Targeting the NF-κB Pathway in Cancer: Mechanisms, Resistance, and Therapeutic Potential Across Tumor Types
Abstract
1. Introduction
2. Methods
3. Overview of NF-κB Pathway
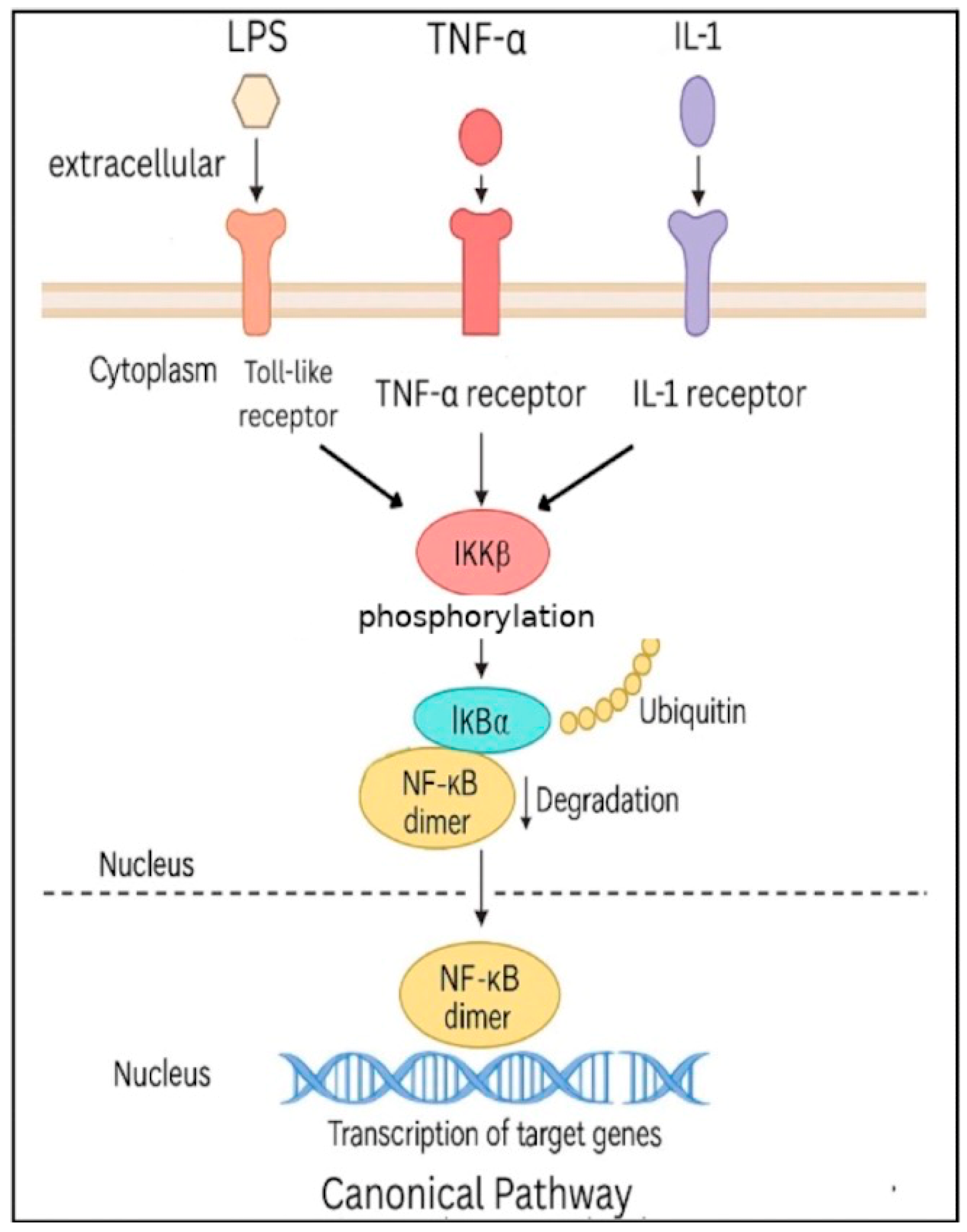
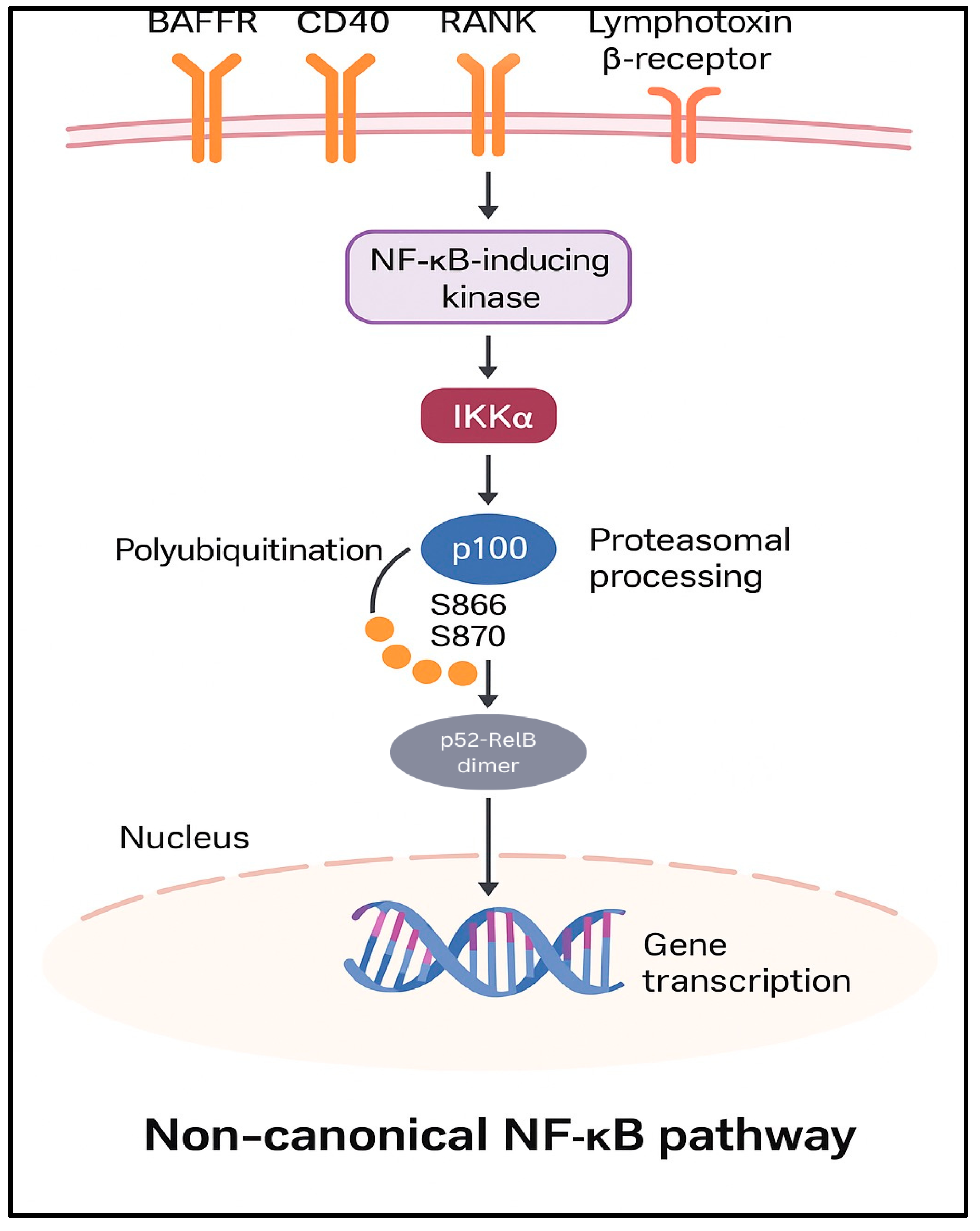
3.1. Canonical and Non-Canonical Pathways
3.2. Role of NF-κB in Different Cellular Pathways
3.3. Role of NF-κB in Cancer Progression
4. NF-κB in Cancer
4.1. NF-κB and Breast Cancer
4.2. NF-κB and Colorectal Cancer
4.3. NF-κB and Lung Cancer
4.4. NF-κB and Melanoma
4.5. NF-κB and Prostate Cancer
4.6. NF-κB and Other Cancers
4.6.1. Gastric Cancer
4.6.2. Nasopharyngeal Cancer
4.6.3. Bladder Cancer
4.6.4. Osteosarcoma, Cervical, and Bone Marrow Cancers
4.6.5. Multiple Cancers and Emerging Therapies
4.6.6. Summary
5. Chemoresistance
6. Strategies for Targeting the NF-κB Pathway to Improve Therapeutic Outcomes
NF-κB and Modulation of the Tumor Microenvironment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ACE | Angiotensin-converting enzyme |
| AKBA | Acetyl-keto-beta boswellic acid |
| AS | Artesunate |
| Bic | Bicalutamide |
| BET | Bromodomain and extra-terminal domain |
| BXD | Banxia Xiexin Decoction |
| CAF | Cancer-associated fibroblasts |
| COX | Cyclooxygenase |
| CRPC | Castrate-resistant prostate cancer |
| DiHEP | Docosahexaenoic acid derivative |
| DMAPT | Dimethylamino parthenolide |
| EBV | Epstein–Barr Virus |
| EGFR | Epidermal growth factor receptor |
| ENZ | Enzalutamide |
| H. pylori | Helicobacter pylori |
| ICI | Immune checkpoint inhibitor |
| IKK | Inhibitory-kappa B kinase |
| IL-1 | Interleukin-1 |
| IL-1R | Interleukin-1 Receptor |
| LMP1 | Latent Membrane Protein 1 |
| LPS | Lipopolysaccharides |
| NF-κB | Nuclear factor kappa B |
| NPC | Nasopharyngeal carcinoma |
| NSCLC | Non-small cell lung cancer |
| PKC | Protein kinase C |
| PTX | Paclitaxel |
| SPP1 | Secreted phosphoprotein 1 |
| TME | Tumor microenvironment |
| TKI | Tyrosine kinase inhibitors |
| TLRs | Toll-like receptors |
| TRIM | Tripartite Motif |
| TNBC | Triple-negative breast cancer |
| TNF-a | Tumor necrosis factor-alpha |
| TNFR | Tumor necrosis factor receptors |
| TRAIL | TNF-related apoptosis-inducing ligand |
| UPP1 | Uridine phosphorylase 1 |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes. Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef]
