Abstract
Garlic (Allium sativum L.) has served as a food source and medicinal agent for over thousands of years. Bioactive constituents, including allicin, diallyl sulfide/disulfide/trisulfide, ajoene, and S-allyl-cysteine, demonstrate antioxidant, anti-inflammatory, antithrombotic, antineoplastic, antimicrobial and neuroprotective properties. Convergent mechanistic evidence suggests the modulation of redox homeostasis, attenuation of pro-inflammatory signaling, regulation of platelet activation, and induction of apoptosis and cell-cycle arrest in tumor models. Computational studies, in conjunction with wet-lab data, offer molecular-level insights and guide candidate prioritization. Density functional theory elucidates radical-scavenging pathways and electronic descriptors that account for redox activity. Structure-based methods, including docking, molecular dynamics, and MM-GBSA, elucidate potential interactions between organosulfur scaffolds and enzymes or receptors pertinent to pharmacological effects. In silico ADME/Tox platforms predict generally favorable oral absorption for hydrophobic allyl sulfides, while polar derivatives exhibit more limited brain penetration. Emerging AI/ML pipelines combine network pharmacology with QSAR to focus on important targets and chemical types, while also spotting potential development. Formulation strategies, including nanoencapsulation and controlled-release systems, are utilized to stabilize labile thiosulfinates and modulate hydrogen-sulfide-releasing profiles, with potential applications in various disease conditions. Significant challenges encompass the standardization of preparations, variability in pharmacokinetics, heterogeneity in dose–response relationships, and interactions between drugs and nutrients or other drugs. The integration of mechanistic, computational, and formulation insights delineates a systematic approach to progress garlic-derived agents from diverse natural products to reproducible, mechanism-guided pharmaceuticals.
1. Introduction
Garlic (Allium sativum L.) is one of the oldest medicinal plants known to humans and has served as both a culinary essential and a therapeutic agent, spanning many civilizations, from ancient Egypt to modern pharmaceutical laboratories. The bioactivity of garlic is primarily attributed to various organosulfur compounds (OSCs), such as reactive thiosulfinates like allicin, lipid-soluble allyl sulfides and polysulfides (e.g., diallyl sulfide, diallyl disulfide, diallyl trisulfide), and water-soluble derivatives present in aged garlic extract (AGE), notably S-allyl-L-cysteine (SAC) and S-allyl-mercaptocysteine (SAMC). These compounds exhibit antimicrobial, antihypertensive, antithrombotic, anticancer, antidiabetic, and neuroprotective properties [1,2,3,4].
The modern pharmaceutical interest in garlic increased significantly after Cavallito and Bailey identified and isolated allicin (diallyl thiosulfinate) in 1944. They demonstrated the potent antimicrobial properties of this OSC and established the initial structure-activity relationships for bioactive constituents [5]. Subsequent decades of research have revealed several OSCs (Figure 1), each contributing unique therapeutic properties through sophisticated biochemical pathways involving enzyme inhibition, gene expression modulation, and cellular signaling pathway regulation [6,7,8,9].
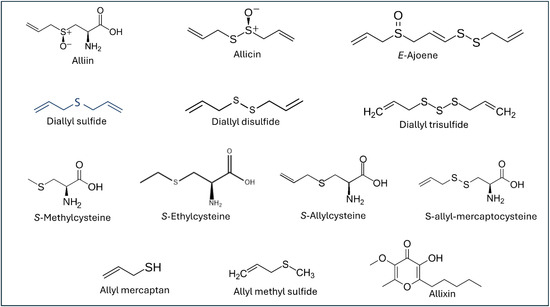
Figure 1.
Chemical structures of the organosulfur compounds of garlic.
Clinical and experimental findings demonstrate consistency for standardized AGE preparations, utilizing water-soluble compounds like SAC, providing enhanced stability and predictable effects. A recent meta-analysis of randomized trials involving hypertensive patients reveals modest yet significant reductions in blood pressure, especially at elevated daily doses [10,11]. Moreover, several studies indicates that garlic may contribute to the prevention and adjunctive management of chronic diseases, such as dyslipidemia, numerous cancers, type 2 diabetes, and infections [12,13,14,15,16,17,18,19]. However, the efficacy is significantly influenced by the processing and delivery methods of garlic. Variations such as raw cloves, dehydrated powders, oils, AGE, and black garlic exhibit distinct chemical profiles, compound stability, and bioavailability, resulting in differing clinical outcomes and tolerability [20,21,22,23,24].
Computational studies increasingly improve wet-lab research by clarifying reactivity, target engagement, and polypharmacology. Density-functional theory has clarified the formation pathways and electrophilicity of allicin [25]. Molecular docking, dynamics, and network pharmacology have identified potential target networks in cardiometabolic, neurodegenerative, infectious, and oncologic contexts, supporting hypotheses related to the thiol-reactive chemistry and H2S signaling of allyl polysulfides [26]. In silico frameworks facilitate the prioritization of scaffolds and support experimental design, addressing the chemical lability and promiscuity associated with OSCs.
The significance of formulation science arises from the fact that the reactivity that facilitates bioactivity simultaneously undermines self-life and systemic dissemination. Encapsulation methods, including cyclodextrin inclusion complexes for odor masking and thermal stability enhancement, nanoemulsions for improved dispersion and antibiofilm efficacy, and liposomal systems for increased solubility and protection of volatile essential-oil polysulfides, are progressing [27,28,29,30,31]. Food and pharmaceutical-grade platforms are starting to align on viable delivery solutions. The efficacy of these technologies will likely influence the translation of promising mechanistic effects into consistent in vivo outcomes [32].
Even with a wealth of reviews, an integrated, current synthesis is required due to the increasing volume of primary research on garlic OSCs and the relative lack of coverage in computational studies and formulation science [11,33,34,35,36,37,38,39,40,41,42,43,44,45]. By unifying advances in clinical efficacy, safety, and translational strategy, we present a coherent framework that reflects the evolving literature and supports the evidence-based development of garlic-derived therapeutics.
2. Pharmacological Activities
2.1. Cardiovascular Health
2.1.1. Blood Pressure Regulation
The role of garlic in regulating blood pressure is multifaceted, involving direct vascular effects, hormonal modulation, and alterations in endothelial function [46]. Evidence from randomized clinical trials and animal models indicates that its bioactive spectrum affects multiple pathways involved in hypertension management [47]. A fundamental mechanism includes the enhanced synthesis of nitric oxide (NO), a vasodilatory molecule produced by endothelial nitric oxide synthase (eNOS). OSCs, including allicin and derivatives from aged garlic extract, can increase NO availability by either upregulating eNOS expression or safeguarding NO from oxidative degradation [8]. Increased vasodilation leads to a decrease in peripheral resistance, resulting in lower systolic blood pressure (SBP) and diastolic blood pressure. In addition to its role in NO-related dilation, garlic influences the renin-angiotensin system by inhibiting angiotensin-converting enzyme (ACE). In vitro studies indicate that γ-glutamyl cysteines present in garlic inhibit ACE activity; however, the extent to which these compounds survive digestion and reach target tissues in an intact form is uncertain, and thus their contribution to in vivo ACE inhibition is yet to be elucidated [48,49]. Inhibiting the conversion of angiotensin I to angiotensin II, a strong vasoconstrictor, leads to a reduction in arterial pressure and a decrease in aldosterone-mediated fluid retention. Preclinical in vivo studies in hypertensive rat models indicate that combining alliin with pharmaceutical ACE inhibitors such as captopril enhances antihypertensive effects compared with monotherapy, without evidence of possible toxicity in these models; however, these findings derive from controlled animal experiments, and dedicated toxicological and clinical studies are still required to establish the safety of such combinations in humans [8].
Experimental findings demonstrate that hydrogen sulfide (H2S), produced from the metabolism of garlic polysulfides, enhances vascular relaxation through the hyperpolarization of smooth muscle [17,50]. H2S functions in conjunction with NO, providing dual gaseous signaling mechanisms that contribute to the maintenance of reduced blood pressure. Variants in enzymes such as cystathionine γ-lyase or deficiencies in cofactors like low vitamin B12 may influence individual responses to garlic interventions. Populations with sub-optimal vitamin B12 levels may show reduced H2S production from garlic consumption, which could account for the variability observed in clinical outcomes.
Meta-analyses indicate varying statistical significance for reductions in systolic and diastolic blood pressure, influenced by study design and dosage form. Pooled analyses indicate significant reductions in hypertensive subgroups, whereas other studies report non-significant changes compared to placebo [51]. This inconsistency may arise from differences in preparation methods: enzymatically processed garlic or aged extracts appear to be more effective than raw powder in specific protocols, likely due to optimized concentrations of stable OSCs such as S-allylcysteine, which offer sustained actions targeting the endothelium.
Increased aortic stiffness raises SBP and pulse pressure by elevating ventricular afterload; garlic’s prostaglandin-like properties may mitigate this stiffness through a reduction in peripheral resistance [48,52]. Decreased vascular tension correlates with enhanced subendocardial perfusion, an essential element in mitigating ischemic injury in hypertensive conditions. Animal research provides mechanistic insights: extracts from related Allium species, such as A. hookeri, have demonstrated antihypertensive activity via simultaneous ACE inhibition and increased NO generation [53]. The data indicate that structural similarities among OSCs across species provide consistent bioactivity pertinent to human applications. Compounds such as S-allyl cysteine (SAC) derivatives influence endothelial function by modulating cyclic GMP pathways downstream of NO signaling, thereby extending their role beyond mere vasodilation to a more precise regulation of vascular tone. In addition, garlic-derived proteins, including methionine–glycine–arginine (MGR) tripeptide, histidine–aspartate–cysteine–phenylalanine (HDCF) tetrapeptide, garlic protein hydrolyzed product by pepsin (GPHP-P), and garlic protein hydrolyzed product by trypsin (GPHP-T), have shown a beneficial impact on cardiovascular system through their ACE inhibitory activity and protective effects against endothelial dysfunction [54]. Moreover, bradykinin, a potent vasodilatory peptide, contributes to cardiovascular health by activating eNOS, which increases NO production, and by enhancing the production of the platelet-aggregation-inhibitory prostaglandin, prostacyclin, through B2 receptor activity. Consequently, enhancing bradykinin activity through the inhibition of its degradation may be essential for promoting beneficial cardiovascular functionality. Garlic-derived proteins and their pepsin- and trypsin-hydrolysates, including GPHP-P and GPHP-T, exhibit antioxidant and ACE-inhibitory activity while maintaining bradykinin activity in hypertensive rats [55].
The variety of mechanisms, (Figure 2) including NO and H2S-mediated dilation, ACE inhibition, enhancement of aortic elasticity, and synergy with current pharmacotherapy, establishes garlic as a comprehensive approach to managing elevated blood pressure [8,17]. Biochemical individuality, influenced by genetic variation and nutrient status, suggests that a universal approach is improbable; personalized strategies informed by clinical profiling may enhance benefits and reduce risks. Future directions may involve computational modeling of organosulfur structure-activity relationships related to specific hypertensive phenotypes and the refinement of extraction technologies to achieve a balance between potency and tolerability.
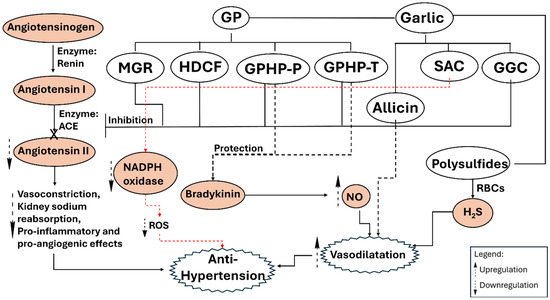
Figure 2.
Garlic constituents protect the cardiovascular system through antihypertensive mechanisms, including ACE inhibition, downregulation of NADPH oxidase, and NO/H2S-driven vasodilation. GGC, gamma-glutamyl cysteine; GP, garlic peptides; GPHP-P, Garlic protein hydrolyzed product by pepsin; GPHP-T, Garlic protein hydrolyzed product by trypsin; HDCF, Histidine–Aspartate–Cysteine–Phenylalanine tetrapeptide; MGR, Methionine–Glycine–Arginine tripeptide; NO, nitric oxide; ROS, Reactive oxygen species; SAC, S-allyl-cysteine.
2.1.2. Lipid Profile Improvement
Compounds derived from garlic have gained significant attention in the context of cardiovascular diseases, especially concerning their ability to alter blood lipid profiles. The clinical evidence includes various study designs and garlic preparations, offering insights into the lipid-modulating effects of this commonly used botanical supplement. The lipid-lowering effects of garlic are largely attributed to OSCs that affect hepatic lipid metabolism by inhibiting key enzymes involved in cholesterol and fatty acid biosynthesis, such as HMG-CoA reductase, fatty acid synthase, and acetyl-CoA carboxylase (Figure 3). Furthermore, these compounds may increase the fecal excretion of bile acids and neutral sterols [56,57,58,59,60].
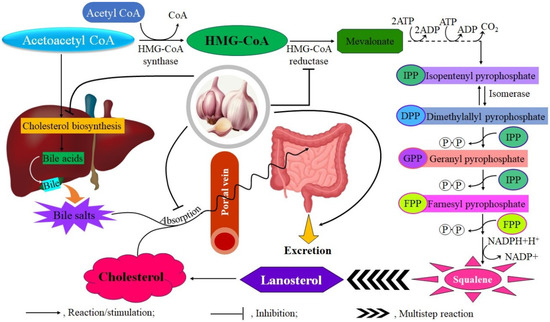
Figure 3.
Garlic offers cardiovascular benefits by inhibiting HMG-CoA reductase, which reduces liver cholesterol biosynthesis, decreasing intestinal absorption, and enhancing elimination.
The clinical research base predominantly comprises controlled trials utilizing diverse garlic formulations. Numerous studies investigated various garlic preparations, such as dried garlic powder tablets, aged garlic extract, steam-distilled garlic oil, and different standardized extracts. Treatment durations varied from 12 weeks to 10 months, with sample sizes ranging from 23 to 115 participants. The evidence indicates generally positive effects on total cholesterol across various preparations. Quantitative reductions varied between 5.7% and 11.5% in studies that offered specific effect size estimates. Sobenin et al. observed an 11.5% reduction compared to placebo with the administration of time-released garlic powder tablets over a 12-week period [61]. Jain et al. reported a 5.7% reduction with the use of standardized garlic powder tablets [62]. A systematic review by Ackermann et al. indicated reductions of 0.03–0.45 mmol/L at 1 month and 0.32–0.66 mmol/L at 3 months [63]. Nonetheless, not all research indicated significant effects, as Neil et al. reported no notable difference over a 6-month period [64], and Berthold et al. observed non-significant outcomes with steam-distilled garlic oil [65].
The impact on low-density lipoprotein (LDL) cholesterol is noteworthy, as multiple studies indicate substantial reductions. Quantitative studies reported LDL reductions of 11% and between 11.8% and 13.8%. Steiner et al. observed a decrease ranging from 4% to 4.6% [66]. Further research indicated notable reductions in LDL levels, though specific numerical values were not provided. In contrast, high-density lipoprotein (HDL) cholesterol exhibited increases in various studies, with Sobenin et al. documenting an 11.5% rise and several other investigations noting significant increases without providing quantitative specifics [61].
Responses of triacylglycerol demonstrated variability across studies. Turner et al. reported a 12% reduction [67]. Numerous studies reported notable reductions but failed to present quantitative effect sizes, whereas Berthold et al. observed non-significant changes. This variability indicates that triacylglycerol responses may be less predictable or necessitate specific conditions to exhibit clinically significant changes [65].
Preparation-specific analyses indicate notable variations in efficacy profiles. Multiple studies utilizing garlic powder tablets indicated modest reductions in total and LDL cholesterol, along with some evidence of increases in HDL levels [67]. The time-released formulation investigated by Sobenin et al. is particularly noteworthy, demonstrating significant improvements in all three major lipid fractions. Standardized extracts, such as aged garlic extract and ethyl acetate preparations, demonstrated reductions in lipid parameters, though effect sizes remained generally modest and reporting consistency varied.
2.1.3. Antithrombotic Effects
Garlic exhibits anti-thrombotic properties that serve as an important adjunct to its lipid-lowering and blood pressure-modulating effects, contributing to a comprehensive strategy for reducing cardiovascular risk. The plant’s ability to modulate platelet activation and aggregation is fundamental to its antithrombotic actions, as these processes are closely associated with thrombus formation and subsequent cardiovascular events, including myocardial infarction and ischemic stroke. Bioactive OSCs, specifically allicin, ajoene, and various diallyl polysulfides, have shown the ability to interfere with platelet aggregation pathways by disrupting the binding of fibrinogen to glycoprotein IIb/IIIa receptors on platelet surfaces [68,69]. This disruption diminishes platelet cross-linking in developing thrombi, consequently decreasing the risk of vessel occlusion. Ajoene, a compound derived from allicin and stable in specific processed forms of garlic, inhibits platelet aggregation by irreversibly modifying fibrinogen receptors and blocking ADP-induced activation [70].
