Next-Generation Hydrogels for Biliary Organoid Engineering
Abstract
1. Introduction: Developmental and Pathological Landscape of the Biliary Tree
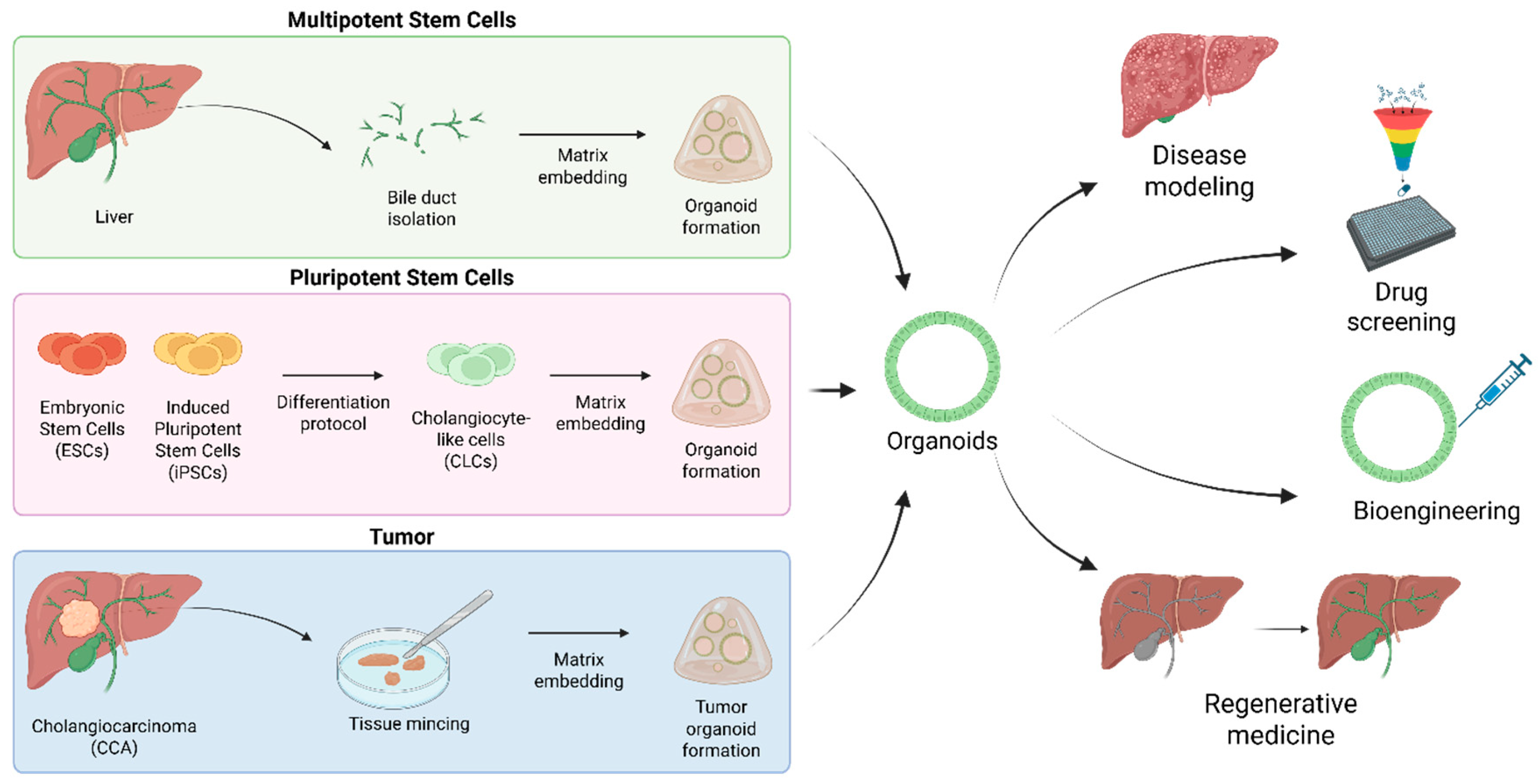
2. Biliary Organoids: From Disease Modeling to Regenerative Medicine
3. Hydrogels: The Next Frontier for Biliary Organoid Engineering
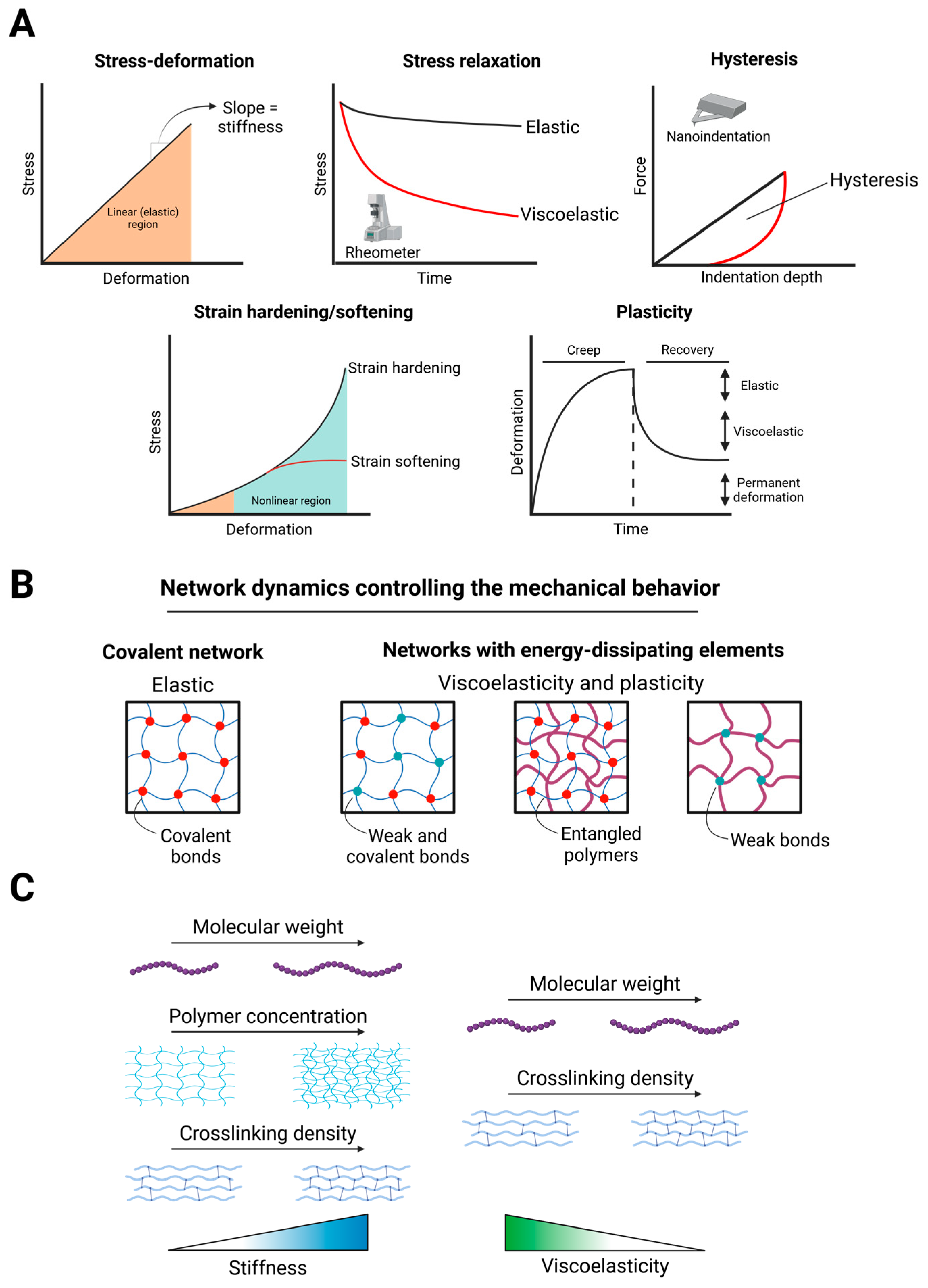
3.1. Mechanical Properties of Hydrogels
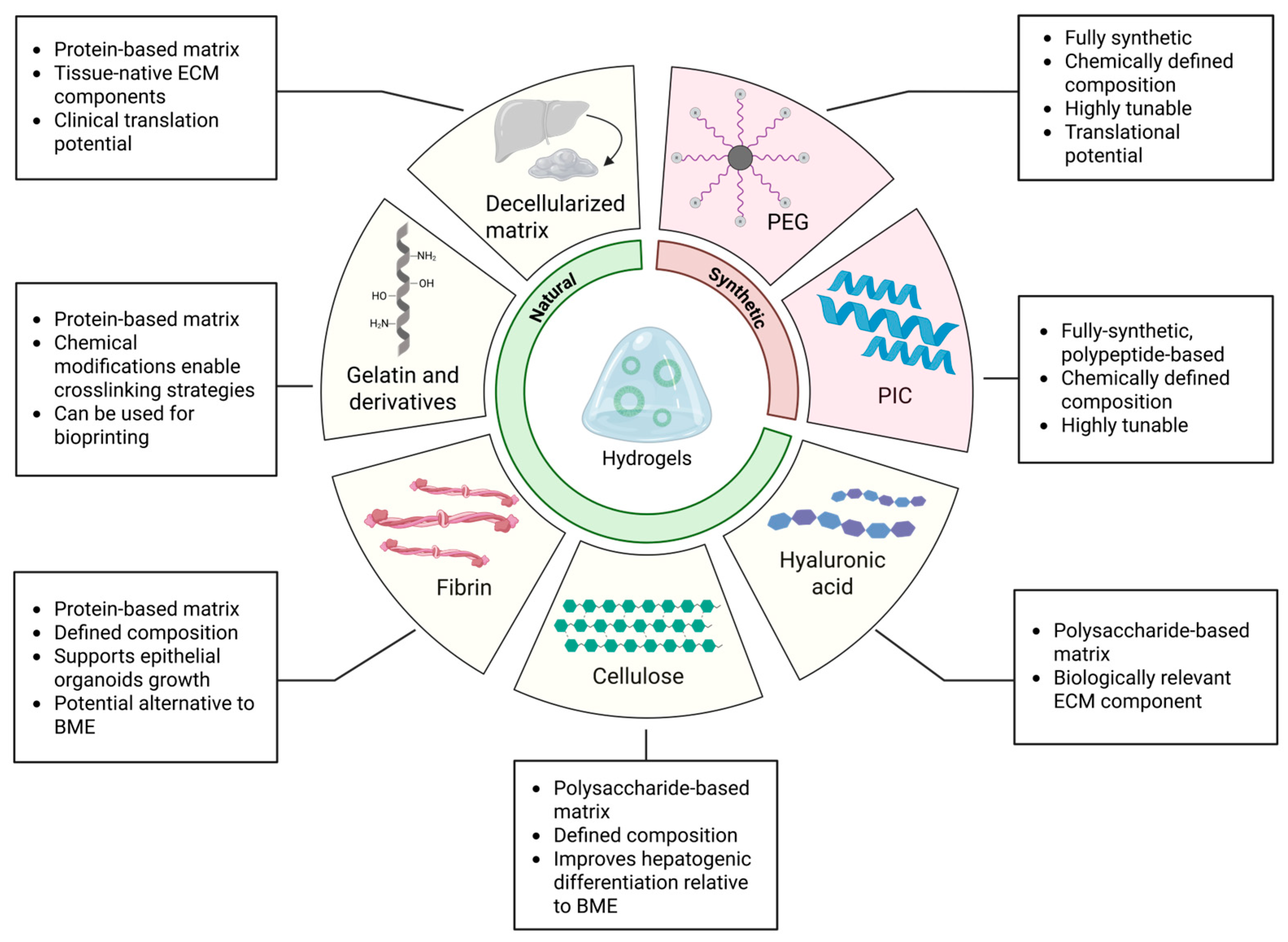
3.2. Natural and Semi-Synthetic Polymers for Hydrogel Fabrication
3.2.1. Decellularized Liver Extracellular Matrix
3.2.2. Gelatin and Derivatives
3.2.3. Cellulose
3.2.4. Hyaluronan
3.2.5. Fibrin
3.3. Synthetic Polymers for Hydrogel Fabrication
3.3.1. Polyisocyanopeptides
3.3.2. Polyethylene Glycol
4. Discussion and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roskams, T.; Desmet, V. Embryology of Extra- and Intrahepatic Bile Ducts, the Ductal Plate. Anat. Rec. 2008, 291, 628–635. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile Formation and Secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef]
- Cardinale, V.; Wang, Y.; Carpino, G.; Mendel, G.; Alpini, G.; Gaudio, E.; Reid, L.M.; Alvaro, D. The Biliary Tree-a Reservoir of Multipotent Stem Cells. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 231–240. [Google Scholar] [CrossRef]
- Spence, J.R.; Lange, A.W.; Lin, S.C.J.; Kaestner, K.H.; Lowy, A.M.; Kim, I.; Whitsett, J.A.; Wells, J.M. Sox17 Regulates Organ Lineage Segregation of Ventral Foregut Progenitor Cells. Dev. Cell 2009, 17, 62–74. [Google Scholar] [CrossRef]
- Lou, C.; Lan, T.; Xu, S.; Hu, X.; Li, J.; Xiang, Z.; Lin, S.; Fan, X.; Chen, J.; Xu, X. Heterogeneity and Plasticity of Cholangiocytes in Liver Injury: A Journey from Pathophysiology to Therapeutic Utility. Gut 2025. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Kim, A.; Lee, S.H.; Shin, D. Liver Progenitor Cell-Driven Liver Regeneration. Exp. Mol. Med. 2020, 52, 1230–1238. [Google Scholar] [CrossRef]
- Tarlow, B.D.; Finegold, M.J.; Grompe, M. Clonal Tracing of Sox9+ Liver Progenitors in Mouse Oval Cell Injury. Hepatology 2014, 60, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S. The Role of Hepatocytes and Oval Cells in Liver Regeneration and Repopulation. Mech. Dev. 2003, 120, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Tarlow, B.D.; Pelz, C.; Naugler, W.E.; Wakefield, L.; Wilson, E.M.; Finegold, M.J.; Grompe, M. Bipotential Adult Liver Progenitors Are Derived from Chronically Injured Mature Hepatocytes. Cell Stem Cell 2014, 15, 605–618. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Larusso, N.F. The Cholangiopathies. Mayo Clin. Proc. 2015, 90, 791–800. [Google Scholar] [CrossRef]
- Squires, R.H.; Ng, V.; Romero, R.; Ekong, U.; Hardikar, W.; Emre, S.; Mazariegos, G.V. Evaluation of the Pediatric Patient for Liver Transplantation: 2014 Practice Guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatolo. Hepatology 2014, 60, 362–398. [Google Scholar] [CrossRef]
- Tam, P.K.H.; Yiu, R.S.; Lendahl, U.; Andersson, E.R. Cholangiopathies—Towards a Molecular Understanding. EBioMedicine 2018, 35, 381–393. [Google Scholar] [CrossRef]
- Sorrentino, G. Microenvironmental Control of the Ductular Reaction: Balancing Repair and Disease Progression. Cell Death Dis. 2025, 16, 246. [Google Scholar] [CrossRef]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Fabris, L.; Cadamuro, M.; Guido, M.; Spirli, C.; Fiorotto, R.; Colledan, M.; Torre, G.; Alberti, D.; Sonzogni, A.; Okolicsanyi, L.; et al. Analysis of Liver Repair Mechanisms in Alagille Syndrome and Biliary Atresia Reveals a Role for Notch Signaling. Am. J. Pathol. 2007, 171, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the Pathogenesis of Primary Sclerosing Cholangitis and Development of Cholangiocarcinoma; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1864, pp. 1390–1400. [Google Scholar]
- Guicciardi, M.E.; Trussoni, C.E.; LaRusso, N.F.; Gores, G.J. The Spectrum of Reactive Cholangiocytes in Primary Sclerosing Cholangitis. Hepatology 2020, 71, 741–748. [Google Scholar] [CrossRef]
- Jalan-Sakrikar, N.; Guicciardi, M.E.; O’Hara, S.P.; Azad, A.; LaRusso, N.F.; Gores, G.J.; Huebert, R.C. Central Role for Cholangiocyte Pathobiology in Cholestatic Liver Diseases. Hepatology 2024, 82, 834–854. [Google Scholar] [CrossRef] [PubMed]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef]
- Greenman, R.; Segal-Salto, M.; Barashi, N.; Hay, O.; Katav, A.; Levi, O.; Vaknin, I.; Aricha, R.; Aharoni, S.; Snir, T.; et al. CCL24 Regulates Biliary Inflammation and Fibrosis in Primary Sclerosing Cholangitis. JCI Insight 2023, 8, e16227. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.; Kangur, T.; Viil, J.; Mäemets-Allas, K.; Minajeva, A.; Vadi, K.; Antsov, M.; Lapidus, N.; Järvekülg, M.; Jaks, V. The Alterations in the Extracellular Matrix Composition Guide the Repair of Damaged Liver Tissue. Sci. Rep. 2016, 6, 27398. [Google Scholar] [CrossRef]