- Brown, M.; Cohen, J.; Arun, P.; Chen, Z.; Van Waes, C. NF-kappaB in carcinoma therapy and prevention. Expert Opin. Ther. Targets 2008, 12, 1109–1122. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, T.; Fujikawa, K.; Nishio, Y.; Mukaida, N.; Matsushima, K.; Kishimoto, T.; Akira, S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 1993, 90, 10193–10197. [Google Scholar] [CrossRef]
- Fonseca, J.E.; Santos, M.J.; Canhão, H.; Choy, E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun. Rev. 2009, 8, 538–542. [Google Scholar] [CrossRef]
- Tahmasbi, M.; Karimpour, A.; Rashidi, M.; Zangooei, M.; Khedri, A.; Panahi, G. Nobiletin can play a role in improving inflammation by inhibiting the NF-kB and MAPK pathways in muscle cells. J. Diabetes Metab. Disord. 2025, 24, 166. [Google Scholar] [CrossRef] [PubMed]
- Burkly, L.; Hession, C.; Ogata, L.; Reilly, C.; Marconi, L.A.; Olson, D.; Tizard, R.; Cate, R.; Lo, D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 1995, 373, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Cheng, S.; Pear, W.S.; Liou, H.C. NF-kB inhibitor blocks B cell development at two checkpoints. Med. Immunol. 2004, 3, 1. [Google Scholar] [CrossRef][Green Version]
- Grumont, R.J.; Rourke, I.J.; O’Reilly, L.A.; Strasser, A.; Miyake, K.; Sha, W.; Gerondakis, S. B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J. Exp. Med. 1998, 187, 663–674. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Sarosiek, S.; Castillo, J.J. Waldenström Macroglobulinemia: Targeted Agents Taking Center Stage. Drugs 2024, 84, 17–25. [Google Scholar] [CrossRef]
- Verzella, D.; Cornice, J.; Arboretto, P.; Vecchiotti, D.; Di Vito Nolfi, M.; Capece, D.; Zazzeroni, F.; Franzoso, G. The NF-κB Pharmacopeia: Novel Strategies to Subdue an Intractable Target. Biomedicines 2022, 10, 2233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Jin, F.; Li, J.G.; Xu, Y.Y.; Dong, H.T.; Liu, Q.; Xing, P.; Zhu, G.L.; Xu, H.; Yin, S.C.; et al. TRIM32 promotes proliferation and confers chemoresistance to breast cancer cells through activation of the NF-κB pathway. J. Cancer 2018, 9, 1349–1356. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Li, G.; Rao, C. The Role of NF-κB/MIR155HG in Regulating the Stemness and Radioresistance in Breast Cancer Stem Cells. Front. Biosci. 2025, 30, 25810. [Google Scholar] [CrossRef]