In vivo evidence supports these biochemical findings. A double-blind, placebo-controlled trial assessed the effects of time-released garlic powder supplementation in patients with coronary heart disease over a 12-month period. The results indicated significant reductions in the calculated 10-year prognostic risk for acute myocardial infarction and sudden death, with a factor of 1.5 in men and 1.3 in women. These outcomes are likely associated with improvements in lipid profiles as well as reduced thrombotic tendencies [6,71]. Parallel reductions in platelet aggregation indices indicate a mechanistic relationship between garlic bioactives and decreased thrombus formation with chronic dietary inclusion. Platelets exhibit sensitivity to oxidative modifications that affect their reactivity threshold at the molecular level. The antioxidant properties of garlic, especially through SAC and phenolic compounds, reduce oxidative stress in circulating platelets by maintaining membrane fluidity and receptor functionality against ROS-induced changes [72]. The decrease in oxidative priming corresponds with diminished responsiveness to pro-aggregatory stimuli, including thromboxane A2 (TXA2). Additionally, allicin inhibits cyclooxygenase activity, thereby reducing TXA2 synthesis and limiting a crucial autocrine signal that facilitates platelet recruitment [73,74]. Moreover, garlic consumption has been linked to increased NO bioavailability, potentially enhancing platelet cGMP levels and reducing platelet adhesion to the endothelium [75]. Elevated cGMP levels result in decreased intracellular calcium concentration, which is essential for actin polymerization during the platelet shape change that occurs prior to aggregation [76]. Garlic offers a dual anti-thrombotic effect through chemical interference with receptor-mediated activation and modulation of endothelial-derived signals that promote an anti-clotting environment (Figure 4).
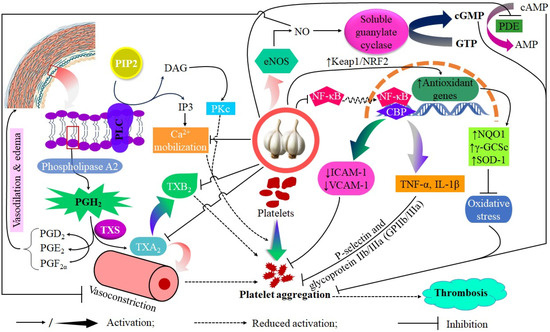
Figure 4.
The proposed antiplatelet mechanisms of garlic include the inhibition of phospholipase A2 (PLA2), modulation of thromboxane A2 (TXA2) signaling resulting in reduced intracellular Ca2+ release and decreased TXB2 formation, attenuation of ADP- and collagen-induced platelet activation, diminished Ca2+ mobilization, and increased nitric oxide (NO) bioavailability.
Studies utilizing animal models provide additional detail; ethanolic extracts of Allium sativum inhibit thrombus formation while preserving physiological hemostasis [70]. Ajoene and diallyl trisulfide demonstrate dose-dependent attenuation profiles, with the former resulting in prolonged inhibition likely due to irreversible enzyme modification in activated platelets. The release of hydrogen sulfide (H2S) mediated by DATS may also provide vasodilatory support, which indirectly diminishes platelet activation induced by shear stress in stenosed vessels. Processing significantly influences potency. Raw garlic, high in allicin, provides immediate but temporary inhibition due to its instability. In contrast, aged garlic extracts depend on stable thiol derivatives such as SAC, which, while appearing less effective against primary aggregation, can have long-term modulatory effects through endothelial conditioning and modification of the oxidative environment [24]. Furthermore, black garlic preparations maintain certain anti-thrombotic properties; however, the mechanisms may transition to phenolic-mediated antioxidant suppression of pro-aggregatory reactive oxygen species rather than direct receptor blockade [68,77,78,79]. These shifts may provide more effective long-term prevention strategies with lower bleeding risk profiles than the consumption of raw garlic in bolus form.
The dosing strategy determines whether antithrombotic responses remain within preventive limits or progress to clinically significant anticoagulation. Low to moderate intakes are likely to confer vascular protection with minimal hemorrhagic risk, whereas higher doses or concentrated supplements may enter pharmacological levels, requiring monitoring similar to that of conventional antithrombotics. Tailored strategies that account for initial clotting status, co-medication profiles, and specific cardiovascular outcomes may enhance this equilibrium.
2.2. Cancer Prevention
Garlic has been identified as a potential natural agent for cancer prevention and treatment. Epidemiological studies often indicate a negative correlation between increased garlic consumption and the occurrence of various cancers [80,81,82,83]. The effects are primarily attributed to the organosulfur constituents of garlic, including allicin, DADS, DATS, SAC, and ajoene, which modulate essential oncogenic pathways. Proposed mechanisms encompass the induction of apoptosis, enforcement of cell-cycle arrest, inhibition of angiogenesis, and suppression of metastatic dissemination [84,85,86]. Moreover, the administration of OSCs in experimental animals decreases lipid peroxidation, suggesting a reduction in oxidative injury [87]. This process simultaneously increases tissue glutathione (GSH) levels and activates stress-responsive MAPK signaling pathways, specifically p38 and JNK [88,89]. Simultaneously, it downregulates pro-survival pathways, as indicated by decreased phosphorylated Akt (p-Akt) levels and reduced Bcl-2 expression [90,91]. These effects collectively enhance apoptotic competence and inhibit tumor initiation and progression in vivo. Figure 5 depicts the mechanistic framework of OSCs in cells together with outcomes from animal models that substantiate these pathways.
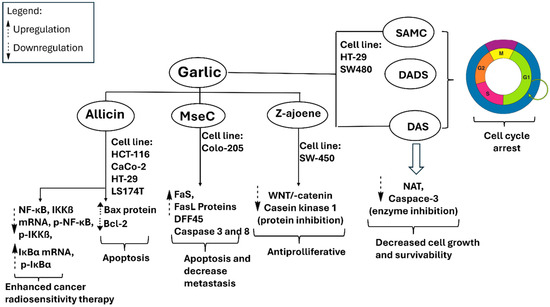
Figure 5.
Metabolites derived from garlic have been evaluated in various cancer cell lines, demonstrating the ability to upregulate Bax and Fas/FasL while downregulating Wnt/β-catenin signaling and casein kinase 1α. Additionally, they inhibit NAT and caspase-3. These effects collectively inhibit cancer cells by enhancing apoptosis, restricting proliferation, diminishing cellular resistance, and augmenting radiosensitivity. DAS, diallyl sulfide; DADS, diallyl disulfide; SAMC, S-allyl-mercapto cysteine; MseC, methyl-L-selenocysteine.
2.2.1. Breast Cancer
Garlic extracts exhibit significant growth-inhibitory effects on breast cancer cells via various cellular pathways. Secondary metabolites, such as allicin, DATS, DADS, and DAS, exhibit a significant inhibitory effect on the proliferation of breast cancer cells, including human MCF-7. Except for allicin, these compounds inhibit cell division at the G0/M phases of the cell cycle, while allicin decreases glutathione (GSH) production [92]. Allicin demonstrates significant activity against the HCC-70 breast cancer cell line in vitro; however, it also shows toxicity to normal cells at elevated concentrations. Allicin functions by downregulating anti-apoptotic proteins such as Bcl-xL and upregulating pro-apoptotic proteins including p21, Bak, and Noxa. This process activates caspase-3 and caspase-8, resulting in apoptosis [93]. Furthermore, DADS enhances apoptotic signaling by elevating PARP cleavage and caspase-3 activity, while simultaneously decreasing TNF-α, a pro-inflammatory cytokine and breast cancer marker, via ERK/MAPK pathways. DATS enhances Ser10 phosphorylation of histone H3 in vivo, a mechanism linked to reduced proliferation of breast cancer cells [94]. DATS enhances AP-1 DNA-binding activity at the transcriptional level [95,96]. AP-1 regulates genes that control cancer cell behaviors such as proliferation, metastasis, and drug resistance [97]. Figure 6 illustrates the anticipated anticancer mechanisms associated with garlic.
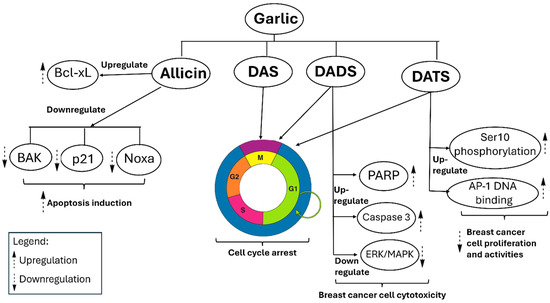
Figure 6.
Proposed mechanisms of garlic compounds against breast cancer cells: induction of apoptosis, cell-cycle arrest, cytotoxicity, and suppression of proliferation.
2.2.2. Colorectal Carcinoma
Laboratory studies consistently show significant anticolorectal cancer effects of garlic compounds. Aged garlic extract markedly reduces colon carcinogenesis induced by 1,2-dimethylhydrazine in animal models, leading to decreased adenoma formation and suppression of dysplastic lesion progression. The mechanism entails a delay in the cell cycle at the G2/M phase via the downregulation of cyclin B1 and CDK1, rather than through direct induction of apoptosis or cell cycle arrest. Compounds in garlic exhibit protective effects against the formation of aberrant crypt foci and demonstrate greater efficacy in preventing high-grade dysplastic adenomas relative to mild or moderate dysplasia [98].
Fleischauer et al. provided a thorough summary of data regarding garlic-derived compounds, indicating significant reductions in colorectal cancer risk factors [99]. Figure 7 illustrates the proposed mechanisms through which garlic constituents inhibit colorectal carcinoma. Bat-Chen and colleagues assessed the effects of allicin on various colorectal cancer cell lines and determined that it promotes apoptosis by increasing Bax levels and enabling cytochrome c release into the cytoplasm while simultaneously reducing B-cell lymphoma-2 (Bcl-2) expression [100]. The simultaneous elevation of Bax and reduction in Bcl-2 serves as a well-established marker of apoptotic activity in colorectal cancer cells [101]. Huang et al. demonstrated that the combination of allicin and X-ray radiotherapy enhances the radiosensitivity of HCT-116 cells by downregulating NF-κB, IKKβ mRNA, p-NF-κB, and p-IKKβ, while upregulating IκBα mRNA and p-IκBα [102]. The modulation of the NF-κB pathway holds clinical significance, as its activity is associated with therapeutic resistance [103].
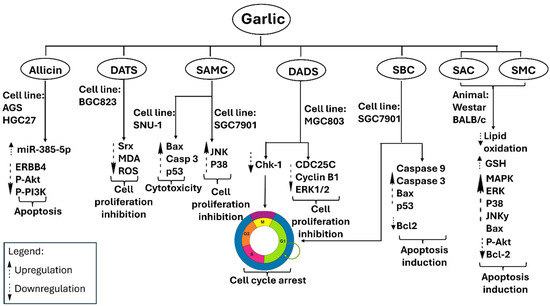
Figure 7.
Metabolites derived from garlic inhibit cancer cells through mechanisms such as apoptosis, antiproliferative activity, cytotoxicity, and cell-cycle arrest, as demonstrated by multiparametric studies.
Z-ajoene exhibits significant antiproliferative effects in SW480 colon cancer cells by inhibiting Ser45 phosphorylation of β-catenin, a Wnt pathway effector modulated by casein kinase 1α (CK1α), and by decreasing the expression of c-Myc and cyclin B1. SAMC, DADS, and DAS exhibit significant antiproliferative activity. SAMC and DADS promote apoptosis in human colon cancer cells, such as HT-29 and SW480, by halting the cell cycle at the G2/M phase and enhancing caspase-3 activity [104]. In contrast, DAS demonstrates anticancer properties through the inhibition of arylamine N-acetyltransferase (NAT). NAT plays a role in carcinogenesis by activating procarcinogens and influencing tumor cell growth and survival [105].
Epidemiological research on colorectal cancer reveals discrepancies between findings from case–control studies and prospective cohort studies. Meta-analyses of case–control studies indicate a risk reduction linked to high garlic consumption. In contrast, prospective cohort studies reveal no significant association [106,107]. A recent meta-analysis concentrating solely on high-quality prospective studies revealed no significant protective association and unexpectedly indicated an increase in risk, although this finding was not statistically significant. The observed discrepancies may indicate variations in garlic preparation methods, consumption patterns, or methodological differences across study designs [108].
2.2.3. Pancreatic Carcinoma
OSCs have been investigated for their anticancer properties concerning pancreatic ductal adenocarcinoma (PDAC) [109]. Evidence from epidemiological studies, preclinical models of PDAC, and the biology of infection and inflammation indicates that garlic and its derivatives operate through several mechanisms: (i) direct modulation of apoptosis, cell-cycle regulation, and oncogenic signaling in PDAC cells; (ii) enhancement of chemosensitivity and reduction in toxicity; and (iii) inhibition of tumor-associated microbes and periodontal inflammation, which are mechanistically linked to the initiation and progression of PDAC [110]. Observational studies indicate that increased consumption of garlic and onions is associated with reduced risk of pancreatic cancer [111]. A small, randomized trial involving patients with advanced gastrointestinal cancers, including pancreatic cancer, demonstrated that AGE supplementation preserved both the number and activity of natural killer cells [112]. However, comprehensive clinical trials focused on PDAC are necessary to determine effective formulations, dosing, pharmacokinetics, and safety, and to evaluate the impact of microbiome-targeted advantages on survival outcomes [44].
2.2.4. Lung Cancer
Consumption of raw garlic exhibits significant protective effects against lung cancer, as evidenced by population-based studies. Case–control studies across various Chinese populations indicate a consistent risk reduction of 44–50% in individuals who consume raw garlic two or more times weekly compared to non-consumers. A comprehensive study involving 1424 lung cancer cases and 4543 controls demonstrated a dose-dependent inverse relationship, with the highest consumption category exhibiting an adjusted odds ratio of 0.56. The protective effect is significant across various demographic subgroups and seems to be independent of smoking status, indicating that garlic may provide protection to both smokers and non-smokers [113,114].
The lung-protective effects of garlic may be attributed to the pulmonary excretion of volatile OSCs, which may offer direct protective contact with lung tissue. In vitro studies indicate that garlic OSCs significantly inhibit the proliferation of lung cancer cell lines, such as A-549 and Calu-1, via various mechanisms, including cell cycle arrest, induction of apoptosis, and inhibition of angiogenesis. Recent studies have identified garlic compounds as potential agents for reversing drug resistance in non-small cell lung cancer, with allicin showing the capability to overcome Taxol resistance via mechanisms of cell cycle regulation [113,114,115].
2.3. Antimicrobial Activity
OSCs derived from garlic demonstrate extensive antimicrobial properties. Allicin exhibits bactericidal and fungicidal properties against both Gram-positive and Gram-negative bacteria, as well as Candida spp. Its primary mechanism of action involves S-thioallylation of cysteine residues in glutathione and various protein targets, such as thioredoxin reductase, alcohol dehydrogenase, and RNA polymerase, which disrupts redox homeostasis and essential metabolic processes [7,116]. Both in vitro and in vivo studies further elucidate these findings. Allicin and DATS demonstrate inhibitory effects on Helicobacter pylori, whereas diallyl sulfides exhibit suppression of methicillin-resistant Staphylococcus aureus (MRSA) in both models and clinical isolates. The potency of these compounds typically increases with the length of the sulfur chain, with the following order: diallyl tetrasulfide > DATS > DADS > DAS [117,118,119]. In addition to direct lethality, garlic components reduce pathogenicity: the vinyl disulfide ajoene acts as a quorum-sensing inhibitor in Pseudomonas aeruginosa, leading to the downregulation of virulence genes and disruption of biofilms; allicin exhibits antibiofilm properties and may enhance the efficacy of conventional treatments in certain scenarios [120,121]. Allicin inhibits bacterial DNA gyrase and, in fungi, enhances oxidative damage when used with amphotericin B, demonstrating complementary pathways for potential therapeutic applications [121,122]. The precursor alliin and stable aged-garlic components like SAC provide antioxidant and immunomodulatory effects, whereas the majority of antimicrobial activity is due to allicin and the more hydrophobic allyl sulfides (DAS, DADS, DATS, and higher polysulfides) [123,124]. Allicin’s volatility facilitates gas-phase activity that influences respiratory pathogens; however, its chemical lability necessitates delivery strategies that produce or stabilize allicin at sites of infection [125,126]. Evidence indicates that allicin is the principal antimicrobial component in garlic, while DADS, DAS, and DATS offer additional bactericidal, antibiofilm, and anti-quorum-sensing properties that may function additively or synergistically with conventional antimicrobials against key pathogens [40].
