Baihe Dihuang Tang Exerts Antidepressant Effects via Modulation of MAOA-Mediated Serotonin Metabolism and Synaptic Plasticity
Abstract
1. Introduction
2. Results
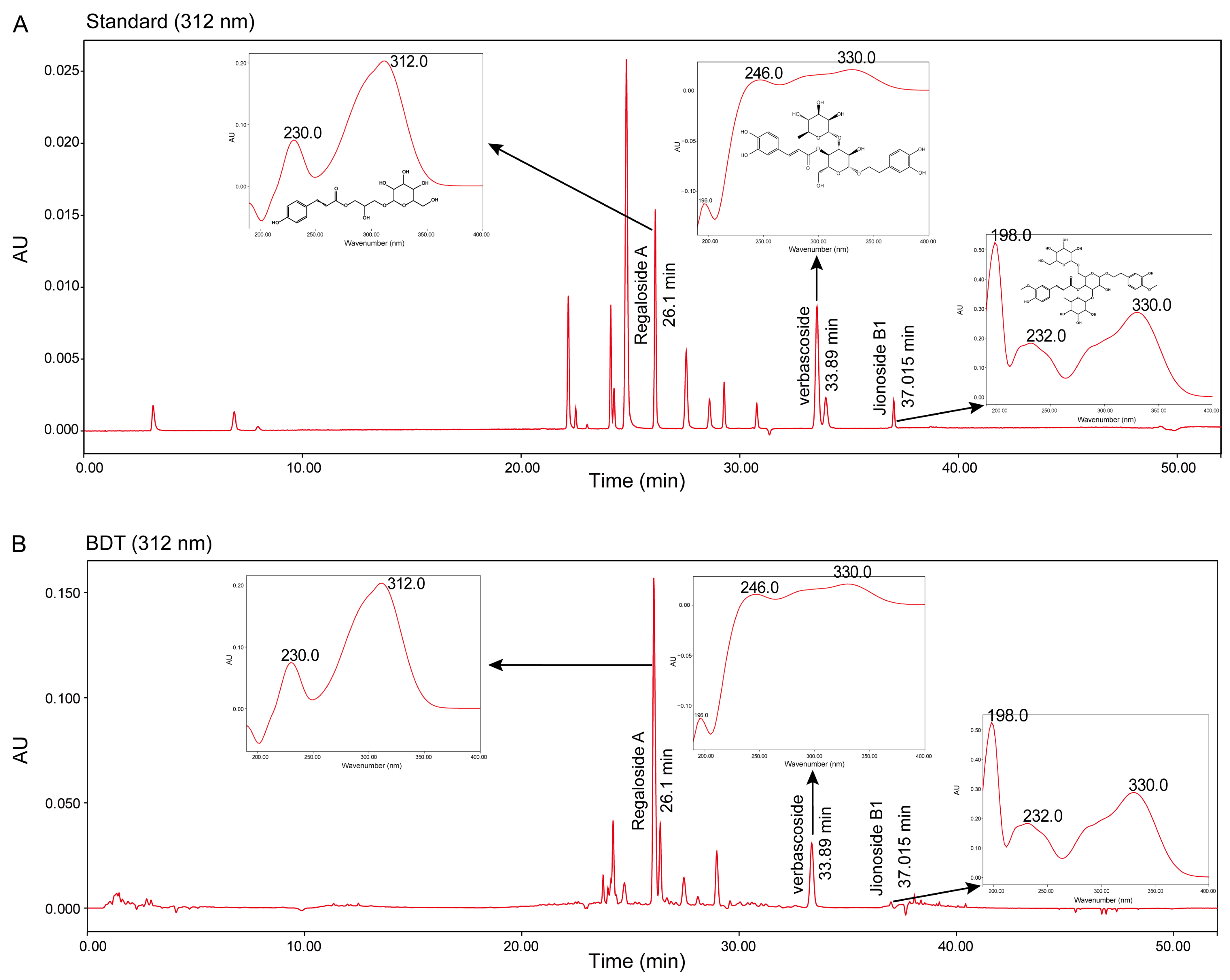
2.1. Results of Quality Control of BDT
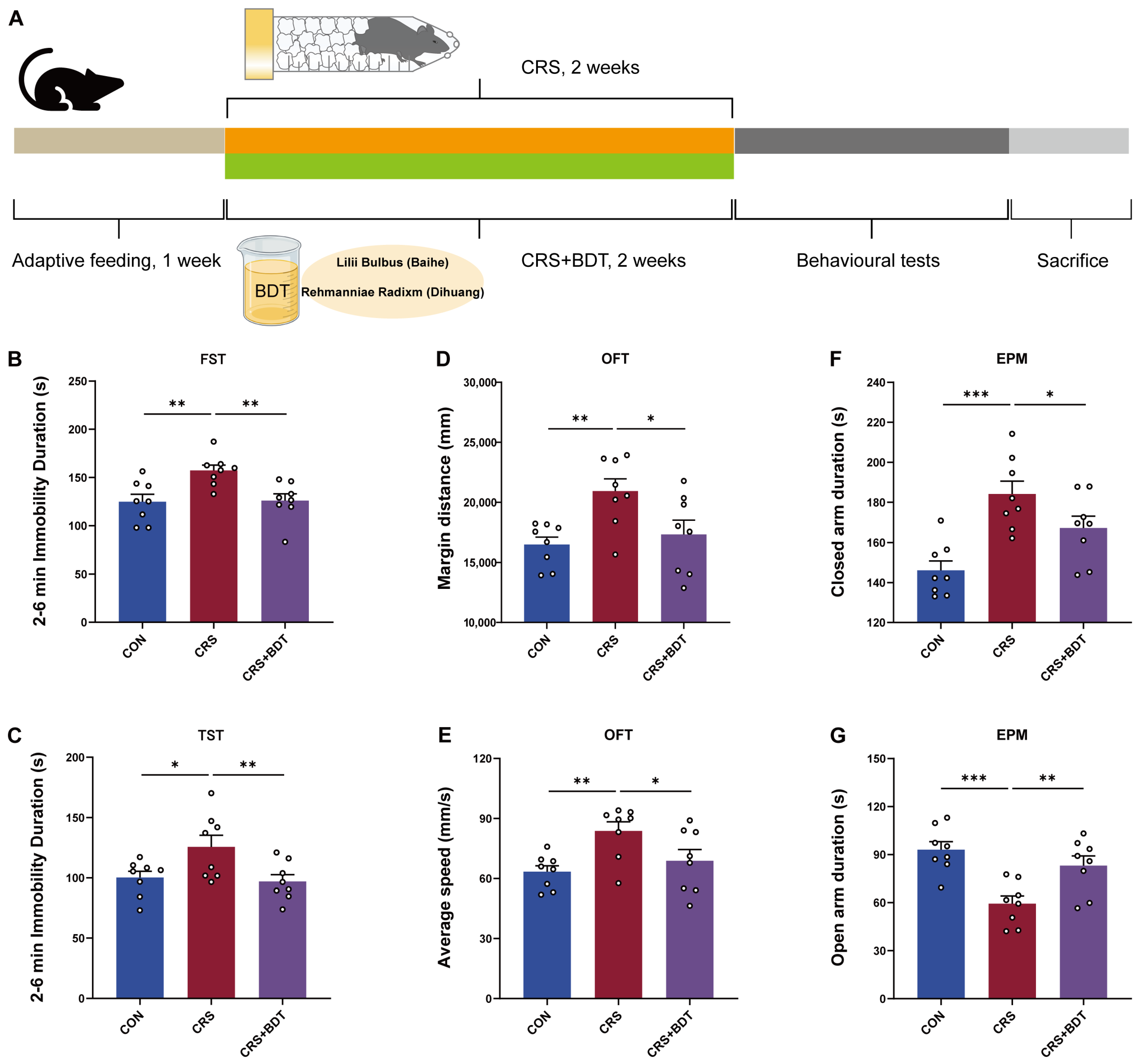
2.2. Results of Behavioral Testing
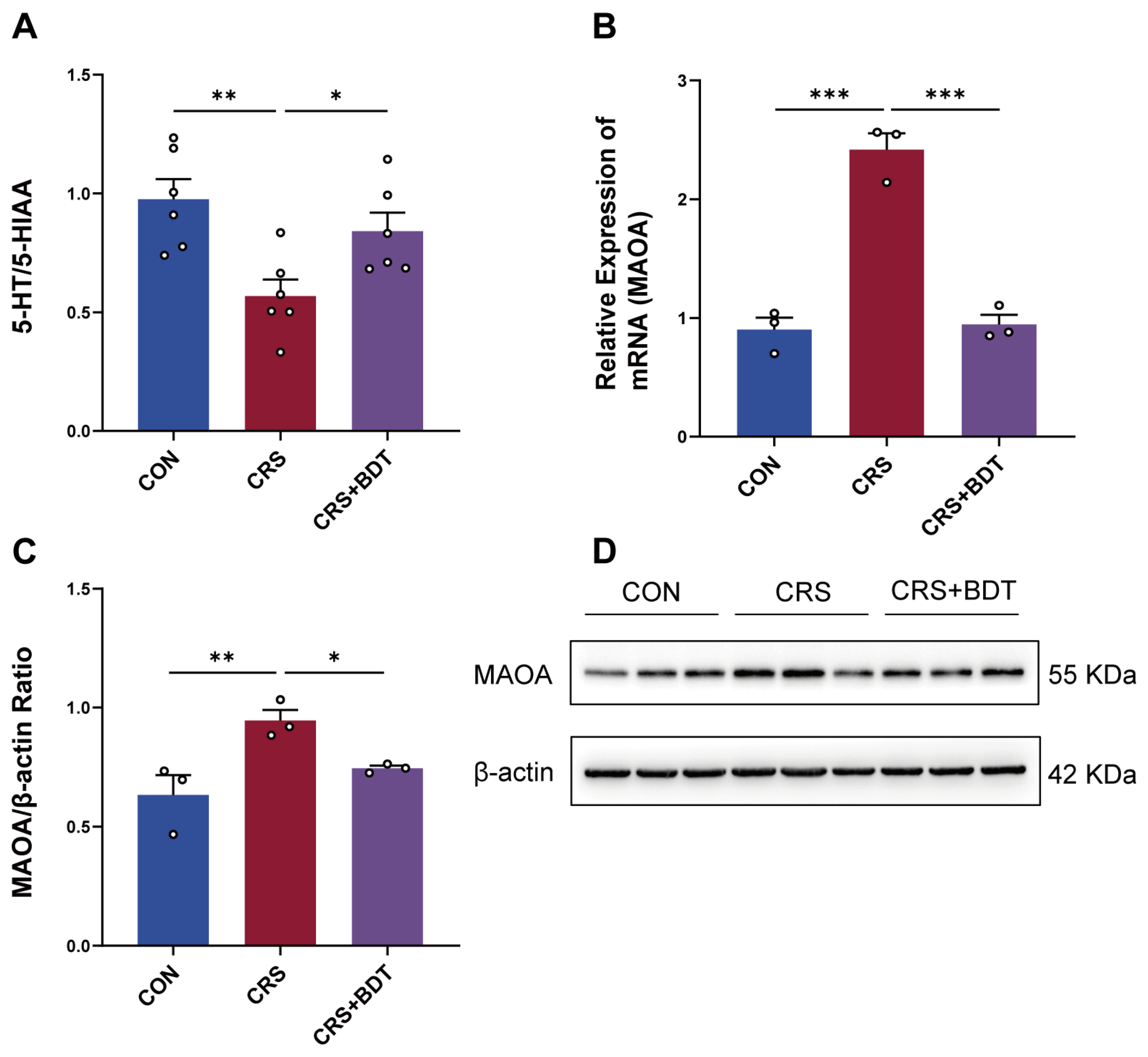
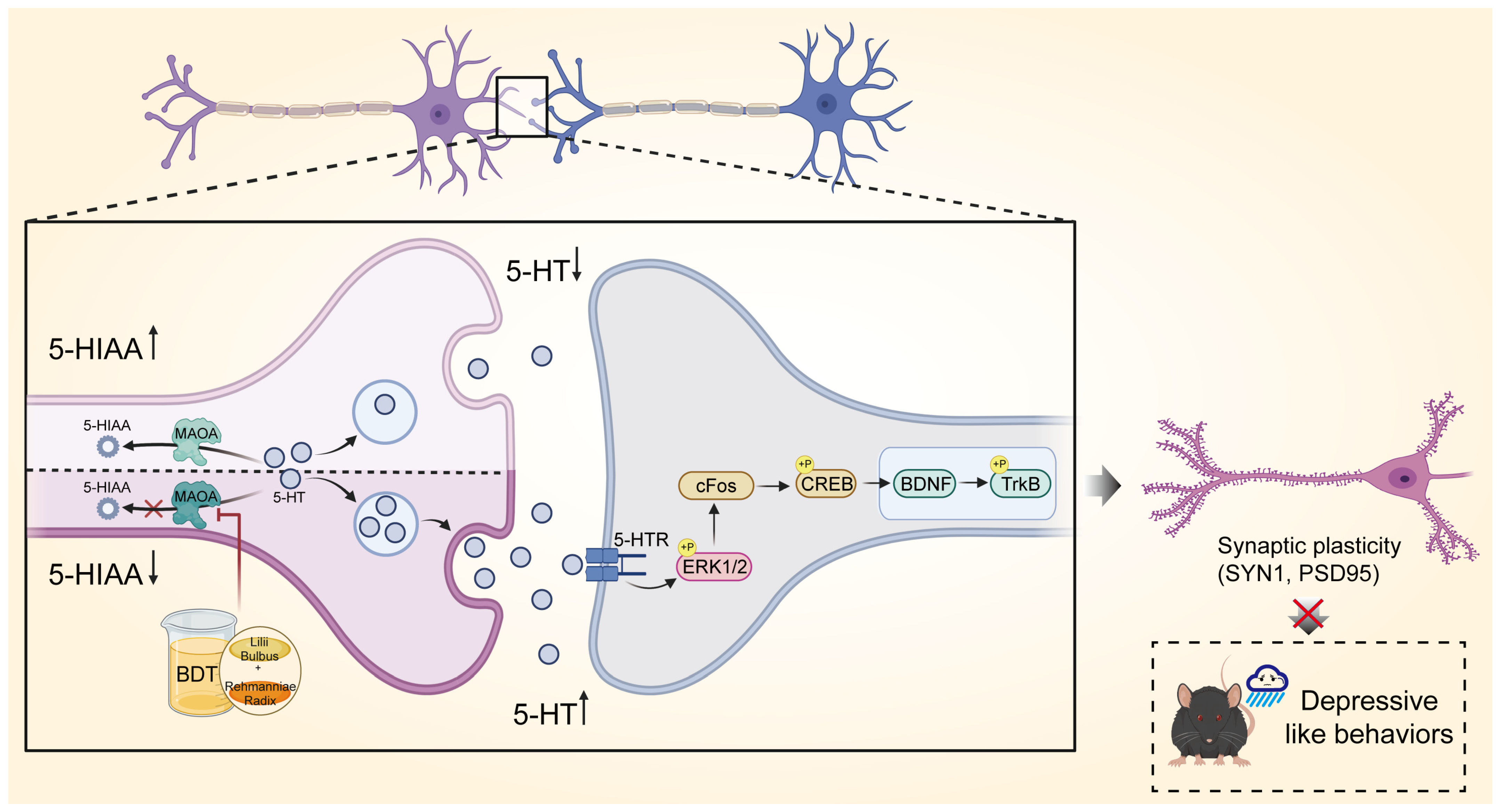
2.3. BDT-Mediated Effects on MAOA
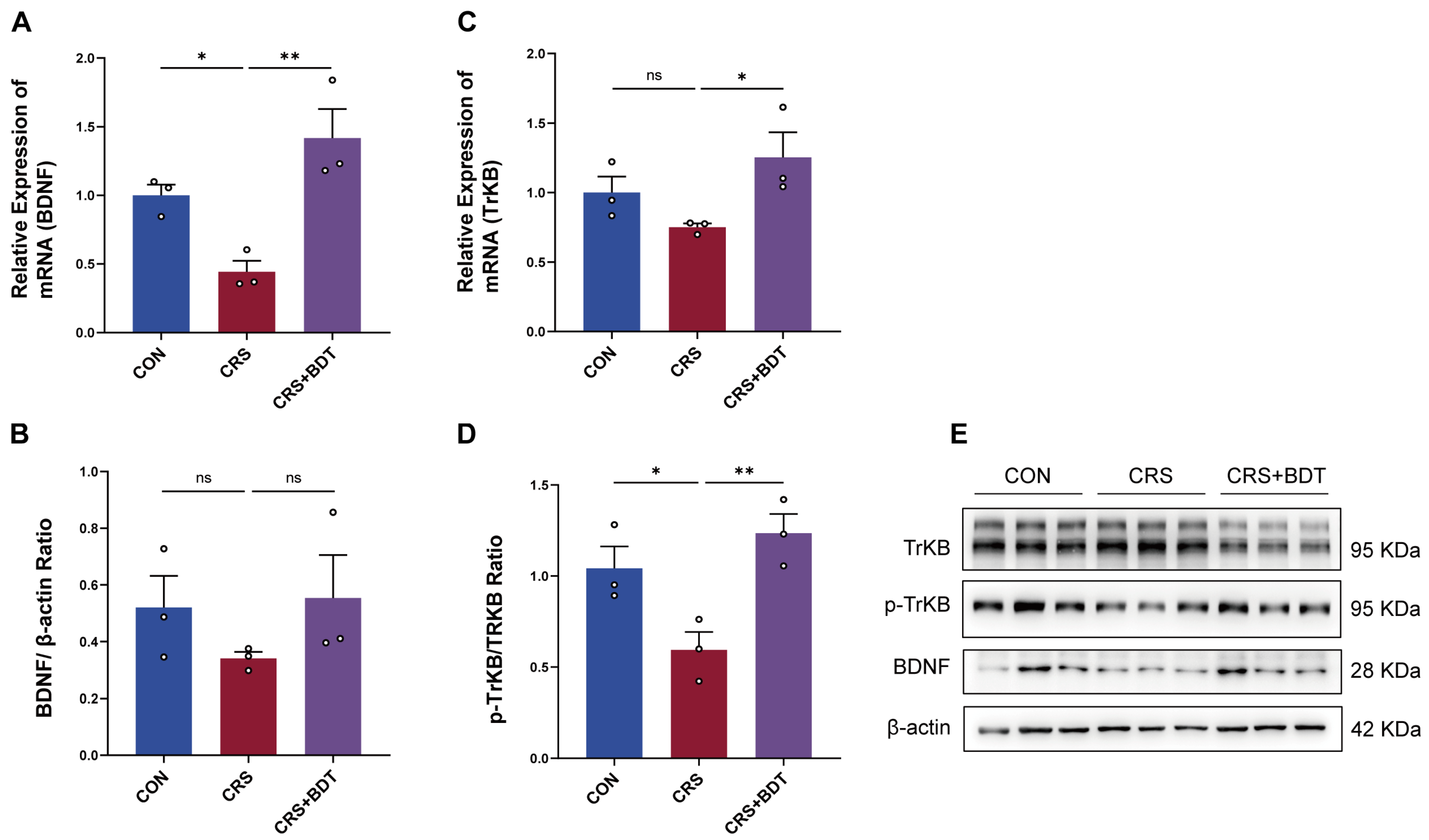
2.4. Impact of Serotonergic Synapse on BDNF/TrkB
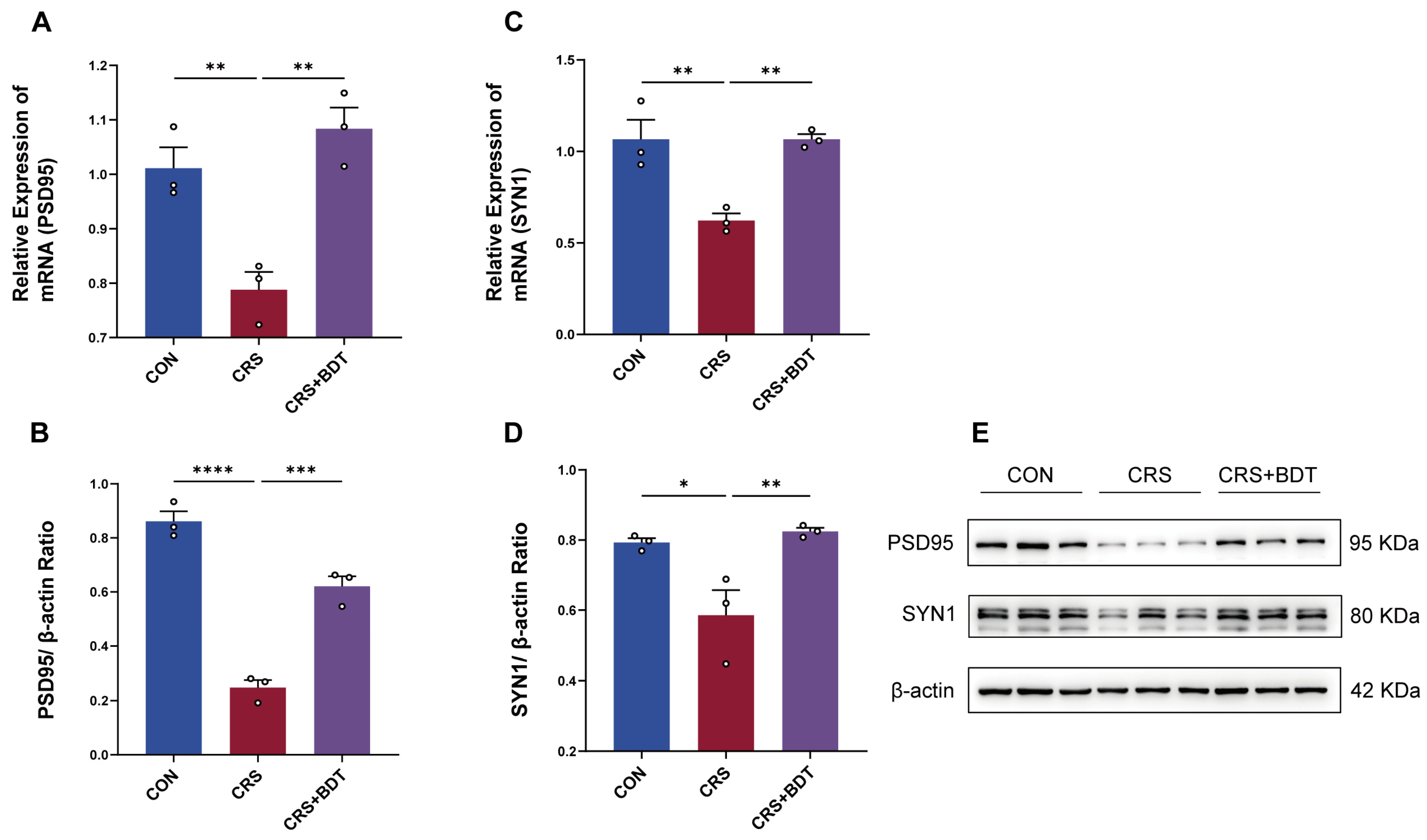
2.5. BDT-Mediated Effects on Synaptic Plasticity
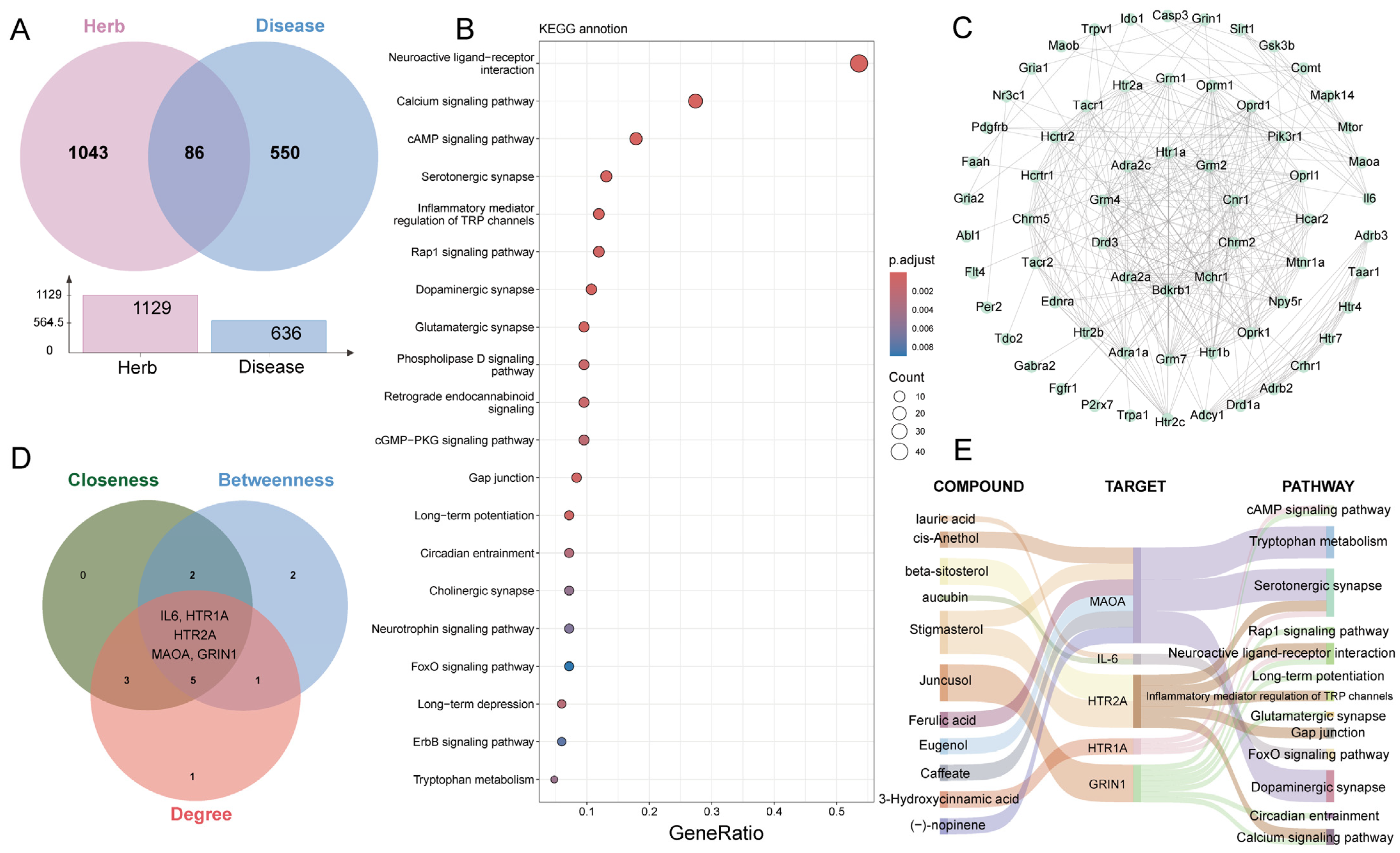
2.6. Network Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of BDT
4.2. Quality Control of BDT
4.3. Animals and Model Construction
4.4. Behavioral Testing
4.4.1. Open Field Test (OFT)
4.4.2. Elevated Plus Maze (EPM)
4.4.3. Forced Swimming Test (FST)
4.4.4. Tail Suspension Test (TST)
4.5. Brain Tissue and Blood Samples Processing
4.6. Examination of 5-HT and 5-HIAA
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.8. Western Blotting
4.9. Network Pharmacology Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDT | Baihe Dihuang Tang |
| 5-HT | Serotonin (5-Hydroxytryptamine) |
| 5-HIAA | 5-Hydroxyindoleacetic acid |
| TCM | Traditional Chinese medicine |
| SSRI | Serotonin reuptake inhibitors |
| NE | Norepinephrine |
| CON | Control Group |
| EPM | Elevated plus maze |
| TST | Tail suspension test |
| PPI | Protein–protein interaction |
| TrKB | Neurotrophic receptor tyrosine kinase 2 |
| SYN1 | Synapsin 1 |