- Sun, S.; Jing, X.; Tong, G.; Chen, C.; Xie, S.; Wang, C.; Chen, D.; Zhao, J.; Qi, Y.; Zhang, W.; et al. Loss of DDX24 inhibits lung cancer progression by stimulating IKBKG splicing-mediated autophagy. Theranostics 2025, 15, 1879–1895. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Lv, C.; Qu, H.; Zhu, W.; Xu, K.; Xu, A.; Jia, B.; Qing, Y.; Li, H.; Wei, H.J.; Zhao, H.Y. Low-Dose Paclitaxel Inhibits Tumor Cell Growth by Regulating Glutaminolysis in Colorectal Carcinoma Cells. Front. Pharmacol. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Lorito, N.; Subbiani, A.; Smiriglia, A.; Bacci, M.; Bonechi, F.; Tronci, L.; Romano, E.; Corrado, A.; Longo, D.L.; Iozzo, M.; et al. FADS1/2 control lipid metabolism and ferroptosis susceptibility in triple-negative breast cancer. EMBO Mol. Med. 2024, 16, 1533–1559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, C.; Jiang, C.; Liu, N.; Yang, Z.; Xing, H. RSL3 induces ferroptosis by activating the NF-κB signalling pathway to enhance the chemosensitivity of triple-negative breast cancer cells to paclitaxel. Sci. Rep. 2025, 15, 1654. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Pal, S.; Singh, R.; Ahmad, K.; Dey, N.; Srivastava, A.; Ahmad, R.; Suliman, M.; Alshahrani, M.Y.; Barkat, M.A.; et al. Antimicrobial peptide moricin induces ROS mediated caspase-dependent apoptosis in human triple-negative breast cancer via suppression of notch pathway. Cancer Cell Int. 2023, 23, 121. [Google Scholar] [CrossRef]
- Sheng, Y.H.; He, Y.; Hasnain, S.Z.; Wang, R.; Tong, H.; Clarke, D.T.; Lourie, R.; Oancea, I.; Wong, K.Y.; Lumley, J.W.; et al. MUC13 protects colorectal cancer cells from death by activating the NF-κB pathway and is a potential therapeutic target. Oncogene 2017, 36, 700–713. [Google Scholar] [CrossRef]
- Chen, J.; Stark, L.A. Aspirin Prevention of Colorectal Cancer: Focus on NF-κB Signalling and the Nucleolus. Biomedicines 2017, 5, 43. [Google Scholar] [CrossRef]
- Airoldi, M.; Bartolini, M.; Fazio, R.; Farinatti, S.; Daprà, V.; Santoro, A.; Puccini, A. First-Line Therapy in Metastatic, RAS Wild-Type, Left-Sided Colorectal Cancer: Should Everyone Receive Anti-EGFR Therapy? Curr. Oncol. Rep. 2024, 26, 1489–1501. [Google Scholar] [CrossRef]