2.4. Metabolic Disorders
OSCs derived from garlic influence key pathophysiological pathways associated with metabolic disorders, such as insulin resistance, dyslipidemia, hepatic steatosis, and mild to moderate inflammation. Clinical evidence indicates that garlic supplementation enhances glycemic control and lipid profiles in individuals with type 2 diabetes. Additionally, trials involving nonalcoholic fatty liver disease (NAFLD/MASLD) demonstrate reductions in steatosis, improvements in liver enzymes, and beneficial improvements in body mass index following garlic-powder treatments [127,128,129,130]. Allicin enhances glucose uptake by activating AMPK through cysteine persulfidation signaling and promotes thermogenic remodeling (beiging) of adipose tissue, indicating increased energy expenditure as a complementary mechanism [131]. Aged garlic extract increases adiponectin levels in humans and enhances vascular-metabolic parameters, supporting the critical function of adiponectin in insulin sensitivity. DATS enhances glycemic control in diabetic models and inhibits adipogenesis in 3T3-L1 cells by modulating lipogenesis and fatty-acid metabolism [132]. In contrast, DADS exhibits variable effects, reducing insulin resistance and hepatic fat in obese mice in vivo, while promoting adipogenesis under certain in vitro conditions, underscoring the importance of dosage, exposure, and cellular state [133,134,135]. Garlic constituents such as alliin, S-allyl cysteine, and SAMC reduce inflammation, modulate insulin-receptor/AMPK signaling, and affect gut microbiota. These effects are combined on the AMPK–SREBP-1c and adipokine pathways, which are associated with enhanced insulin sensitivity and lipid homeostasis [136,137,138,139,140]. Human trials and mechanistic studies indicate that allicin and allyl sulfides, enhanced by aged-garlic constituents, provide multifactorial benefits for metabolic syndrome components, NAFLD, and insulin resistance. However, there is notable heterogeneity in preparations, doses, and endpoints [141].
2.5. Neuroprotection
Garlic-derived OSCs demonstrate neuroprotective effects in both acute and chronic neurological injury models. These effects are mediated through the modulation of oxidative stress, neuroinflammation, proteostasis, and mitochondrial resilience. SAC consistently provides protection against ischemic and neurodegenerative insults among water-soluble constituents. It activates Nrf2-dependent antioxidant defenses, mitigates endoplasmic reticulum stress, reduces infarct burden and neurological deficits following experimental stroke, and interferes with amyloid-β aggregation while enhancing learning and memory in rodent models of Alzheimer’s disease [142,143,144].
Lipophilic allyl polysulfides exhibit significant anti-neuroinflammatory properties in microglia. In LPS-activated BV2 cells, DATS and DADS inhibit nitric oxide and pro-inflammatory cytokines through NF-κB suppression, with DATS demonstrating the highest activity. In vivo, DATS mitigates neuroinflammation and enhances recovery following traumatic brain injury via PGK1/Nrf2 signaling [145,146]. Allicin exhibits neuroprotective effects in cerebral ischemia–reperfusion, as well as in traumatic and hemorrhagic brain injuries, by reducing oxidative damage, inhibiting TLR4–NF-κB/MAPK pathways, maintaining blood–brain barrier integrity, and enhancing functional recovery [147,148,149]. Recent findings indicate that alliin, the stable precursor of allicin, may mitigate neuronal injury in preclinical models; however, its efficacy in microglial inflammation models appears to be less pronounced compared to DATS/DADS [146,150]. Aged garlic extract, which is rich in SAC and related hydrophiles, has been shown to reduce Aβ-induced cognitive impairment and neuroinflammation in rodent models, as well as reverse significant endotoxin-induced transcriptomic alterations in microglia [151,152]. Furthermore, the neuroprotective profile of allyl polysulfides, particularly DATS, is enhanced by their ability to donate hydrogen sulfide (H2S). This gasotransmitter activates Nrf2/HO-1, reduces microglial activation, and enhances mitochondrial function in models of neurodegeneration [153].
Garlic has been extensively investigated in pharmacological research, mostly due to its bioactive OSCs. A summary table of preclinical data is presented below, covering in vitro, in vivo, and in silico research, listing the main molecular targets and mechanisms, the model systems used, and notable results, together with relevant references (Table 1).

Table 1.
An overview of the molecular targets and mechanisms of action of garlic organosulfur compounds, and the key findings from relevant studies.
3. Formulations of Organosulfur Compounds
Several formulations of OSCs are aimed at enhancing aqueous dispersion and solubility, providing protection against hydrolysis, volatilization, and oxidation, reducing odor, and improving bioavailability and tissue targeting.
3.1. Nanoemulsions and High Internal Phase Emulsions (HIPEs)
Nanoemulsions significantly enhance the dispersion, stability, and pharmacological efficacy of highly volatile or chemically labile garlic actives by protecting against premature decomposition [191]. Recent studies demonstrate that allicin nanoemulsions work synergistically with ε-polylysine to inhibit both planktonic and biofilm forms of E. coli, exhibiting a low fractional inhibitory concentration (FIC) index [27]. Additionally, these nanoemulsions modulate cell surface charge, indicating that nanodroplets enhance agent-cell contact and efficacy. DADS has been nanoemulsified using soy-protein emulsifiers, resulting in effective droplet formation, enhanced physicochemical stability, and suitability for complex food systems [192]. HIPEs extend beyond conventional emulsions by physically entrapping allicin within a fiber-stabilized interface that resists coalescence and facilitates thermo-responsive release. This approach offers an alternative method for confining this thiosulfinate under conditions of thermal and oxidative stress [193]. Complementary studies indicate that garlic essential oil nanoemulsions, comprising a garlic-only mix of DAS/DADS/DATS/ajoene, exhibit broad-spectrum antibacterial and antibiofilm activity, along with enhanced kinetic stability compared to coarse dispersions. These findings endorse nanoemulsification as an effective formulation strategy for OSCs [194,195].
3.2. Liposomes
Phospholipid bilayers protect sulfur functionalities from hydrolysis and oxidation, facilitating sustained, cell-interactive delivery of specific garlic actives. A pivotal study on allicin nanoliposomes indicated the formation of approximately 145 nm vesicles exhibiting a negative ζ-potential of −40 mV and sustained in vitro release [196]. This study demonstrates the efficient entrapment and controlled release of a single, labile thiosulfinate. Recent studies indicate that DADS co-loaded with cisplatin into nanoliposomes enhances cytotoxicity compared to either free drug in breast and lung cancer cell lines [197], suggesting that allyl disulfides may function as synergistic payloads within clinically established vesicles. Furthermore, PEGylated liposomes containing DATS improved doxorubicin chemosensitization, demonstrating high entrapment efficiency and significant antitumor activity in a colorectal cancer model [198]. In addition, liposomal DADS enhanced in vitro activity and was investigated in conjunction with oxaliplatin [199]. Supporting evidence for liposomal cisplatin platforms and recent reviews of liposome technology highlight the stability, adjustable release, and membrane fusion benefits relevant to allicin/DADS/DATS formulations [200,201,202,203].
3.3. Solid Lipid Nanoparticles and Related Lipid Carriers
Solid lipid nanoparticles (SLNs) provide solid-state matrices that reduce diffusion, limit volatility, and protect reactive sulfur moieties. Allicin-loaded solid lipid nanoparticles (SLN) functionalized with folic acid and chitosan demonstrated high encapsulation efficiency, a size range of approximately 80–90 nm, and enhanced in vitro anticancer activity compared to free allicin. This indicates that lipid solids can effectively stabilize and deliver a labile thiosulfinate [204]. Folic-acid–decorated DATS-SLN effectively targeted triple-negative breast cancer cells, enhancing uptake and efficacy. This approach mitigated the issues of DATS hydrophobicity and short half-life while maintaining the integrity of the garlic-only payload [205]. Comprehensive evaluations of lipid nanoparticles in the delivery of natural products and cancer treatment highlight SLN/NLC platforms as viable methods for converting DADS/DATS/allicin into constructs that are either tumor-targeted or enhanced for bioavailability [206,207].
3.4. Polymeric Nanoparticles and pH-Responsive Matrices
Biopolymers enhance mucoadhesion and facilitate site-selective release of polar garlic compounds. SAC, a water-soluble amino acid derived from garlic, has been encapsulated in chitosan nanoparticles for intranasal administration. This formulation enhances brain bioavailability in ischemia models, demonstrating that both lipophilic allyl sulfides and ionic garlic compounds can benefit from nanoscale carriers [208]. The allicin precursor, alliin, is encapsulated in pH-responsive gel beads that protect against gastric acidity, facilitating prolonged intestinal release. This mechanism aligns the exposure window with the targeted intestinal biophase while minimizing acid-catalyzed decomposition [209]. Previous research on alliin/alliinase tablets and their release analytics supports these encapsulation strategies and highlights the formulation challenge of producing allicin in situ within physiological constraints [210].
3.5. Cyclodextrin Inclusion Complexes
The complexation of host and guest molecules with cyclodextrins diminishes odor, enhances apparent solubility, and stabilizes volatile sulfides. Allicin complexes with α- and β-cyclodextrin effectively mask odor and stabilize the thiosulfinate. A recent study on β-cyclodextrin achieved approximately 50% drug loading with around 86% entrapment, enhancing in vitro performance and addressing allicin’s instability through host-guest interactions [30,211]. A study conducted by Qian et al. focused on encapsulating DADS and garlic oil within β-cyclodextrin to reduce soil interactions and minimize oxidation, degradation, and evaporative loss of sulfur volatiles [212]. Numerous studies on garlic-oil/β-CD inclusion, involving mixtures of DAS, DADS, DATS, and ajoene, demonstrate enhanced chemical stability, water compatibility, and odor masking, thereby validating CDs as effective scalable carriers for garlic bioactive compounds [213,214].
3.6. Hydrogels, Films, and Nanofibers
Soft matrices effectively localize garlic actives at moist interfaces, facilitating controlled release and enhancing tissue interaction [215]. A hyaluronic-acid methacryloyl hydrogel that delivers allicin-enhanced multiterritory perforator flap survival in rats and activates PI3K/AKT and HO-1/NRF2 signaling pathways, exhibiting a sustained and biocompatible release of a volatile thiosulfinate under in vivo-like conditions [216]. In addition, a collagen hydrogel incorporating allicin-derived Ag nanoparticles utilized allicin from garlic as the exclusive organic active/capping agent, demonstrating antimicrobial and wound-healing properties [217]. Furthermore, electrospun core-sheath fibers infused with garlic extract demonstrate the viability of nanofibrous dressings that retain exclusive garlic composition [215].
3.7. Ethosomes and Niosomes
Ethanol-enriched ethosomes and nonionic-surfactant niosomes enhance stratum corneum penetration and decrease volatility. Garlic-oil ethosomes exhibit notable, broad-spectrum antibacterial activity against clinical isolates and enhanced skin distribution relative to conventional carriers [218]. Additional studies suggest antidermatophytic and wound-healing capabilities associated with the garlic formulation [219]. Prototype niosomes encapsulating allicin, prepared via thin-film hydration, illustrate the cost-effectiveness of vesicular systems for thiosulfinates. Additionally, extensive reviews on niosomes emphasize carrier characteristics relevant to allicin, DAS, DADS, DATS, and ajoene [220,221].
4. Computational Insights
Recent advancements in computational chemistry have significantly enhanced our understanding of garlic bioactive compounds, especially organosulfur molecules like allicin, DAS, DADS, and DATS. The combination of molecular docking, quantum mechanical calculations, quantitative structure-activity relationships, molecular dynamics simulations, and artificial intelligence methodologies has significantly enhanced our comprehension of garlic’s bioactive components and their molecular mechanisms of action [198,199]. The small size, high polarizability, and soft Lewis basicity render them suitable ligands for metallo-enzymes and redox-sensitive sites, prompting significant computational studies [222].
4.1. Molecular Docking
Molecular docking functions at the intersection of structural biology and computational chemistry, with the objective of mathematically predicting the fitting of a ligand into a protein’s active site and its binding affinity. This process fundamentally models the spatial and energetic compatibility between a small molecule, typically a bioactive compound like allicin, diallyl disulfide, or alliin, and a macromolecular target, which may include an enzyme, receptor, or transporter. Interactions are evaluated using scoring functions that estimate free energy changes resulting from hydrogen bonding, hydrophobic contacts, van der Waals forces, electrostatic complementarity, and variations in conformational entropy.
Molecular docking studies have identified various biological targets for garlic OSCs. Molecular docking using AutoDock Vina yielded predicted binding scores ranging from −3.7 to −8.3 kcal/mol across different protein families, such as EGFR mutants T790M/C797S [223], bacterial topoisomerase IV [224], and ferroptosis-related proteins [225]. In the estrogen receptor-α docking study [226], alliin demonstrated the most favorable AutoDock Vina binding score of −4.8 kcal/mol compared to the other tested ligands.
Alrumaihi et al. [198] utilized AutoDock Vina to assess the docking of diallyl trisulfide (DATS) in comparison to the reference drug doxorubicin (DOXO) across twelve potential anticancer targets. The docking results identified MMP-9 as the most favorable target for DATS, exhibiting a binding energy of −4.6 kcal/mol, while DOXO demonstrated a stronger binding affinity to the same protein at −8.9 kcal/mol. DATS exhibited significant affinities for CDK2 (−4.3 kcal/mol), Bcl-2 (−4.2 kcal/mol), and MMP-2 (−4.0 kcal/mol). In contrast, DOXO exhibited the strongest interaction with JAK2 (−9.6 kcal/mol), while DATS demonstrated only a moderate engagement with this protein (−3.5 kcal/mol). Figure 8 presents interaction maps for DATS with CDK2 and Bcl-2. DATS exclusively formed hydrophobic contacts with all macromolecular targets, whereas DOXO established both polar and non-polar interactions. The interacting residues for MMP-9 comprised L188, A189, W210, L222, V223, H226, E241, A242, L243, M244, Y245, P246, M247, Y248, R249, T251, P254, and P255 (Figure 9). DOXO, recognized as a DNA intercalator, exhibited moderate affinity for nucleic acid docking (−5.5 kcal/mol), while DATS demonstrated weak DNA binding (−2.1 kcal/mol).
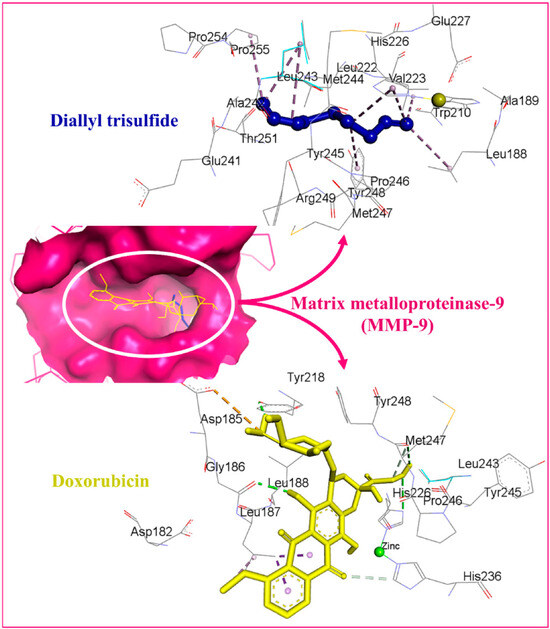
Figure 8.
Docked DATS (blue) and doxorubicin (yellow) within the matrix metalloproteinase-9 (MMP-9) active site. The binding pocket is shown as a dark-pink surface, interacting residues as gray sticks, and intermolecular interactions as dashed lines. Adapted from Alrumaihi et al. [198].
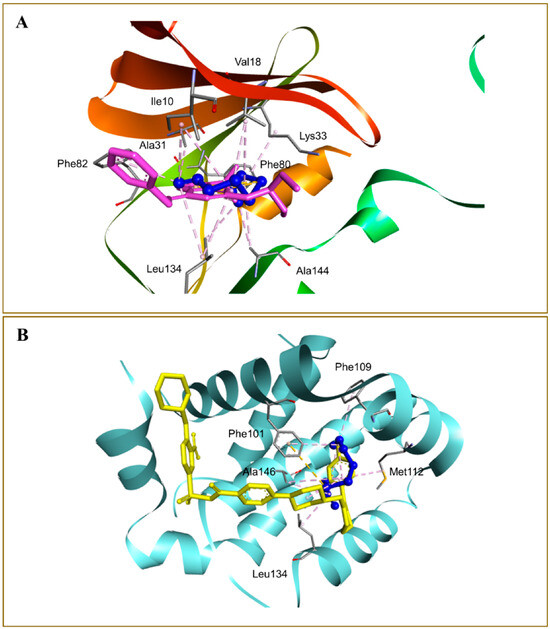
Figure 9.
DATS (blue, ball-and-stick) is docked in the binding pockets of cyclin-dependent kinase 2 (A) and the apoptosis regulator Bcl-2 (B). Native co-crystallized ligands are represented as purple and yellow sticks. Nonbonded interactions are represented by dashed lines. Adapted from Alrumaihi et al. [198].
A separate study conducted by the same group [199] investigated the effects of liposomal diallyl disulfide (DADS) and oxaliplatin (OXA), a standard drug, on the proliferation of colorectal cancer cells. DADS and OXA were docked to sixteen targets associated with anticancer drug discovery.