- Manns, M.P.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Muir, A.J.; Ponsioen, C.; Trauner, M.; Wong, G.; Younossi, Z.M. Primary Sclerosing Cholangitis. Nat. Rev. Dis. Primers 2025, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, B.; Davenport, M. Biliary Atresia: A Comprehensive Review. J. Autoimmun. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Tam, P.K.H.; Wells, R.G.; Tang, C.S.M.; Lui, V.C.H.; Hukkinen, M.; Luque, C.D.; De Coppi, P.; Mack, C.L.; Pakarinen, M.; Davenport, M. Biliary Atresia. Nat. Rev. Dis. Primers 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.A.; Spinner, N.B. Alagille Syndrome: Genetics and Functional Models. Curr. Pathobiol. Rep. 2017, 5, 233–241. [Google Scholar] [CrossRef]
- Li, L.; Krantz, I.D.; Deng, Y.; Genin, A.; Banta, A.B.; Collins, C.C.; Qi, M.; Trask, B.J.; Kuo, W.L.; Cochran, J.; et al. Alagille Syndrome Is Caused by Mutations in Human Jagged1, Which Encodes a Ligand for Notch1. Nat. Genet. 1997, 16, 243–251. [Google Scholar] [CrossRef]
- Warthen, D.M.; Moore, E.C.; Kamath, B.M.; Morrissette, J.J.D.; Sanchez, P.; Piccoli, D.A.; Krantz, I.D.; Spinner, N.B. Jagged1 (JAG1) Mutations in Alagille Syndrome: Increasing the Mutation Detection Rate. Hum. Mutat. 2006, 27, 436–443. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Durie, P.R. Cystic Fibrosis from the Gastroenterologist’s Perspective. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 175–185. [Google Scholar] [CrossRef]
- Olaizola, P.; Rodrigues, P.M.; Caballero-Camino, F.J.; Izquierdo-Sanchez, L.; Aspichueta, P.; Bujanda, L.; Larusso, N.F.; Drenth, J.P.H.; Perugorria, M.J.; Banales, J.M. Genetics, Pathobiology and Therapeutic Opportunities of Polycystic Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 585–604. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Dageforde, L.A. Cholangiocarcinoma. Surg. Clin. N. Am. 2019, 99, 315–335. [Google Scholar] [CrossRef]
- Cai, X.; Zhai, J.; Kaplan, D.E.; Zhang, Y.; Zhou, L.; Chen, X.; Qian, G.; Zhao, Q.; Li, Y.; Gao, L.; et al. Background Progenitor Activation Is Associated with Recurrence after Hepatectomy of Combined Hepatocellular-Cholangiocarcinoma. Hepatology 2012, 56, 1804–1816. [Google Scholar] [CrossRef]
- Komuta, M.; Spee, B.; Borght, S.V.; De Vos, R.; Verslype, C.; Aerts, R.; Yano, H.; Suzuki, T.; Matsuda, M.; Fujii, H.; et al. Clinicopathological Study on Cholangiolocellular Carcinoma Suggesting Hepatic Progenitor Cell Origin. Hepatology 2008, 47, 1544–1556. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk Factors for Intrahepatic and Extrahepatic Cholangiocarcinoma in the United States: A Population-Based Study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef]
- Sirica, A.E.; Strazzabosco, M.; Cadamuro, M. Intrahepatic Cholangiocarcinoma: Morpho-Molecular Pathology, Tumor Reactive Microenvironment, and Malignant Progression. Adv. Cancer Res. 2021, 149, 321–387. [Google Scholar] [CrossRef]
- Cadamuro, M.; Stecca, T.; Brivio, S.; Mariotti, V.; Fiorotto, R.; Spirli, C.; Strazzabosco, M.; Fabris, L. The Deleterious Interplay between Tumor Epithelia and Stroma in Cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Gores, G.J. Desmoplastic Stroma and Cholangiocarcinoma: Clinical Implications and Therapeutic Targeting. Hepatology 2014, 59, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Roos, F.J.M.; Verstegen, M.M.A.; Clevers, H.; Vallier, L.; Takebe, T.; Huch, M.; Peng, W.C.; et al. Building Consensus on Definition and Nomenclature of Hepatic, Pancreatic, and Biliary Organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef]
- Dianat, N.; Dubois-Pot-Schneider, H.; Steichen, C.; Desterke, C.; Leclerc, P.; Raveux, A.; Combettes, L.; Weber, A.; Corlu, A.; Dubart-Kupperschmitt, A. Generation of Functional Cholangiocyte-like Cells from Human Pluripotent Stem Cells and HepaRG Cells. Hepatology 2014, 60, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Ramli, M.N.B.; Lim, Y.S.; Koe, C.T.; Demircioglu, D.; Tng, W.; Gonzales, K.A.U.; Tan, C.P.; Szczerbinska, I.; Liang, H.; Soe, E.L.; et al. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology 2020, 159, 1471–1486.e12. [Google Scholar] [CrossRef]
- Sampaziotis, F.; De Brito, M.C.; Madrigal, P.; Bertero, A.; Saeb-Parsy, K.; Soares, F.A.C.; Schrumpf, E.; Melum, E.; Karlsen, T.H.; Bradley, J.A.; et al. Cholangiocytes Derived from Human Induced Pluripotent Stem Cells for Disease Modeling and Drug Validation. Nat. Biotechnol. 2015, 33, 845–852. [Google Scholar] [CrossRef]
- Ogawa, M.; Ogawa, S.; Bear, C.E.; Ahmadi, S.; Chin, S.; Li, B.; Grompe, M.; Keller, G.; Kamath, B.M.; Ghanekar, A. Directed Differentiation of Cholangiocytes from Human Pluripotent Stem Cells. Nat. Biotechnol. 2015, 33, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Jiang, J.X.; Xia, S.; Yang, D.; Ding, A.; Laselva, O.; Hernandez, M.; Cui, C.; Higuchi, Y.; Suemizu, H.; et al. Generation of Functional Ciliated Cholangiocytes from Human Pluripotent Stem Cells. Nat. Commun. 2021, 12, 6504. [Google Scholar] [CrossRef] [PubMed]
