Aqueous Extracts and Flavonoids Obtained from Annona cherimola Miller as Antidiabetic Treatments Alone and in Combination with Antidiabetic Drugs: In Vivo and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. Acute Oral Toxicity
2.2. Acute Evaluation of the Aqueous Extracts from Annona cherimola and Its Combinations
2.3. Subchronic Evaluation of Aqueous Extract of A. cherimola Leaves and Its Combinations
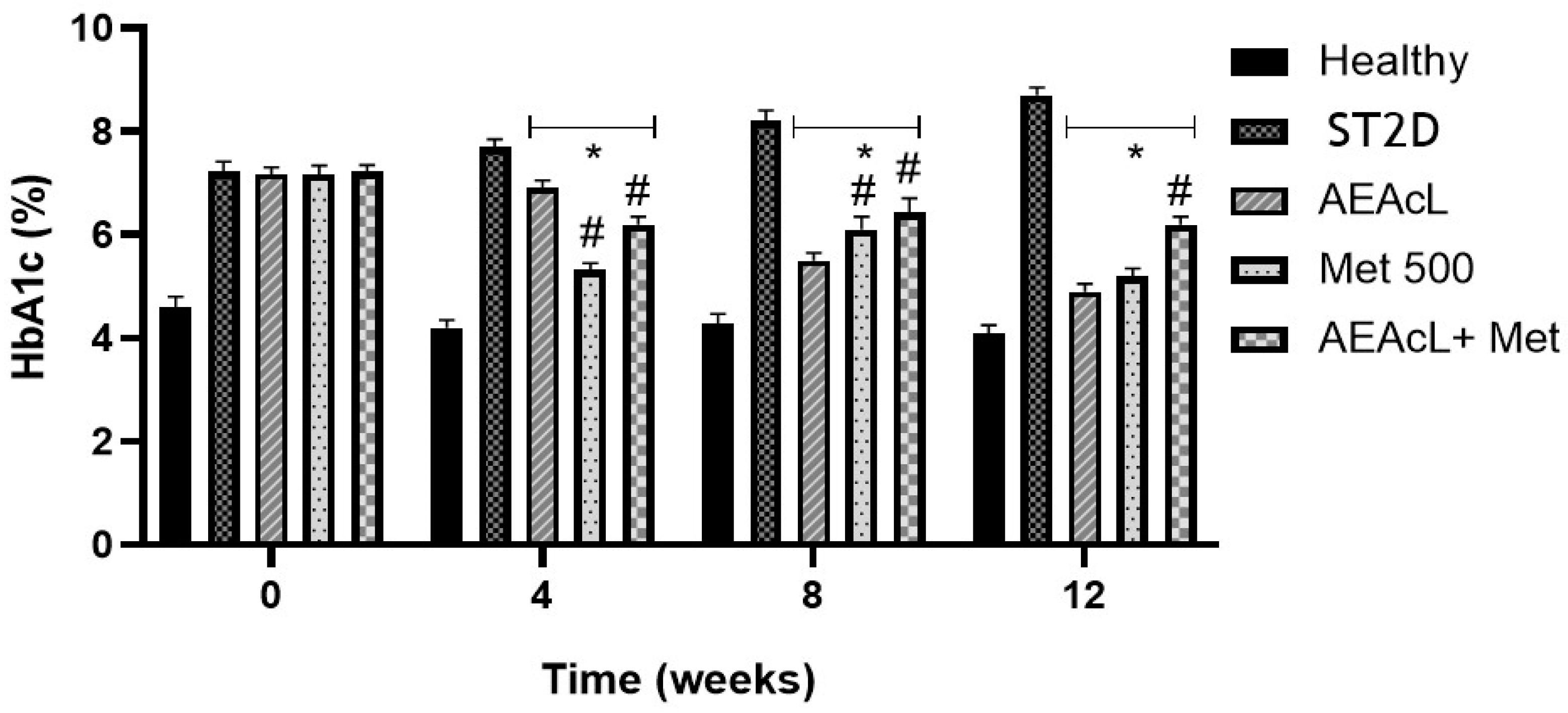
2.4. Effects on % Glycated Hemoglobin After Subchronical Administration of Aqueous Extract of A. cherimola Leaves and Its Combinations
2.5. Effect on Lipid Profile After Subchronical Administration of Aqueous Extract of A. cherimola Leaves and Its Combinations
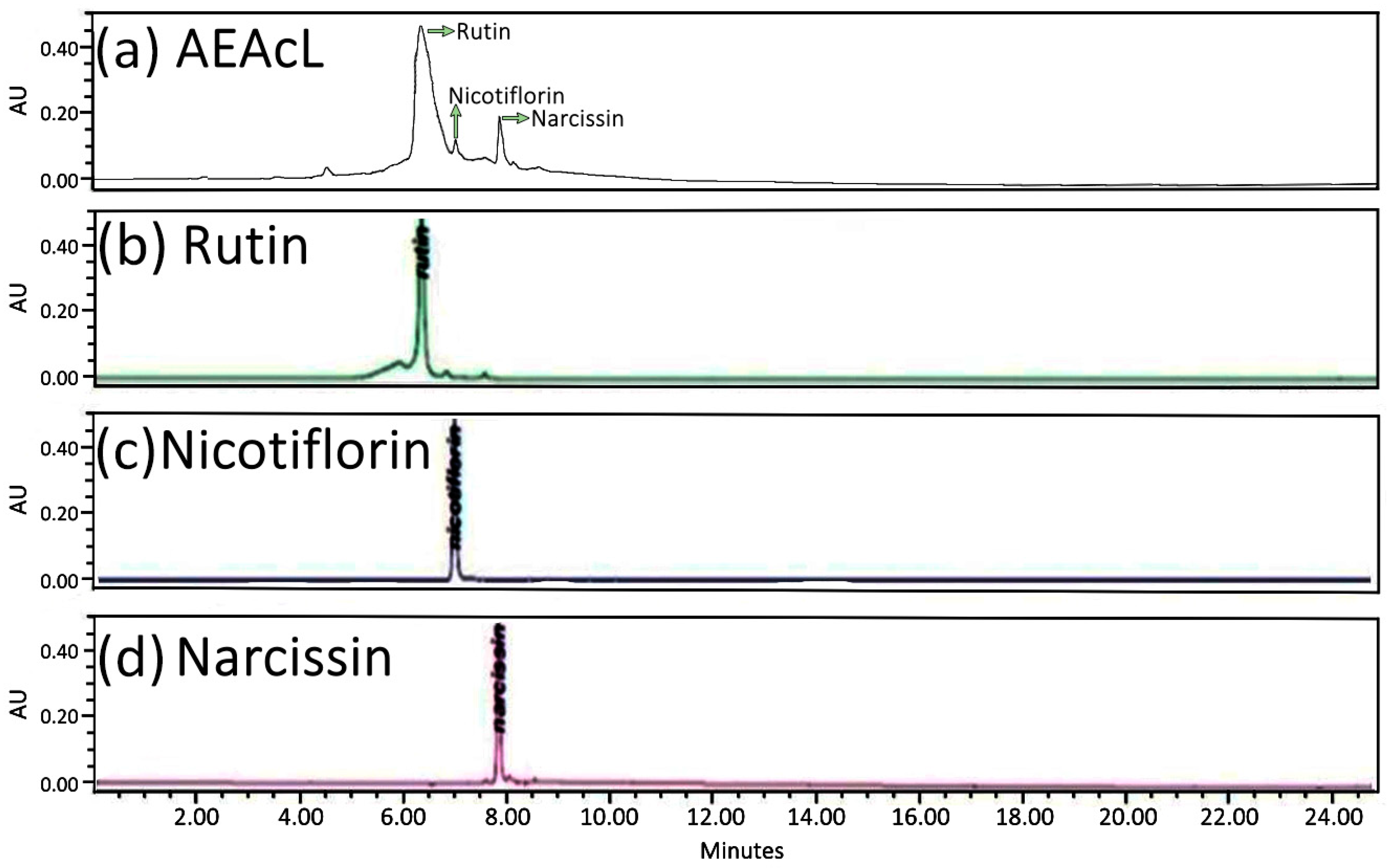