DADS exhibited the highest affinity for CDK2 at −5.23 kcal/mol, while OXA demonstrated a weaker binding affinity of −2.11 kcal/mol for CDK2. In contrast, Hsp90 exhibited the highest binding affinity for OXA (−7.06 kcal/mol), whereas DADS showed a moderate affinity for Hsp90 (−3.84 kcal/mol). The compounds established both hydrophilic and hydrophobic interactions with the macromolecular targets. The CDK2 binding pocket, which includes R126, D127, L128, K129, T165, L166, W167, Y168, R169, I173, and V184, serves as a strong interface for DADS and OXA (Figure 10). In Hsp90, N51 formed a hydrogen bond with OXA, while DADS did not exhibit similar polar interactions. Non-polar contacts were associated with M98, L103, L107, G135, V136, F138, Y139, V150, and W162 (Figure 11).
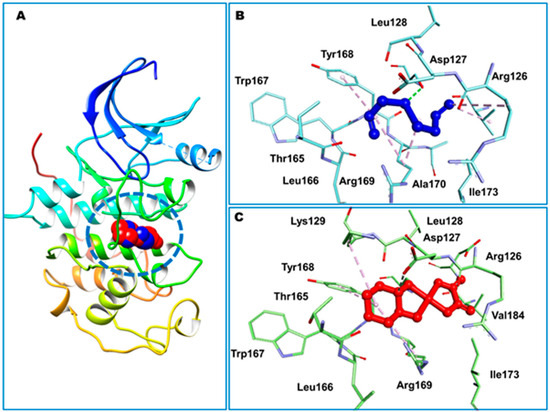
Figure 10.
Docked DADS (blue) and OXA (red) within cyclin-dependent kinase 2 (CDK2; (A)). CDK2 is illustrated as ribbons, with ligands occupying the binding site represented in space-filling (CPK) format. The minimum-energy conformations of DADS (B) and OXA (C) are depicted using a ball-and-stick model, with interacting residues represented as sticks. Dashed lines represent intermolecular interactions. Adapted from Alrumaihi et al. [199].
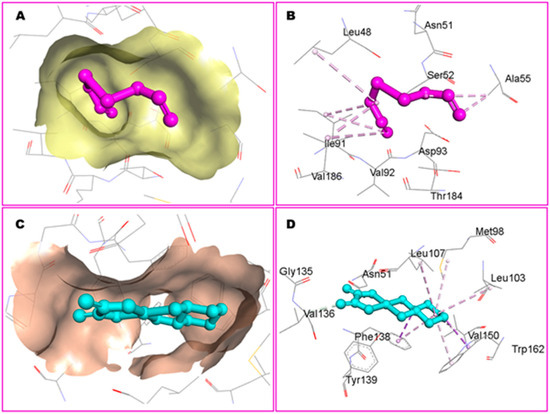
Figure 11.
The binding site of heat shock protein 90 (Hsp90) is depicted as a molecular surface (A,C), which accommodates the docked DADS (purple) and OXA (cyan). Binding-site residues are illustrated using line representation (B,D). Adapted from Alrumaihi et al. [199].
Furthermore, Zeng et al. [26] assessed the neuroprotective potential of allicin using molecular docking by the CB-DOCK2 program to predict binding poses and affinities for three targets: the dopamine transporter (DAT), protein kinase A (PKA), and apolipoprotein E (APOE). The binding energies predicted were −4.5 kcal·mol−1 for PKA, −3.5 kcal·mol−1 for DAT, and −3.4 kcal·mol−1 for APOE. Hydrogen bonds were detected in all complexes, suggesting favorable ligand–target interactions (Figure 12).
Figure 12.
Molecular docking of allicin (ALC) with three targets. (A) ALC bound to protein kinase A. (B) ALC bound to the dopamine transporter. (C) ALC bound to apolipoprotein E. Adapted from Zeng et al. [26].
Bouamrane et al. [227] employed molecular docking to evaluate garlic-derived compounds targeting Aspergillus fumigatus 14α-sterol demethylase (CYP51B). Alliin exhibited a predicted binding energy of −5.2 kcal mol−1 and established polar interactions with residues Y68, Y122, H374, and S375 (Figure 13).
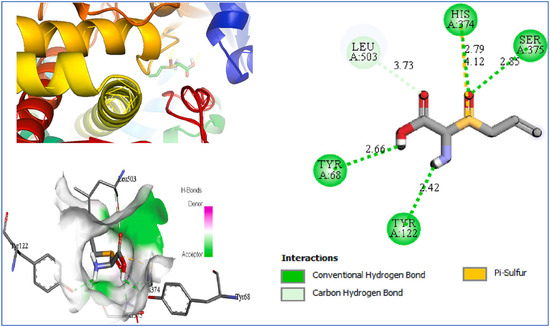
Figure 13.
Alliin bound in the CYP51B active site pocket. Adapted from Bouamrane et al. [227].
Yazdani et al. conducted docking studies of allicin within the ligand-binding pocket of the SidA quorum-sensing receptor [228]. The highest-ranked pose exhibited a predicted affinity of −5.1 kJ mol−1, suggesting a favorable binding profile (Figure 14). The model includes hydrogen bonds between the sulfur center of allicin and residues S39 and S130, as well as an electrostatic interaction with D76. The combination of bonding and non-bonding interactions explains the accommodation of allicin within SidA’s pocket and may facilitate competitive engagement of the receptor site.
Figure 14.
Best-scoring docking pose of allicin in SidA. Allicin (yellow, ball-and-stick) is embedded in the SidA binding pocket (shown as blue surface style); the protein is depicted as a solid ribbon. Adapted from Yazdani et al. [228].
4.2. Molecular Dynamics Simulation
Molecular dynamics (MD) simulation is integral to structure-guided investigations of garlic phytochemicals, as it effectively captures protein-ligand flexibility, solvent interactions, and the durability of critical contacts beyond static docking. This capability enhances mechanistic hypotheses and facilitates the prioritization of leads before experimental validation [229,230,231,232]. In various disease areas, MD has been employed to analyze canonical stability descriptors, including root-mean-square deviation (RMSD) and root-mean-square fluctuation (RMSF) of protein backbones, hydrogen-bond duration, radius of gyration (Rg), and solvent-accessible surface area (SASA) for OSCs like allicin, alliin, and related sulfides [233].
In oncology pipelines, garlic-derived candidates identified through docking against NSCLC targets (EGFR, HER2, EML4-ALK) were refined using MD to confirm pose robustness and interaction networks, demonstrating that dynamics enhance the reliability of virtual screening results [234]. MD is also used in neuroprotective applications. A 100 ns study demonstrated that alliin and allicin form structurally stable complexes with α-synuclein, with MM-GBSA energy computations, supporting alliin and in vitro validation in SH-SY5Y cells confirming anti-aggregation effects [235].
In antiviral research, MD has confirmed docking-derived poses for garlic constituents at the SARS-CoV-2 main protease (Mpro), specifically γ-glutamyl-S-allylcysteine and SAC, and has also been utilized for complexes involving the spike/ACE2 interface [236,237]. In addition to virology, toxicology research integrated docking with MD to elucidate allicin’s binding mode in CYP2E1, correlating the predicted stable complex with diminished reactive oxygen species values in vitro [238]. Furthermore, antifungal workflows have integrated reverse docking with MD to assess the stability of garlic metabolites in relation to fungal CYP51, transitioning from score-based ranking to time-resolved interaction analyses [227]. MD has elucidated host-directed mechanisms, with simulations indicating that S-1-propenyl-L-cysteine engages TLR4, aligning with the observed anti-inflammatory signaling [239]. Recent computational and experimental analyses of topoisomerase IV employed MD, utilizing RMSD, Rg, SASA, and hydrogen bond tracking to demonstrate stable allicin-enzyme complexes and coordinated motions, thereby reinforcing the proposed antibacterial mechanisms of garlic OSCs [224].
4.3. Network Pharmacology
Network pharmacology research consistently illustrates that allicin and its derivatives function as multi-target agents influencing lipid metabolism, inflammation, oxidative stress, and apoptotic pathways. In palmitate-induced steatosis, allicin was associated with PPAR signaling nodes and confirmed by qPCR to up-regulate PPARA and FABP6 while down-regulating FABP4 and PPARG, demonstrating a compound-target-pathway framework anchored in the mechanism [240]. Alliin has been identified as a multitarget autophagy modulator through the integration of target prediction, PPI-network analysis, GO/KEGG enrichment, docking, and experimental results. In addition to its role in metabolic regulation, the phytochemistry of garlic has been examined in relation to complex diseases [241]. Network pharmacology, along with docking and experimental validation, suggests that garlic compounds may play a role in alleviating alcoholic liver disease and atherosclerosis [242], particularly through ferroptosis-related pathways [225,243]. Recent studies in oncology have identified allicin as a potential therapeutic agent for non-small cell lung cancer [244]. Phenotypic anti-infective effects correspond with these networks; for instance, DADS inhibits quorum sensing, virulence factors, and biofilm formation in Pseudomonas aeruginosa, supporting broader evidence that garlic organosulfurs function as bactericidal, antibiofilm, anti-toxin, and anti-QS agents against various pathogens [40,245,246].
4.4. Quantitative Structure–Activity Relationships (QSAR)
QSAR modeling of allicin analogs utilizes topological, electronic, and lipophilicity descriptors to elucidate antiviral potencies and prioritize substitutions, while docking offers complementary target-binding hypotheses. Structure-activity analyses of allicin-derived disulfides for antibacterial applications reveal coherent SAR trends, indicating that heteroaryl or quinazolinyl disulfides enhance both potency and stability [247,248]. These studies frequently support thiol-reactive mechanisms via thiol-disulfide exchange. Chain-length effects in allyl polysulfides elucidate activity, where an increase in sulfur atoms (DAS < DADS < DATS and higher) is typically linked to a stronger biological response, aligning with improved thiol-modifying ability and hydrogen-sulfide–releasing potential [249]. QSAR and SAR identify substituent patterns, sulfur chain length, and electrophilicity as critical factors for enhancing potency, selectivity, and developability in antimicrobial, metabolic, cardiovascular, and anticancer applications [250,251].
4.5. Density Functional Theory Computations
Recent studies employing density functional theory (DFT) have elucidated the roles of essential OSCs in garlic as antioxidants, redox modulators, and reaction intermediates. In this regard, Molski assessed thermodynamic and global reactivity descriptors for 2-propenesulfenic acid and allyl mercaptan using B3LYP/cc-pVQZ with implicit water [25]. The findings indicate that in aqueous environments, the sequential proton loss-electron transfer pathway is preferred, whereas hydrogen atom transfer in the gas phase may prevail. These results elucidate the enhanced radical-trapping ability of sulfenic acids derived from allicin (Figure 15). In addition to intrinsic reactivity, DFT has played an important role in delineating chemically significant transformations of garlic polysulfides. Cai and Hu described the hydrogen sulfide-releasing mechanism of diallyl di- and trisulfide through thiolate attack, elucidating the greater H2S donation capacity of diallyl trisulfide compared to the disulfide [252].
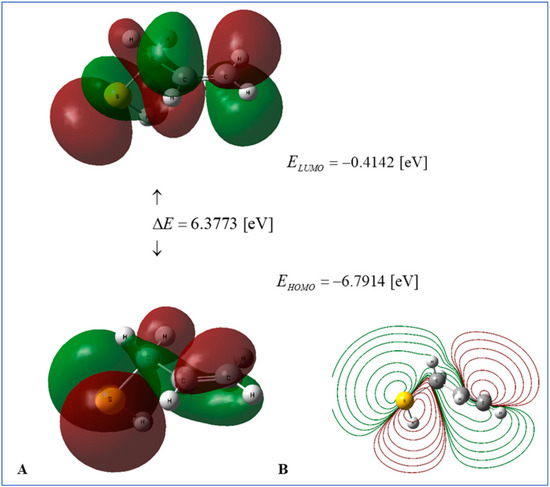
Figure 15.
Frontier orbitals and electrostatic potential of allyl mercaptan. (A) HOMO–LUMO energy levels and isosurfaces in water (DFT B3LYP/cc-pVQZ, C-PCM). (B) Molecular electrostatic potential (MEP) contoured on the electron-density surface. Adapted from Molski 2024 [25].
DFT study was used to elucidate the gas-phase dehydration mechanisms of the allicin radical cation compared to its protonated form, corroborating experimentally observed pathway preferences [253]. Furthermore, recent multiscale DFT studies of allicin-nanocage complexes indicate increased stability, reactivity, and target engagement, proposing formulation strategies that could enhance bioavailability [254]. Likewise, DFT has been employed to elucidate the geometry and isomer preference in metal complexes of S-allyl-L-cysteine, establishing a connection between structure and antibacterial activity [255].
Arroio et al. employed density functional theory to compute and correlate essential electronic descriptors of ajoene with its proposed antioxidant properties [256]. The findings indicate that the E-isomer is thermodynamically preferred compared to the Z-isomer and demonstrates significant electron-accepting properties. The electrophilic nature and ability for charge-transfer interactions establish a mechanistic foundation for the redox activity of E-ajoene, indicating its potential to interact with biological targets via donor–acceptor pathways [257].
Benchmarking of S–S bond descriptions indicates that disulfide-containing systems, such as thiosulfinates and disulfides, necessitate the selection of appropriate modern functionals to ensure accurate structural and vibrational properties [258]. In conjunction with kinetic DFT studies indicating that 2-propenesulfenic acid exhibits significantly greater reactivity toward peroxyl radicals compared to allicin, these results establish DFT as a pivotal instrument for the mechanistic understanding and development of garlic-derived pharmaceuticals [259].
4.6. ADMET Studies
Computational ADME/Tox screening is routinely utilized for garlic OSCs to prioritize candidates prior to laboratory assessments. Standard workflows integrate property and liability prediction tools, including SwissADME [260], pkCSM [261], admetSAR 2.0 [262], and ADMETlab 2.0 [263], as well as toxicity classifiers such as ProTox-II [264]. These tools are utilized to estimate gastrointestinal absorption, blood–brain barrier (BBB) penetration, P-glycoprotein interaction, and potential cytochrome P450 inhibition. The studies of garlic indicate significant predicted gastrointestinal absorption for various organosulfurs, with allicin and allyl sulfides (DAS, DADS, DATS) showing potential for BBB penetration [265]. In contrast, more polar compounds, such as SAC, exhibit reduced likelihood of crossing the BBB. Permeability modeling integrating immobilized artificial membrane (IAM) chromatography with chemometric/QSAR analysis indicates a strong correlation between hydrophobic descriptors (e.g., log k′(IAM)) and predicted human intestinal absorption, BBB permeation, and skin permeability, which illustrates the importance of lipophilicity in this chemotype [266]. Recent benchmarking of ADMET tools highlights variability across endpoints and suggests that the selection of tools and interpretation of endpoints should be aligned with emerging experimental data [267].
5. Safety Considerations and Drug Interactions
Safety assessments of garlic’s primary OSCs suggest a largely favorable profile at culinary or standard supplemental levels; however, significant risks arise with concentrated formulations, elevated dosages, parenteral administration, inappropriate topical use, or particular drug interactions [40,268,269]. Allicin exhibits chemical reactivity and potential mucosal irritation. Recent reviews provide a summary of its absorption, metabolism, and dose-dependent adverse effects, including gastrointestinal disturbances and contact sensitivity, along with the preparation type and stability [270]. Regulatory and expert panels evaluating allyl sulfides as flavoring substances typically identify no safety concerns with reported dietary intakes, while also noting data gaps for specific members of the class [271,272].
Recent toxicological research on DATS indicates that prolonged high-dose administration may present risks. A report of Wu et al., which compiled acute and subacute studies, proposed a conservative upper intake estimate for adults of approximately 359 mg of DATS per day (equivalent to about 84.5 g of garlic), emphasizing that apoptosis-related mechanisms are associated with supradietary exposure [273]. Topical application of raw garlic is associated with various cutaneous injuries, ranging from irritant or allergic contact dermatitis to second-degree burns. A systematic review has compiled numerous cases of burns, and recent reports highlight incidents of infant injuries resulting from garlic poultices [268,274]. Hypersensitivity reactions vary from fingertip pulpitis and occupational asthma to rare instances of anaphylaxis [275,276].
Diallyl disulfide is identified as a contact allergen, with contemporary reviews highlighting that raw handling poses a greater risk than exposure to cooked forms. Garlic derivatives, particularly ajoene, have demonstrated both mechanistic and experimental evidence for platelet inhibition. However, dietary garlic does not significantly affect platelet function in healthy individuals. Despite this, anesthesia societies advise discontinuing herbal supplements, including garlic, one to two weeks prior to surgery to reduce the risk of bleeding complications [269,277,278,279].
Clinically relevant drug interactions are a critical safety concern. Aged garlic extract did not elevate haemorrhagic events in patients monitored while on warfarin [280]. However, a controlled crossover trial indicated that garlic capsules significantly decreased plasma saquinavir concentrations, highlighting potential impacts on xenobiotic metabolism and the necessity for individual assessments with narrow-therapeutic-index medications [281]. Moreover, hepatic adverse events are infrequent but may occur, as evidenced by case reports of reversible hepatotoxicity following high-dose commercial garlic products. Animal studies suggest liver injury at significantly elevated daily intakes, necessitating the avoidance of excessive doses and careful monitoring in individuals with liver disease [282,283].