| HTR2A | 5-hydroxytryptamine receptor 2A |
| MAOA | Monoamine oxidase A |
| CUS | Chronic unpredictable stress |
| SST | Somatostatin |
| CRS | Chronic restraint stress |
| DA | Dopamine |
| BC | Betweenness Centrality |
| CC | Closeness Centrality |
| OFT | Open field test |
| FST | Forced swimming test |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BDNF | Brain-derived neurotrophic factor |
| PSD95 | Postsynaptic density protein 95 |
| HTR1A | 5-hydroxytryptamine receptor 1A |
References
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Yasenok, V.; Baumer, A.M.; Petrashenko, V.; Kaufmann, M.; Frei, A.; Rüegger, S.; Ballouz, T.; Loboda, A.; Smiianov, V.; Seifritz, E.; et al. Mental health burden of persons living in Ukraine and Ukrainians displaced to Switzerland: The mental health assessment of the Ukrainian population (MAP) studies. BMJ Glob. Health 2025, 10, e019557. [Google Scholar] [CrossRef]
- Gałecki, P.; Mossakowska-Wójcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 291–294. [Google Scholar] [CrossRef]
- Xue, X.; Pan, J.; Zhang, H.; Lu, Y.; Mao, Q.; Ma, K. Baihe Dihuang (Lilium Henryi Baker and Rehmannia Glutinosa) decoction attenuates somatostatin interneurons deficits in prefrontal cortex of depression via miRNA-144-3p mediated GABA synthesis and release. J. Ethnopharmacol. 2022, 292, 115218. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.F.; Guo, D.Y.; Zhai, B.T.; Liang, Y.J.; Chen, Z.Z.; Zou, J.B.; Shi, Y.J. Evaluation of the Clinical Efficacy of the Classic Prescription “Baihe Dihuang Decoction” Based on Meta-Analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 8559176. [Google Scholar] [CrossRef]
