Chemical, Biochemical, Antimicrobial, and Pharmacological Assessment of Postdistillation Waste Material Extracts of Mentha x piperita †
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Chemical Composition
2.2. Antioxidant Potential
2.2.1. Antioxidant Activity In Vitro
2.2.2. Antioxidant Activity Ex Vivo
2.3. Antibacterial Testing
2.4. Anticholinesterase Activity
2.5. Pharmacodynamic Studies
2.5.1. Rotarod Performance Test
2.5.2. Tail Suspension Test
2.5.3. Elevated Plus Maze
2.5.4. Novel Object Recognition Test
3. Materials and Methods
3.1. Plant Material, Extract Preparation (P1–P6) and Extraction Yield
3.2. Experimental Animals
3.3. Chemical Composition
3.4. Antioxidant Potential
3.4.1. In Vitro Antioxidant Activity
3.4.2. Ex Vivo Antioxidant Activity
- Total protein content;
- Activity of xanthine oxidase (XOD);
- Activity of superoxide dismutase (SOD);
- Lipid peroxidation (LP);
- Glutathione cycle, determined by
- Total glutathione content (GSH);
- Activity of glutathione peroxidase (GSH-Px);
- Activity of glutathione reductase (GSH-R);
- Activity of glutathione-S-transpherase (GSH-(S)T) [47].
3.5. Antibacterial Testing
3.5.1. Bacterial Strains and Growth Conditions
3.5.2. Broth Microdilution Method
3.6. Anticholinesterase Activity
3.7. Pharmacodynamic Studies
3.8. Rotarod Performance Test
3.8.1. Tail Suspension Test (TST)
3.8.2. Elevated Plus Maze Test (EPM)
3.8.3. Novel Object Recognition Test (NOR)
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, H. A Descriptive Overview of the Medical Uses Given to Mentha Aromatic Herbs throughout History. Biology 2020, 9, 484. [Google Scholar] [CrossRef]
- Yousefian, S.; Esmaeili, F.; Lohrasebi, T. A Comprehensive Review of the Key characteristics of the Genus Mentha, Natural Compounds and Biotechnological Approaches for the Production of Secondary Metabolites. Iran. J. Biotechnol. 2023, 21, e3605. [Google Scholar]
- Takhtajan, A. Flowering Plants; Springer: Berlin, Germany, 2009. [Google Scholar]
- European Medicines Agency (EMEA). Community Herbal Monograph on Mentha × piperita L., folium. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 8 June 2025).
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B. Mentha L. Species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008, 14, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horcinová Sedlácková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoea. Monographs on Herbal Drugs and Herbal Drug Preparations, 10th ed.; European Directorate for the Quality of Medicines & Health Care, Council of Europe: Strasbourgh, France, 2019; Volume 1, pp. 1577–1580. [Google Scholar]
- Voigt, V.; Franke, H.; Lachenmeier, D. Risk Assessment of Pulegone in Foods Based on Benchmark Dose–Response Modeling. Foods 2024, 13, 2906. [Google Scholar] [CrossRef]
- Sadowska, U.; Villavicencio, R.A.; Dziadek, K.; Skoczylas, J.; Sadowski, S.K.; Kopec, A. The Identification of Polyphenolic Compounds and the Determination of Antioxidant Activity in Extracts and Infusions of Peppermint, Lemon Balm and Lavender. Appl. Sci. 2024, 14, 699. [Google Scholar] [CrossRef]
- Rosmalena; Putri, N.A.; Yazid, F.; Ambarwati, N.S.S.; Omar, H.; Ahmad, I. Phytochemical, in vitro radical scavenging and in vivo oxidative stress analysis of peppermint (Mentha piperita L.) leaves extract. J. Adv. Pharm. Technol. Res. 2022, 13, 133–137. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Klimek-Szczykutowicz, M.; El-Ansary, D.O.; Mahmoud, E.A. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × piperita and Mentha longifolia Populations in Northern Saudi Arabia. Processes 2020, 8, 479. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Van Vuuren, S.F.; Viljoem, A.M. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S. Afr. J. Bot. 2011, 77, 80–85. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and antioxidant activity of Mentha piperita. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- YuXian, L.; YiBo, L.; AiQin, M.; Yong, B.; Man, W.; ZhenLiang, S. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Protsenko, M.A.; Mazurkova, N.A.; Filippova, E.I.; Kukushkina, T.A.; Lobanova, I.E.; Pshenichkina, Y.A.; Vysochina, G.I. Anti-Influenza activity of extracts from plants of the Lamiaceae familx. Russ. J. Bioorg. Chem. 2022, 48, 1534–1541. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Jain, D.; Pathak, N.; Khan, S.; Raghuram, G.V.; Bhargava, A.; Samarth, R.; Mishra, P.K. Evaluation of cytotoxicity and anticarcinogenesis potential of Mentha leaf extracts. Int. J. Toxicol. 2011, 30, 225–236. [Google Scholar] [CrossRef]
- Samojlik, I.; Petković, S.; Mimica-Dukić, N.; Božin, B. Acute and chronic pretreatment with essential oil of peppermint (Mentha × piperita L., Lamiaceae) influences drug effects. Phytother. Res. 2012, 26, 820–825. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Taylor, R.; LeQuang, J.A.; Raffa, R.B.; NEMA Research Group. The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef]
- Anjum, R.; Raza, C.; Faheem, M.; Ullah, A.; Chaudhry, M. Neuroprotective potential of Mentha piperita extract prevents motor dysfunctions in mouse model of Parkinson’s disease through anti-oxidant capacities. PLoS ONE 2024, 19, e0302102. [Google Scholar] [CrossRef]
- Food and Drug Administration, Department of Health and Human Services. Spices and Other Natural Seasonings and Flavorings. Available online: https://www.ecfr.gov/current/title-21/part-182/section-182.10 (accessed on 21 September 2025).