6. Future Perspectives
The future prospects of garlic as a therapeutic agent signify a transition from conventional folk medicine to advanced, evidence-based therapeutic strategies that utilize modern biotechnology and precision medicine techniques. Recent studies indicate that garlic is evolving into next-generation therapeutics via advanced nanotechnology-based drug delivery systems, such as silver nanoparticle conjugates and microencapsulation technologies, which improve bioavailability and targeted therapeutic efficacy. The incorporation of artificial intelligence in computer-aided drug design is transforming garlic-based drug discovery. Computational models are identifying optimal bioactive compounds and predicting therapeutic interactions for significant health issues, including COVID-19, antimicrobial resistance, and cancer. Future applications are expected to include personalized medicine strategies that exploit pharmacogenomics to enhance garlic-derived treatments according to individual genetic profiles. Additionally, organ-on-chip technologies offer novel platforms for evaluating garlic therapeutics in physiologically relevant human tissue models. The integration of garlic research with microbiome science, immunomodulation, and precision nutrition is advancing these historical therapeutic agents within modern healthcare. Current clinical trials are investigating their roles in optimizing gut health, protecting cardiovascular function, and enhancing immune system performance through evidence-based, bioengineered formulations that preserve traditional efficacy while adhering to contemporary pharmaceutical standards.
7. Conclusions
Garlic demonstrates consistent effectiveness in treating cardiovascular and metabolic disorders, notably in lowering blood pressure, enhancing lipid profiles, and regulating inflammatory markers. Clinical trials and meta-analyses provide evidence for its supplementary role in the management of hypertension, dyslipidemia, and elements of metabolic syndrome. Recent data indicate potential neuroprotective effects, particularly in Alzheimer’s and other neurodegenerative models, as well as significant anticancer properties via multi-targeted apoptosis induction and cell cycle regulation. Its antimicrobial properties contribute to its extensive range of therapeutic applications. Clinical validation for neurodegenerative and oncological indications is constrained by small sample sizes, variability in garlic preparations, and a lack of large-scale randomized controlled trials.
In addition to basic preparations, various formulations seek to standardize dosage and enhance the bioavailability of garlic’s OSCs. Aged garlic extract, stabilized allicin products, and garlic oil are delivered as enteric-coated tablets, capsules, or odor-masked liquids to limit gastric degradation and enhance intestinal release; newer systems such as nanoemulsions, cyclodextrin inclusions, liposomes, solid-lipid nanoparticles, polymeric nanoparticles and pH-responsive matrices, hydrogels, films, ethosomes, and niosomes seek improved solubility, permeability, and sustained release. Simultaneously, computational studies are progressively informing target selection and scaffold optimization: docking and molecular dynamics elucidate multi-target engagement, while DFT analyses of HOMO–LUMO gaps, electrophilicity, and sulfur redox states clarify the covalent and non-covalent reactivity of allicin, ajoene, alliin, and allyl mercaptan. QSAR, network pharmacology, and in silico ADMET screenings are utilized to prioritize leads and formulation strategies prior to in vitro validation. Taken together, pharmacological evidence, formulation innovations, and computational workflows are collectively guiding the field toward reproducible, mechanism-based garlic therapeutics. Nevertheless, standardized preparation characterization and prospective pharmacokinetic–pharmacodynamic studies are essential for clinical translation.
Author Contributions
F.A.: Writing—original draft, Writing—review and editing. M.J.A.: Writing—review and editing. J.K.: Writing—review and editing. S.A.A.: Supervision. A.-H.E.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Open access publication of this article was made possible through funding of the article processing charge by the Deanship of Graduate Studies and Scientific Research at Qassim University (QU-APC-2025).
Data Availability Statement
No data was used for the research described in the article.
Acknowledgments
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; El-Mageed, T.A.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic bioactive substances and their therapeutic applications for improving human health: A comprehensive review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Tattelman, E. Health effects of garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar]
- Tsai, C.-W.; Chen, H.-W.; Sheen, L.-Y.; Lii, C.-K. Garlic: Health benefits and actions. BioMedicine 2012, 2, 17–29. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Seki, T.; Hosono, T. Functionality of garlic sulfur compounds (Review). Biomed. Rep. 2025, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Khan, M.M.U.; Khalilullah, H.; Eid, E.E.M.; Azam, F.; Khan, M.A.; Khan, A.; Siddiqui, N.A.; Mahmood, T.; Ahsan, F.; Khan, W.U.; et al. A Dig Deep to Scout the Pharmacological and Clinical Facet of Garlic (Allium sativum). Curr. Tradit. Med. 2022, 8, 1–19. [Google Scholar] [CrossRef]
- Saadh, M.J.; Kariem, M.; Shukla, M.; Ballal, S.; Kumar, A.; Chahar, M.; Saini, S.; Kapila, I.; Hasaanzadeh, S. Effects of aged garlic extract on blood pressure in hypertensive patients: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Other Lipid Mediat. 2024, 175, 106914. [Google Scholar] [CrossRef]
- Bashiri, S.; TaghipourSheshdeh, F.; Foshati, S.; Askarpour, M.; Ahmadi, A.; Babajafari, S. The Effect of Aged Garlic Supplementation on Blood Pressure and Lipid Profile: A Dose-Response Grade-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. In Phytotherapy Research; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Ansary, J.; Forbes-Hernández, T.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gándara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Tudu, C.K.; Dutta, T.; Ghorai, M.; Biswas, P.; Samanta, D.; Olekšák, P.; Jha, N.; Kumar, M.; Radha; Proćków, J.; et al. Traditional uses, phytochemistry, pharmacology and toxicology of garlic (Allium sativum), a storehouse of diverse phytochemicals: A review of research from the last decade focusing on health and nutritional implications. Front. Nutr. 2022, 9, 949554. [Google Scholar] [CrossRef] [PubMed]
- Saikat, A.S.M.; Hossain, R.; Mina, F.; Das, S.; Khan, I.; Mubarak, M.; Islam, M. Antidiabetic Effect of Garlic. Rev. Bras. Farm. 2021, 32, 1–11. [Google Scholar] [CrossRef]
- Farhat, Z.; Hershberger, P.; Freudenheim, J.; Mammen, M.; Blair, R.H.; Aga, D.; Mu, L. Types of garlic and their anticancer and antioxidant activity: A review of the epidemiologic and experimental evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef]
- Sobenin, I.; Myasoedova, V.; Iltchuk, M.; Zhang, D.-W.; Orekhov, A. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 2019, 17, 721–728. [Google Scholar] [CrossRef]
- Sleiman, C.; Daou, R.-M.; Al Hazzouri, A.; Hamdan, Z.; Ghadieh, H.E.; Harbieh, B.; Romani, M. Garlic and Hypertension: Efficacy, Mechanism of Action, and Clinical Implications. Nutrients 2024, 16, 2895. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Li, X.; Luo, W.; Zhu, J.; Zhao, J.; Wang, M.; Sang, L.; Chang, B.; Wang, B.-Y. Allicin in Digestive System Cancer: From Biological Effects to Clinical Treatment. Front. Pharmacol. 2022, 13, 903259. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yun, W.; Wang, G.; Li, A.; Gao, J.; He, Q.-Y. Roles and mechanisms of garlic and its extracts on atherosclerosis: A review. Front. Pharmacol. 2022, 13, 954938. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Dhiman, A.; Kumar, S.; Suhag, R. Garlic (Allium sativum L.): A review on bio-functionality, allicin’s potency and drying methodologies. S. Afr. J. Bot. 2024, 171, 129–146. [Google Scholar] [CrossRef]
- Ryu, J.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Zhao, M.; Wu, J.; Niu, Z.; Zhang, X.; Simal-Gándara, J.; Süntar, I.; Jafari, S.; Qiao, X.; et al. Improving the bioavailability and bioactivity of garlic bioactive compounds via nanotechnology. Crit. Rev. Food Sci. Nutr. 2021, 62, 8467–8496. [Google Scholar] [CrossRef]
- Ozma, M.A.; Abbasi, A.; Rezaee, A.; Hosseini, H.; Hosseinzadeh, N.; Sabahi, S.; Noori, S.; Sepordeh, S.; Khodadadi, E.; Lahouty, M.; et al. A Critical Review on the Nutritional and Medicinal Profiles of Garlic’s (Allium sativum L.) Bioactive Compounds. Food Rev. Int. 2022, 39, 6324–6361. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef] [PubMed]
- Molski, M. Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives. Molecules 2024, 29, 668. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Liu, Y.; Liu, X.; Qi, Z. Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition. Molecules 2025, 30, 3265. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bai, Y.; Feng, M.; Wang, X.; Ni, L.; Cai, L.; Cao, Y. The synergistic antibacterial effects of allicin nanoemulsion and ε-polylysine against Escherichia coli in both planktonic and biofilm forms. Food Chem. 2025, 472, 142949. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Saleem, M.F.; Hassanzadeh, H. Optimization of solvent evaporation method in liposomal nanocarriers loaded-garlic essential oil (Allium sativum): Based on the encapsulation efficiency, antioxidant capacity, and instability. IET Nanobiotechnol. 2023, 17, 438–449. [Google Scholar] [CrossRef]
- Tavares, L.; Santos, L.; Noreña, C.P.Z. Bioactive compounds of garlic: A comprehensive review of encapsulation technologies, characterization of the encapsulated garlic compounds and their industrial applicability. Trends Food Sci. Technol. 2021, 114, 232–244. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, J.; Peng, H.; Guo, T.; Xiao, J.; Zhu, W.; Qian, W.; Zhang, J.; Wu, L. Allicin inclusions with α-cyclodextrin effectively masking its odor: Preparation, characterization, and olfactory and gustatory evaluation. J. Food Sci. 2021, 86, 4026–4036. [Google Scholar] [CrossRef]
- Gaikwad, A.R.; Borse, S.L. A Comprehensive Review on Garlic Oil as an Anti-inflammatory Nanoemulsion. Curr. Nanomed. 2025, 15, 485–491. [Google Scholar] [CrossRef]
- Deng, Y.; Ho, C.-T.; Lan, Y.; Xiao, J.; Lu, M. Bioavailability, Health Benefits, and Delivery Systems of Allicin: A Review. J. Agric. Food Chem. 2023, 71, 19207–19220. [Google Scholar] [CrossRef]
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic constituents for cancer prevention and therapy: From phytochemistry to novel formulations. Pharmacol. Res. 2022, 175, 105837. [Google Scholar] [CrossRef] [PubMed]
- Bataduwaarachchi, V.R.; Liyanage, D.C.J.; Hansanie, S.M.N.; Perera, H.; D’Cruz, L.G. Therapeutic potential of Garlic (Allium sativum L.) on new models of asthma immune pathobiology: A review. Phytomed. Plus 2025, 5, 100749. [Google Scholar] [CrossRef]
- Wang, C.; Han, N.; Mao, C.; Chen, J.; Cheng, N.; Zhao, J.; Song, Y.; Sun, X. The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2025, 26, 8257. [Google Scholar] [CrossRef]
- Agostinelli, E.; Marzaro, G.; Gambari, R.; Finotti, A. Potential applications of components of aged garlic extract in mitigating pro-inflammatory gene expression linked to human diseases (Review). Exp. Ther. Med. 2025, 30, 134. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, V.; Zahroodi, M.; Clark, C.C.T.; Mir, E.; Atashi, N.; Rivaz, R. Effects of Garlic Supplementation on Cardiovascular Risk Factors in Adults: A Comprehensive Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025, nuaf090. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Int. J. Mol. Sci. 2024, 25, 1801. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100075. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Okoro, B.C.; Dokunmu, T.M.; Okafor, E.; Sokoya, I.A.; Israel, E.N.; Olusegun, D.O.; Bella-Omunagbe, M.; Ebubechi, U.M.; Ugbogu, E.A.; Iweala, E.E.J. The ethnobotanical, bioactive compounds, pharmacological activities and toxicological evaluation of garlic (Allium sativum): A review. Pharmacol. Res. Mod. Chin. Med. 2023, 8, 100273. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Morales-González, J.A.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Valadez-Vega, M.D.; Álvarez-González, I.; Morales-González, Á.; Madrigal-Santillán, E. Garlic (Allium sativum L.): A Brief Review of Its Antigenotoxic Effects. Foods 2019, 8, 343. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nussbaum, G. Therapeutic potential of garlic, aged garlic extract and garlic-derived compounds on pancreatic cancer (Review). Biomed. Rep. 2025, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Mandge, H.M.; Rehal, J.; Goswami, D.; Choudhary, A.; Kaur, P. Unraveling phytochemistry and biofunctionality of black garlic: A systematic review. Food Sci. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Qin, G.; Liang, S.; Yin, J.; Jiang, H.; Liu, M.; Li, X. Therapeutic potentials of allicin in cardiovascular disease: Advances and future directions. Chin. Med. 2024, 19, 93. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Jia, J. The effect of garlic on the lowering of blood pressure in the patients with hypertension: An updated meta-analysis and trial sequential analysis. Asian Biomed. 2025, 19, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Biju, R. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [CrossRef][Green Version]
- Sendl, A.; Elbl, G.; Steinke, B.; Redl, K.; Breu, W.; Wagner, H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992, 58, 1–7. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Gadidala, S.K.; Johny, E.; Thomas, C.; Nadella, M.; Undela, K.; Adela, R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: A systematic review and meta-analysis. Phyther. Res. 2023, 37, 2242–2254. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef]
- Singh, N.; Gusain, A.; Nigam, M.; Mishra, A.P. The pharmacological and therapeutic versatility of Allium species: A comprehensive exploration of bioactive constituents and biological activities. Discov. Appl. Sci. 2025, 7, 349. [Google Scholar] [CrossRef]
- Xiang, L.; Qiu, Z.; Qiao, X.; Zhang, B.; Li, L.; Chen, H.; Zheng, Z.; Sun-Waterhouse, D. Synthesis of a garlic protein-derived ACE inhibitory peptide and exploration of its antihypertensive mechanisms using integrated network pharmacology, transcriptomics, and in vitro cellular experiments. J. Future Foods 2025. [Google Scholar] [CrossRef]
- Gao, X.; Xue, Z.; Ma, Q.; Guo, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism. J. Food Biochem. 2020, 44, e13126. [Google Scholar] [CrossRef]
- Gupta, N.; Porter, T.D. Garlic and garlic-derived compounds inhibit human squalene monooxygenase. J. Nutr. 2001, 131, 1662–1667. [Google Scholar] [CrossRef]