- Tong, Y.; Dong, L.; Shu, H.; Yang, Y.; Bai, Y.; Wen, J. Preclinical evidence evaluation of Xiaoyao san in treating chronic unpredictable mild stress model of depression based on meta-analysis. Phytomedicine 2023, 119, 154991. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bi, Y.; Zeng, S.; Liu, Y.; Shao, M.; Liu, K.; Deng, Y.; Wen, G.; Sun, X.; Zeng, P.; et al. Modified Xiaoyao San ameliorates depressive-like behaviors by triggering autophagosome formation to alleviate neuronal apoptosis. Biomed. Pharmacother. 2019, 111, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, H.; Zhao, H.; Huang, S.; Liu, H.; Zhang, S.; Tang, L. Antidepressant mechanism of Baihe Dihuang Decoction based on metabolomics and network pharmacology. China J. Chin. Mater. Medica 2024, 50, 10–20. [Google Scholar]
- Cao, L.-H.; Wang, Z.-Z.; Zhao, H.; Tian, S.; He, H.-J.; Miao, J.-X.; Huang, S.-n.; Wang, X.-Y.; Song, Y.-G.; Kang, L.; et al. The microglial state transition as a novel mechanism by which fresh Baihe Dihuang decoction prevents depression by regulating SIRT1/HMGB1 signaling. Phytomedicine 2025, 141, 156718. [Google Scholar] [CrossRef]
- Tang, L.; Liu, J.; Yang, H.; Zhao, H.Q.; Hu, C.; Ma, S.J.; Qing, Y.H.; Yang, L.; Zhou, R.R.; Zhang, S.H. Microbiome Metabolomic Analysis of the Anxiolytic Effect of Baihe Dihuang Decoction in a Rat Model of Chronic Restraint Stress. Drug Des. Dev. Ther. 2024, 18, 2227–2248. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef]