2.6. Flavonoids Isolated from A. cherimola Leaves Aqueous Extract: Identification and Characterization
2.6.1. HPLC-DAD Analysis of the Aqueous Extract of the Leaves of Annona cherimola
2.6.2. H and 13C-NMR Spectra Analysis of Rutin, Nicotiflorin, and Narcissin
2.7. Acute Evaluation of Rutin, Nicotiflorin and Narcissin
2.8. Molecular Docking Studies of Rutin, Nicotiflorin, and Narcissin
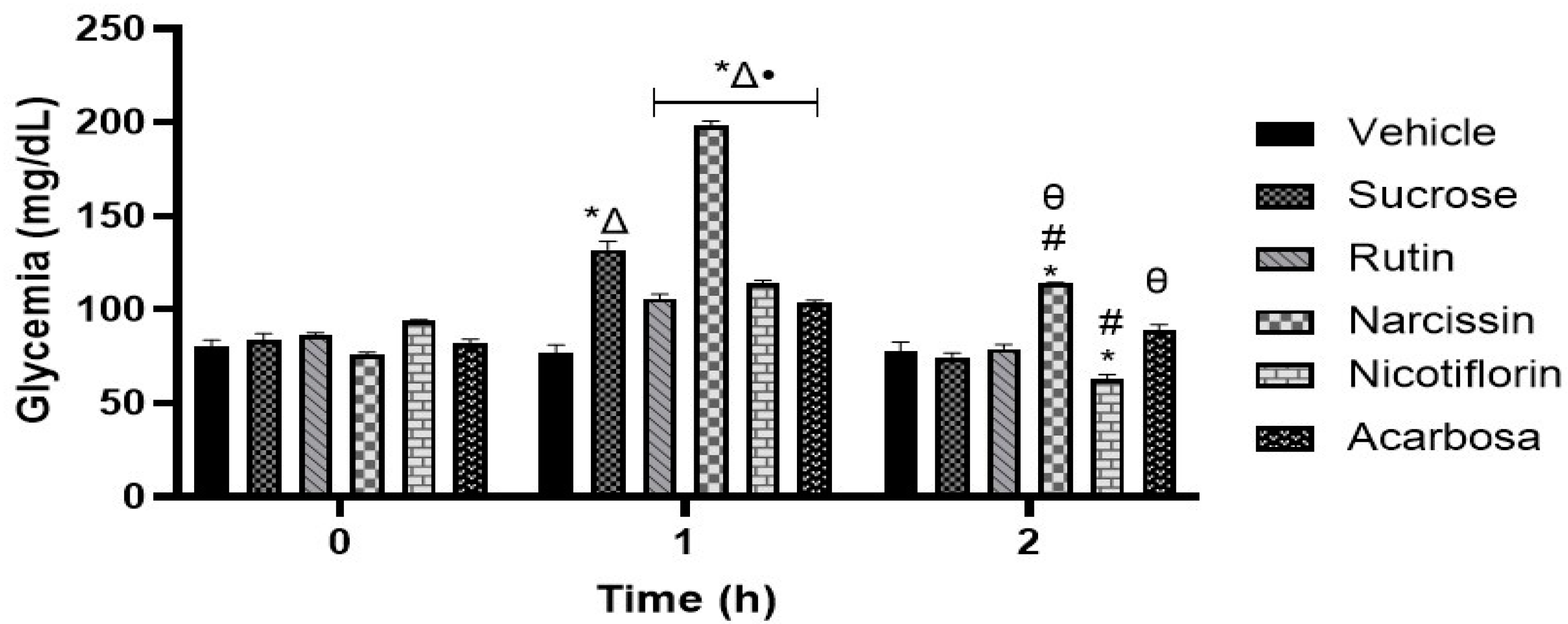
2.9. Oral Sucrose Tolerance Test (OSTT)
2.10. Oral Glucose Tolerance Test (OGTT)
2.11. Toxicoinformatic and Pharmaceutical Analysis of Flavonoids
3. Discussion
4. Materials and Methods
4.1. Reagents, Drugs, and Chemicals
4.2. Plant Material
4.3. Obtention of Aqueous Extracts of the Leaves and Stems from Annona cherimola
4.4. Isolation, Extraction, and Identification of Flavonoids
4.5. In Vivo Assays
4.5.1. Animals
4.5.2. Acute Oral Toxicity
4.5.3. Experimental Type 2 Diabetes Induction
4.6. Grouping
4.6.1. Acute Evaluation of Aqueous Extracts from Annona cherimola and Its Combinations
4.6.2. Subchronic Evaluation of Aqueous Leaf Extract of Annona cherimola in the ST2D Mouse Model
4.6.3. Measurement of % HbA1c
4.6.4. Lipid Profile Measurement
4.6.5. Oral Sucrose Tolerance Assay
4.6.6. Oral Glucose Tolerance Assay
4.7. Studies of Molecular Docking of Flavonoids
4.8. In Silico Toxicology and Pharmaceutical Properties
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/es/news-room/fact-sheets/detail/diabetes (accessed on 7 April 2025).
