Bisphenol A Alters the Expression of Genes Involved in Lipogenesis, Inflammation, and Oxidative Stress in the Liver of Adult Zebrafish
Abstract
1. Introduction
2. Results
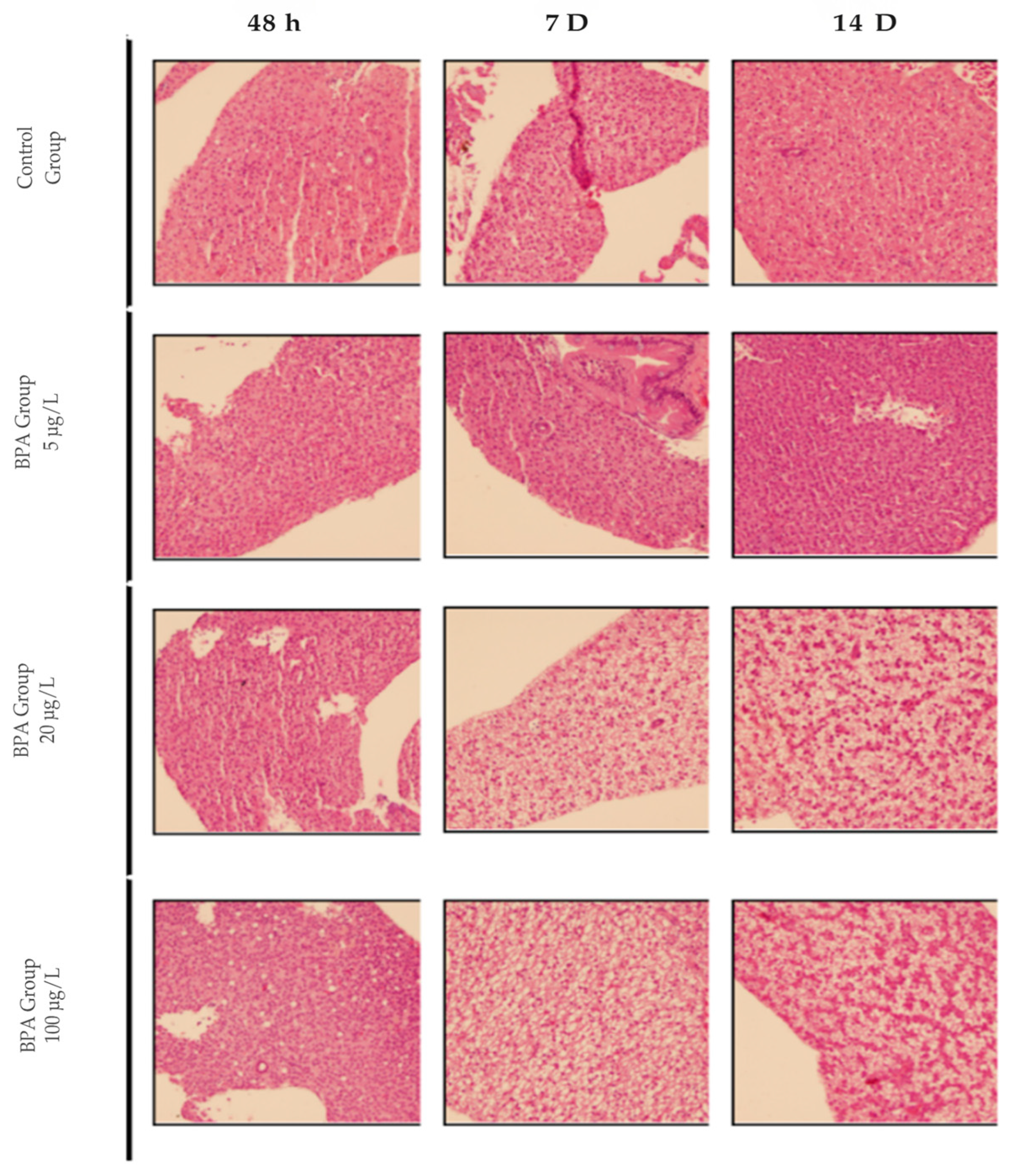
2.1. Histological Analysis of the Pilot Test
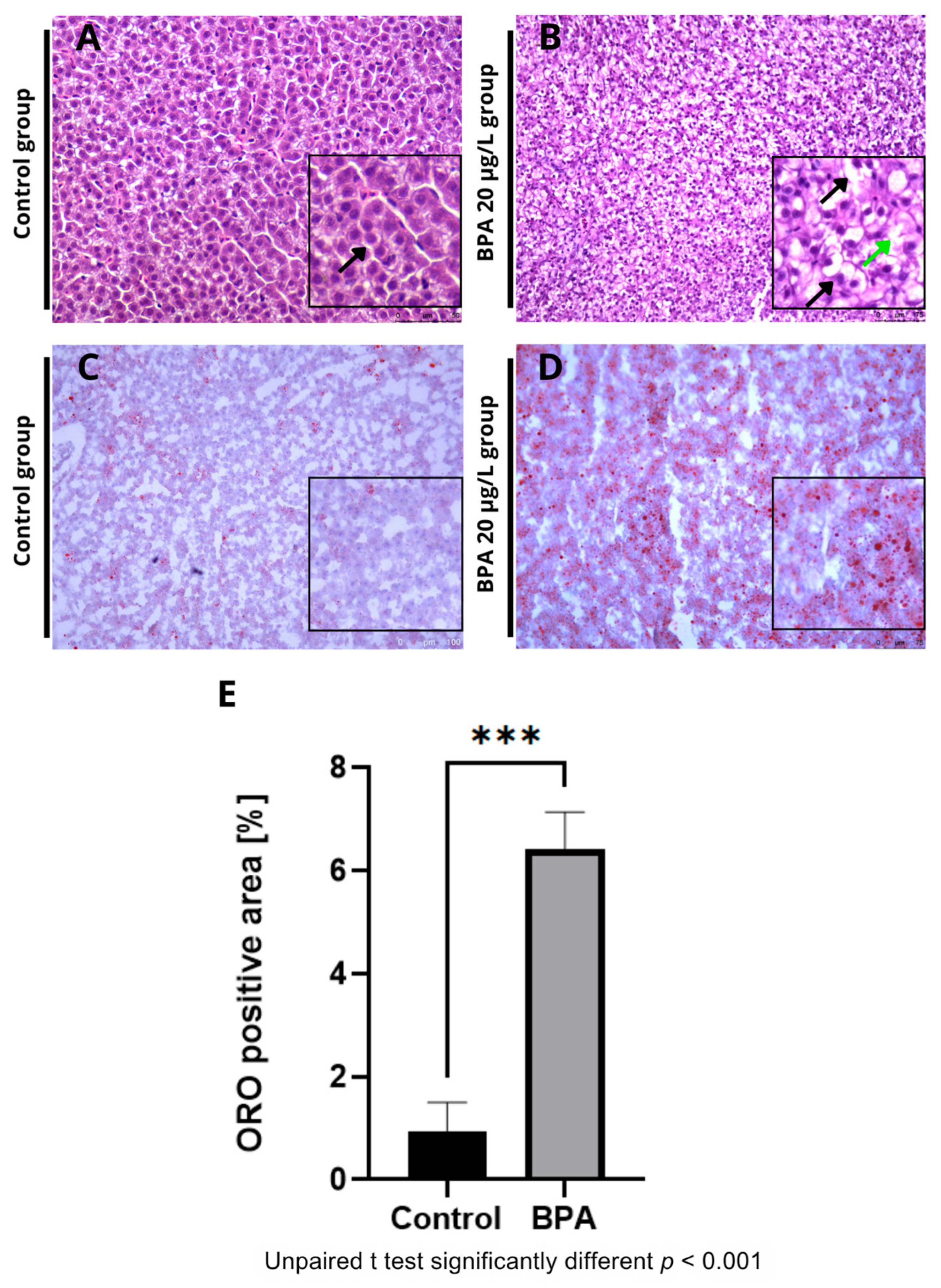
2.2. Histological and Histochemical Analysis of Zebrafish Liver Exposed to 20 µg/L of BPA
2.3. Expression of Genes Related to Lipid Metabolism
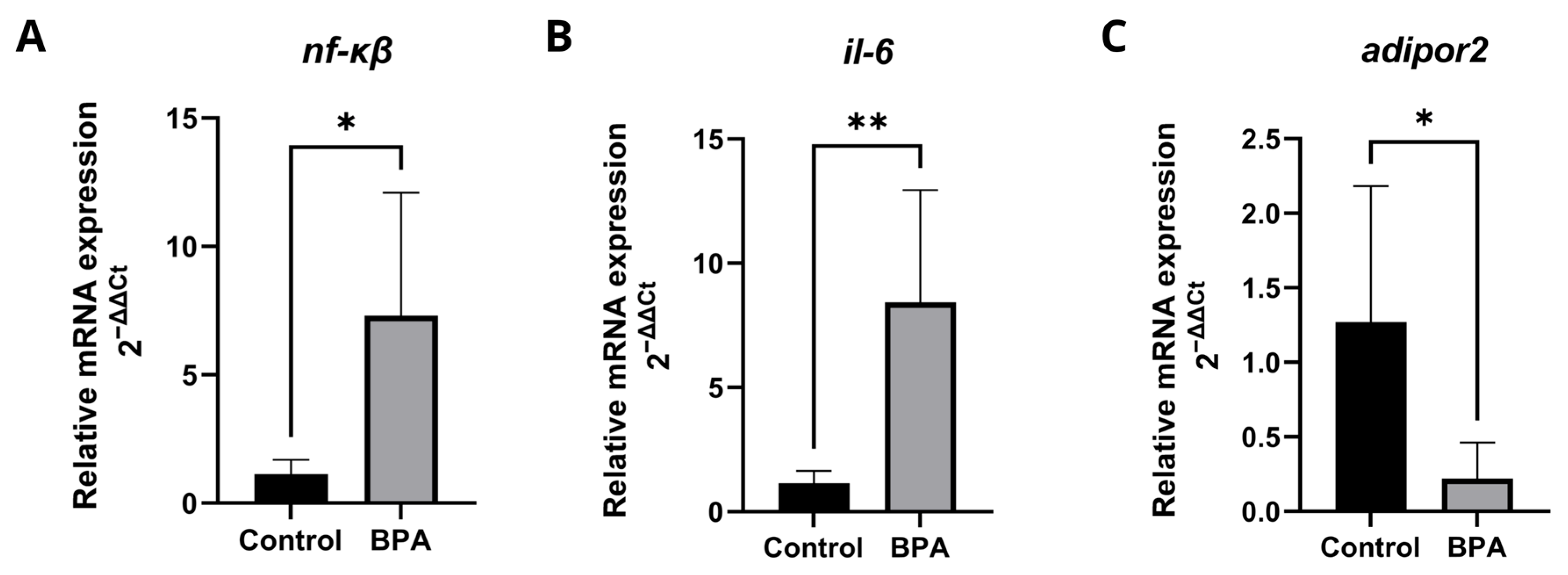
2.4. Expression of Genes Related to Immune Response, Inflammation, and Metabolism
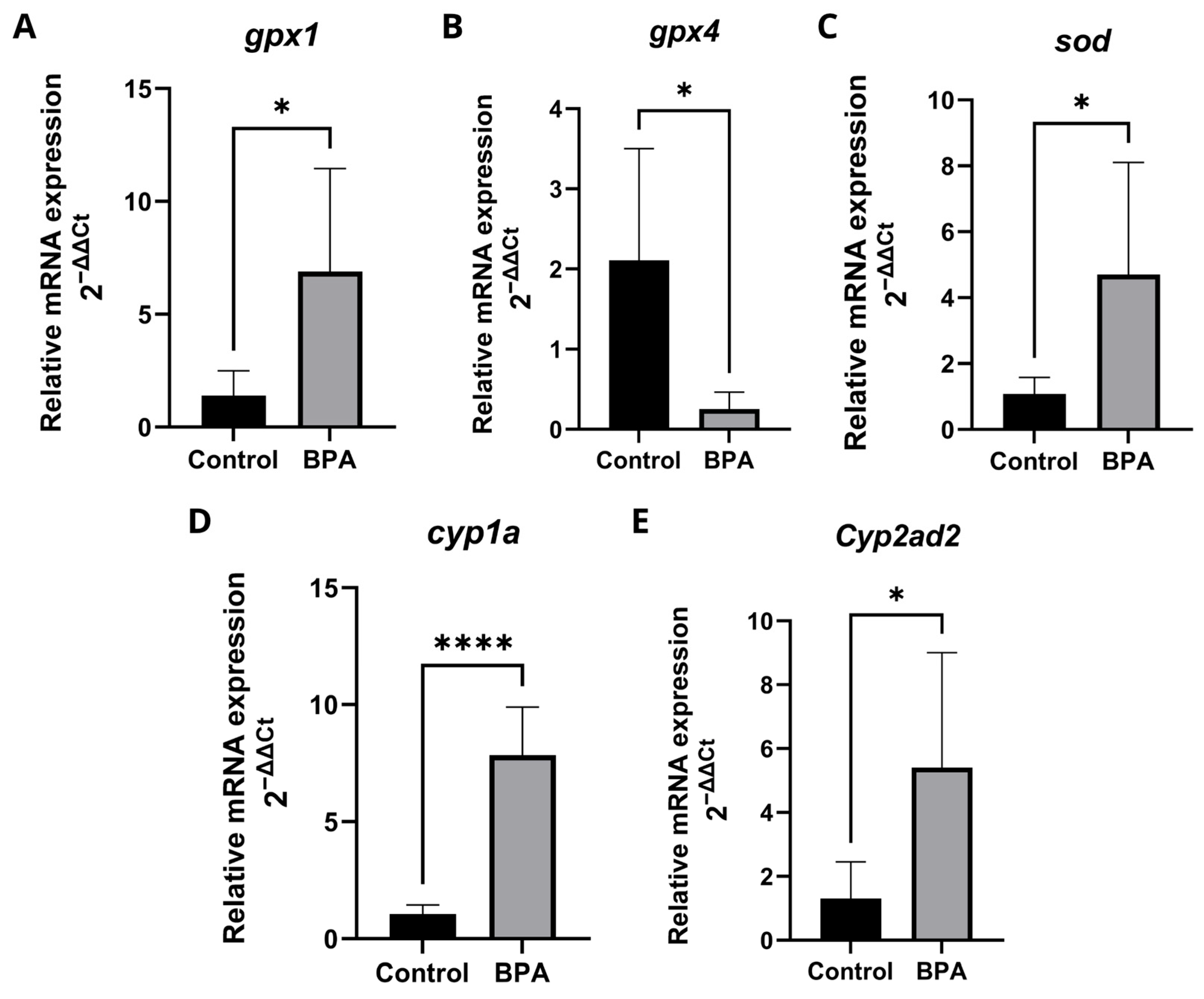
2.5. Expression of Genes Related to Oxidative Stress and Biotransformation of Xenobiotics
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemical Preparation
4.3. Experimental Design
4.4. Histological Analysis
4.5. Histochemical Analysis
4.6. Biochemical Analysis
4.7. Detection of mRNA Levels of Target Genes by Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Jiang, Y.; Miao, X.-M.; Tao, Y.-X.; Xie, L.; Li, Y. A Model Construction of Starvation Induces Hepatic Steatosis and Transcriptome Analysis in Zebrafish Larvae. Biology 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Valerio, C.M.; Villela-Nogueira, C.A.; Cercato, C.; Gerchman, F.; Lottenberg, A.M.P.; Godoy-Matos, A.F.; Oliveira, R.d.A.; Mello, C.E.B.; Álvares-da-Silva, M.R.; et al. Brazilian Evidence-Based Guideline for Screening, Diagnosis, Treatment, and Follow-up of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Adult Individuals with Overweight or Obesity: A Joint Position Statement from the Brazilian Society of Endocrinology and Metabolism (SBEM), Brazilian Society of Hepatology (SBH), and Brazilian Association for the Study of Obesity and Metabolic Syndrome (Abeso). Arch. Endocrinol. Metab. 2023, 67, e230123. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Torres-García, J.L.; Ahuactzin-Pérez, M.; Fernández, F.J.; Cortés-Espinosa, D.V. Bisphenol A in the Environment and Recent Advances in Biodegradation by Fungi. Chemosphere 2022, 303, 134940. [Google Scholar] [CrossRef]
- Pathak, R.K.; Jung, D.-W.; Shin, S.-H.; Ryu, B.-Y.; Lee, H.-S.; Kim, J.-M. Deciphering the Mechanisms and Interactions of the Endocrine Disruptor Bisphenol A and Its Analogs with the Androgen Receptor. J. Hazard. Mater. 2024, 469, 133935. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Harner, T. Bisphenol A and Its Analogues in Outdoor and Indoor Air: Properties, Sources and Global Levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Gravina, A.G.; Diano, N.; Errico, S.; Masarone, M.; Romeo, M.; Tuccillo, C.; Stiuso, P.; Morisco, F.; et al. The Bisphenol A Induced Oxidative Stress in Non-Alcoholic Fatty Liver Disease Male Patients: A Clinical Strategy to Antagonize the Progression of the Disease. Int. J. Environ. Res. Public Health 2020, 17, 3369. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Navarro-Martín, L.; van Antro, M.; Fuertes, I.; Casado, M.; Barata, C.; Piña, B. Changes in Lipid Profiles Induced by Bisphenol A (BPA) in Zebrafish Eleutheroembryos during the Yolk Sac Absorption Stage. Chemosphere 2020, 246, 125704. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Jiang, Y.; Li, Y.; Wan, Y.; Liu, J.; Ma, Y.; Mao, Z.; Chang, H.; Li, G.; Xu, B.; et al. Early-Life Exposure to Bisphenol A Induces Liver Injury in Rats Involvement of Mitochondria-Mediated Apoptosis. PLoS ONE 2014, 9, e90443. [Google Scholar] [CrossRef]
- Linillos-Pradillo, B.; Rancan, L.; Paredes, S.D.; Schlumpf, M.; Lichtensteiger, W.; Vara, E.; Tresguerres, J.Á.F. Low Dose of BPA Induces Liver Injury through Oxidative Stress, Inflammation and Apoptosis in Long–Evans Lactating Rats and Its Perinatal Effect on Female PND6 Offspring. Int. J. Mol. Sci. 2023, 24, 4585. [Google Scholar] [CrossRef]
- Santangeli, S.; Notarstefano, V.; Maradonna, F.; Giorgini, E.; Gioacchini, G.; Forner-Piquer, I.; Habibi, H.R.; Carnevali, O. Effects of Diethylene Glycol Dibenzoate and Bisphenol A on the Lipid Metabolism of Danio rerio. Sci. Total Environ. 2018, 636, 641–655. [Google Scholar] [CrossRef]
- Zhu, Z.; Long, X.; Wang, J.; Cao, Q.; Yang, H.; Zhang, Y. Bisphenol A Has a Sex-Dependent Disruptive Effect on Hepatic Lipid Metabolism in Zebrafish. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2023, 268, 109616. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, S.; Sujitha, M.V.; Alphonse, C.R.W.; Kalaiarasan, R.; Kannan, R.R. Bisphenol-A Alters Hematopoiesis through EGFR/ERK Signaling to Induce Myeloblastic Condition in Zebrafish Model. Sci. Total Environ. 2021, 787, 147530. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, D.; Wan, M.; Li, X.; Zhang, L.; Yang, D.; Liu, F.; Liu, J.; Li, K.; et al. BPA Induces Hepatotoxicity in Zebrafish through Oxidative Stress and Apoptosis Pathways. Fish Physiol. Biochem. 2024, 50, 403–412. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Srivastava, R.; Eswar, K.; Ramesh, S.S.R.; Prajapati, A.; Sonpipare, T.; Basa, A.; Gubige, M.; Ponnapalli, S.; Thatikonda, S.; Rengan, A.K. Zebrafish as a Versatile Model Organism: From Tanks to Treatment. MedComm–Future Med. 2025, 4, e70028. [Google Scholar] [CrossRef]
- Katoch, S.; Patial, V. Zebrafish: An Emerging Model System to Study Liver Diseases and Related Drug Discovery. J. Appl. Toxicol. 2021, 41, 33–51. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; Yang, J.; Liu, H.; Cao, Z.; He, Y.; Feng, D. Curcumin Protects against Bisphenol A-Induced Hepatic Steatosis by Inhibiting Cholesterol Absorption and Synthesis in CD-1 Mice. Food Sci. Nutr. 2023, 11, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Peña de la Sancha, P.; Wieser, B.I.; Schauer, S.; Reicher, H.; Sattler, W.; Breinbauer, R.; Schweiger, M.; Lechleitner, M.; Frank, S.; Zechner, R.; et al. Lipolysis-Derived Fatty Acids Are Needed for Homeostatic Control of Sterol Element-Binding Protein-1c Driven Hepatic Lipogenesis. Commun. Biol. 2025, 8, 588. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef]
