||
|---|---|---|---|
| Independent Parameters | Minimum (−1) | Moderate (0) | Maximum (1) |
| X1: Span 60 amount (mg) | 50 | 75 | 100 |
| X2: Stearic (mg) | 50 | 125 | 200 |
| X3: Cholesterol amount (mg) | 20 | 60 | 100 |
| Responses (dependent parameters) | limitations | ||
| Y1: Encapsulation Efficiency (%) | Maximize | ||
| Y2: Vesicle Size (nm) | Minimize | ||
| Y3: Zeta Potential (mv) | Maximize | ||
| Y4: polydispersity index | Minimize | ||
| Y5: Percentage of drug release within 8 h (%) | Maximize | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, I.A.; Abu-Elsaoud, A.M.; Sabry, S.A.; Elghany, M.A.; Khodeer, D.; Abdelgawad, F.E.; Nasr, A.M. Formulation and Characterization of Oxiconazole-Loaded Novasomes to Enhance the Treatment of Fungus Infections: Experimentally Induced Candidiasis in Rat. Pharmaceuticals 2025, 18, 1803. https://doi.org/10.3390/ph18121803
Mousa IA, Abu-Elsaoud AM, Sabry SA, Elghany MA, Khodeer D, Abdelgawad FE, Nasr AM. Formulation and Characterization of Oxiconazole-Loaded Novasomes to Enhance the Treatment of Fungus Infections: Experimentally Induced Candidiasis in Rat. Pharmaceuticals. 2025; 18(12):1803. https://doi.org/10.3390/ph18121803
Chicago/Turabian StyleMousa, Ibrahim A., Abdelghafar M. Abu-Elsaoud, Shereen A. Sabry, Mahmoud Abd Elghany, Dina Khodeer, Fathy E. Abdelgawad, and Ali M. Nasr. 2025. "Formulation and Characterization of Oxiconazole-Loaded Novasomes to Enhance the Treatment of Fungus Infections: Experimentally Induced Candidiasis in Rat" Pharmaceuticals 18, no. 12: 1803. https://doi.org/10.3390/ph18121803
APA StyleMousa, I. A., Abu-Elsaoud, A. M., Sabry, S. A., Elghany, M. A., Khodeer, D., Abdelgawad, F. E., & Nasr, A. M. (2025). Formulation and Characterization of Oxiconazole-Loaded Novasomes to Enhance the Treatment of Fungus Infections: Experimentally Induced Candidiasis in Rat. Pharmaceuticals, 18(12), 1803. https://doi.org/10.3390/ph18121803