- Instituto Nacional de Estadística y Geografía. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2025/edr/edr2024_en-jun_RR.pdf (accessed on 7 April 2025).
- Zhong, J. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Rad. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Gudoor, R.; Suits, A.; Shubrook, J.H. Perfecting the Puzzle of Pathophysiology: Exploring Combination Therapy in the Treatment of Type 2 Diabetes. Diabetology 2023, 4, 379–392. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Gutiérrez-Mariscal, F.M.; Gómez-Luna, P.; Alcalá-Diaz, J.F.; Pérez-Martinez, P.; López-Miranda, J. Dietary modulation of advanced glycation end products metabolism on carotid intima-media thickness in type 2 diabetes patients: From the CORDIOPREV study. Clín. Investig. Arterioscler. 2023, 35, 105–114. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Kumar, P.R.; Bhansali, A.; Ravikiran, M.; Bhansali, S.; Dutta, P.; Thakur, J.S.; Walia, R. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: A community-based study. J. Clin. Endocrinol. Metab. 2010, 95, 2832–2835. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes mellitus and its metabolic complications: The role of adipose tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Erion, D.M.; Park, H.J.; Lee, H.Y. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016, 49, 139. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.R.; Sesso, H.D.; Lee, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Gaziano, J.M. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004, 292, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Venkatasamy, V.V.; Pericherla, S.; Manthuruthil, S.; Mishra, S.; Hanno, R. Effect of Physical activity on Insulin Resistance, Inflammation and Oxidative Stress in Diabetes Mellitus. J. Clin. Diagn. Res. 2013, 7, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Fuchsberger, C.; Flannick, J.; Teslovich, T.M.; Mahajan, A.; Agarwala, V.; Gaulton, K.J.; Ma, C.; Fontanillas, P.; Moutsianas, L.; McCarthy, D.J.; et al. The genetic architecture of type 2 diabetes. Nature 2016, 536, 41–47. [Google Scholar] [CrossRef]
- Flannick, J.; Florez, J.C. Type 2 diabetes: Genetic data sharing to advance complex disease research. Nat. Rev. Genet. 2016, 17, 535–549. [Google Scholar] [CrossRef]
- Barrientos-Ávalos, J.; Morel-Cerda, E.; Félix-Téllez, F.; Vidrio-Huerta, B.E.; Aceves-Ayala, A.R.; Flores-Rendón, Á.R.; Velarde-Ruiz Velasco, J.A. Gastrointestial adverse effects of old and new antidiabetics: How do we deal with them in real life? Rev. Gastroenterol. Mex. (Engl. Ed.) 2024, 89, 521–532. [Google Scholar]
- Soccio, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal medicines for diabetes management and its secondary complications. Curr. Diabetes Rev. 2021, 17, 437–456. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Al Kazman, B.S.; Harnett, J.E.; Hanrahan, J.R. Traditional uses, phytochemistry and pharmacological activities of annonacae. Molecules 2022, 27, 3462. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical profile and biological activity of cherimoya (Annona cherimola Mill.) and atemoya (Annona atemoya) leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Vinci, S.; Ramazzotti, M.; Francesconi, A.; Manetti, F.; Degl’Innocenti, D. Annona cherimola Miller fruit as a promising candidate against diabetic complications: An in vitro study and preliminary clinical results. Foods 2020, 9, 1350. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Araujo, A. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J. Ethnopharmacol. 2012, 139, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ammoury, C.; Younes, M.; El-Khoury, M. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement. Altern. Med. 2019, 19, 365. [Google Scholar]
- Verma, A.; Kumar, A.; Shekar, R.; Kumar, K.; Chakrapani, R. Pharmacological Screening of Annona cherimola for Antihyperlipidemic Potential. J. Basic. Clin. Pharm. 2011, 2, 63–69. [Google Scholar] [PubMed]