- Soroka, C.J.; Assis, D.N.; Boyer, J.L. Patient-Derived Organoids from Human Bile: An in Vitro Method to Study Cholangiopathies. In Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1981, pp. 363–372. ISBN 978-1-4939-9420-5. [Google Scholar]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In Vitro Expansion of Single Lgr5 + Liver Stem Cells Induced by Wnt-Driven Regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; Van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; Van Wenum, M.; Fuchs, S.A.; De Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and Establishment of Self-Renewing Human and Mouse Adult Liver and Pancreas 3D Organoids and Their Genetic Manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Chen, C.; Jochems, P.G.M.; Salz, L.; Schneeberger, K.; Penning, L.C.; Van De Graaf, S.F.J.; Beuers, U.; Clevers, H.; Geijsen, N.; Masereeuw, R.; et al. Bioengineered Bile Ducts Recapitulate Key Cholangiocyte Functions. Biofabrication 2018, 10, 034103. [Google Scholar] [CrossRef]
- Nguyen, R.; Da Won Bae, S.; Qiao, L.; George, J. Developing Liver Organoids from Induced Pluripotent Stem Cells (IPSCs): An Alternative Source of Organoid Generation for Liver Cancer Research. Cancer Lett. 2021, 508, 13–17. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Lemaigre, F.P.; Duncan, S.A. Organogenesis and Development of the Liver. Dev. Cell 2010, 18, 175–189. [Google Scholar] [CrossRef]
- Soroka, C.J.; Assis, D.N.; Alrabadi, L.S.; Roberts, S.; Cusack, L.; Jaffe, A.B.; Boyer, J.L. Bile-Derived Organoids From Patients With Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology 2019, 70, 871–882. [Google Scholar] [CrossRef]
- Babu, R.O.; Lui, V.C.H.; Chen, Y.; Yiu, R.S.W.; Ye, Y.; Niu, B.; Wu, Z.; Zhang, R.; Yu, M.O.N.; Chung, P.H.Y.; et al. Beta-Amyloid Deposition around Hepatic Bile Ducts Is a Novel Pathobiological and Diagnostic Feature of Biliary Atresia. J. Hepatol. 2020, 73, 1391–1403. [Google Scholar] [CrossRef]
- Bijvelds, M.J.C.; Roos, F.J.M.; Meijsen, K.F.; Roest, H.P.; Verstegen, M.M.A.; Janssens, H.M.; van der Laan, L.J.W.; de Jonge, H.R. Rescue of Chloride and Bicarbonate Transport by Elexacaftor-Ivacaftor-Tezacaftor in Organoid-Derived CF Intestinal and Cholangiocyte Monolayers. J. Cyst. Fibros. 2022, 21, 537–543. [Google Scholar] [CrossRef]
- Waisbourd-Zinman, O.; Koh, H.; Tsai, S.; Lavrut, P.M.; Dang, C.; Zhao, X.; Pack, M.; Cave, J.; Hawes, M.; Koo, K.A.; et al. The Toxin Biliatresone Causes Mouse Extrahepatic Cholangiocyte Damage and Fibrosis through Decreased Glutathione and SOX17. Hepatology 2016, 64, 880–893. [Google Scholar] [CrossRef]
- Fried, S.; Gilboa, D.; Har-Zahav, A.; Lavrut, P.M.; Du, Y.; Karjoo, S.; Russo, P.; Shamir, R.; Wells, R.G.; Waisbourd-Zinman, O. Extrahepatic Cholangiocyte Obstruction Is Mediated by Decreased Glutathione, Wnt and Notch Signaling Pathways in a Toxic Model of Biliary Atresia. Sci. Rep. 2020, 10, 7599. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.R.; Chivukula, I.V.; Hankeova, S.; Sjöqvist, M.; Tsoi, Y.L.; Ramsköld, D.; Masek, J.; Elmansuri, A.; Hoogendoorn, A.; Vazquez, E.; et al. Mouse Model of Alagille Syndrome and Mechanisms of Jagged1 Missense Mutations. Gastroenterology 2018, 154, 1080–1095. [Google Scholar] [CrossRef]
- Iqbal, A.; Van Hul, N.; Belicova, L.; Corbat, A.A.; Hankeova, S.; Andersson, E.R.; Van Hul, N.; Belicova, L.; Corbat, A.A.; Hankeova, S.; et al. Spatially Segregated Defects and IGF1-Responsiveness of Hilar and Peripheral Biliary Organoids from a Model of Alagille Syndrome. Liver Int. 2024, 44, 541–558. [Google Scholar] [CrossRef]
- Gribben, C.; Galanakis, V.; Calderwood, A.; Williams, E.C.; Chazarra-Gil, R.; Larraz, M.; Frau, C.; Puengel, T.; Guillot, A.; Rouhani, F.J.; et al. Acquisition of Epithelial Plasticity in Human Chronic Liver Disease. Nature 2024, 630, 166–173. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human Primary Liver Cancer-Derived Organoid Cultures for Disease Modeling and Drug Screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Li, L.; Knutsdottir, H.; Hui, K.; Weiss, M.J.; He, J.; Philosophe, B.; Cameron, A.M.; Wolfgang, C.L.; Pawlik, T.M.; Ghiaur, G.; et al. Human Primary Liver Cancer Organoids Reveal Intratumor and Interpatient Drug Response Heterogeneity. JCI Insight 2019, 4, e121490. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Tateishi, K.; Misumi, K.; Hayashi, A.; Igarashi, K.; Kato, H.; Nakatsuka, T.; Suzuki, N.; Yamamoto, K.; Kudo, Y.; et al. Mutant IDH1 Confers Resistance to Energy Stress in Normal Biliary Cells through PFKP-Induced Aerobic Glycolysis and AMPK Activation. Sci. Rep. 2019, 9, 18859. [Google Scholar] [CrossRef] [PubMed]