- Romaniello, D.; Dall’Olio, L.; Mazzeschi, M.; Francia, A.; Pagano, F.; Gelfo, V.; D’Uva, G.; Giampieri, E.; Lauriola, M. NF-kB oscillation profiles decode response to anti-EGFR monoclonal antibodies. SLAS Discov. 2025, 31, 100219. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Zhao, D.; Tian, X.; Zou, J.; Yu, J. Type I Diabetes Mellitus impairs cytotoxic immunity through CEACAM5 upregulation in colorectal cancer: Exploring the intersection of autoimmune dysfunction and cancer progression: The role of NF-κB p65 in colorectal cancer. J. Mol. Histol. 2024, 55, 1285–1293. [Google Scholar] [CrossRef]
- Gupta, B.K.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Koch, M.D.; Lynch, D.W.; Bohlmeyer, T.; Watanabe, A.; Aburatani, H.; Puumala, S.E.; et al. Increased expression and aberrant localization of mucin 13 in metastatic colon cancer. J. Histochem. Cytochem. 2012, 60, 822–831. [Google Scholar] [CrossRef]
- Su, Y.; Choi, H.S.; Choi, J.H.; Kim, H.S.; Jang, Y.S.; Seo, J.W. 7S,15R-Dihydroxy-16S,17S-epoxy-docosapentaenoic Acid Overcomes Chemoresistance of 5-Fluorouracil by Suppressing the Infiltration of Tumor-Associated Macrophages and Inhibiting the Activation of Cancer Stem Cells in a Colorectal Cancer Xenograft Model. Mar. Drugs 2023, 21, 80. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Xu, Y.; Liu, Y.; Li, W.; Cai, J.; Zhang, Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wang, J.M.; Lai, H.Z.; Xiao, C.; You, F.M.; Kuang, Q.X.; Jiang, Y.F. Banxia Xiexin Decoction suppresses malignant phenotypes of colon cancer cells via PARG/PARP1/NF-κB signaling pathway. Zhongguo Zhong Yao Za Zhi 2025, 50, 496–506. [Google Scholar] [CrossRef]
- Priyadarshi, K.; Shirsath, K.; Waghela, N.B.; Sharma, A.; Kumar, A.; Pathak, C. Surface modified PAMAM dendrimers with gallic acid inhibit, cell proliferation, cell migration and inflammatory response to augment apoptotic cell death in human colon carcinoma cells. J. Biomol. Struct. Dyn. 2021, 39, 6853–6869. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, W.; Du, B.; Zang, S.; Wang, X.; Mao, X.; Hu, Z. TRIM32 overexpression improves chemoresistance through regulation of mitochondrial function in non-small-cell lung cancers. OncoTargets Ther. 2018, 11, 7841–7852. [Google Scholar] [CrossRef]