- Kosar, M.; Dorman, H.J.D.; Hiltunen, R. Effect of acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chem. 2005, 91, 522–533. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour. Bioprocess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Berktas, S.; Cam, M. Peppermint leaves hydrodistillation by-products: Bioactive properties and incorporation into icecream formulations. J. Food Sci. Technol. 2020, 58, 4282–4293. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varietes and cultivars. J. Agric. Food Chem. 2003, 52, 4563–4569. [Google Scholar] [CrossRef]
- Kosar, M.; Dorman, H.J.D.; Husnu Can Baser, K.; Hiltunen, R. Screening of free radical scavenging compounds in water extracts of Mentha samples using a postcolumn derivatization method. J. Agric. Food Chem. 2004, 52, 5004–5010. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Kosar, M.; Husnu, C.; Baser, K.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha × piperita (Peppermint) extracts. Nat. Prod. Comm. 2009, 4, 535–542. [Google Scholar] [CrossRef]
- Gavarić, N.; Kladar, N.; Bogavac, M.; Mijatović Jovin, V.; Samojlik, I.; Božin, B. Anxyolitic effect of deodorised extracts of Mentha × piperita L., Lamiaceae. In Abstract Book, The 15th International Congress of the International Society for Ethno-Pharmacology, Petra, Jordan, 5–8 May 2015; International Society for Ethno-Pharmacology: Strasbourg, France, 2015. [Google Scholar]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.I.; Oprean, R.; Benedec, D.; Hanganu, D.; Duma, M.; Oniga, I.; Vlase, L. LC-MS analysis, antioxidant and antimicrobial activity for five species of Mentha cultivated in Romania. Dig. J. Nanomater. Biostruct. 2014, 9, 559–566. [Google Scholar]
- Hostetler, G.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of spectrophotometric methods for assessing antioxidant potential in plant food samples—A critical approach. Appl. Sci. 2025, 15, 5925. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic disease and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Moazzen, A.; Oztinen, N.; Ak-Sakalli, E.; Kosar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Guimares, R.; Barros, L.; Carvalho, A.M.; Ferreira, I. Infusions and decoctions of mixed herbs used in folk medicine: Synergism of antioxidant potential. Phytother. Res. 2011, 25, 1209–1212. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Taqui, R.; Debnath, M.; Ahmed, S.; Ghosh, A. Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomed. Plus. 2022, 2, 100184. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef]
- Srief, M.; Moustafa, B.; Mokrani, E.H.; Mennai, I.; Hamdi, M.; Boumechhour, A.; Mustapha, M.A.; Derdour, M.; Kerkatou, M.; El-Shazly, M.; et al. Evaluation on in vitro and in silico anti-alzheimer potential of nonpolar extracts and essential oil from Mentha piperita. Foods 2023, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Bruckmueller, H.; Cascorbi, I. Interaction of herbal products with prescribed medications: A systematic review and meta-analysis. Pharmacol. Res. 2019, 141, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Gavarić, N.; Radovanović, K.; Kladar, N.; Hitl, M.; Srđenović Čonić, B.; Mijatović Jovin, V.; Samojlik, I. Can we use Melissa officinalis (lemon balm) postdistillation waste extracts in pharmacy? In vivo pharmacodynamic studies. S. Afr. J. Bot. 2024, 172, 396–406. [Google Scholar] [CrossRef]
- Moghari, F.; Esmaeili, S.; Sarahroodi, S. The aqueous extract of Mentha piperita (Peppermint) can alter depression parameters: A behavioral study in mice. Avicenna J. Pharm. Res. 2023, 4, 44–49. [Google Scholar]
- Walf, A.; Frye, C. The use of elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, J. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crops Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Mandić, A.; Sakač, M.; Milovanović, I.; Sedej, I. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Centr. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Božin, B.; Mimica-Dukić, N.; Samojlik, I.; Anačkov, G.; Igić, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Božin, B.; Mimica-Dukić, N.; Simin, N.; Anačkov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Milić-Torres, V.; Poša, M.; Srđenović, B.; Simplici, A.L. Solubilization of fullerene C60 in micellar solutions of different solubilizers. Coll. Surf. B 2011, 82, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Samojlik, I.; Lakić, N.; Mimica-Dukić, N.; Đaković-Švajcer, K.; Božin, B. Antioxidant and Hepatoprotective Potential of Essential Oils of Coriander (Coriandrum sativum L.) and Caraway (Carum carvi L.) (Apiaceae). J. Agric. Food Chem. 2010, 58, 8848–8853. [Google Scholar] [CrossRef]
- Gavarić, N.; Kovač, J.; Kretschmer, N.; Kladar, N.; Smole Možina, S.; Bucar, F.; Bauer, R.; Božin, B. Natural products as antibacterial agents—Antibacterial potential and safety of postdistillation and waste material from Thymus vulgaris L., Lamiaceae. In Concepts, Compounds and the Alternatives of Antibacterials; InTech: London, UK, 2015; ISBN 978-953-51-2232-6. [Google Scholar]
- Kovač, J.; Gavarić, N.; Bucar, F.; Smole Možina, S. Antimicrobial and resistance modulatory activity of Alpinia katsumadai seed phenolic extract, essential oil and post-distillation extract. Food Techn. Biotechnol. 2014, 52, 248–254. [Google Scholar]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; SmoleMožina, S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Srđenović Čonić, B. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’ Wort species (Hypericum spp., Hypericaceae) from Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Carr, M.; Bekku, N.; Yoshimura, H. Identification of anxiolytic ingredients in ginseng root using elevated plus-maze test in mice. Eur. J. Pharmacol. 2006, 531, 160–165. [Google Scholar] [CrossRef]
- de Bruin, N.; Pozet, B. Beneficial effects of galantamine on performance in the object recognition task in Swiss mice: Deficits induced by scopolamine and by prolonging the retention interval. Pharmacol. Biochem. Behav. 2006, 85, 253–260. [Google Scholar] [CrossRef]
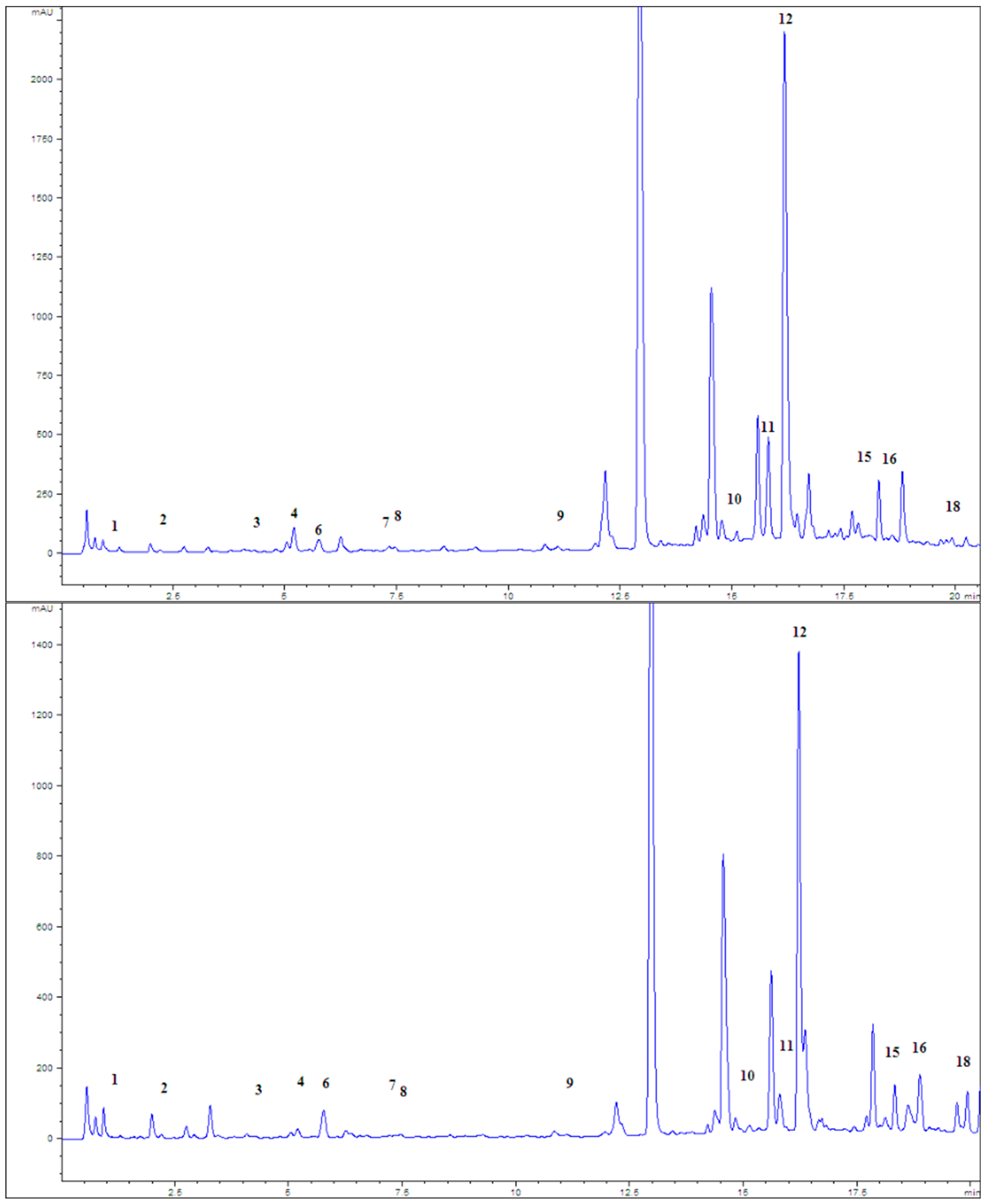
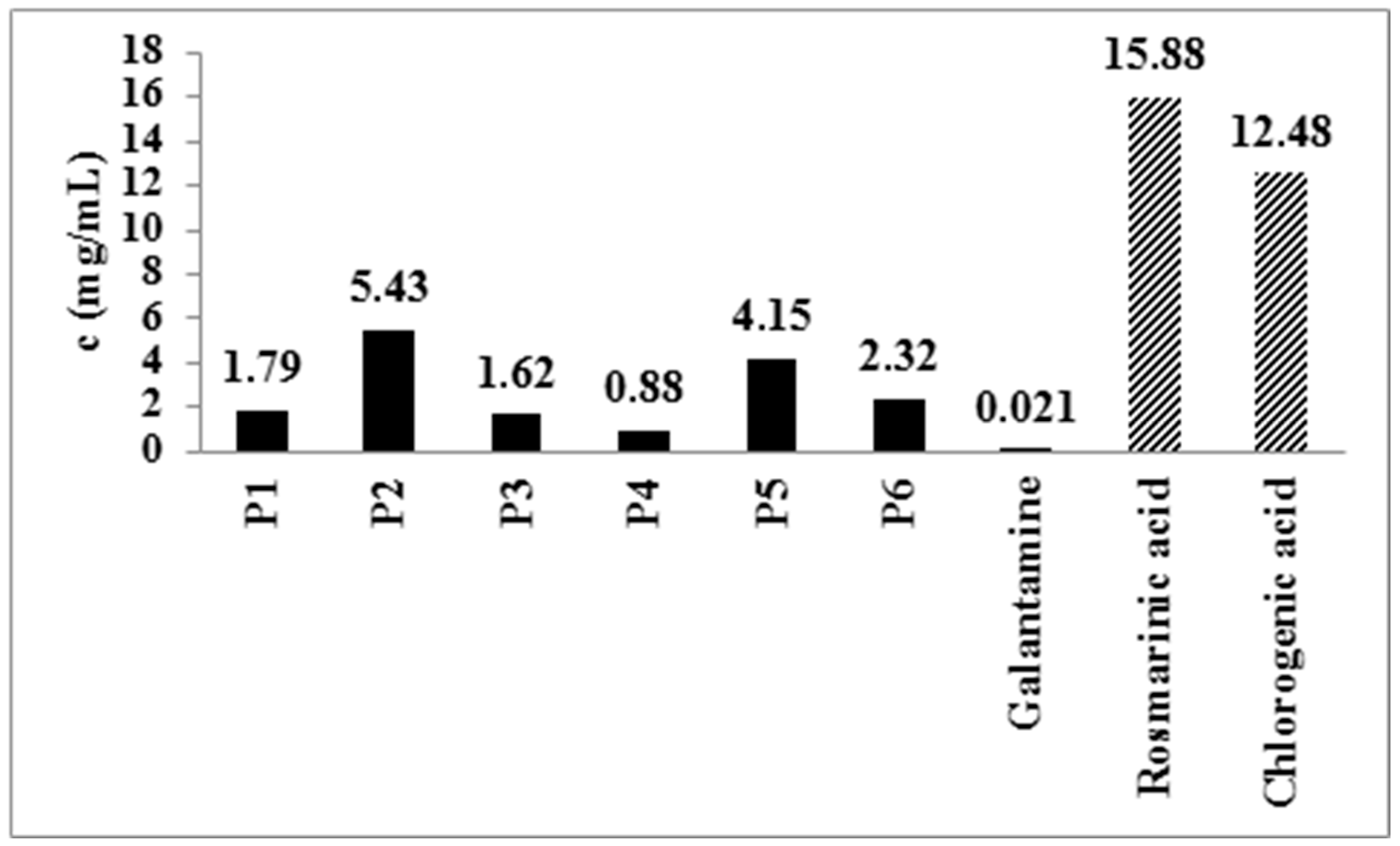