- Moneva-Sakelarieva, M.; Kobakova, Y.; Konstantinov, S.; Momekov, G.; Ivanova, S.; Atanasova, V.; Chaneva, M.; Tododrov, R.; Bashev, N.; Atanasov, P. The Role of the Transcription Factor NF-kB in the Pathogenesis of Inflammation and Carcinogenesis. Modulation Capabilities. Pharmacia 2025, 72, 1–13. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Hebbard, L.; Ranscht, B. Multifaceted Roles of Adiponectin in Cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 59–69. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The Role of Glutathione Peroxidase-1 in Health and Disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, J.; Chen, L.; Li, S.; Yuan, M.; Tian, X.; Cao, P.; Qiu, Z. Ferroptosis as an Emerging Therapeutic Target in Liver Diseases. Front. Pharmacol. 2023, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, K.; Nie, Y.; Guo, Y.; Ma, Q. A Visual Electrochemiluminescence Biosensor Based on CuInZnS Quantum Dots for Superoxide Dismutase Detection. Anal. Bioanal. Chem. 2020, 412, 1893–1899. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Zhang, Y.; Zhang, Y.; Wang, W.; Han, H.; Yang, C.; Dong, X. Superoxide Dismutase Ameliorates Oxidative Stress and Regulates Liver Transcriptomics to Provide Therapeutic Benefits in Hepatic Inflammation. PeerJ 2023, 11, e15829. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Non-Alcoholic Fatty Liver Disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Sangro, P.; de la Torre Aláez, M.; Sangro, B.; D’Avola, D. Metabolic Dysfunction–Associated Fatty Liver Disease (MAFLD): An Update of the Recent Advances in Pharmacological Treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Zhi, X.; Du, L.; Zhang, P.; Guo, X.; Li, W.; Wang, Y.; He, Q.; Wu, P.; Lei, X.; Qu, B. BPA Induces Testicular Damage in Male Rodents via Apoptosis, Autophagy, and Ferroptosis. Food Chem. Toxicol. 2024, 193, 114984. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yan, S.; Meng, Z.; Huang, S.; Sun, W.; Jia, M.; Teng, M.; Zhou, Z.; Zhu, W. New Insights into Bisphenols Induced Obesity in Zebrafish (Danio rerio): Activation of Cannabinoid Receptor CB1. J. Hazard. Mater. 2021, 418, 126100. [Google Scholar] [CrossRef]
- Sun, L.; Ling, Y.; Jiang, J.; Wang, D.; Wang, J.; Li, J.; Wang, X.; Wang, H. Differential Mechanisms Regarding Triclosan vs. Bisphenol A and Fluorene-9-Bisphenol Induced Zebrafish Lipid-Metabolism Disorders by RNA-Seq. Chemosphere 2020, 251, 126318. [Google Scholar] [CrossRef]