- Spies, M.; Murgaš, M.; Vraka, C.; Philippe, C.; Gryglewski, G.; Nics, L.; Balber, T.; Baldinger-Melich, P.; Hartmann, A.M.; Rujescu, D.; et al. Impact of genetic variants within serotonin turnover enzymes on human cerebral monoamine oxidase A in vivo. Transl. Psychiatry 2023, 13, 208. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Y.; Liu, B.; Wang, D.; Liu, L.; Zhu, L.; Liu, H.; Zhang, C.; Yang, W. Metabonomics Study on the Intervention of Baihe Dihuang Decoction in Depressed rats. Acta Chin. Med. Pharmacol. 2023, 51, 36–45. [Google Scholar]
- Zhao, H.; Tang, L.; Liu, Y.; Jiang, J.; Lyu, R.; LIiu, J.; Long, H.; Wang, Y. Mechanism of Baihe Dihuang Decoction activating AMPA receptor to improve anxiety and depression-like behavior in chronic unpredictability stress mice. China J. Tradit. Chin. Med. Pharm. 2023, 38, 1955–1960. [Google Scholar]
- Mao, Q.; Zhang, H.; Zhang, Z.; Lu, Y.; Pan, J.; Guo, D.; Huang, L.; Tian, H.; Ma, K. Co-decoction of Lilii bulbus and Radix Rehmannia Recens and its key bioactive ingredient verbascoside inhibit neuroinflammation and intestinal permeability associated with chronic stress-induced depression via the gut microbiota-brain axis. Phytomedicine 2024, 129, 155510. [Google Scholar] [CrossRef]
- Liu, F.; Jia, Y.; Zhao, L.; Xiao, L.-n.; Cheng, X.; Xiao, Y.; Zhang, Y.; Zhang, Y.; Yu, H.; Deng, Q.-e.; et al. Escin ameliorates CUMS-induced depressive-like behavior via BDNF/TrkB/CREB and TLR4/MyD88/NF-κB signaling pathways in rats. Eur. J. Pharmacol. 2024, 984, 177063. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, K.; Zhang, Z. Effects of the 5-HT1A receptor antagonist on synaptic plasticity in sevoflurane-induced cognitive dysfunction in aged rats and its mechanism. J. China Med. Univ. 2024, 53, 60–66, 74. [Google Scholar]