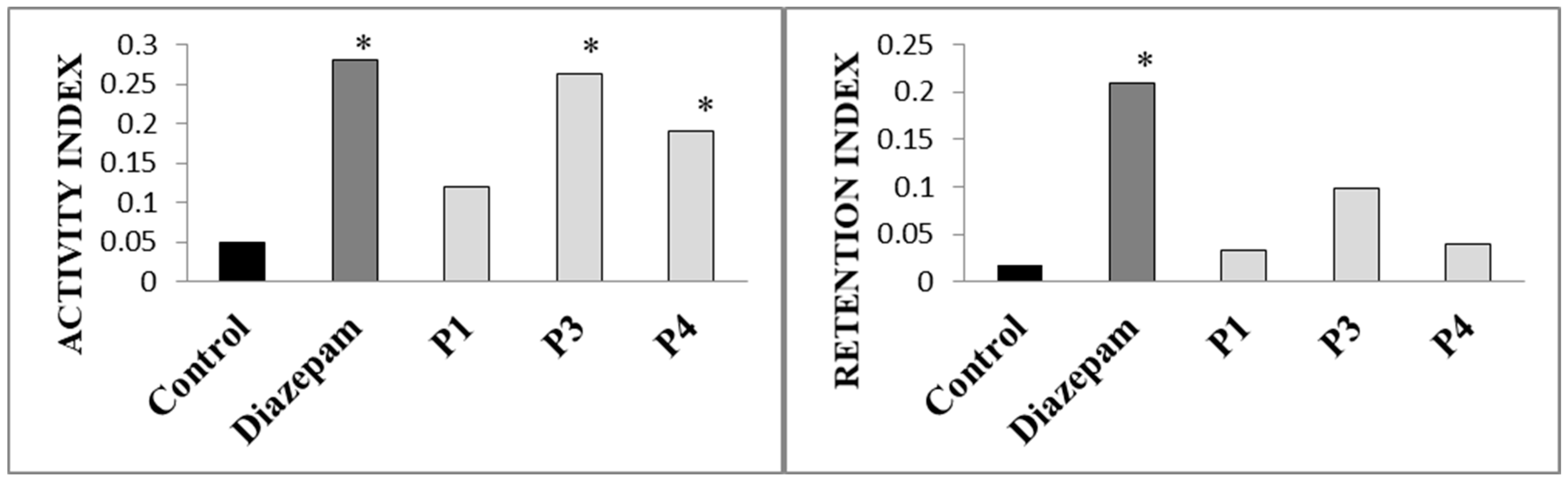
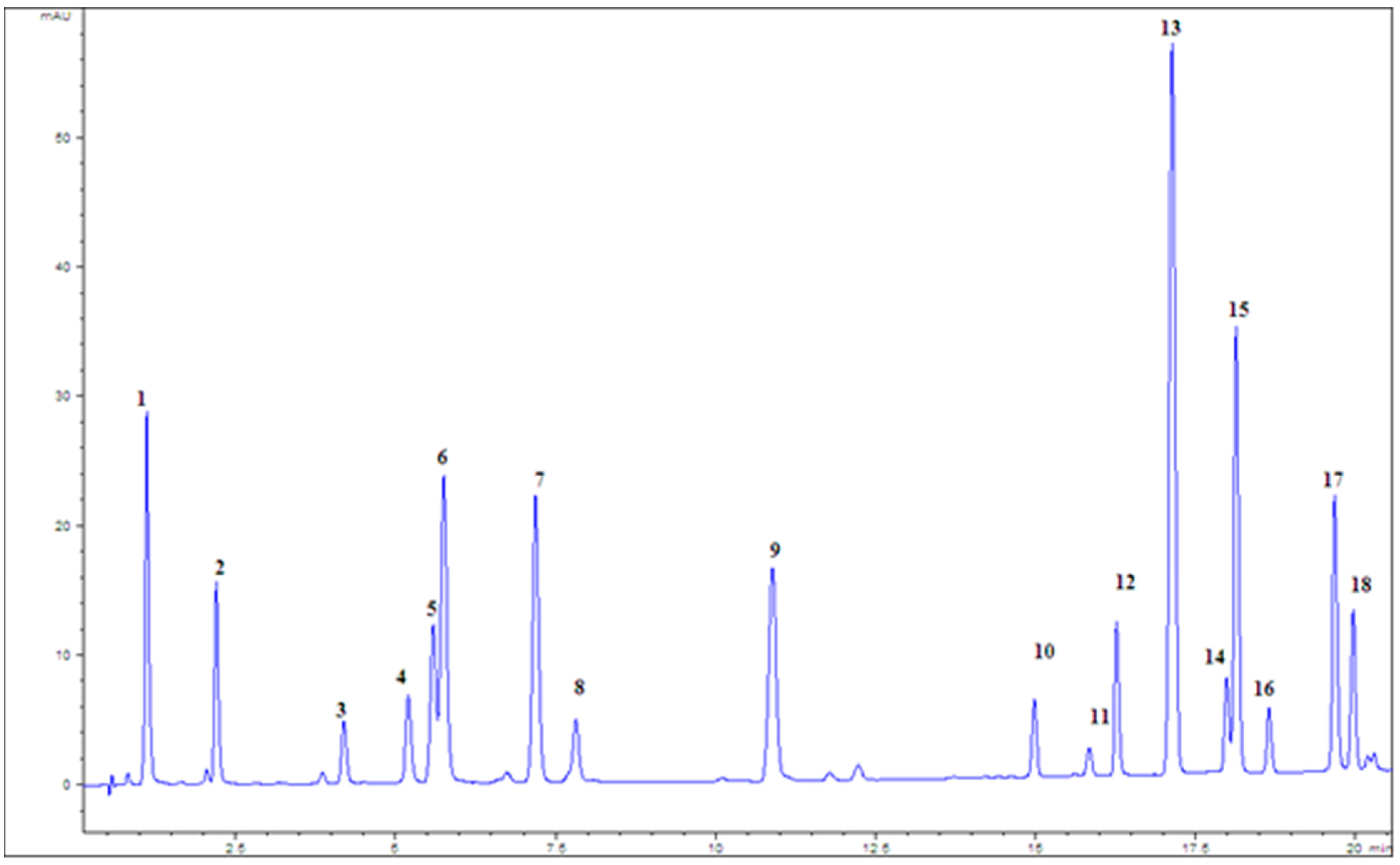

| Type of Extract | % Dry Extract (g d.e./100 g of Plant Material) | Total Phenolic Compounds (mg GAE/g d.e.) | Total Flavonoid Content (µg QE/g d.e.) |
|---|---|---|---|
| P1 | 21.45 ± 0.1 | 31.65 ± 0.03 | 39.25 ± 0.002 |
| P2 | 24.17 ± 0.2 | 36.68 ± 0.002 | 31.02 ± 0.003 |
| P3 | 7.95 ± 0.06 | 36.75 ± 0.01 | 48.35 ± 0.004 |
| P4 | 6.43 ± 0.12 | 38.73 ± 0.01 | 67.63 ± 0.007 |
| P5 | 7.19 ± 0.07 | 24.14 ± 0.03 | 10.44 ± 0.003 |
| P6 | 6.88 ± 0.07 | 34.29 ± 0.003 | 19.84 ± 0.006 |
| Type of Extract Concentration (mg/g d.e.) | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| Gallic acid | 0.15 | 0.09 | 0.08 | 0.08 | 0.11 | 0.13 |
| Protocatechuic acid | 0.27 | 0.32 | 0.39 | 0.46 | 0.25 | 0.36 |
| Catechin | 0.46 | 0.32 | 0.88 | 0.39 | 1.41 | 1.82 |
| Caffeic acid | 4.29 | 1.61 | 2.09 | 2.27 | 2.47 | 2.53 |
| Chlorogenic acid | 0.56 | 0.66 | 1.12 | 1.42 | 0.82 | 0.56 |
| Syringic acid | 0.07 | 0.06 | 0.09 | 0.12 | 0.09 | 0.13 |
| Epicatechin | 0.35 | 0.32 | 0.47 | 0.58 | 0.45 | 0.63 |
| Ferulic acid | 0.24 | 0.14 | 0.18 | 0.22 | 0.13 | 0.18 |
| Rutin | 0.61 | 0.45 | 0.66 | 0.76 | 0.5 | 1.25 |
| Rosmarinic acid | 20.51 | 11.99 | 18.89 | 21.19 | 7.05 | 10.19 |
| Naringenin | 0.18 | 0.13 | 0.73 | 0.72 | 0.03 | 0.14 |
| Apigenin | 0.51 | 0.31 | 3.07 | 2.98 | 0.31 | 0.6 |
| Sample/Assay | DPPH | AAI | OH• | LP |
|---|---|---|---|---|
| P1 | 2.85 | 3.11 | 51.21 | 3.98 |
| P2 | 2.25 | 3.94 | 65.19 | 2.49 |
| P3 | 3.22 | 2.75 | 23.25 | 3.05 |
| P4 | 2.2 | 4.02 | 48.28 | 1.64 |
| P5 | 8.45 | 1.05 | 66.06 | 9.19 |
| P6 | 3.03 | 2.92 | 53.61 | 4.31 |
| BHT | 6.95 | 1.3 | / | 14.71 |
| Rutin | 1.22 | 7.28 | / | / |
| Rosmarinic acid | 0.55 | 16.16 | / | 5.73 |
| Parameter | Control | Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| P1 | P3 | P4 | ||||||
| Treatment | ||||||||
| - | CCl4 | - | CCl4 | - | CCl4 | - | CCl4 | |
| proteins (mg/g of liver homogenate) | 318 ± 40.2 | 397.4 ± 35.4 | 317.8 ± 27.1 | 360.4 ± 11.3 | 317.3 ± 32.0 | 359.2 ± 14.0 | 351.2 ± 13.2 | 392.7 ± 53.2 |