- Qu, H.; Fang, Y.; Zhang, F.; Liu, W.; Xia, S.; Duan, W.; Zou, K. CD146 promotes resistance of NSCLC brain metastases to pemetrexed via the NF-κB signaling pathway. Front. Pharmacol. 2024, 15, 1502165. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.; Jo, A.; Eum, H.H.; Kim, H.K.; Lee, K.; Cho, J.H.; Ku, B.M.; Jung, H.A.; Sun, J.M.; et al. Unveiling the influence of tumor and immune signatures on immune checkpoint therapy in advanced lung cancer. Elife 2024, 13, RP98366. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zeng, F.; Su, J.; Zhao, S.; Hu, R.; Zhu, W.; Hu, S.; Chen, X.; Yin, M. BET inhibitor suppresses melanoma progression via the noncanonical NF-κB/SPP1 pathway. Theranostics 2020, 10, 11428–11443. [Google Scholar] [CrossRef]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Rogut, M.; Mielczarek-Lewandowska, A.; Wozniak, M.; Czyz, M. 17-Aminogeldanamycin Inhibits Constitutive Nuclear Factor-Kappa B (NF-κB) Activity in Patient-Derived Melanoma Cell Lines. Int. J. Mol. Sci. 2020, 21, 3749. [Google Scholar] [CrossRef]
- Rodriguez-Baena, F.J.; Marquez-Galera, A.; Ballesteros-Martinez, P.; Castillo, A.; Diaz, E.; Moreno-Bueno, G.; Lopez-Atalaya, J.P.; Sanchez-Laorden, B. Microglial reprogramming enhances antitumor immunity and immunotherapy response in melanoma brain metastases. Cancer Cell 2025, 43, 413–427.e419. [Google Scholar] [CrossRef]
- Amiri, K.I.; Richmond, A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005, 24, 301–313. [Google Scholar] [CrossRef]
- Barzegar-Fallah, A.; Alimoradi, H.; Dunlop, J.L.; Torbati, E.; Baird, S.K. Serotonin type-3 receptor antagonists selectively kill melanoma cells through classical apoptosis, microtubule depolymerisation, ERK activation, and NF-κB downregulation. Cell Biol. Toxicol. 2023, 39, 1119–1135. [Google Scholar] [CrossRef]
- Ferraz, C.A.A.; Grougnet, R.; Nicolau, E.; Picot, L.; de Oliveira Junior, R.G. Carotenoids from Marine Microalgae as Antimelanoma Agents. Mar. Drugs 2022, 20, 618. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.Y.; Dreyer, J.; Emran, A.A.; Gunatilake, D.; Pirozyan, M.; Cullinane, C.; Dutton-Regester, K.; Rizos, H.; Hayward, N.K.; McArthur, G.; et al. Co-targeting bromodomain and extra-terminal proteins and MCL1 induces synergistic cell death in melanoma. Int. J. Cancer 2020, 147, 2176–2189. [Google Scholar] [CrossRef]
- Tudor, D.V.; Bâldea, I.; Olteanu, D.E.; Fischer-Fodor, E.; Piroska, V.; Lupu, M.; Călinici, T.; Decea, R.M.; Filip, G.A. Celecoxib as a Valuable Adjuvant in Cutaneous Melanoma Treated with Trametinib. Int. J. Mol. Sci. 2021, 22, 4387. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 25 May 2025).