- Li, C.-L.; Yao, Z.-Y.; Zhang, Y.-F.; Cui, X.-T.; Sun, A.; Cao, J.-Y.; Wang, Z.-S. Bisphenols Exposure and Non-Alcoholic Fatty Liver Disease: From Environmental Trigger to Molecular Pathogenesis. Front. Endocrinol. 2025, 16, 1606654. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Romeo, S.; Pajvani, U. A Genetic Hypothesis for Burnt-out Steatohepatitis. Liver Int. 2021, 41, 2816–2818. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Choudhary, N.S.; Mishra, S.K. Pathophysiological Mechanisms Underlying MAFLD. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1875–1887. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Yang, W.; Xiao, C.; Fu, S.; Deng, Q.; Ding, H.; Wang, Z.; Liu, G.; Li, X. SREBP-1c Overexpression Induces Triglycerides Accumulation through Increasing Lipid Synthesis and Decreasing Lipid Oxidation and VLDL Assembly in Bovine Hepatocytes. J. Steroid Biochem. Mol. Biol. 2014, 143, 174–182. [Google Scholar] [CrossRef]
- Su, F.; Koeberle, A. Regulation and Targeting of SREBP-1 in Hepatocellular Carcinoma. Cancer Metastasis Rev. 2024, 43, 673–708. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-zad, Z. Role of SREBPs in Liver Diseases: A Mini-Review. J. Clin. Transl. Hepatol. 2018, 6, 332–338. [Google Scholar] [CrossRef]
- Byrne, C.D. Fatty Liver: Role of Inflammation and Fatty Acid Nutrition. Prostagland. Leukot. Essent. Fat. Acids 2010, 82, 265–271. [Google Scholar] [CrossRef]
- Silva-Marrero, J.I.; Villasante, J.; Rashidpour, A.; Palma, M.; Fàbregas, A.; Almajano, M.P.; Viegas, I.; Jones, J.G.; Miñarro, M.; Ticó, J.R.; et al. The Administration of Chitosan-Tripolyphosphate-DNA Nanoparticles to Express Exogenous SREBP1a Enhances Conversion of Dietary Carbohydrates into Lipids in the Liver of Sparus Aurata. Biomolecules 2019, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Y.; Dunfield, J.; Roderique, T.; Ni, H.-M. Liver-Adipose Tissue Crosstalk in Alcohol-Associated Liver Disease: The Role of mTOR. Liver Res. 2022, 6, 227–237. [Google Scholar] [CrossRef]
- Wu, M.; Chen, P.; Wang, Y.; Wang, X.; Bao, Y.; Fan, L.; Rao, Y.; Song, X.; Zhang, J. Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae. Toxics 2025, 13, 449. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Q.; Chen, W.; Gao, Y.; Ye, J.; Chen, Y.; Wang, T.; Gao, L.; Liu, Y.; Yang, Y. New Advances of Adiponectin in Regulating Obesity and Related Metabolic Syndromes. J. Pharm. Anal. 2024, 14, 100913. [Google Scholar] [CrossRef]
- García García, M.; Picó, Y.; Morales-Suárez-Varela, M. Effects of Bisphenol A on the Risk of Developing Obesity. Nutrients 2024, 16, 3740. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted Disruption of AdipoR1 and AdipoR2 Causes Abrogation of Adiponectin Binding and Metabolic Actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible Overexpression of Adiponectin Receptors Highlight the Roles of Adiponectin-Induced Ceramidase Signaling in Lipid and Glucose Homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Gamberi, T.; Magherini, F.; Modesti, A.; Fiaschi, T. Adiponectin Signaling Pathways in Liver Diseases. Biomedicines 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Kawai, Y.K.; Yamashita, N.; Lee, J.S.; Kondoh, D.; Zhang, S.; Nishi, Y.; Suzuki, K.; Kitazawa, T.; Teraoka, H. Transcriptional Profiling of Cytochrome P450 Genes in the Liver of Adult Zebrafish, Danio rerio. J. Toxicol. Sci. 2019, 44, 347–356. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Y.; Xiong, Y.; Wu, J.; Wang, M.; Sun, X.; Ding, X.; Yang, L.; Sun, X.; Ge, G. CYP1A Inhibitors: Recent Progress, Current Challenges, and Future Perspectives. Med. Res. Rev. 2024, 44, 169–234. [Google Scholar] [CrossRef]
- Gu, Z.; Jia, R.; He, Q.; Cao, L.; Du, J.; Feng, W.; Jeney, G.; Xu, P.; Yin, G. Alteration of Lipid Metabolism, Autophagy, Apoptosis and Immune Response in the Liver of Common Carp (Cyprinus carpio) after Long-Term Exposure to Bisphenol A. Ecotoxicol. Environ. Saf. 2021, 211, 111923. [Google Scholar] [CrossRef]
- Jia, M.; Qin, D.; Zhao, C.; Chai, L.; Yu, Z.; Wang, W.; Tong, L.; Lv, L.; Wang, Y.; Rehwinkel, J.; et al. Redox Homeostasis Maintained by GPX4 Facilitates STING Activation. Nat. Immunol. 2020, 21, 727–735. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An Insight on Superoxide Dismutase (SOD) from Plants for Mammalian Health Enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, Ferroptosis, and Diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Schwarz, M.; Löser, A.; Cheng, Q.; Wichmann-Costaganna, M.; Schädel, P.; Werz, O.; Arnér, E.S.J.; Kipp, A.P. Side-by-Side Comparison of Recombinant Human Glutathione Peroxidases Identifies Overlapping Substrate Specificities for Soluble Hydroperoxides. Redox Biol. 2023, 59, 102593. [Google Scholar] [CrossRef]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A Marvel of High-Throughput Biology for 21st Century Toxicology. Curr. Environ. Health Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef]
- Anderson, J.L.; Carten, J.D.; Farber, S.A. Zebrafish Lipid Metabolism: From Mediating Early Patterning to the Metabolism of Dietary Fat and Cholesterol. Methods Cell. Biol. 2011, 101, 111–141. [Google Scholar] [CrossRef]
- Molina, A.M.; Lora, A.J.; Blanco, A.; Monterde, J.G.; Ayala, N.; Moyano, R. Endocrine-Active Compound Evaluation: Qualitative and Quantitative Histomorphological Assessment of Zebrafish Gonads after Bisphenol-A Exposure. Ecotoxicol. Environ. Saf. 2013, 88, 155–162. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Qin, J.; Wei, P.; Jia, Y.; Wang, J.; Ru, S. Long-Term Bisphenol S Exposure Induces Fat Accumulation in Liver of Adult Male Zebrafish (Danio rerio) and Slows Yolk Lipid Consumption in F1 Offspring. Chemosphere 2019, 221, 500–510. [Google Scholar] [CrossRef]