| LP (nmol MDA/mg of proteins) | 0.47 ± 0.13 | 0.98 ± 0.12 | 0.66 ± 0.07 § | 1.26 ± 0.3 # | 0.73 ± 0.16 | 0.97 ± 0.54 | 0.13 ± 0.03 #§ | 1.14 ± 0.17 |
| XOD (nmol/mg of proteins min−1) | 4.71 ± 0.98 | 11.84 ± 1.45 | 7.5 ± 1.52 § | 11.34 ± 0.72 # | 7.63 ± 0.9 § | 11.41 ± 2.16 # | 7.37 ± 2.49 § | 9.54 ± 1.84 # |
| SOD (IU/mg of proteins) a | 0.57 ± 0.11 | 1.24 ± 0.33 # | 0.85 ± 0.29 | 1.35 ± 0.47 | 0.86 ± 0.45 | 1.12 ± 0.29 | 0.52 ± 0.22 § | 0.9 ± 0.42 |
| GSH (nmol/mg of proteins) | 1.05 ± 0.31 | 0.05 ± 0.04 # | 1.6 ± 0.53 § | 0.29 ± 0.14 # | 1.58 ± 0.05 § | 0.3 ± 0.08 *§ | 2.75 ± 0.49 #§ | 0.96 ± 0.28 § |
| GSH-Px (IU/g of proteins) a | 1.93 ± 0.75 | 3.16 ± 0.86 | 3.03 ± 1.17 | 1.41 ± 0.94 | 2.08 ± 1.58 | 2.27 ± 1.03 | 1 ± 0.23 § | 2.14 ± 0.88 |
| GSH-R * | 12.61 ± 5.58 | 18.94 ± 0.93 | 9.49 ± 8.33 | 19.31 ± 11.86 | 6.64 ± 2.31 § | 18.34 ± 10.41 | 10.89 ± 12.69 | 13.52 ± 5.72 |
| GSH-(S)T ** | 5.48 ± 2.84 | 16.31 ± 8.87 | 10.59 ± 6.66 | 10.12 ± 5.44 | 3.34 ± 1.16 | 14.43 ± 10.63 | 5.49 ± 1.89 | 7.39 ± 3.64 |
| Staphylococcus aureus | Salmonella Infantis | Escherichia coli | Bacillus cereus | |
|---|---|---|---|---|
| P1 | 0.12 | 0.125 | 0.26 | 0.35 |
| P2 | 0.15 | 0.15 | 0.3 | 0.59 |
| P3 | 0.07 | 0.15 | 0.18 | 0.07 |
| P4 | 0.15 | 0.21 | 0.15 | 0.11 |
| P5 | 0.13 | 0.39 | 0.20 | 0.27 |
| P6 | 0.28 | 0.55 | 0.19 | 0.28 |
| BHT (mg/mL) | 0.55 | 0.55 | 0.55 | 0.28 |
| Rosmarinic acid (mg/mL) | 5 | 2.5 | 2.5 | 5 |
| Campylobacter jejuni | |
|---|---|
| P1 | 0.13 |
| P3 | 0.07 |
| Rosmarinic acid | 1.25 |
| Ciprofloxacin | 0.25 |
| Erythromycin | 0.25 |
| Extract | Timeframe After Midazolam Application (min) | ||||
|---|---|---|---|---|---|
| 1–4 | 5–8 | 10–13 | 15–18 | 20–23 | |
| P1-control | 74.78 ± 79.31 | 134.5 ± 69.59 | 180.0 ± 0.0 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P1-drug | 88.5 ± 81.4 # | 115.33 ± 75.3 | 132.8 ± 74.1 | 149.7 ± 60.46 | 170.7 ± 27.26 |