- Jin, R.; Yi, Y.; Yull, F.E.; Blackwell, T.S.; Clark, P.E.; Koyama, T.; Smith, J.A., Jr.; Matusik, R.J. NF-κB gene signature predicts prostate cancer progression. Cancer Res. 2014, 74, 2763–2772. [Google Scholar] [CrossRef]
- Liang, Y.; Jeganathan, S.; Marastoni, S.; Sharp, A.; Figueiredo, I.; Marcellus, R.; Mawson, A.; Shalev, Z.; Pesic, A.; Sweet, J.; et al. Emergence of Enzalutamide Resistance in Prostate Cancer is Associated with BCL-2 and IKKB Dependencies. Clin. Cancer Res. 2021, 27, 2340–2351. [Google Scholar] [CrossRef]
- Kulasegaran, T.; Oliveira, N. Metastatic Castration-Resistant Prostate Cancer: Advances in Treatment and Symptom Management. Curr. Treat Options Oncol. 2024, 25, 914–931. [Google Scholar] [CrossRef]
- Morel, K.L.; Hamid, A.A.; Clohessy, J.G.; Pandell, N.; Ellis, L.; Sweeney, C.J. NF-κB Blockade with Oral Administration of Dimethylaminoparthenolide (DMAPT), Delays Prostate Cancer Resistance to Androgen Receptor (AR) Inhibition and Inhibits AR Variants. Mol. Cancer Res. 2021, 19, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.J.; Pandey, S.K.; Yadav, A.; Goel, S.; Ateeq, B. Targeting NF-kappa B Signaling by Artesunate Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 2017, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Kiran, M.; Kuscu, C.; Chatrath, A.; Wotton, D.; Mayo, M.W.; Dutta, A. Long Noncoding RNA DRAIC Inhibits Prostate Cancer Progression by Interacting with IKK to Inhibit NF-κB Activation. Cancer Res. 2020, 80, 950–963. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, T.C.; van Aswegen, C.H.; du Plessis, D.J. Influence of acetylsalicylic acid and metabolites on DU-145 prostatic cancer cell proliferation. Oncology 1995, 52, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Brusselaers, N. Maintenance use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk. Prostate Cancer Prostatic Dis. 2018, 21, 147–152. [Google Scholar] [CrossRef]
- Assayag, J.; Pollak, M.N.; Azoulay, L. The use of aspirin and the risk of mortality in patients with prostate cancer. J. Urol. 2015, 193, 1220–1225. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.Q.; Xie, L.; Wang, J.; Li, T.; He, Y.; Gao, Y.; Qin, X.; Li, S. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: A systematic review and meta-analysis. BMC Med. 2014, 12, 55. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, B.; Wang, S.; Jiang, X.; Ping, Y.; Xia, J.; Yu, F.; Li, Y.; Zhang, M.; Ding, Y. Intestinal metaplasia key molecules and UPP1 activation via Helicobacter pylori /NF-kB: Drivers of malignant progression in gastric cancer. Cancer Cell Int. 2024, 24, 399. [Google Scholar] [CrossRef]
- Wan, L.; Cao, D.; Zeng, J.; Yan, R.; Pizzorno, G. Modulation of uridine phosphorylase gene expression by tumor necrosis factor-alpha enhances the antiproliferative activity of the capecitabine intermediate 5′-deoxy-5-fluorouridine in breast cancer cells. Mol. Pharmacol. 2006, 69, 1389–1395. [Google Scholar] [CrossRef]
- Zhai, J.; Shen, J.; Xie, G.; Wu, J.; He, M.; Gao, L.; Zhang, Y.; Yao, X.; Shen, L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019, 454, 37–43. [Google Scholar] [CrossRef]
- Kong, F.B.; Deng, Q.M.; Deng, H.Q.; Dong, C.C.; Li, L.; He, C.G.; Wang, X.T.; Xu, S.; Mai, W. Siva-1 regulates multidrug resistance of gastric cancer by targeting MDR1 and MRP1 via the NF-κB pathway. Mol. Med. Rep. 2020, 22, 1558–1566. [Google Scholar] [CrossRef]