- Le Mentec, H.; Monniez, E.; Legrand, A.; Monvoisin, C.; Lagadic-Gossmann, D.; Podechard, N. A New In Vivo Zebrafish Bioassay Evaluating Liver Steatosis Identifies DDE as a Steatogenic Endocrine Disruptor, Partly through SCD1 Regulation. Int. J. Mol. Sci. 2023, 24, 3942. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Qian, M.; Zhang, B.; Deng, X.; Mao, P.; Fan, Z.; Fang, X.; Cheng, L.; Liu, X.; et al. Multi-Omics Analysis Reveals BPF Exposure Causes Hepatic Glucose and Lipid Metabolism Disorder in Rats by Disrupting Energy Homeostasis. Toxicology 2025, 515, 154130. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jiang, X.; Yin, J.; Jiang, R.; Zhu, J.; Zou, S. Bisphenol S Accelerates the Progression of High Fat Diet-Induced NAFLD by Triggering Ferroptosis via Regulating HMGCS2. J. Hazard. Mater. 2025, 487, 137166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Tao, J.; Wang, S.; Hu, M.; Wang, X.; Yu, Z.; Xu, L.; Lin, Y.; Wu, W.; et al. Mystery of Bisphenol F-Induced Nonalcoholic Fatty Liver Disease-like Changes: Roles of Drp1-Mediated Abnormal Mitochondrial Fission in Lipid Droplet Deposition. Sci. Total Environ. 2023, 904, 166831. [Google Scholar] [CrossRef]
- Moon, M.K. Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab. J. 2019, 43, 46–48. [Google Scholar] [CrossRef]
- Hyun, M.; Rathor, L.; Kim, H.-J.; McElroy, T.; Hwang, K.H.; Wohlgemuth, S.; Curry, S.; Xiao, R.; Leeuwenburgh, C.; Heo, J.-D.; et al. Comparative Toxicities of BPA, BPS, BPF, and TMBPF in the Nematode Caenorhabditis elegans and Mammalian Fibroblast Cells. Toxicology 2021, 461, 152924. [Google Scholar] [CrossRef]
- Sangwan, S.; Bhattacharyya, R.; Banerjee, D. Plastic Compounds and Liver Diseases: Whether Bisphenol A Is the Only Culprit. Liver Int. 2024, 44, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Eldefrawy, F.; Guo, K.M. Liver Toxicity Induced by Exposure to Bisphenol Analogs at Environmentally Relevant Levels: Insights from a Literature Review on Multiple Species. Livers 2025, 5, 24. [Google Scholar] [CrossRef]
- Ohko, Y.; Ando, I.; Niwa, C.; Tatsuma, T.; Yamamura, T.; Nakashima, T.; Kubota, Y.; Fujishima, A. Degradation of Bisphenol A in Water by TiO2 Photocatalyst. Environ. Sci. Technol. 2001, 35, 2365–2368. [Google Scholar] [CrossRef]
- Nath, A.; Biswas, S.; Pal, A. A Comprehensive Review on BPA Degradation by Heterogeneous Fenton-like Processes. Water Sci. Technol. 2022, 86, 714–745. [Google Scholar] [CrossRef]
- Chemical Study on Bisphenol A|Hydrotheek. Available online: https://library.wur.nl/WebQuery/hydrotheek/1630288 (accessed on 3 November 2025).
- Bukola Shittu, F.; Iqbal, A.; Norazmi Ahmad, M.; Rahimi Yusop, M.; Mohamad Ibrahim, M.N.; Sabar, S.; Wilson, L.D.; Yuli Yanto, D.H. Insight into the Photodegradation Mechanism of Bisphenol-A by Oxygen Doped Mesoporous Carbon Nitride under Visible Light Irradiation and DFT Calculations. RSC Adv. 2022, 12, 10409–10423. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A Review of the Environmental Fate, Effects, and Exposures of Bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Reis, L.d.P.G.; Lora-Benítez, A.J.; Molina-López, A.M.; Mora-Medina, R.; Ayala-Soldado, N.; Moyano-Salvago, M.d.R. Evaluation of the Toxicity of Bisphenol A in Reproduction and Its Effect on Fertility and Embryonic Development in the Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2022, 19, 962. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 2007. Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 21 October 2025).