- Cristinziano, G.; Porru, M.; Lamberti, D.; Buglioni, S.; Rollo, F.; Amoreo, C.A.; Manni, I.; Giannarelli, D.; Cristofoletti, C.; Russo, G.; et al. FGFR2 Fusion Proteins Drive Oncogenic Transformation of Mouse Liver Organoids towards Cholangiocarcinoma. J. Hepatol. 2021, 75, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Roos, F.J.M.; van Tienderen, G.S.; Wu, H.; Bordeu, I.; Vinke, D.; Albarinos, L.M.; Monfils, K.; Niesten, S.; Smits, R.; Willemse, J.; et al. Human Branching Cholangiocyte Organoids Recapitulate Functional Bile Duct Formation. Cell Stem Cell 2022, 29, 776–794.e13. [Google Scholar] [CrossRef]
- Sampaziotis, F.; Muraro, D.; Tysoe, O.C.; Sawiak, S.; Beach, T.E.; Godfrey, E.M.; Upponi, S.S.; Brevini, T.; Wesley, B.T.; Garcia-Bernardo, J.; et al. Cholangiocyte Organoids Can Repair Bile Ducts after Transplantation in the Human Liver. Science 2021, 371, 839–846. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, W.; Wang, Z.; Chen, D.; Wang, J.; Ren, H. Constructing Biomimetic Microenvironments for Liver Regeneration. J. Nanobiotechnology 2025, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Elci, B.S.; Nikolaev, M.; Rezakhani, S.; Lutolf, M.P. Bioengineered Tubular Biliary Organoids. Adv. Heal. Healthc. Mater. 2024, 13, 2302912. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, J.; Lee, J.S.; Min, S.; Kim, S.; Ahn, D.H.; Kim, Y.G.; Cho, S.W. Vascularized Liver Organoids Generated Using Induced Hepatic Tissue and Dynamic Liver-Specific Microenvironment as a Drug Testing Platform. Adv. Funct. Mater. 2018, 28, 1801954. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Wang, J.; Filppula, A.M.; Zhang, H.; Zhao, Y. Ion-Specific Hydrogel Microcarriers with Biomimetic Niches for Bioartifical Liver System. Adv. Funct. Mater. 2024, 34, 2402999. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Reed, J.; Walczak, W.J.; Petzold, O.N.; Gimzewski, J.K. In Situ Mechanical Interferometry of Matrigel Films. Langmuir 2009, 25, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The Elastic Modulus of MatrigelTM as Determined by Atomic Force Microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour; Nature Research: London, UK, 2020; Volume 584, pp. 535–546. [Google Scholar]
- Chaudhuri, O.; Koshy, S.T.; Branco Da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular Matrix Stiffness and Composition Jointly Regulate the Induction of Malignant Phenotypes in Mammary Epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Kumar, P.; Smith, T.; Raeman, R.; Chopyk, D.M.; Brink, H.; Liu, Y.; Sulchek, T.; Anania, F.A. Periostin Promotes Liver Fibrogenesis by Activating Lysyl Oxidase in Hepatic Stellate Cells. J. Biol. Chem. 2018, 293, 12781–12792. [Google Scholar] [CrossRef]
- Yin, M.; Woollard, J.; Wang, X.; Torres, V.E.; Harris, P.C.; Ward, C.J.; Glaser, K.J.; Manduca, A.; Ehman, R.L. Quantitative Assessment of Hepatic Fibrosis in an Animal Model with Magnetic Resonance Elastography. Magn. Reson. Med. 2007, 58, 346–353. [Google Scholar] [CrossRef]
- Fan, W.; Adebowale, K.; Váncza, L.; Li, Y.Y.; Rabbi, M.F.; Kunimoto, K.; Chen, D.; Mozes, G.; Chiu, D.K.C.; Li, Y.Y.; et al. Matrix Viscoelasticity Promotes Liver Cancer Progression in the Pre-Cirrhotic Liver. Nature 2024, 626, 635–642. [Google Scholar] [CrossRef]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix Stiffness Modulates Proliferation, Chemotherapeutic Response, and Dormancy in Hepatocellular Carcinoma Cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef]
- Georges, P.C.; Hui, J.J.; Gombos, Z.; McCormick, M.E.; Wang, A.Y.; Uemura, M.; Mick, R.; Janmey, P.A.; Furth, E.E.; Wells, R.G. Increased Stiffness of the Rat Liver Precedes Matrix Deposition: Implications for Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The Role of Matrix Stiffness in Regulating Cell Behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Ahmed, S.; Alshehri, E.; Nazneen, S.; Attia, F.; Obeid, D.; Almuzaini, H.; Alzahrani, A.; Salma, J.; Fujitsuka, I.; Assiri, A.M.; et al. Current Advances and Prospects in Biomaterials-Guided Tools for Liver Organoids Research. Eng. Regen. 2025, 6, 203–217. [Google Scholar] [CrossRef]
- Zhao, J.; Zhi, Y.; Ren, H.; Wang, J.; Zhao, Y. Emerging Biotechnologies for Engineering Liver Organoids. Bioact. Mater. 2025, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Gupta, A.; Najibi, A.J.; Seo, B.R.; Garry, R.; Tringides, C.M.; de Lázaro, I.; Darnell, M.; Gu, W.; Zhou, Q.; et al. Matrix Viscoelasticity Controls Spatiotemporal Tissue Organization. Nat. Mater. 2023, 22, 117–127. [Google Scholar] [CrossRef]
- Bonakdar, N.; Gerum, R.; Kuhn, M.; Spörrer, M.; Lippert, A.; Schneider, W.; Aifantis, K.E.; Fabry, B. Mechanical Plasticity of Cells. Nat. Mater. 2016, 15, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Erk, K.A.; Henderson, K.J.; Shull, K.R. Strain Stiffening in Synthetic and Biopolymer Networks. Biomacromolecules 2010, 11, 1358–1363. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S.J. Stress-Strain Experiments on Individual Collagen Fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Hu, K.H.; Butte, M.J.; Chaudhuri, O. Strain-Enhanced Stress Relaxation Impacts Nonlinear Elasticity in Collagen Gels. Proc. Natl. Acad. Sci. USA 2016, 113, 5492–5497. [Google Scholar] [CrossRef]