- Gebhardt, R.; Beck, H.; Wagner, K.G. Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cells. Biochim. Biophys. Acta 1994, 1213, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Porter, T.D. Inhibition of sterol 4alpha-methyl oxidase is the principal mechanism by which garlic decreases cholesterol synthesis. J. Nutr. 2006, 136, 759S–764S. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yeh, Y.Y. Water-soluble organosulfur compounds of garlic inhibit fatty acid and triglyceride syntheses in cultured rat hepatocytes. Lipids 2001, 36, 395–400. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.Y. Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic. Lipids 2000, 35, 197–203. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Andrianova, I.V.; Demidova, O.N.; Gorchakova, T.; Orekhov, A.N. Lipid-lowering effects of time-released garlic powder tablets in double-blinded placebo-controlled randomized study. J. Atheroscler. Thromb. 2008, 15, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Vargas, R.; Gotzkowsky, S.; McMahon, F.G. Can garlic reduce levels of serum lipids? A controlled clinical study. Am. J. Med. 1993, 94, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.T.; Mulrow, C.D.; Ramirez, G.; Gardner, C.D.; Morbidoni, L.; Lawrence, V.A. Garlic shows promise for improving some cardiovascular risk factors. Arch. Intern. Med. 2001, 161, 813–824. [Google Scholar] [CrossRef]
- Neil, H.A.; Silagy, C.A.; Lancaster, T.; Hodgeman, J.; Vos, K.; Moore, J.W.; Jones, L.; Cahill, J.; Fowler, G.H. Garlic powder in the treatment of moderate hyperlipidaemia: A controlled trial and meta-analysis. J. R. Coll. Physicians Lond. 1996, 30, 329–334. [Google Scholar] [CrossRef]
- Berthold, H.K.; Sudhop, T.; von Bergmann, K. Effect of a garlic oil preparation on serum lipoproteins and cholesterol metabolism: A randomized controlled trial. JAMA 1998, 279, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Khan, A.H.; Holbert, D.; Lin, R.I. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am. J. Clin. Nutr. 1996, 64, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.; Mølgaard, C.; Marckmann, P. Effect of garlic (Allium sativum) powder tablets on serum lipids, blood pressure and arterial stiffness in normo-lipidaemic volunteers: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2004, 92, 701–706. [Google Scholar] [CrossRef]
- Apitz-Castro, R.; Ledezma, E.; Escalante, J.; Jain, M.K. The molecular basis of the antiplatelet action of ajoene: Direct interaction with the fibrinogen receptor. Biochem. Biophys. Res. Commun. 1986, 141, 145–150. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012, 91, 1275–1280. [Google Scholar] [CrossRef]
- Clark-Montoya, I.; Milán-Segovia, R.D.; Lemus-Rojero, O.; Jaramillo-Morales, O.A.; Júarez-Flores, B.; Terán-Figueroa, Y. Preclinical Evaluation of the Anticoagulant Effect of an Aqueous Extract of Snow Mountain Garlic. Medicina 2025, 61, 429. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Pryanishnikov, V.V.; Kunnova, L.M.; Rabinovich, Y.A.; Martirosyan, D.M.; Orekhov, A.N. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010, 9, 119. [Google Scholar] [CrossRef]
- Tian, H.; Sun, S.; Qiu, X.; Wang, J.; Gao, Y.; Chen, J.; Han, X.; Bao, Z.; Guo, X.; Sun, Y.; et al. Diallyl Trisulfide From Garlic Regulates RAB18 Phase Separation to Inhibit Lipophagy and Induce Cuproptosis in Hepatic Stellate Cells for Antifibrotic Effects. Adv. Sci. 2025, 12, 2415325. [Google Scholar] [CrossRef]
- Ali, M.; Angelo-Khattar, M.; Farid, A.; Hassan, R.A.; Thulesius, O. Aqueous extracts of garlic (Allium sativum) inhibit prostaglandin synthesis in the ovine ureter. Prostaglandins. Leukot. Essent. Fat. Acids 1993, 49, 855–859. [Google Scholar] [CrossRef]
- Ali, M. Mechanism by which garlic (Allium sativum) inhibits cyclooxygenase activity. Effect of raw versus boiled garlic extract on the synthesis of prostanoids. Prostaglandins. Leukot. Essent. Fat. Acids 1995, 53, 397–400. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M.; Smith, S. Aged Garlic Extract Inhibits Human Platelet Aggregation by Altering Intracellular Signaling and Platelet Shape Change. J. Nutr. 2016, 146, 410S–415S. [Google Scholar] [CrossRef]
- Rahman, K. Effects of garlic on platelet biochemistry and physiology. Mol. Nutr. Food Res. 2007, 51, 1335–1344. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Z.; Liu, L.; Mu, D.; Wu, J.; Li, X.; Wu, X. Changes of Physicochemical Properties in Black Garlic during Fermentation. Fermentation 2022, 8, 653. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Qin, L.; Lin, S. Garlic-derived compounds: Epigenetic modulators and their antitumor effects. Phyther. Res. 2024, 38, 1329–1344. [Google Scholar] [CrossRef]
- Bhuker, S.; Kaur, A.; Rajauria, K.; Tuli, H.S.; Saini, A.K.; Saini, R.V.; Gupta, M. Allicin: A promising modulator of apoptosis and survival signaling in cancer. Med. Oncol. 2024, 41, 210. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Saini, V.; Deswal, G.; Chopra, B.; Grewal, A.S.; Dhingra, A.K. Harnessing Therapeutic Potential of Allicin Against Cancer: An Exploratory Review. Anti-Cancer Agents Med. Chem. 2025, 25, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Zurani, N.; Tukiran, N.A.; Yazik, A. The Benefit of Garlic from Islamic Perspective and Malay Practise. Malays. J. Health Sci. Sains Kesihat. Malays. 2024, 22, 117–123. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 213. [Google Scholar] [CrossRef]
- Yan, J.-Y.; Tian, F.-M.; Hu, W.-N.; Zhang, J.-H.; Cai, H.-F.; Li, N. Apoptosis of human gastric cancer cells line SGC 7901 induced by garlic-derived compound S-allylmercaptocysteine (SAMC). Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 745–751. [Google Scholar]
- Kang, J.S.; Kim, S.O.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Chang, Y.-C.; Kim, W.-J.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.-Y.; Zhang, Z.-H.; Bian, H.-L.; Lin, G. Garlic-derived compound S-allylmercaptocysteine inhibits cell growth and induces apoptosis via the JNK and p38 pathways in human colorectal carcinoma cells. Oncol. Lett. 2014, 8, 2591–2596. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Li, A.; Sun, Y.; Liu, Y.; Sun, X.; Feng, X.; Li, S.; Zhao, Z. S-allylmercaptocysteine suppresses the growth of human gastric cancer xenografts through induction of apoptosis and regulation of MAPK and PI3K/Akt signaling pathways. Biochem. Biophys. Res. Commun. 2017, 491, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Xia, Q.; Li, J.; Wang, Z. Allicin suppresses growth and metastasis of gastric carcinoma: The key role of microRNA-383-5p-mediated inhibition of ERBB4 signaling. Biosci. Biotechnol. Biochem. 2020, 84, 1997–2004. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, J.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef]
- Rosas-González, V.C.; Téllez-Bañuelos, M.C.; Hernández-Flores, G.; Bravo-Cuellar, A.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Haramati, J.; Solorzano-Ibarra, F.; Ortiz-Lazareno, P.C. Differential effects of alliin and allicin on apoptosis and senescence in luminal A and triple-negative breast cancer: Caspase, ΔΨm, and pro-apoptotic gene involvement. Fundam. Clin. Pharmacol. 2020, 34, 671–686. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bhat, Z.R.; Tikoo, K. MicroRNA-941 regulates the proliferation of breast cancer cells by altering histone H3 Ser 10 phosphorylation. Sci. Rep. 2020, 10, 17954. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front. Pharmacol. 2023, 14, 1154034. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Kim, E.-H.; Choi, M.-A.; Park, J.-M.; Kim, D.-H.; Surh, Y.-J. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem. Pharmacol. 2012, 84, 1241–1250. [Google Scholar] [CrossRef]
- Song, D.; Lian, Y.; Zhang, L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front. Immunol. 2023, 14, 1224892. [Google Scholar] [CrossRef]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged garlic extract inhibits 1,2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Fleischauer, A.T.; Poole, C.; Arab, L. Garlic consumption and cancer prevention: Meta-analyses of colorectal and stomach cancers. Am. J. Clin. Nutr. 2000, 72, 1047–1052. [Google Scholar] [CrossRef]
- Bat-Chen, W.; Golan, T.; Peri, I.; Ludmer, Z.; Schwartz, B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2010, 62, 947–957. [Google Scholar] [CrossRef]
- Cahyadi, A.; Ugrasena, I.D.G.; Andarsini, M.R.; Larasati, M.C.S.; Aryati, A.; Arumsari, D.K. Relationship between Bax and Bcl-2 Protein Expression and Outcome of Induction Phase Chemotherapy in Pediatric Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2022, 23, 1679–1685. [Google Scholar] [CrossRef]
- Huang, W.-L.; Wu, S.-F.; Xu, S.-T.; Ma, Y.-C.; Wang, R.; Jin, S.; Zhou, S. Allicin enhances the radiosensitivity of colorectal cancer cells via inhibition of NF-κB signaling pathway. J. Food Sci. 2020, 85, 1924–1931. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist. Updates 2024, 73, 101042. [Google Scholar] [CrossRef]
- Shirin, H.; Pinto, J.T.; Kawabata, Y.; Soh, J.W.; Delohery, T.; Moss, S.F.; Murty, V.; Rivlin, R.S.; Holt, P.R.; Weinstein, I.B. Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res. 2001, 61, 725–731. [Google Scholar] [PubMed]
- Chung, J.G.; Lu, H.F.; Yeh, C.C.; Cheng, K.C.; Lin, S.S.; Lee, J.H. Inhibition of N-acetyltransferase activity and gene expression in human colon cancer cell lines by diallyl sulfide. Food Chem. Toxicol. 2004, 42, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, P.; Wu, Y.; Liu, D.; Ji, M.; Li, H.; Wang, Y. Association and mechanism of garlic consumption with gastrointestinal cancer risk: A systematic review and meta-analysis. Oncol. Lett. 2022, 23, 125. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Hu, Y.-W.; Zhou, J.-J.; Zhang, M.-W.; Li, D.; Zheng, S. Consumption of garlic and risk of colorectal cancer: An updated meta-analysis of prospective studies. World J. Gastroenterol. 2014, 20, 15413–15422. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Sun, H.; Liu, J.; Lin, Y.; Zhu, Z.; Han, X.; Sun, X.; Li, X.; Zhang, H.; Tang, Z. Effects of garlic oil on pancreatic cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 5905–5910. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.A.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A.; et al. Garlic (Allium sativum L.): Its Chemistry, Nutritional Composition, Toxicity, and Anticancer Properties. Curr. Top. Med. Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef]
- Chan, J.M.; Wang, F.; Holly, E.A. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2093–2097. [Google Scholar] [CrossRef]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136, 816S–820S. [Google Scholar] [CrossRef] [PubMed]
- Myneni, A.A.; Chang, S.-C.; Niu, R.; Liu, L.; Swanson, M.K.; Li, J.; Su, J.; Giovino, G.A.; Yu, S.; Zhang, Z.-F.; et al. Raw Garlic Consumption and Lung Cancer in a Chinese Population. Cancer Epidemiol. Biomark. Prev. 2016, 25, 624–633. [Google Scholar] [CrossRef]
- Jin, Z.-Y.; Wu, M.; Han, R.-Q.; Zhang, X.-F.; Wang, X.-S.; Liu, A.-M.; Zhou, J.-Y.; Lu, Q.-Y.; Zhang, Z.-F.; Zhao, J.-K. Raw garlic consumption as a protective factor for lung cancer, a population-based case-control study in a Chinese population. Cancer Prev. Res. 2013, 6, 711–718. [Google Scholar] [CrossRef]
- Gao, X.; Santhanam, R.K.; Huang, Z.; Liu, M.; Hou, S.; Song, A.; Ren, T.; Zhao, Q. Allicin-Mediated Cell Cycle Regulation Reverses Taxol Resistance in NSCLC: Molecular Insights and Therapeutic Potentia. J. Food Biochem. 2023, 2023, 9910431. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Tsao, S.; Hsu, C.; Yin, M. Garlic extract and two diallyl sulphides inhibit methicillin-resistant Staphylococcus aureus infection in BALB/cA mice. J. Antimicrob. Chemother. 2003, 52, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Yin, M.-C. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 2001, 50, 646–649. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef]
- Zhi, T.; Di, G.; Jiayue, T.; Jing, W.; Siqi, L.; Qiaoxia, W.; Feng, X.; Shengyuan, X.; Rufeng, W. Synergistic Antibacterial Effect and Mechanism of Allicin and an Enterobacter cloacae Bacteriophage. Microbiol. Spectr. 2022, 11, e0315522. [Google Scholar] [CrossRef]
- Xin, Y.; Shuang, B.; Jiamin, W.; Yunlong, F.; Yuekun, Z.; Zhikuan, X.; Junhong, A.; Tong, C.; Mingwang, Z.; Rongya, Y. Antifungal Activity and Potential Action Mechanism of Allicin against Trichosporon asahii. Microbiol. Spectr. 2023, 11, e00907–e00923. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef] [PubMed]
- Mašková, L.; Závišová, L.; Kašpar, O.; Knejzlík, Z.; Rimpelová, S.; Tokárová, V. Design and evaluation of composite films for in situ synthesis and antibacterial activity of allicin vapour. J. Mater. Sci. 2024, 59, 13614–13631. [Google Scholar] [CrossRef]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, R.; Wang, C.; Zhang, C.; Chu, C.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W.; Zhang, H.; et al. Effects of garlic supplementation on non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2022, 99, 105294. [Google Scholar] [CrossRef]
- Soleimani, D.; Paknahad, Z.; Askari, G.; Iraj, B.; Feizi, A. Effect of garlic powder consumption on body composition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Adv. Biomed. Res. 2016, 5, 2. [Google Scholar] [CrossRef]
- Soleimani, D.; Paknahad, Z.; Rouhani, M.H. Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Diabetes. Metab. Syndr. Obes. 2020, 13, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Lan, H.; Wang, W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): A meta-analysis of randomized controlled trials. Food Nutr. Res. 2017, 61, 1377571. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Uyanga, V.A.; Wang, X.; Jiao, H.; Zhao, J.; Zhou, Y.; Li, H.; Lin, H. Allicin Promotes Glucose Uptake by Activating AMPK through CSE/H(2)S-Induced S-Sulfhydration in a Muscle-Fiber Dependent Way in Broiler Chickens. Mol. Nutr. Food Res. 2024, 68, e2300622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, K.-A.; Kwon, K.-B.; Kim, E.-K.; Lee, Y.-R.; Song, M.-Y.; Koo, J.-H.; Ka, S.-O.; Park, J.-W.; Park, B.-H. Diallyl disulfide accelerates adipogenesis in 3T3-L1 cells. Int. J. Mol. Med. 2007, 20, 59–64. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Negishi, T.; Yukawa, K. Effects of diallyl disulfide administration on insulin resistance in high-fat diet-fed mice. Nutrition 2024, 118, 112292. [Google Scholar] [CrossRef]
- Chang, W.-T.; Wu, C.-H.; Hsu, C.-L. Diallyl trisulphide inhibits adipogenesis in 3T3-L1 adipocytes through lipogenesis, fatty acid transport, and fatty acid oxidation pathways. J. Funct. Foods 2015, 16, 414–422. [Google Scholar] [CrossRef]
- Liu, C.-T.; Hse, H.; Lii, C.-K.; Chen, P.-S.; Sheen, L.-Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005, 516, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Ettehad-Marvasti, F.; Ejtahed, H.-S.; Siadat, S.-D.; Soroush, A.-R.; Hoseini-Tavassol, Z.; Hasani-Ranjbar, S.; Larijani, B. Effect of garlic extract on weight loss and gut microbiota composition in obese women: A double-blind randomized controlled trial. Front. Nutr. 2022, 9, 1007506. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, M.; Zhang, R.; Zhang, H.; Liu, Y.; Li, W.; Sun, X.; Yu, Q.; Tipoe, G.L.; Xiao, J. S-Allylmercaptocysteine improves alcoholic liver disease partly through a direct modulation of insulin receptor signaling. Acta Pharm. Sin. B 2021, 11, 668–679. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, an Allium sativum Nutraceutical, ReducesMetaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, T.; Xia, H.; Yang, Y.; Wang, S. Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients 2024, 16, 1692. [Google Scholar] [CrossRef]
- Kosuge, Y. Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease. Exp. Ther. Med. 2020, 19, 1565–1569. [Google Scholar] [CrossRef]
- Ashafaq, M.; Khan, M.M.; Raza, S.S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2012, 32, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Rao, K.S.J. Anti-amyloidogenic activity of S-allyl-l-cysteine and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Neurosci. Lett. 2007, 429, 75–80. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, J.; Chen, Y.; Liang, Y.; Zhang, Z.; Wang, Z. Diallyl trisulfide regulates PGK1/Nrf2 expression and reduces inflammation to alleviate neurological damage in mice after traumatic brain injury. Brain Res. 2024, 1843, 149116. [Google Scholar] [CrossRef]