- Zainuddin, A.H.; Roslan, M.Q.J.; Razak, M.R.; Yusoff, F.M.; Haron, D.E.M.; Aris, A.Z. Occurrence, Distribution, and Ecological Risk of Bisphenol Analogues in Marine Ecosystem of Urbanized Coast and Estuary. Mar. Pollut. Bull. 2023, 192, 115019. [Google Scholar] [CrossRef] [PubMed]
- Fabrello, J.; Matozzo, V. Bisphenol Analogs in Aquatic Environments and Their Effects on Marine Species—A Review. J. Mar. Sci. Eng. 2022, 10, 1271. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U. Endocrine Disrupting Compounds (Nonylphenol and Bisphenol A)–Sources, Harmfulness and Laccase-Assisted Degradation in the Aquatic Environment. Microorganisms 2022, 10, 2236. [Google Scholar] [CrossRef]
- Kang, J.-H.; Aasi, D.; Katayama, Y. Bisphenol A in the Aquatic Environment and Its Endocrine-Disruptive Effects on Aquatic Organisms. Crit. Rev. Toxicol. 2007, 37, 607–625. [Google Scholar] [CrossRef]
- Liu, D.; Kang, G.; Zhang, Y.; Shi, L.; Ma, B.; Zhang, S.; Lu, G. Exploring the Distribution and Fate of Bisphenol A in an Aquatic Microcosm Combined with a Multimedia Model. Ecotoxicol. Environ. Saf. 2025, 290, 117752. [Google Scholar] [CrossRef]
- Smorodinskaya, S.; Kochetkov, N.; Gavrilin, K.; Nikiforov-Nikishin, D.; Reznikova, D.; Vatlin, A.; Klimuk, A.; Odorskaya, M.; Nikiforov-Nikishin, A.; Ponomarev, A.; et al. The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio). Animals 2023, 13, 3685. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.X.; Vieira, L.; Souza, J.A.C.R.; Silva, M.G.F.; Muniz, M.S.; Souza, T.; Queiroga, F.R.; Machado, M.R.F.; da Silva, P.M.; Farias, D. Exposure to 2,4-D Herbicide Induces Hepatotoxicity in Zebrafish Larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109110. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lechón, M.J.; Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Castell, J.V.; O’Connor, J.-E. A Human Hepatocellular in Vitro Model to Investigate Steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, Z.; Ran, C.; Ding, Q.; Liu, H.; Xie, M.; Yang, Y.; Xie, Y.; Gao, C.; Zhang, H.; Zhou, Z. Ability of Prebiotic Polysaccharides to Activate a HIF1α-Antimicrobial Peptide Axis Determines Liver Injury Risk in Zebrafish. Commun. Biol. 2019, 2, 274. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Harper, S.L.; Yun, S.-I. In Vivo Toxicological Assessment of Biologically Synthesized Silver Nanoparticles in Adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef]