- Gardel, M.L.; Shin, J.H.; MacKintosh, F.C.; Mahadevan, L.; Matsudaira, P.; Weitz, D.A. Elastic Behavior of Cross-Linked and Bundled Actin Networks. Science 2004, 304, 1301–1305. [Google Scholar] [CrossRef]
- Storm, C.; Pastore, J.J.; MacKintosh, F.C.; Lubensky, T.C.; Janmey, P.A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.K.R.; Rizzi, L.G. Revisiting the Strain-Induced Softening Behaviour in Hydrogels. Soft Matter 2024, 20, 5616–5624. [Google Scholar] [CrossRef]
- Wisdom, K.M.; Adebowale, K.; Chang, J.; Lee, J.Y.; Nam, S.; Desai, R.; Rossen, N.S.; Rafat, M.; West, R.B.; Hodgson, L.; et al. Matrix Mechanical Plasticity Regulates Cancer Cell Migration through Confining Microenvironments. Nat. Commun. 2018, 9, 4144. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Lee, J.; Brownfield, D.G.; Chaudhuri, O. Viscoplasticity Enables Mechanical Remodeling of Matrix by Cells. Biophys. J. 2016, 111, 2296–2308. [Google Scholar] [CrossRef]
- Bertsch, P.; Sacco, P. The Role of Non-Linear Viscoelastic Hydrogel Mechanics in Cell Culture and Transduction. Mater. Today Bio. 2025, 34, 102188. [Google Scholar] [CrossRef]
- Parada, G.A.; Zhao, X. Ideal Reversible Polymer Networks. Soft Matter 2018, 14, 5186–5196. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, K.; Allan, C.; Ha, B.; Saraswathibhatla, A.; Zhu, J.; Indana, D.; Popescu, M.C.; Demirdjian, S.; Martinez, H.A.; Esclamado, A.; et al. Monocytes Use Protrusive Forces to Generate Migration Paths in Viscoelastic Collagen-Based Extracellular Matrices. Proc. Natl. Acad. Sci. USA 2025, 122, e2309772122. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Soto, J.; Hoffman, T.; Lin, X.; Zhang, A.; Chen, S.; Massad, R.N.; Han, X.; Qi, D.; et al. Viscoelastic Extracellular Matrix Enhances Epigenetic Remodeling and Cellular Plasticity. Nat. Commun. 2025, 16, 4054. [Google Scholar] [CrossRef]
- Adu-Berchie, K.; Liu, Y.; Zhang, D.K.Y.; Freedman, B.R.; Brockman, J.M.; Vining, K.H.; Nerger, B.A.; Garmilla, A.; Mooney, D.J. Generation of Functionally Distinct T-Cell Populations by Altering the Viscoelasticity of Their Extracellular Matrix. Nat. Biomed. Eng. 2023, 7, 1374–1391. [Google Scholar] [CrossRef]
- Zwirner, J.; Devananthan, P.; Kabaliuk, N.; Docherty, P.D.; Ondruschka, B. The Use of Liver Biomechanics in Forensic Pathology. Int. J. Leg. Med. 2025, 1–8. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Seremeta, K.P.; Sosnik, A. Natural and Semi-Natural Polymers; Springer: Cham, Switzerland, 2023; pp. 55–70. ISBN 978-3-031-36135-7. [Google Scholar]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical Applications of Decellularized Extracellular Matrices for Tissue Engineering and Regenerative Medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, X.; Xia, S.; Liu, S.; Ren, H. Orthotopic Implantable Liver Decellularized Scaffold for Acute Liver Failure. Eng. Regen. 2023, 4, 12–19. [Google Scholar] [CrossRef]
- Tomofuji, K.; Fukumitsu, K.; Kondo, J.; Horie, H.; Makino, K.; Wakama, S.; Ito, T.; Oshima, Y.; Ogiso, S.; Ishii, T.; et al. Liver Ductal Organoids Reconstruct Intrahepatic Biliary Trees in Decellularized Liver Grafts. Biomaterials 2022, 287, 121614. [Google Scholar] [CrossRef]
- Krüger, M.; Samsom, R.A.; Oosterhoff, L.A.; van Wolferen, M.E.; Kooistra, H.S.; Geijsen, N.; Penning, L.C.; Kock, L.M.; Sainz-Arnal, P.; Baptista, P.M.; et al. High Level of Polarized Engraftment of Porcine Intrahepatic Cholangiocyte Organoids in Decellularized Liver Scaffolds. J. Cell Mol. Med. 2022, 26, 4949–4958. [Google Scholar] [CrossRef]
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson, G.G.; Schneeberger, K.; et al. Hydrogels Derived from Decellularized Liver Tissue Support the Growth and Differentiation of Cholangiocyte Organoids. Biomaterials 2022, 284, 121473. [Google Scholar] [CrossRef]
- Lewis, P.L.; Su, J.; Yan, M.; Meng, F.; Glaser, S.S.; Alpini, G.D.; Green, R.M.; Sosa-Pineda, B.; Shah, R.N. Complex Bile Duct Network Formation within Liver Decellularized Extracellular Matrix Hydrogels. Sci. Rep. 2018, 8, 12220. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, A.; Klein, O.; Nebrich, G.; Weinhart, M.; Tang, P.; Arnold, A.; Ullah, I.; Pohl, J.; Moosburner, S.; Raschzok, N.; et al. The Human Liver Matrisome—Proteomic Analysis of Native and Fibrotic Human Liver Extracellular Matrices for Organ Engineering Approaches. Biomaterials 2020, 257, 120247. [Google Scholar] [CrossRef]