- Al-Bahlani, S.; Burney, I.A.; Al-Dhahli, B.; Al-Kharusi, S.; Al-Kharousi, F.; Al-Kalbani, A.; Ahmed, I. Boswellic acid sensitizes gastric cancer cells to Cisplatin-induced apoptosis via p53-mediated pathway. BMC Pharmacol. Toxicol. 2020, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.; Lo, K.W.; Tsao, S.W.; Wong, H.L.; Hui, J.W.; To, K.F.; Hayward, D.S.; Chui, Y.L.; Lau, Y.L.; Takada, K.; et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 2006, 8, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, R.J.; Tong, S.; Zhang, G.; Zong, J.; Chen, Y.; Jin, D.Y.; Chen, M.R.; Pan, J.; Chen, H. NF-κB Signaling Regulates Expression of Epstein-Barr Virus BART MicroRNAs and Long Noncoding RNAs in Nasopharyngeal Carcinoma. J. Virol. 2016, 90, 6475–6488. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, L.; Lin, W.; Yip, Y.L.; Lo, K.W.; Lau, V.M.Y.; Zhu, D.; Tsang, C.M.; Zhou, Y.; Deng, W.; et al. Epstein-Barr Virus-Encoded Latent Membrane Protein 1 Upregulates Glucose Transporter 1 Transcription via the mTORC1/NF-κB Signaling Pathways. J. Virol. 2017, 91, e02168-16. [Google Scholar] [CrossRef]
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Liu, S.Y.; Wu, B. TRIM29 Overexpression Promotes Proliferation and Survival of Bladder Cancer Cells through NF-κB Signaling. Cancer Res. Treat 2016, 48, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, X.; Chen, Y.; Han, Y.; Yu, W.; Zhang, S.; Yang, B.; Zhang, J.; Chen, Y. An unfolded protein response (UPR)-signature regulated by the NFKB-miR-29b/c axis fosters tumor aggressiveness and poor survival in bladder cancer. Front. Mol. Biosci. 2025, 12, 1542650. [Google Scholar] [CrossRef]
- Giliberti, G.; Marrapodi, M.M.; Di Feo, G.; Pota, E.; Di Martino, M.; Di Pinto, D.; Rossi, F.; Di Paola, A. Curcumin and Methotrexate: A Promising Combination for Osteosarcoma Treatment via Hedgehog Pathway Inhibition. Int. J. Mol. Sci. 2024, 25, 11300. [Google Scholar] [CrossRef]
- Ou, J.; Meng, F.; Liu, J.; Li, D.; Cao, H.; Sun, B. Ovatodiolide exerts anticancer effects on human cervical cancer cells via mitotic catastrophe, apoptosis and inhibition of NF-kB pathway. J. Buon. 2020, 25, 87–92. [Google Scholar]
- Pearson, S.; Blance, R.; Yan, F.; Hsieh, Y.C.; Geary, B.; Amaral, F.M.R.; Somervaille, T.C.P.; Kirschner, K.; Whetton, A.D.; Pierce, A. Identification of curaxin as a potential new therapeutic for JAK2 V617F mutant patients. PLoS ONE 2023, 18, e0286412. [Google Scholar] [CrossRef]
- Batool, S.; Asim, L.; Qureshi, F.R.; Masood, A.; Mushtaq, M.; Saleem, R.S.Z. Molecular Targets of Plant-based Alkaloids and Polyphenolics in Liver and Breast Cancer- An Insight into Anticancer Drug Development. Anticancer Agents Med. Chem. 2025, 25, 295–312. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Tan, T.Z.; Ampasala, D.R.; Kumar, A.P. Peruvoside targets apoptosis and autophagy through MAPK Wnt/β-catenin and PI3K/AKT/mTOR signaling pathways in human cancers. Life Sci. 2020, 241, 117147. [Google Scholar] [CrossRef]
- Kato, S.; Adashek, J.J.; Subbiah, V.; Fu, S.; Sun, M.; Nguyen, L.; Brown, E.J.; Yap, T.A.; Karp, D.D.; Piha-Paul, S.A.; et al. A phase i study of ixazomib and erlotinib in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 99–105. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sedukhina, A.S.; Okamoto, N.; Nagasawa, S.; Suzuki, N.; Ohta, T.; Hattori, H.; Roche-Molina, M.; Narváez, A.J.; Jeyasekharan, A.D.; et al. NF-κB signaling mediates acquired resistance after PARP inhibition. Oncotarget 2015, 6, 3825–3839. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Middleton, J.; Kim, T.; Laganà, A.; Piovan, C.; Secchiero, P.; Nuovo, G.J.; Cui, R.; Joshi, P.; Romano, G.; et al. A set of NF-κB-regulated microRNAs induces acquired TRAIL resistance in lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E3355–E3364. [Google Scholar] [CrossRef]
- Galvani, E.; Sun, J.; Leon, L.G.; Sciarrillo, R.; Narayan, R.S.; Sjin, R.T.; Lee, K.; Ohashi, K.; Heideman, D.A.; Alfieri, R.R.; et al. NF-κB drives acquired resistance to a novel mutant-selective EGFR inhibitor. Oncotarget 2015, 6, 42717–42732. [Google Scholar] [CrossRef]
- Cao, Y.; Yi, Y.; Han, C.; Shi, B. NF-κB signaling pathway in tumor microenvironment. Front. Immunol. 2024, 15, 1476030. [Google Scholar] [CrossRef]