- Ma, Y.; Chen, H.; Li, H.; Zhao, Z.; An, Q.; Shi, C. Targeting monoamine oxidase A: A strategy for inhibiting tumor growth with both immune checkpoint inhibitors and immune modulators. Cancer Immunol. Immunother. 2024, 73, 48. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Xie, W.; Fu, X.Y.; Li, H.Y.; Jiang, X.H.; Qiu, M.-H. Experimental study on abnormal expression of TPH2,DDC and MAO-A involved in depression-like behaviors of rats induced by CUS. Chin. Pharmacol. Bull. 2016, 32, 1677–1683. [Google Scholar]
- Suchting, R.; Tirumalaraju, V.; Gareeb, R.; Bockmann, T.; de Dios, C.; Aickareth, J.; Pinjari, O.; Soares, J.C.; Cowen, P.J.; Selvaraj, S. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: A systematic review and network meta-analysis. J. Affect. Disord. 2021, 282, 1153–1160. [Google Scholar] [CrossRef]
- de Morais, H.; de Souza, C.P.; da Silva, L.M.; Ferreira, D.M.; Werner, M.F.; Andreatini, R.; da Cunha, J.M.; Zanoveli, J.M. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav. Brain Res. 2014, 258, 52–64. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef]
- Tao, G.; Irie, Y.; Li, D.-J.; Keung, W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005, 13, 4777–4788. [Google Scholar] [CrossRef]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Wu, X.; Yao, J.; Ding, M.; Shi, Z.-S.; Xu, F.-L.; Zhang, J.-J.; Wang, B.-J. 5-HT1A receptor (HTR1A) 5′ region haplotypes significantly affect protein expression in vitro. Neurosci. Lett. 2017, 638, 51–54. [Google Scholar] [CrossRef]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Hervig, M.E.