- van Tienderen, G.S.; Koerkamp, B.G.; Ijzermans, J.N.M.; van der Laan, L.J.W.; Verstegen, M.M.A. Recreating Tumour Complexity in a Dish: Organoid Models to Study Liver Cancer Cells and Their Extracellular Environment. Cancers 2019, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Overi, D.; Melandro, F.; Grimaldi, A.; Cardinale, V.; Matteo, S.D.; Mennini, G.; Rossi, M.; Alvaro, D.; Barnaba, V.; et al. Matrisome Analysis of Intrahepatic Cholangiocarcinoma Unveils a Peculiar Cancer-Associated Extracellular Matrix Structure. Clin. Proteom. 2019, 16, 37. [Google Scholar] [CrossRef]
- Lee, J.I.; Campbell, J.S. Role of Desmoplasia in Cholangiocarcinoma and Hepatocellular Carcinoma. J. Hepatol. 2014, 61, 432–434. [Google Scholar] [CrossRef] [PubMed]
- van Tienderen, G.S.; Rosmark, O.; Lieshout, R.; Willemse, J.; de Weijer, F.; Elowsson Rendin, L.; Westergren-Thorsson, G.; Doukas, M.; Groot Koerkamp, B.; van Royen, M.E.; et al. Extracellular Matrix Drives Tumor Organoids toward Desmoplastic Matrix Deposition and Mesenchymal Transition. Acta Biomater. 2023, 158, 115–131. [Google Scholar] [CrossRef]
- Milton, L.A.; Davern, J.W.; Hipwood, L.; Chaves, J.C.S.; McGovern, J.; Broszczak, D.; Hutmacher, D.W.; Meinert, C.; Toh, Y.C. Liver Click DECM Hydrogels for Engineering Hepatic Microenvironments. Acta Biomater. 2024, 185, 144–160. [Google Scholar] [CrossRef]
- Xue, T.; Zhang, J.; Li, F.; Chen, G.; Yi, K.; Chen, X.; Zhang, Y.; Xu, Y.; Wang, H.; Ju, E.; et al. Tunable Biomechanical Niches Regulate Hepatic Differentiation of Mesenchymal Stem Cells for Acute Liver Failure Therapy. Biomaterials 2026, 324, 123458. [Google Scholar] [CrossRef]
- Carpentier, N.; Ye, S.; Delemarre, M.D.; Van der Meeren, L.; Skirtach, A.G.; van der Laan, L.J.W.; Schneeberger, K.; Spee, B.; Dubruel, P.; Van Vlierberghe, S. Gelatin-Based Hybrid Hydrogels as Matrices for Organoid Culture. Biomacromolecules 2024, 25, 590–604. [Google Scholar] [CrossRef]
- Lin, C.C.; Ki, C.S.; Shih, H. Thiol-Norbornene Photoclick Hydrogels for Tissue Engineering Applications. J. Appl. Polym. Sci. 2015, 132, 41563. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, N.; Van der Meeren, L.; Skirtach, A.G.; Devisscher, L.; Van Vlierberghe, H.; Dubruel, P.; Van Vlierberghe, S. Gelatin-Based Hybrid Hydrogel Scaffolds: Toward Physicochemical Liver Mimicry. Biomacromolecules 2023, 24, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Buchholz, M.B.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; et al. Bioprinting of Human Liver-Derived Epithelial Organoids for Toxicity Studies. Macromol. Biosci. 2021, 21, 2100327. [Google Scholar] [CrossRef]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G.; Samsom, R.A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, 2110054. [Google Scholar] [CrossRef]
- Chen, Y.X.; Cain, B.; Soman, P. Gelatin Methacrylate-Alginate Hydrogel with Tunable Viscoelastic Properties. AIMS Mater. Sci. 2017, 4, 363–369. [Google Scholar] [CrossRef]
- Lipari, S.; Marfoglia, A.; Sorrentino, G.; Cazalbou, S.; Pilloux, L.; Sacco, P.; Donati, I. Thermally Cured Gelatin-Methacryloyl Hydrogels Form Mechanically Modulating Platforms for Cell Studies. Biomacromolecules 2025, 26, 5086–5095. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the Elastic Modulus of Cellulose Nanofibril Hydrogels—Scaffolds with Potential in Tissue Engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D Bioprinting of Dual-Crosslinked Nanocellulose Hydrogels for Tissue Engineering Applications. J. Mater. Chem. B 2021, 9, 6163–6175. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.W.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Heal. Healthc. Mater. 2020, 9, 1901658. [Google Scholar] [CrossRef]
- Yang, J.; Shao, C.; Meng, L. Strain Rate-Dependent Viscoelasticity and Fracture Mechanics of Cellulose Nanofibril Composite Hydrogels. Langmuir 2019, 35, 10542–10550. [Google Scholar] [CrossRef]
- Curvello, R.; Kerr, G.; Micati, D.J.; Chan, W.H.; Raghuwanshi, V.S.; Rosenbluh, J.; Abud, H.E.; Garnier, G. Engineered Plant-Based Nanocellulose Hydrogel for Small Intestinal Organoid Growth. Adv. Sci. 2021, 8, 2002135. [Google Scholar] [CrossRef]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seki, E. Hyaluronan in Liver Fibrosis: Basic Mechanisms, Clinical Implications, and Therapeutic Targets. Hepatol. Commun. 2023, 7, e0083. [Google Scholar] [CrossRef]
- Yang, Y.M.; Noureddin, M.; Liu, C.; Ohashi, K.; Kim, S.Y.; Ramnath, D.; Powell, E.E.; Sweet, M.J.; Roh, Y.S.; Hsin, I.F.; et al. Hyaluronan Synthase 2-Mediated Hyaluronan Production Mediates Notch1 Activation and Liver Fibrosis. Sci. Transl. Med. 2019, 11, eaat9284. [Google Scholar] [CrossRef] [PubMed]
- Petit, N.; Chang, Y.J.; Lobianco, F.A.; Hodgkinson, T.; Browne, S. Hyaluronic Acid as a Versatile Building Block for the Development of Biofunctional Hydrogels: In Vitro Models and Preclinical Innovations. Mater. Today Bio 2025, 31, 101596. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Y.; Li, J.; Wang, J.; Yu, Y.; Zhao, Y. Tailoring Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2306554. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kumar, S. CD44-Mediated Adhesion to Hyaluronic Acid Contributes to Mechanosensing and Invasive Motility. Mol. Cancer Res. 2014, 12, 1416–1429. [Google Scholar] [CrossRef]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the Mechanobiology of Malignant Brain Tumors Using a Brain Matrix-Mimetic Hyaluronic Acid Hydrogel Platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef]