- Ho, S.-C.; Su, M.-S. Evaluating the Anti-Neuroinflammatory Capacity of Raw and Steamed Garlic as Well as Five Organosulfur Compounds. Molecules 2014, 19, 17697–17714. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Chang, T.; Cai, W.-K.; Zhang, Z.; Yang, Y.-X.; Sun, C.; Li, Z.-Y.; Li, W.-X. Post-injury administration of allicin attenuates ischemic brain injury through sphingosine kinase 2: In vivo and in vitro studies. Neurochem. Int. 2015, 89, 92–100. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Shi, X.; Qiao, S.; Liu, B.; Wang, Z.; Huo, H.; Liang, F.; Shen, L.; Zhu, L.; He, B.; et al. Allicin promotes functional recovery in ischemic stroke via glutathione peroxidase-1 activation of Src-Akt-Erk. Cell Death Discov. 2023, 9, 335. [Google Scholar] [CrossRef]
- Meng, J.; Wen, C.; Lu, Y.; Fan, X.; Dang, R.; Chu, J.; Jiang, P.; Han, W.; Feng, L. Alliin from garlic as a neuroprotective agent attenuates ferroptosis in vitro and in vivo via inhibiting ALOX15. Food Funct. 2025, 16, 5278–5300. [Google Scholar] [CrossRef]
- Song, H.; Cui, J.; Mossine, V.V.; Michael, C.G.; Fritsche, K.; Sun, Y.G.; Gu, Z. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration (Review). Exp. Ther. Med. 2020, 19, 1554–1559. [Google Scholar] [CrossRef]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef]
- Sharif, A.H.; Iqbal, M.; Manhoosh, B.; Gholampoor, N.; Ma, D.; Marwah, M.; Sanchez-Aranguren, L. Hydrogen Sulphide-Based Therapeutics for Neurological Conditions: Perspectives and Challenges. Neurochem. Res. 2023, 48, 1981–1996. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M. Modulation of Keap1/Nrf2/ARE signaling pathway by curcuma-and garlic-derived hybrids. Front. Pharmacol. 2020, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Li, S.; Dong, Z.; Li, C.; Sun, Y.; Li, A.; Zhao, Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharmacol. 2020, 81, 106273. [Google Scholar] [CrossRef]
- Zhan, X.; Peng, W.; Wang, Z.; Liu, X.; Dai, W.; Mei, Q.; Hu, X. Polysaccharides from garlic protect against liver injury in DSS-induced inflammatory bowel disease of mice via suppressing pyroptosis and oxidative damage. Oxid. Med. Cell. Longev. 2022, 2022, 2042163. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tsuneyoshi, T.; Ogawa, T.; Morihara, N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2–related factor 2–antioxidant response element signaling pathway in human endothelial cells. Nutr. Res. 2016, 36, 143–149. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Nakanishi, E.; Kuwata, H.; Chen, J.; Nakasone, Y.; He, X.; He, J.; Liu, X.; Zhang, S.; Zhang, B. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol. Nutr. Food Res. 2013, 57, 2049–2060. [Google Scholar] [CrossRef]
- Hitchcock, J.K.; Mkwanazi, N.; Barnett, C.; Graham, L.M.; Katz, A.A.; Hunter, R.; Schäfer, G.; Kaschula, C.H. The garlic compound Z—Ajoene, S—Thiolates COX2 and STAT3 and dampens the inflammatory response in RAW264. 7 macrophages. Mol. Nutr. Food Res. 2021, 65, 2000854. [Google Scholar] [CrossRef]
- Du, M.; Zhao, K.; Chen, A.; Shi, D.; Wu, Z.; Yan, T.; Wang, Y.; Bai, B. Inhibitory mechanisms of lipoxygenase by garlic sulfides: “adsorption-embolism” effect of DAS and DADS and “quasi-domain-separation” effect of DATS and Allicin. Int. J. Biol. Macromol. 2025, 304, 140874. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.-H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Fateminasab, F.; Moradi, M. Screening cyclooxygenase-2 inhibitors from Allium sativum L. compounds: In silico approach. J. Mol. Model. 2022, 28, 24. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Li, S.; Deng, L.; Li, Y.; Li, H. Effect of allicin against ischemia/hypoxia-induced H9c2 myoblast apoptosis via eNOS/NO pathway-mediated antioxidant activity. Evid. Based Complement. Altern. Med. 2018, 2018, 3207973. [Google Scholar] [CrossRef]
- Weiss, N.; Papatheodorou, L.; Morihara, N.; Hilge, R.; Ide, N. Aged garlic extract restores nitric oxide bioavailability in cultured human endothelial cells even under conditions of homocysteine elevation. J. Ethnopharmacol. 2013, 145, 162–167. [Google Scholar] [CrossRef]
- Kim, K.-M.; Chun, S.-B.; Koo, M.-S.; Choi, W.-J.; Kim, T.-W.; Kwon, Y.-G.; Chung, H.-T.; Billiar, T.R.; Kim, Y.-M. Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic. Biol. Med. 2001, 30, 747–756. [Google Scholar] [CrossRef]
- Das, I.; Khan, N.S.; Sooranna, S.R. Nitric oxide synthase activation is a unique mechanism of garlic action. Biochem. Soc. Trans. 1995, 23, 136S. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef]
- Ho, C.; Weng, C.; Jhang, J.; Cheng, Y.; Huang, S.; Yen, G. Diallyl sulfide as a potential dietary agent to reduce TNF-α-and histamine-induced proinflammatory responses in A 7r5 cells. Mol. Nutr. Food Res. 2014, 58, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, Q.; Li, X.; Nie, Y.; Sun, S.; Deng, X.; Wang, L.; Lu, J.; Tang, Y.; Luo, F. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol. Nutr. Food Res. 2017, 61, 1601013. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; He, W.; Chen, R.; Zhuang, R. Effects of alliin on LPS-induced acute lung injury by activating PPARγ. Microb. Pathog. 2017, 110, 375–379. [Google Scholar] [CrossRef]
- Yue, L.; Zhu, X.; Li, R.; Chang, H.; Gong, B.; Tian, C.; Liu, C.; Xue, Y.; Zhou, Q.; Xu, T. S-allyl-cysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy. Int. J. Mol. Med. 2019, 44, 1943–1951. [Google Scholar] [CrossRef]
- Ji, S.T.; Kim, M.-S.; Park, H.R.; Lee, E.; Lee, Y.; Jang, Y.J.; Kim, H.S.; Lee, J. Diallyl disulfide impairs hippocampal neurogenesis in the young adult brain. Toxicol. Lett. 2013, 221, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soto, C.Y.; Rangel-López, E.; Galván-Arzate, S.; Colín-González, A.L.; Silva-Palacios, A.; Zazueta, C.; Pedraza-Chaverri, J.; Ramírez, J.; Chavarria, A.; Túnez, I. S-Allylcysteine protects against excitotoxic damage in rat cortical slices via reduction of oxidative damage, activation of Nrf2/ARE binding, and BDNF preservation. Neurotox. Res. 2020, 38, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, S.; Li, B.; Cheng, L.; Jiang, P.; Tian, Z.; Sun, S. Protective Effects of Garlic-Derived S-Allylmercaptocysteine on IL-1β-Stimulated Chondrocytes by Regulation of MMPs/TIMP-1 Ratio and Type II Collagen Expression via Suppression of NF-κB Pathway. Biomed. Res. Int. 2017, 2017, 8686207. [Google Scholar] [CrossRef]
- Shin, S.-S.; Song, J.-H.; Hwang, B.; Noh, D.-H.; Park, S.L.; Kim, W.T.; Park, S.-S.; Kim, W.-J.; Moon, S.-K. HSPA6 augments garlic extract-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; Implication for cell cycle dysregulation, signaling pathway alteration, and transcription factor-associated MMP-9 regulation. PLoS ONE 2017, 12, e0171860. [Google Scholar] [CrossRef]
- Aly, S.M.; Fetaih, H.A.; Hassanin, A.A.I.; Abomughaid, M.M.; Ismail, A.A. Protective effects of garlic and cinnamon oils on hepatocellular carcinoma in albino rats. Anal. Cell. Pathol. 2019, 2019, 9895485. [Google Scholar] [CrossRef] [PubMed]
- Maitisha, G.; Aimaiti, M.; An, Z.; Li, X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021, 48, 7261–7272. [Google Scholar] [CrossRef]
- Lee, W.S.; Yi, S.M.; Yun, J.W.; Jung, J.H.; Kim, D.H.; Kim, H.J.; Chang, S.-H.; Kim, G.; Ryu, C.H.; Shin, S.C. Polyphenols isolated from Allium cepa L. induces apoptosis by induction of p53 and suppression of Bcl-2 through inhibiting PI3K/Akt signaling pathway in AGS human cancer cells. J. Cancer Prev. 2014, 19, 14. [Google Scholar] [CrossRef]
- Roy, N.; Nazeem, P.A.; Babu, T.D.; Abida, P.S.; Narayanankutty, A.; Valsalan, R.; Valsala, P.A.; Raghavamenon, A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-L-Cysteine Sulfoxide. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 686–693. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Gu, Y.; Chen, Y. Preventive effect of Diallyl Trisulfide on cutaneous toxicities induced by EGFR inhibitor. Int. Immunopharmacol. 2019, 69, 79–87. [Google Scholar] [CrossRef]
- Zhang, W.; Ha, M.; Gong, Y.; Xu, Y.; Dong, N.; Yuan, Y. Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol. Rep. 2010, 24, 1585–1592. [Google Scholar] [CrossRef]
- Kanamori, Y.; Dalla Via, L.; Macone, A.; Canettieri, G.; Greco, A.; Toninello, A.; Agostinelli, E. Aged garlic extract and its constituent, S-allyl-L-cysteine, induce the apoptosis of neuroblastoma cancer cells due to mitochondrial membrane depolarization. Exp. Ther. Med. 2020, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.A.; Cha, Y.Y.; Park, M.S.; Kim, J.M.; Lim, Y.C. Diallyl sulfide induces growth inhibition and apoptosis of anaplastic thyroid cancer cells by mitochondrial signaling pathway. Oral Oncol. 2010, 46, e15–e18. [Google Scholar] [CrossRef]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef]
- Huang, L.; Song, Y.; Lian, J.; Wang, Z. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol. Lett. 2017, 14, 468–474. [Google Scholar] [CrossRef][Green Version]
- Fujii, D.; Nango, H.; Ohtani, M. Aged garlic extract enhances the production of β-defensin 4 via activation of the Wnt/β-catenin pathway in mouse gingiva. Exp. Ther. Med. 2025, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; He, H.; Li, Y.-K.; Xia, H.; Liu, F.; Zeng, Y.; Zeng, X.; Ling, H.; Su, B.; Su, Q. Diallyl disulfide inhibits proliferation, epithelial-mesenchymal transition, and invasion through RORα-mediated downregulation of Wnt1/β-catenin pathway in gastric cancer cells. J. Food Drug Anal. 2022, 30, 479. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y.; Xie, C.; Zhu, J.; Wang, X.; Li, Y.; Geng, S.; Wu, J.; Zhong, C.; Li, M. Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J. Cell. Biochem. 2018, 119, 4134–4141. [Google Scholar] [CrossRef]
- Li, T.; Shi, H.-Y.; Hua, Y.-X.; Gao, C.; Xia, Q.; Yang, G.; Li, B. Effects of allicin on the proliferation and cell cycle of chondrocytes. Int. J. Clin. Exp. Pathol. 2015, 8, 12525. [Google Scholar]
- Jeong, J.-W.; Park, S.; Park, C.; Chang, Y.-C.; Moon, D.-O.; Kim, S.O.; Kim, G.-Y.; Cha, H.-J.; Kim, H.-S.; Choi, Y.-W. N-benzyl-N-methyldecan-1-amine, a phenylamine derivative isolated from garlic cloves, induces G2/M phase arrest and apoptosis in U937 human leukemia cells. Oncol. Rep. 2014, 32, 373–381. [Google Scholar] [CrossRef]
- Xu, J.; Huang, J.; Yu, Y.; Hu, D.; Zhang, Y.; Dai, H.; You, L.; Xu, F.; Shen, J.; Wang, C. Garlic-derived nanoparticles inhibit tumor by activating tumor-infiltrating γδT cells. Nano Res. 2025, 18, 94907145. [Google Scholar] [CrossRef]
- Wan, X.; Li, D.; Lu, J.; Yan, Y.; He, Z.; Chen, J.; Jiao, Y.; Li, J.; Li, W. The construction of garlic diallyl disulfide nano-emulsions and their effect on the physicochemical properties and heterocyclic aromatic amines formation in roasted pork. Food Chem. 2023, 408, 135159. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, S.; Peng, S.; Chen, X.; Zou, L.; Liang, R.; Ruan, R.; Dai, L.; Liu, W. Fiber complex-stabilized high-internal-phase emulsion for allicin encapsulation: Microstructure, stability, and thermal-responsive properties. J. Sci. Food Agric. 2025, 105, 1116–1125. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Feng, M.; Bai, Y.; Huang, J.; Cao, Y. Antibacterial, anti-biofilm activity and underlying mechanism of garlic essential oil in water nanoemulsion against Listeria monocytogenes. LWT 2024, 196, 115847. [Google Scholar] [CrossRef]
- Hassan, K.A.M.; Mujtaba, M.D.A. Antibacterial efficacy of garlic oil nano-emulsion. AIMS Agric. Food 2019, 4, 194–205. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci. Technol. 2014, 57, 686–695. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Vasamsetti, B.M.K.; Thangavelu, P.; Natesan, K.; Mujyambere, B.; Sundaram, V.; Jayaraj, R.; Kim, Y.-J.; Samiappan, S.; Choi, J.-W. Cytotoxic Effects of Nanoliposomal Cisplatin and Diallyl Disulfide on Breast Cancer and Lung Cancer Cell Lines. Biomedicines 2023, 11, 1021. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 2192. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; AlSuhaymi, N.; Alsugoor, M.H.; et al. The Effect of Liposomal Diallyl Disulfide and Oxaliplatin on Proliferation of Colorectal Cancer Cells: In Vitro and In Silico Analysis. Pharmaceutics 2022, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Baliga, V.; Londhe, V.Y. Liposomal Formulations: A Recent Update. Pharmaceutics 2025, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lv, J.; Zhu, S.; Wang, S.; Su, J.; Xu, C. Enzyme-responsive liposomes for controlled drug release. Drug Discov. Today 2024, 29, 104014. [Google Scholar] [CrossRef] [PubMed]
- Fidan, Y.; Muçaj, S.; Timur, S.S.; Gürsoy, R.N. Recent advances in liposome-based targeted cancer therapy. J. Liposome Res. 2024, 34, 316–334. [Google Scholar] [CrossRef]
- Chai, C.; Park, J. Food liposomes: Structures, components, preparations, and applications. Food Chem. 2024, 432, 137228. [Google Scholar] [CrossRef]
- Alyasiri, F.J.; Ghobeh, M.; Tabrizi, M.H. Preparation and Characterization of Allicin-Loaded Solid Lipid Nanoparticles Surface-Functionalized with Folic Acid-Bonded Chitosan: In Vitro Anticancer and Antioxidant Activities. Front. Biosci. 2023, 28, 135. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Roychowdhury, P.; Bhuyan, N.R.; Ko, Y.T.; Singh, S.K.; Dua, K.; Kuppusamy, G. Folic Acid Functionalized Diallyl Trisulfide–Solid Lipid Nanoparticles for Targeting Triple Negative Breast Cancer. Molecules 2023, 28, 1393. [Google Scholar] [CrossRef]
- Llaguno-Munive, M.; Vazquez-Lopez, M.I.; Garcia-Lopez, P. Solid Lipid Nanoparticles, an Alternative for the Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 10712. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals 2024, 17, 329. [Google Scholar] [CrossRef]
- Khan, M.F.; Ahmad, N.; Alkholifi, F.K.; Ullah, Z.; Khalid, M.S.; Akhtar, S.; Farooqui, S.; Khan, N.; Chaudhary, A.A.; Alawam, A.S.; et al. Preparation of novel S-allyl cysteine chitosan based nanoparticles for use in ischemic brain treatment. RSC Adv. 2024, 14, 160–180. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Yao, Y.; Li, X.; Wang, M. pH-responsive alliin delivery system: Sustained intestinal release. J. Food Eng. 2026, 402, 112670. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, J.-J.; Zhang, Q.-B.; Wang, Z.-X.; Yin, Z.-N.; Li, X.-X.; Chen, J.; Ye, L.-M. Release test of alliin/alliinase double-layer tablet by HPLC-Allicin determination. J. Pharm. Anal. 2013, 3, 187–192. [Google Scholar] [CrossRef]
- Patil, H.I.; Koshti, G.; Doijad, S.; Wadkar, K.A. The Inclusion Complex of Allicin with Β-Cyclodextrin as A Drug-Delivery System for Enhanced Solubility in the Treatment of Breast Cancer. Palest. Med. Pharm. J. 2025, 11, 1–10. [Google Scholar] [CrossRef]
- Qian, Y.L.; Hua, G.K.; Dung, J.K.S.; Qian, M. Encapsulation of garlic oil and diallyl disulfide with β-cyclodextrin for white rot control in Allium crops. Crop Prot. 2024, 178, 106597. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Liu, Y.; Qiu, H.; Gao, W.; Che, K.; Zhou, B.; Liu, R.; Hu, W. Characterization of garlic oil/β-cyclodextrin inclusion complexes and application. Front. Nutr. 2023, 10, 1308787. [Google Scholar] [CrossRef]
- Majd, H.; Gultekinoglu, M.; Bayram, C.; Karaosmanoğlu, B.; Taşkıran, E.Z.; Kart, D.; Erol, Ö.D.; Harker, A.; Edirisinghe, M. Biomedical Efficacy of Garlic-Extract-Loaded Core-Sheath Plasters for Natural Antimicrobial Wound Care. Macromol. Mater. Eng. 2024, 309, 2400014. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Fu, K.; Zhao, X.; Hu, X.; Cheng, S.; Jiang, R.; Dong, C.; Wang, L.; Wu, H.; et al. Allicin-loaded hydrogel enhances the viability of multiterritory perforator flap. Mater. Today Bio 2025, 33, 102026. [Google Scholar] [CrossRef] [PubMed]