| P1-extract | 142.2 ± 61.03 | 169.8 ± 30.67 | 174.4 ± 16.67 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P1-interaction | 146.7 ± 67.5 | 152.55 ± 59.2 | 166.67 ± 40.0 | 170.9 ± 23.33 | 180.0 ± 0.0 |
| P3-control | 79.6 ± 75.8 | 134 ± 74.32 | 159.2 ± 45.53 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P3-drug | 80.7 ± 75.4 # | 112.6 ± 86.75 | 140.3 ± 69.06 | 164.6 ± 48.7 | 180.0 ± 0.0 |
| P3-extract | 180.0 ± 0.0 | 180.0 ± 0.0 | 180.0 ± 0.0 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P3-interaction | 118.8 ± 73.13 | 146.3 ± 71.11 | 178.5 ± 4.74 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P4-control | 97.9 ± 76.4 | 148 ± 67.48 | 164.3 ± 49.65 | 168.3 ± 37.0 | 180.0 ± 0.0 |
| P4-drug | 180.0 ± 0.0 # | 180.0 ± 0.0 # | 180.0 ± 0.0 | 180.0 ± 0.0 | 180.0 ± 0.0 |
| P4-extract | 83.7 ± 77.49 | 95.4 ± 87.37 | 151.6 ± 48.02 | 169.2 ± 34.15 | 170.6 ± 29.73 |
| P4-interaction | 112.9 ± 75.18 | 127.6 ± 84.39 | 141.1 ± 67.72 | 177.7 ± 7.27 | 180.0 ± 0.0 |
| Type of Treatment | Immobilization Time (s) |
|---|---|
| P1—control | 118.33 ± 36.6 |
| P1—drug | 51.25 ± 31.9 # |
| P1—extract | 101.25 ± 32.59 |
| P1—drug + extract | 62.12 ± 42.8 # |
| P3—control | 112.8 ± 51.8 |
| P3—drug | 69.8 ± 49.3 # |
| P3—extract | 109 ± 50.3 |
| P3—drug + extract | 74.1 ± 53.0 # |
| P4—control | 105.2 ± 46.15 |
| P4—drug | 59.6 ± 50.9 # |
| P4—extract | 97.1 ± 52.7 |
| P4—drug + extract | 56 ± 53.3 # |
| Extracts | EPM Parameters | ||||
|---|---|---|---|---|---|
| nO | nE | tO (s) | tE (s) | tCP (s) | |
| Control | 0.57 ± 0.79 | 9.57 ± 1.81 | 2.71 ± 3.64 | 154.71 ± 26.46 | 142.57 ± 26.02 |
| Drug | 6.43 ± 6.97 # | 12.14 ± 3.39 | 38.43 ± 50.73 # | 128.57 ± 50.04 | 118.71 ± 35.96 |
| P1-extract | 1.57 ± 1.62 | 10.14 ± 1.86 | 6.14 ± 6.41 | 164.43 ± 41.66 | 129.43 ± 44.97 |
| P3-extract | 3 ± 2.08 # | 8 ± 2.24 § | 14.57 ± 14.42 # | 144.86 ± 35.82 | 140.57 ± 27.96 |
| P4-extract | 2 ± 1.73 # | 8.71 ± 3.15 § | 6.14 ± 8.23 § | 142.86 ± 43.33 | 151 ± 44.86 |
| Abbreviations | Extract Preparation |
|---|---|
| P1 | maceration of the leaves in 45% ethanol for 24 h—officially prepared (standard) extract |