- Chopra, A.; Murray, M.E.; Byfield, F.J.; Mendez, M.G.; Halleluyan, R.; Restle, D.J.; Raz-Ben Aroush, D.; Galie, P.A.; Pogoda, K.; Bucki, R.; et al. Augmentation of Integrin-Mediated Mechanotransduction by Hyaluronic Acid. Biomaterials 2014, 35, 71–82. [Google Scholar] [CrossRef]
- He, Y.; Wu, G.D.; Sadahiro, T.; Noh, S.I.; Wang, H.; Talavera, D.; Wang, H.; Vierling, J.M.; Klein, A.S. Interaction of CD44 and Hyaluronic Acid Enhances Biliary Epithelial Proliferation in Cholestatic Livers. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 305–312. [Google Scholar] [CrossRef]
- Kon, J.; Ooe, H.; Oshima, H.; Kikkawa, Y.; Mitaka, T. Expression of CD44 in Rat Hepatic Progenitor Cells. J. Hepatol. 2006, 45, 90–98. [Google Scholar] [CrossRef]
- Rizwan, M.; Ling, C.; Guo, C.; Liu, T.; Jiang, J.X.; Bear, C.E.; Ogawa, S.; Shoichet, M.S. Viscoelastic Notch Signaling Hydrogel Induces Liver Bile Duct Organoid Growth and Morphogenesis. Adv. Heal. Healthc. Mater. 2022, 11, 2200880. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological Advances in Fibrin for Tissue Engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Broguiere, N.; Isenmann, L.; Hirt, C.; Ringel, T.; Placzek, S.; Cavalli, E.; Ringnalda, F.; Villiger, L.; Züllig, R.; Lehmann, R.; et al. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30, e1801621. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xu, J.; Van Dam, E.P.; Giubertoni, G.; Rezus, Y.L.A.; Hammink, R.; Bakker, H.J.; Zhan, Y.; Rowan, A.E.; Xing, C.; et al. Strategies to Increase the Thermal Stability of Truly Biomimetic Hydrogels: Combining Hydrophobicity and Directed Hydrogen Bonding. Macromolecules 2017, 50, 9058–9065. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; de Almeida, P.; ven den Boomen, O.; Eksteen-Akeroyd, Z.H.; Hammink, R.; Jaspers, M.; Kragt, S.; Mabesoone, M.F.J.; Nolte, R.J.M.; Rowan, A.E.; et al. Controlling the Gelation Temperature of Biomimetic Polyisocyanides. Chin. Chem. Lett. 2018, 29, 281–284. [Google Scholar] [CrossRef]
- Vandaele, J.; Louis, B.; Liu, K.; Camacho, R.; Kouwer, P.H.J.; Rocha, S. Structural Characterization of Fibrous Synthetic Hydrogels Using Fluorescence Microscopy. Soft Matter 2020, 16, 4210–4219. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; Koepf, M.; Le Sage, V.A.A.; Jaspers, M.; Van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive Biomimetic Networks from Polyisocyanopeptide Hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef]
- Das, R.K.; Gocheva, V.; Hammink, R.; Zouani, O.F.; Rowan, A.E. Stress-Stiffening-Mediated Stem-Cell Commitment Switch in Soft Responsive Hydrogels. Nat. Mater. 2016, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mihaila, S.M.; Rowan, A.; Oosterwijk, E.; Kouwer, P.H.J. Synthetic Extracellular Matrices with Nonlinear Elasticity Regulate Cellular Organization. Biomacromolecules 2019, 20, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Boeter, J.W.B.; Mihajlovic, M.; van Steenbeek, F.G.; van Wolferen, M.E.; Oosterhoff, L.A.; Marsee, A.; Caiazzo, M.; van der Laan, L.J.W.; Penning, L.C.; et al. A Chemically Defined Hydrogel for Human Liver Organoid Culture. Adv. Funct. Mater. 2020, 30, 2000893. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, S.; van der Laan, L.J.W.; Schneeberger, K.; Masereeuw, R.; Spee, B. Chemically Defined Organoid Culture System for Cholangiocyte Differentiation. Adv. Heal. Healthc. Mater. 2024, 13, 2401511. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic Matrix Metalloproteinase-Sensitive Hydrogels for the Conduction of Tissue Regeneration: Engineering Cell-Invasion Characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, S.; Gjorevski, N.; Lutolf, M.P. Low-Defect Thiol-Michael Addition Hydrogels as Matrigel Substitutes for Epithelial Organoid Derivation. Adv. Funct. Mater. 2020, 30, 2000761. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- McKinnon, D.D.; Domaille, D.W.; Cha, J.N.; Anseth, K.S. Biophysically Defined and Cytocompatible Covalently Adaptable Networks as Viscoelastic 3d Cell Culture Systems. Adv. Mater. 2014, 26, 865–872. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; Quirós, M.; Farkas, A.E.; Dedhia, P.H.; Huang, S.; Siuda, D.; García-Hernández, V.; Miller, A.J.; Spence, J.R.; Nusrat, A.; et al. Synthetic Hydrogels for Human Intestinal Organoid Generation and Colonic Wound Repair. Nat. Cell Biol. 2017, 19, 1326–1335. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; Quirós, M.; Huang, S.; Siuda, D.; Spence, J.R.; Nusrat, A.; García, A.J. PEG-4MAL Hydrogels for Human Organoid Generation, Culture, and in Vivo Delivery. Nat. Protoc. 2018, 13, 2102–2119. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Sorrentino, G.; Rezakhani, S.; Yildiz, E.; Nuciforo, S.; Heim, M.H.; Lutolf, M.P.; Schoonjans, K. Mechano-Modulatory Synthetic Niches for Liver Organoid Derivation. Nat. Commun. 2020, 11, 3416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Riediger, L.; Rao, Q.; Xu, X.; Nie, Y.; Zhou, Y.; Zhang, J.; Tang, P.; Wang, W.; Tacke, F.; et al. Tunable Synthetic Hydrogel Modulates Hepatic Lineage Specification of Human Liver Organoid. Adv. Funct. Mater. 2025, 14, e08430. [Google Scholar] [CrossRef]