- Atanasova, V.S.; de Jesus Cardona, C.; Hejret, V.; Tiefenbacher, A.; Mair, T.; Tran, L.; Pfneissl, J.; Draganić, K.; Binder, C.; Kabiljo, J.; et al. Mimicking Tumor Cell Heterogeneity of Colorectal Cancer in a Patient-derived Organoid-Fibroblast Model. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1391–1419. [Google Scholar] [CrossRef]
- Yang, J.P.; Kulkarni, N.N.; Yamaji, M.; Shiraishi, T.; Pham, T.; Do, H.; Aiello, N.; Shaw, M.; Nakamura, T.; Abiru, A.; et al. Unveiling immune cell response disparities in human primary cancer-associated fibroblasts between two- and three-dimensional cultures. PLoS ONE 2024, 19, e0314227. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, P.; Wang, Y.; Si, Y.; Yin, D.; Weber, C.R.; Fishel, M.L.; Pollok, K.E.; Qiu, B.; Xiao, F.; et al. A novel triptolide analog downregulates NF-κB and induces mitochondrial apoptosis pathways in human pancreatic cancer. Elife 2023, 12, e85862. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Xu, T.; Zhang, Q.; Ma, T.; Li, S.; Wang, X. A comprehensive multi-omics analysis identifies a robust scoring system for cancer-associated fibroblasts and intervention targets in colorectal cancer. J. Cancer Res. Clin. Oncol. 2024, 150, 124. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cai, Q.; Wang, L.; Ji, J.; Sun, Y.; Jiang, J.; Wang, C.; Wu, J.; Zhang, B.; Zhao, L.; et al. Paracrine activin B-NF-κB signaling shapes an inflammatory tumor microenvironment in gastric cancer via fibroblast reprogramming. J. Exp. Clin. Cancer Res. 2023, 42, 269. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem. Cell Res. Ther. 2015, 6, 87. [Google Scholar] [CrossRef]
- Hernandez, R.; Zhou, C. Recent Advances in Understanding the Role of IKKβ in Cardiometabolic Diseases. Front. Cardiovasc. Med. 2021, 8, 752337. [Google Scholar] [CrossRef]
- Fridmacher, V.; Kaltschmidt, B.; Goudeau, B.; Ndiaye, D.; Rossi, F.M.; Pfeiffer, J.; Kaltschmidt, C.; Israël, A.; Mémet, S. Forebrain-specific neuronal inhibition of nuclear factor-kappaB activity leads to loss of neuroprotection. J. Neurosci. 2003, 23, 9403–9408. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, J.; Shui, Y.; Wu, Q.; Wang, J.; Xia, X. NF-κB-activated oncogene inhibition strategy for cancer gene therapy. Cancer Gene Ther. 2024, 31, 1632–1645. [Google Scholar] [CrossRef]
| NF-κB pathway Molecules in Cancer Types | |
|---|---|
| Cancer Type | NF-κB Activation Pathway/Key Molecules |
| Breast Cancer | Wnt, TRIM32, RSL3 |
| Colorectal Cancer | MUC13, EGFR |
| Lung Cancer | DDX24, TRIM32, CD146 |
| Melanoma | P65, SPP1, IL-8, VEGF, BET, MCL1 |
| Prostate Cancer | IκKβ, BCL-2 |
| Gastric Cancer | P65, IL-8, Siva-1 |
| Nasopharyngeal Cancer | STAT3, LMP1, miR-125b, BamHI-A |
| Bladder Cancer | Survivin, miR-29b, TRIM29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukas, K.; Nguyen, J.; Necas, C.; Dave, K.; Venketaraman, V. Targeting the NF-κB Pathway in Cancer: Mechanisms, Resistance, and Therapeutic Potential Across Tumor Types. Pharmaceuticals 2025, 18, 1764. https://doi.org/10.3390/ph18111764
Lukas K, Nguyen J, Necas C, Dave K, Venketaraman V. Targeting the NF-κB Pathway in Cancer: Mechanisms, Resistance, and Therapeutic Potential Across Tumor Types. Pharmaceuticals. 2025; 18(11):1764. https://doi.org/10.3390/ph18111764
Chicago/Turabian StyleLukas, Kara, Jessica Nguyen, Clare Necas, Kushal Dave, and Vishwanath Venketaraman. 2025. "Targeting the NF-κB Pathway in Cancer: Mechanisms, Resistance, and Therapeutic Potential Across Tumor Types" Pharmaceuticals 18, no. 11: 1764. https://doi.org/10.3390/ph18111764
APA StyleLukas, K., Nguyen, J., Necas, C., Dave, K., & Venketaraman, V. (2025). Targeting the NF-κB Pathway in Cancer: Mechanisms, Resistance, and Therapeutic Potential Across Tumor Types. Pharmaceuticals, 18(11), 1764. https://doi.org/10.3390/ph18111764