-S.; Zühlsdorff, K.; Olesen, S.F.; Phillips, B.; Božič, T.; Dalley, J.W.; Cardinal, R.N.; Alsiö, J.; Robbins, T.W. 5-HT 2A and 5-HT 2C receptor antagonism differentially modulate reinforcement learning and cognitive flexibility: Behavioural and computational evidence. Psychopharmacology 2024, 241, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.T.; Asada, H.; Inoue, A.; Kadji, F.M.N.; Im, D.; Mori, C.; Arakawa, T.; Hirata, K.; Nomura, Y.; Nomura, N.; et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat. Struct. Mol. Biol. 2019, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Leitão, E.; Kuechler, A.; Villard, L.; Goizet, C.; Courdier, C.; Bayat, A.; Rossi, A.; Julia, S.; Bruel, A.-L.; et al. The different clinical facets of SYN1-related neurodevelopmental disorders. Front. Cell Dev. Biol. 2022, 10, 1019715. [Google Scholar] [CrossRef]
- Gu, Y.; Pope, A.; Smith, C.; Carmona, C.; Johnstone, A.; Shi, L.; Chen, X.; Santos, S.; Bacon-Brenes, C.C.; Shoff, T.; et al. BDNF and TRiC-inspired reagent rescue cortical synaptic deficits in a mouse model of Huntington’s disease. Neurobiol. Dis. 2024, 195, 106502. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, D.; Sun, B.; Yang, J.; Fu, Y.; Ma, S.; Zhu, G. Research Progress and Quality Marker Prediction of Famous Classical Formula Baihe Dihuangtang. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 235–242. [Google Scholar]