- Xu, R.; Ooi, H.S.; Bian, L.; Ouyang, L.; Sun, W. Dynamic Hydrogels for Biofabrication: A Review. Biomaterials 2025, 320, 123266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Dong, L.; Cheng, Y.; Huang, X.; Xue, B.; Jiang, C.; Cao, Y.; Yang, J. Hydrogel-Based Strategies for Liver Tissue Engineering. Chem. Bio Eng. 2024, 1, 887–915. [Google Scholar] [CrossRef]
| Hydrogel | Composition | Mechanical Profile | Application | Pros | Cons | Reference |
|---|---|---|---|---|---|---|
| Decellularized ECM (dECM) | Decellularized liver ECM | Stiffness: ~250 Pa (human liver), ~650 Pa (porcine liver) Viscoelastic | Organoid culture and differentiation | Tissue-specific ECM; supports organoid growth and differentiation; ECM components are preserved; possibility of clinical applications | Requires modification to tune mechanical properties; relies on tissue availability | [111,117] |
| Decellularized CCA ECM | Stiffness: n/a1 Viscoelastic | Organoid culture | Cancer-specific ECM; CCA patient-derived organoids contribute to desmoplasia in non-tumoral dECM hydrogels | |||
| GelMA, GelSH | Hydrolyzed collagen–chemically modified | Stiffness: 2.0–9.6 kPa (GelMA, tunable); 6.2–32.5 kPa (GelSH, depending on modification) Elastic | Organoid culture and differentiation; organoid volumetric bioprinting | Cell adhesive; suitable for hepatogenic differentiation; stiffness can be tuned to support organoid growth | Gelatin requires modification to form mechanically stable hydrogels at low concentrations; elastic (strategies to make viscoelastic hydrogels are possible) | [120,123,124] |
| Cellulose | TEMPO-oxidized cellulose nanofibrils | Stiffness: ~255 Pa Viscoelastic | Organoid differentiation | Widely available; improves hepatogenic differentiation of biliary organoids | Low mechanical properties; doesn’t allow for organoid growth | [131] |
| Hyaluronan (HA) | HA functionalized with RGD peptides and laminin | Stiffness: 0.2–1.0 kPa Viscoelastic (tunable with laminin addition) | Organoid culture | Mechanical properties can be independently tuned | Does not possess integrin-binding sequences | [145] |
| Fibrin | Fibrin supplemented with laminin | Stiffness: 30–430 Pa Viscoelastic | Organoid culture | Defined composition; stiffness can be tuned to support optimal organoid growth | Requires laminin to support organoid proliferation | [147] |
| Polyisocyanopeptides (PIC) | Fully synthetic; peptide-based polymer; functionalized with cell adhesive sequences | Stiffness: ~18–~83 Pa; Non-linear elastic | Organoid culture and differentiation | Chemically defined; xeno-free; stiffness can be tuned to support optimal organoid growth | Requires modification to introduce cell-adhesive ligands | [155,156] |
| Polyethylene glycol (PEG) | Fully synthetic; functionalized with cell adhesive sequences | 1.3–4.0 kPa (PEG); Elastic | Organoid culture and differentiation | Chemically defined; xeno-free; support long-term culture and hepatogenic differentiation; stiffness can be tuned to support optimal organoid growth | Elastic (strategies to produce viscoelastic and viscoplastic hydrogels are available); requires modification to introduce cell-adhesive ligands | [165,166] |
| 270 Pa–1.4 kPa (pNIPAAm-co-PEG-N3/dPG-BCN) Viscoelastic | Organoid culture and differentiation | Hydrogel design allows to control hepatoblasts differentiation; stiffness can be tuned to support optimal organoid growth | Requires modification to introduce cell-adhesive ligands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marfoglia, A.; Sorrentino, G. Next-Generation Hydrogels for Biliary Organoid Engineering. Pharmaceuticals 2025, 18, 1781. https://doi.org/10.3390/ph18121781
Marfoglia A, Sorrentino G. Next-Generation Hydrogels for Biliary Organoid Engineering. Pharmaceuticals. 2025; 18(12):1781. https://doi.org/10.3390/ph18121781
Chicago/Turabian StyleMarfoglia, Andrea, and Giovanni Sorrentino. 2025. "Next-Generation Hydrogels for Biliary Organoid Engineering" Pharmaceuticals 18, no. 12: 1781. https://doi.org/10.3390/ph18121781
APA StyleMarfoglia, A., & Sorrentino, G. (2025). Next-Generation Hydrogels for Biliary Organoid Engineering. Pharmaceuticals, 18(12), 1781. https://doi.org/10.3390/ph18121781