- Subashini, R.; Tabbasum, M.T.D.; Dharshan, A.H.; Varsha, S.S. Development of collagen-based hydrogel derived from allicin-silver nanoparticles for wound healing. Naturwissenschaften 2025, 112, 67. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.R.; Prakash, S.N.J.; Kumar, S.; Bhagirathi; Ahuja, D.; Abhishek; Singh, P. Study of antimicrobial efficacy of garlic oil loaded ethosome against clinical microbial isolates of diverse origin. J. Herb. Med. 2024, 43, 100824. [Google Scholar] [CrossRef]
- Rana, M.; Kumar, A.; Raguvaran, R.; Kumar, R.; Kumar, P.; Prajapati, S.K.; Abhishek; Gupta, M.; Shukla, R. Evaluation of antidermatophytic and wound-healing effect of garlic essential oil-loaded ethosomes formulation. J. Drug Deliv. Sci. Technol. 2025, 113, 107328. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Artificial Intelligence in Natural Product Drug Discovery: Current Applications and Future Perspectives. J. Med. Chem. 2025, 68, 3948–3969. [Google Scholar] [CrossRef] [PubMed]
- Çağlar, Y.S. Molecular Docking and Reactive Sites Identification (Homo–Lumo, Mep) of Allicin and Diallyl Disulfide: Potential Anticancer Inhibitor TT—Allisin ve Diallil Disülfitin Moleküler Yerleştirme ve Reaktif Bölgelerinin Tanımlanması (Homo–Lumo, Mep): Potansiy. Karadeniz Fen. Bilim. Derg. 2023, 13, 1523–1539. [Google Scholar] [CrossRef]
- Almansoori, A.K.K.; Maaroof, R.J.A.; Al-Oudah, G.A.; Hindi, N.K.K.; Yu, L.H.; Jasim, H.M.; Adnan, M.; Abdullah, M.H. Novel Molecular Mechanism of Allicin-Mediated Inhibition of Bacterial Topoisomerase IV: A Combined Computational and Experimental Analysis. Nat. Prod. Commun. 2025, 20, 1934578X251368993. [Google Scholar] [CrossRef]
- Gao, T.; Gao, S.; Wang, H.; Wang, S.; Li, L.; Hu, J.; Yan, S.; Zhang, R.; Zhou, Y.; Dong, H. Garlic ameliorates atherosclerosis by regulating ferroptosis pathway: An integrated strategy of network pharmacology, bioinformatic and experimental verification. Front. Pharmacol. 2024, 15, 1388540. [Google Scholar] [CrossRef]
- Sarkar, D.; Maiti, A.K. Virtual screening and molecular docking studies with organosulfur and flavonoid compounds of garlic targeting the estrogen receptor protein for the therapy of breast cancer. Biointerface Res. Appl. Chem. 2023, 13, 49. [Google Scholar]
- Bouamrane, S.; Khaldan, A.; Alaqarbeh, M.; Sbai, A.; Ajana, M.A.; Bouachrine, M.; Lakhlifi, T.; Maghat, H. Garlic as an effective antifungal inhibitor: A combination of reverse docking, molecular dynamics simulation, ADMET screening, DFT, and retrosynthesis studies. Arab. J. Chem. 2024, 17, 105642. [Google Scholar] [CrossRef]
- Yazdani, M.; Beihaghi, M.; Ataee, N.; Zabetian, M.; Khaksar, S.; Nasrizadeh, H.; Chaboksavar, M. Anti-quorum sensing effects of SidA protein on Escherichia coli receptors: In silico analysis. J. Biomol. Struct. Dyn. 2024, 43, 7235–7246. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Azam, F.; Almahmoud, S.A.; Ali, A.; Ali, A. Targeting Mitochondrial Drp1 Dynamics in Neurodegenerative Diseases: A Comprehensive Drug Design Approach Using AutoDock Vina-GPU, DFT, and Molecular Dynamics Simulations. ChemistrySelect 2025, 10, e202406041. [Google Scholar] [CrossRef]
- Gupta, A.; Parveen, D.; Azam, F.; Shaquiquzzaman, M.; Akhter, M.; Jaremko, M.; Emwas, A.-H.; Khan, M.A.; Parvez, S.; Khanna, S.; et al. Mechanistic insights into novel cyano-pyrimidine pendant chalcone derivatives as LSD1 inhibitors by docking, ADMET, MM/GBSA, and molecular dynamics simulation. Biochem. Biophys. Rep. 2025, 41, 101937. [Google Scholar] [CrossRef]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef]
- Bari, S.; Reza, N.; Marma, M.; Ahmed, S.; Hussain, M.; Jabed, M.; Hossain, M.; Manzoor, M.; Alam, M. Computational analysis of Allium sativum compounds to identify thermol abile hemolysin inhibitors against Vibrio alginolyticus in shrimp. J. Adv. Biotechnol. Exp. Ther. 2025, 8, 1–17. [Google Scholar] [CrossRef]
- Padmini, R.; Maheshwari, V.U.; Saravanan, P.; Lee, K.W.; Razia, M.; Alwahibi, M.S.; Ravindran, B.; Elshikh, M.S.; Kim, Y.O.; Kim, H.; et al. Identification of novel bioactive molecules from garlic bulbs: A special effort to determine the anticancer potential against lung cancer with targeted drugs. Saudi J. Biol. Sci. 2020, 27, 3274–3289. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Dawria, A.; Ahmad, M.F.; Salih, A. Allium sativum-Derived Alliin and Allicin Stably Bind to α-Synuclein and Prevent Its Cytotoxic Aggregation. Proteins 2025, 93, 1905–1920. [Google Scholar] [CrossRef]
- Li, T.; Cheng, B. Analysis of the mechanism of alliin and allicin against SARS-CoV-2S/ACE2 and SARS-CoV-2 Mpro based on molecular docking and molecular dynamics. In Proceedings of the 2023 4th International Symposium on Artificial Intelligence for Medicine Science, Chengdu, China, 27–29 October 2023; Association for Computing Machinery: New York, NY, USA, 2024; pp. 1325–1331. [Google Scholar]
- Parashar, A.; Shukla, A.; Sharma, A.; Behl, T.; Goswami, D.; Mehta, V. Reckoning γ-Glutamyl-S-allylcysteine as a potential main protease (mpro) inhibitor of novel SARS-CoV-2 virus identified using docking and molecular dynamics simulation. Drug Dev. Ind. Pharm. 2021, 47, 699–710. [Google Scholar] [CrossRef]
- Nan, B.; Yang, C.; Li, L.; Ye, H.; Yan, H.; Wang, M.; Yuan, Y. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem. Toxicol. 2021, 148, 111937. [Google Scholar] [CrossRef]
- Papi, C.; Gasparello, J.; Marzaro, G.; Macone, A.; Zurlo, M.; Di Padua, F.; Fino, P.; Agostinelli, E.; Gambari, R.; Finotti, A. Aged garlic extract major constituent S-1-propenyl-l-cysteine inhibits proinflammatory mRNA expression in bronchial epithelial IB3-1 cells exposed to the BNT162b2 vaccine. Exp. Ther. Med. 2025, 30, 153. [Google Scholar] [CrossRef]
- Cheng, B.; Li, T.; Li, F. Use of Network Pharmacology to Investigate the Mechanism by Which Allicin Ameliorates Lipid Metabolism Disorder in HepG2 Cells. Evid. Based Complement. Alternat. Med. 2021, 2021, 3956504. [Google Scholar] [CrossRef]
- Cheng, B.; Li, T.; Li, F. Study on the multitarget mechanism of alliin activating autophagy based on network pharmacology and molecular docking. J. Cell. Mol. Med. 2022, 26, 5590–5601. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lv, D.; Tao, Y.; Wang, J. The therapeutic effects of natural organosulfur compounds on atherosclerosis and their potential mechanisms: A comprehensive review. Front. Cardiovasc. Med. 2025, 12, 1599154. [Google Scholar] [CrossRef]
- Gao, S.; Gao, T.; Li, L.; Wang, S.; Hu, J.; Zhang, R.; Zhou, Y.; Dong, H. Exploring the therapeutic potential of garlic in alcoholic liver disease: A network pharmacology and experimental validation study. Genes Nutr. 2024, 19, 13. [Google Scholar] [CrossRef]
- Teng, L.; Chen, H. Network pharmacology and experimental verification reveals the anti-growth and anti-migration roles of allicin in non-small cell lung cancer. Lett. Drug Des. Discov. 2025, 22, 100098. [Google Scholar] [CrossRef]
- Li, W.-R.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Sun, T.-L.; Peng, H.; Huang, X.-M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 102, 7555–7564. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Yu, F.; Ye, F.; Tan, W.; Hao, L.; Li, S.; Deng, J.; Hu, X. Garlic-Derived Quorum Sensing Inhibitors: A Novel Strategy Against Fungal Resistance. Drug Des. Dev. Ther. 2024, 18, 6413–6426. [Google Scholar] [CrossRef]
- Wang, J.-R.; Hu, Y.-M.; Zhou, H.; Li, A.-P.; Zhang, S.-Y.; Luo, X.-F.; Zhang, B.-Q.; An, J.-X.; Zhang, Z.-J.; Liu, Y.-Q. Allicin-Inspired Heterocyclic Disulfides as Novel Antimicrobial Agents. J. Agric. Food Chem. 2022, 70, 11782–11791. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Long, X.; Wang, C.; Ouyang, G.; Wang, Z. Antibacterial Activity of Allicin-Inspired Disulfide Derivatives against Xanthomonas axonopodis pv. citri. Int. J. Mol. Sci. 2022, 23, 11947. [Google Scholar] [CrossRef]
- Hosono, T.; Sato, A.; Nakaguchi, N.; Ozaki-Masuzawa, Y.; Seki, T. Diallyl Trisulfide Inhibits Platelet Aggregation through the Modification of Sulfhydryl Groups. J. Agric. Food Chem. 2020, 68, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Das, R.; Emran, T.B.; Labib, R.K.; Tabassum, N.E.; Islam, F.; Sharma, R.; Ahmad, I.; Nainu, F.; Chidambaram, K.; et al. Diallyl Disulfide: A Bioactive Garlic Compound with Anticancer Potential. Front. Pharmacol. 2022, 13, 943967. [Google Scholar] [CrossRef]
- Novakovic, J.; Muric, M.; Bradic, J.; Ramenskaya, G.; Jakovljevic, V.; Jeremic, N. Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 9831. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-R.; Hu, C.-H. Computational Study of H2S Release in Reactions of Diallyl Polysulfides with Thiols. J. Phys. Chem. B 2017, 121, 6359–6366. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, C. Theoretical studies on the dehydration reaction of the allicin radical cation in the gas phase. Comput. Theor. Chem. 2011, 972, 75–80. [Google Scholar] [CrossRef]
- Mozafari, E.S.; Baei, M.T.; Lemeski, E.T. Computational study of the therapeutic properties of allicin and its nanocomplexes using DFT and molecular docking techniques. Sci. Rep. 2025, 15, 23034. [Google Scholar] [CrossRef]
- Spera, M.B.M.; Quintão, F.A.; Ferraresi, D.K.D.; Lustri, W.R.; Magalhães, A.; Formiga, A.L.B.; Corbi, P.P. Palladium(II) complex with S-allyl-L-cysteine: New solid-state NMR spectroscopic measurements, molecular modeling and antibacterial assays. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Arroio, A.; Honório, K.M.; de Mello, P.H.; Weber, K.C.; da Silva, A.B.F. A density functional theory study on the role of electronic properties in the antioxidant activity of the ajoene molecule. Internet Electron. J. Mol. Des. 2004, 3, 308–315. [Google Scholar]
- Bouyahya, A.; Kamari, F.; El Omari, N.; Touhtouh, J.; Mssillou, I.; Aanniz, T.; Benali, T.; Khalid, A.; Abdalla, A.N.; Iesa, M.A.M.; et al. Unlocking the natural chemical sources, nutritional properties, biological effects, and molecular actions of Ajoene. J. Funct. Foods 2025, 127, 106714. [Google Scholar] [CrossRef]
- Alessandrini, S.; Ye, H.; Biczysko, M.; Puzzarini, C. Describing the Disulfide Bond: From the Density Functional Theory and Back through the “Lego Brick” Approach. J. Phys. Chem. A 2024, 128, 9383–9397. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Francisco-Marquez, M. Peroxyl-radical-scavenging activity of garlic: 2-propenesulfenic acid versus allicin. J. Phys. Chem. B 2009, 113, 16077–16081. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Ouma, S.; Kagia, R.; Kamakia, F. Determination of pharmacological activity of bioactives in Allium sativum using computational analysis. F1000Research 2023, 12, 151. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Federici, M.F.; Altamirano, J.C.; Camargo, A.B.; Luco, J.M. Permeability Data of Organosulfur Garlic Compounds Estimated by Immobilized Artificial Membrane Chromatography: Correlation Across Several Biological Barriers. Front. Chem. 2021, 9, 690707. [Google Scholar] [CrossRef]
- Gadaleta, D.; Serrano-Candelas, E.; Ortega-Vallbona, R.; Colombo, E.; Lomana, M.G.D.; Biava, G.; Aparicio-Sánchez, P.; Roncaglioni, A.; Gozalbes, R.; Benfenati, E. Comprehensive benchmarking of computational tools for predicting toxicokinetic and physicochemical properties of chemicals. J. Cheminform. 2024, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavarić, N.; Čonić, B.S.; Božin, B. Garlic burn injuries—A systematic review of reported cases. Am. J. Emerg. Med. 2021, 44, 5–10. [Google Scholar] [CrossRef]
- Lazo, O.L.E.; White, P.F.; Lee, C.; Eng, H.C.; Matin, J.M.; Lin, C.; Cid, F.D.; Yumul, R. Use of herbal medication in the perioperative period: Potential adverse drug interactions. J. Clin. Anesth. 2024, 95, 111473. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.; Azzini, E.; Adetunji, C.; Mohammed, S.; Banerjee, S.; Sharopov, F.; Rigano, D.; Rad, J.S.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Silano, V.; Bolognesi, C.; Castle, L.; Chipman, K.; Cravedi, J.-P.; Engel, K.-H.; Fowler, P.; Franz, R.; Grob, K.; Gürtler, R.; et al. Scientific Opinion on Flavouring Group Evaluation 74, Revision 4 (FGE.74Rev4): Consideration of aliphatic sulphides and thiols evaluated by JECFA (53rd and 61st meeting) structurally related to aliphatic and alicyclic mono-, di-, tri- and polysulphides with or without additional oxygenated functional groups from chemical group 20 evaluated by EFSA in FGE.08Rev5. EFSA J. 2018, 16, e05167. [Google Scholar] [CrossRef]
- Maged, Y.; Gabriele, A.; Laurence, C.; Karl-Heinz, E.; Paul, F.; Fernandez, F.; Jose, M.; Peter, F.; Ursula, G.-R.; Rainer, G.; et al. Scientific Opinion on Flavouring Group Evaluation 91, Revision 3 (FGE.91Rev3): Consideration of aliphatic, aromatic and α,β-unsaturated sulfides and thiols evaluated by JECFA (53rd, 61st, 68th and 76th meetings), structurally related to substances in FGE.08Rev5. EFSA J. 2020, 18, e06154. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, B.; Li, S.; Chen, J.; Shen, P.; Zhou, W.; Ma, Z.; Tang, X.; Xiao, C.; Wang, Y.; et al. Safety assessment and exploration of the mechanism of toxic effects of diallyl trisulfide: Based on acute and subacute toxicity and proteomics experiments. J. Ethnopharmacol. 2025, 338, 119102. [Google Scholar] [CrossRef]
- Lina, S.A.; Yee, C.H.; Yap, Y.C. Unforeseen sequela of a traditional remedy: A garlic burn case report. Malays. Fam. Physician 2025, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, W.Y.; Casale, T.B. Self-reported garlic allergy. World Allergy Organ. J. 2024, 17, 100959. [Google Scholar] [CrossRef] [PubMed]
- Armentia, A.; Martín-Armentia, S.; Pineda, F.; Martín-Armentia, B.; Castro, M.; Fernández, S.; Moro, A.; Castillo, M. Allergic hypersensitivity to garlic and onion in children and adults. Allergol. Immunopathol. 2020, 48, 232–236. [Google Scholar] [CrossRef]
- Scharbert, G.; Kalb, M.L.; Duris, M.; Marschalek, C.; Kozek-Langenecker, S.A. Garlic at Dietary Doses Does Not Impair Platelet Function. Anesth. Analg. 2007, 105, 1214–1218. [Google Scholar] [CrossRef]
- Cummings, K.C.; Keshock, M.; Ganesh, R.; Sigmund, A.; Kashiwagi, D.; Devarajan, J.; Grant, P.J.; Urman, R.D.; Mauck, K.F. Preoperative Management of Surgical Patients Using Dietary Supplements: Society for Perioperative Assessment and Quality Improvement (SPAQI) Consensus Statement. Mayo Clin. Proc. 2021, 96, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Apitz-Castro, R.; Badimon, J.J.; Badimon, L. Effect of ajoene, the major antiplatelet compound from garlic, on platelet thrombus formation. Thromb. Res. 1992, 68, 145–155. [Google Scholar] [CrossRef]
- Macan, H.; Uykimpang, R.; Alconcel, M.; Takasu, J.; Razon, R.; Amagase, H.; Niihara, Y. Aged garlic extract may be safe for patients on warfarin therapy. J. Nutr. 2006, 136, 793S–795S. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Burstein, A.H.; Welden, N.; Gallicano, K.D.; Falloon, J. The Effect of Garlic Supplements on the Pharmacokinetics of Saquinavir. Clin. Infect. Dis. 2002, 34, 234–238. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Tischer, S.; Choi, E.K.; Fontana, R.J. Good for the lung but bad for the liver? Garlic-induced hepatotoxicity following liver transplantation. J. Clin. Pharm. Ther. 2017, 42, 646–648. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Singh, K. Garlic hepatotoxicity: Safe dose of garlic. Trop. Gastroenterol. 2006, 27, 26–30. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).