- Pettem, C.M.; Weber, L.P.; Janz, D.M. Cardiac and Metabolic Effects of Dietary Selenomethionine Exposure in Adult Zebrafish. Toxicol. Sci. 2017, 159, 449–460. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, P.-D.; Quetin-Leclercq, J.; Muller, M.; Ly Huong, D.T.; Pham, H.T.; Kestemont, P. Developmental Toxicity of Clerodendrum Cyrtophyllum Turcz Ethanol Extract in Zebrafish Embryo. J. Ethnopharmacol. 2021, 267, 113538. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Abbate, F.; Navarra, M.; Guerrera, M.C.; Laura, R.; Vega, J.A.; Levanti, M.; Germanà, A. Melatonin Treatment Suppresses Appetite Genes and Improves Adipose Tissue Plasticity in Diet-Induced Obese Zebrafish. Endocrine 2018, 62, 381–393. [Google Scholar] [CrossRef]
- Safari, R.; Roosta, Z.; Vakili, F.; Rahmani, E.; Sakhawat Hossain, M.; Raeisi, M.; Van Doan, H.; Paolucci, M.; Hoseinifar, S.H. Dietary Dragonhead Effects on Growth, Immunity and Antioxidant and Related Genes Expression in Zebrafish (Danio rerio). Aquac. Rep. 2022, 27, 101384. [Google Scholar] [CrossRef]


| Gene | FP Sequence (5′-3′) | RP Sequence (5′-3′) |
|---|---|---|
| 18s [11] | TCGAATGTCTGCCCTATCAACT | AGACTTGCCCTCCAATGGATC |
| acc1 [87] | GCGTGGCCGAACAATGGCAG | GCAGGTCCAGCTTCCCTGCG |
| fas [87] | GGAGCAGGCTGCCTCTGTGC | TTGCGGCCTGTCCCACTCCT |
| srebp-1c [87] | CAGAGGGTGGGCATGCTGGC | CAGAGGGTGGGCATGCTGGC |
| il-6 [87] | TCAACTTCTCCAGCGTGATG | TCTTTCCCTCTTTTCCTCCTG |
| cyp2ad2 [50] | CCCCAGACACTTTCAACC | AGAGCACATTACGAGCCA |
| cyp1a [50] | AAGTTGGAAGGCGAGAAGG | GCCAGGAACAGGAAGACTTC |
| Nfkb [88] | AGAGAGCGCTTGCGTCCTT | TTGCCTTTGGTTTTTCGGTAA |
| adipor2 [54] | GACCCCACCCAAACATCA | CCTCCTCGCATGAAGACAGT |
| gpx1 [89] | AGCATGGCAGGAACCATGAA | GAAGCCATTTCCAGGACGGA |
| gpx4 [90] | TGAGAAGGGTTTACGCATCCTG | TGTTGTTCCCCAGTGTTCCT |
| β–actin [91] | AAGATCAAGATCATTGCTCC | CCAGACTCATCGTACTCCT |
| Sod [92] | GGGTGGCAATGAGGAAAG | GCCCACATAGAAATGCACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salau, E.A.d.H.; Diglio, D.; Guimarães, G.R.; Furtado-Filho, O.V.; Porawski, M. Bisphenol A Alters the Expression of Genes Involved in Lipogenesis, Inflammation, and Oxidative Stress in the Liver of Adult Zebrafish. Pharmaceuticals 2025, 18, 1765. https://doi.org/10.3390/ph18111765
Salau EAdH, Diglio D, Guimarães GR, Furtado-Filho OV, Porawski M. Bisphenol A Alters the Expression of Genes Involved in Lipogenesis, Inflammation, and Oxidative Stress in the Liver of Adult Zebrafish. Pharmaceuticals. 2025; 18(11):1765. https://doi.org/10.3390/ph18111765
Chicago/Turabian StyleSalau, Eronides Anathan de Heberle, Daniela Diglio, Giuliano Rizzotto Guimarães, Orlando Vieira Furtado-Filho, and Marilene Porawski. 2025. "Bisphenol A Alters the Expression of Genes Involved in Lipogenesis, Inflammation, and Oxidative Stress in the Liver of Adult Zebrafish" Pharmaceuticals 18, no. 11: 1765. https://doi.org/10.3390/ph18111765
APA StyleSalau, E. A. d. H., Diglio, D., Guimarães, G. R., Furtado-Filho, O. V., & Porawski, M. (2025). Bisphenol A Alters the Expression of Genes Involved in Lipogenesis, Inflammation, and Oxidative Stress in the Liver of Adult Zebrafish. Pharmaceuticals, 18(11), 1765. https://doi.org/10.3390/ph18111765