- He, Y.; Ren, Y.; Chen, X.; Wang, Y.; Yu, H.; Cai, J.; Wang, P.; Ren, Y.; Xie, P. Neural and molecular investigation into the paraventricular thalamus for chronic restraint stress induced depressive-like behaviors. J. Adv. Res. 2024, 75, 521–537. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Wang, D.; Zhuang, X.; Li, Y.; Guo, C.; Chu, H.; Zhu, F.; Wang, J.; Wang, X.; et al. Programmed cell death 4 as an endogenous suppressor of BDNF translation is involved in stress-induced depression. Mol. Psychiatry 2021, 26, 2316–2333. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, S.; Xia, T.; Liu, Y.; Zhou, Y.; Liu, X.; Pan, R.; Liao, Y.; Yan, M.; Chang, Q. Nelumbinis Stamen Ameliorates Chronic Restraint Stress-Induced Muscle Dysfunction and Fatigue in Mice by Decreasing Serum Corticosterone Levels and Activating Sestrin2. J. Agric. Food Chem. 2022, 70, 16188–16200. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, M.; Wang, J.; Li, M.D.; Yang, Z. CRISPR/Cas9 based gene editing of Frizzled class receptor 6 (FZD6) reveals its role in depressive symptoms through disrupting Wnt/β-catenin signaling pathway. J. Adv. Res. 2024, 58, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, D.S.N.; Thiruselvam, P.; Sundararajan, V.; Ravindran, R.; Gunasekaran, S.; Madathil, D.; Kaliamurthi, S.; Peslherbe, G.H.; Selvaraj, G.; Sudhakaran, S.L. Insights into dietary phytochemicals targeting Parkinson’s disease key genes and pathways: A network pharmacology approach. Comput. Biol. Med. 2024, 172, 108195. [Google Scholar] [CrossRef]
| Parameter | Data |
|---|---|
| HPLC Model | Agilent 1290-Infinity II (Santa Clara, CA, USA) |
| Column | Waters CORTECS T3 (100 mm × 2.1 mm, 1.6 μm) (Milford, MA, USA) |
| Column Temperature | 35 °C |