| P2 | decoction (water extract) of the remaining after hydro-distillation of peppermint leaves |
| P3 | maceration of deodorized peppermint leaves (essential oil was removed with hydro-distillation) in 45% ethanol for 24 h |
| P4 | maceration of deodorized peppermint leaves (essential oil was removed with hydro-distillation) in 75% ethanol for 24 h |
| P5 | maceration of ground peppermint stems in 45% ethanol for 24 h |
| P6 | maceration of ground peppermint stems in 75% ethanol for 24 h |
| Bacterial Strain | Source |
|---|---|
| Staphylococcus aureus | ATCC 25923 |
| Bacillus cereus | ŽMJ 164 |
| Salmonella Infantis | ŽMJ 106 |
| Escherichia coli | ŽMJ 370 |
| Campylobacter jejuni | NCTC 11168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavarić, N.; Radovanović, K.; Milošević, N.; Jovičić-Bata, J.; Lalić-Popović, M.; Možina, S.S.; Samojlik, I. Chemical, Biochemical, Antimicrobial, and Pharmacological Assessment of Postdistillation Waste Material Extracts of Mentha x piperita. Pharmaceuticals 2025, 18, 1782. https://doi.org/10.3390/ph18121782
Gavarić N, Radovanović K, Milošević N, Jovičić-Bata J, Lalić-Popović M, Možina SS, Samojlik I. Chemical, Biochemical, Antimicrobial, and Pharmacological Assessment of Postdistillation Waste Material Extracts of Mentha x piperita. Pharmaceuticals. 2025; 18(12):1782. https://doi.org/10.3390/ph18121782
Chicago/Turabian StyleGavarić, Neda, Katarina Radovanović, Nataša Milošević, Jelena Jovičić-Bata, Mladena Lalić-Popović, Sonja Smole Možina, and Isidora Samojlik. 2025. "Chemical, Biochemical, Antimicrobial, and Pharmacological Assessment of Postdistillation Waste Material Extracts of Mentha x piperita" Pharmaceuticals 18, no. 12: 1782. https://doi.org/10.3390/ph18121782
APA StyleGavarić, N., Radovanović, K., Milošević, N., Jovičić-Bata, J., Lalić-Popović, M., Možina, S. S., & Samojlik, I. (2025). Chemical, Biochemical, Antimicrobial, and Pharmacological Assessment of Postdistillation Waste Material Extracts of Mentha x piperita. Pharmaceuticals, 18(12), 1782. https://doi.org/10.3390/ph18121782