| Mobile Phase A:B | A: Water (0.1% formic acid, v/v) B: Acetonitrile |
| Flow Rate | 0.3 mL/min |
| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0–6 | 100 | 0 |
| 6–11 | 100–97 | 0–3 |
| 11–17 | 97 | 3 |
| 17–22 | 97–90 | 3–10 |
| 22–29 | 90–85 | 10–15 |
| 29–35 | 85 | 15 |
| 35–45 | 85–35 | 15–65 |
| 45–48 | 35–10 | 65–90 |
| 48.5 | 10–100 | 90–0 |
| 52 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tie, D.; Wang, Y.; Zhou, J.; Zhang, Y.; Ji, H.; Yu, Y.; Han, H.; Xiang, Z.; Li, W. Baihe Dihuang Tang Exerts Antidepressant Effects via Modulation of MAOA-Mediated Serotonin Metabolism and Synaptic Plasticity. Pharmaceuticals 2025, 18, 1786. https://doi.org/10.3390/ph18121786
Tie D, Wang Y, Zhou J, Zhang Y, Ji H, Yu Y, Han H, Xiang Z, Li W. Baihe Dihuang Tang Exerts Antidepressant Effects via Modulation of MAOA-Mediated Serotonin Metabolism and Synaptic Plasticity. Pharmaceuticals. 2025; 18(12):1786. https://doi.org/10.3390/ph18121786
Chicago/Turabian StyleTie, Defu, Yuting Wang, Jieru Zhou, Yiting Zhang, Hua Ji, Yue Yu, Haijun Han, Zheng Xiang, and Wenlong Li. 2025. "Baihe Dihuang Tang Exerts Antidepressant Effects via Modulation of MAOA-Mediated Serotonin Metabolism and Synaptic Plasticity" Pharmaceuticals 18, no. 12: 1786. https://doi.org/10.3390/ph18121786
APA StyleTie, D., Wang, Y., Zhou, J., Zhang, Y., Ji, H., Yu, Y., Han, H., Xiang, Z., & Li, W. (2025). Baihe Dihuang Tang Exerts Antidepressant Effects via Modulation of MAOA-Mediated Serotonin Metabolism and Synaptic Plasticity. Pharmaceuticals, 18(12), 1786. https://doi.org/10.3390/ph18121786