- Díaz-de-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Solares-Pascasio, J.I.; Ordoñez-Razo, R.M.; Velazquez, C.; Barbosa, E.; García-Hernández, N.; Correa-Basurto, J. Antihyperglycemic activity of the leaves from Annona cherimola miller and rutin on alloxan-induced diabetic rats. Pharmacogn. Res. 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Valdés, M. Antihyperglycemic and antilipidemic properties of a tea infusion of the leaves from Annona cherimola miller on streptozocin-induced type 2 diabetic mice. Molecules 2021, 26, 2408. [Google Scholar] [CrossRef]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Gutiérrez-Meza, J.M. Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves. Plants 2022, 11, 3224. [Google Scholar] [CrossRef]
- Valdes, M.; Calzada, F.; Martínez-Solís, J.; Martínez-Rodríguez, J. Antihyperglycemic effects of Annona cherimola miller and the flavonoid rutin in combination with oral antidiabetic drugs on streptozocin-induced diabetic mice. Pharmaceuticals 2023, 16, 112. [Google Scholar] [CrossRef]
- Calzada, F.; Valdes, M.; Martínez-Solís, J.; Velázquez, C.; Barbosa, E. Annona cherimola Miller and Its Flavonoids, an Important Source of Products for the Treatment of Diabetes Mellitus: In Vivo and In Silico Evaluations. Pharmaceuticals 2023, 16, 724. [Google Scholar] [CrossRef]
- Perrone, A.; Yousefi, S.; Salami, A.; Papini, A.; Martinelli, F. Botanical, genetic, phytochemical and pharmaceutical aspects of Annona cherimola Mill. Sci. Hortic. 2022, 296, 110896. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert. Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Zhao, M.; Li, N.; Zhou, H. SGLT1: A potential drug target for cardiovascular disease. Drug Des. Dev. Ther. 2023, 17, 2011–2023. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef]
- Schuster, D.P.; Duvuuri, V. Diabetes mellitus. Clin. Podiatr. Med. Surg. 2002, 19, 79–107. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Drug therapies for diabetes. Inter. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef]
- Kane, J.P.; Pullinger, C.R.; Goldfine, I.D.; Malloy, M.J. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr. Opin. Pharm. 2021, 61, 21–27. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Encuesta Nacional de Salud y Nutrición. Available online: https://ensanut.insp.mx. (accessed on 7 April 2025).
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Yi, X.; Pan, Y.; Peng, H.; Ren, M.; Jia, Q.; Wang, B. The optimal dose of metformin to control conversion to diabetes in patients with prediabetes: A meta-analysis. J. Diabetes Complicat. 2024, 38, 108846. [Google Scholar] [CrossRef]
- Tian, J.; Li, C.; Dong, Z.; Yang, Y.; Xing, J.; Yu, P.; Xin, Y.; Xu, F.; Wang, L.; Mu, Y.; et al. Inactivation of the antidiabetic drug acarbose by human intestinal microbial-mediated degradation. Nat. Metab. 2023, 5, 896–909. [Google Scholar] [CrossRef]
- Hadi, F.; Sardar, H.; Al-Otaibi, J.S.; Pirzada, A.S.; Alam, W.; Khan, H. Comprehensive in vivo Antidiabetic Investigations and antioxidant potential of Caralluma edulis whole Plant. Curr. Res. Nutr. Food Sci. J. 2025, 13, 179–192. [Google Scholar] [CrossRef]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.; Ferreira, C.; Maruzzella, F.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L. Evaluation of cholesterol absorption and biosynthesis by decoctions of Annona cherimola leaves. J. Ethnopharmacol. 2013, 150, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Met. 2015, 12, 60. [Google Scholar] [CrossRef]
- Hanis, N.; Ismail, N.A.; Ali, E.Z. Systematic review on effectiveness of flavonoids against hypercholesterolemia: Insights from in-silico, in-vitro, and in-vivo studies. Food Chem. Adv. 2025, 7, 100981. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetol. Croat. 2010, 15–35. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Tahsin, M.R.; Tithi, T.I.; Mim, S.R.; Haque, E.; Sultana, A.; Bahar, N.B.; Amran, M.S. In vivo and in silico assessment of diabetes ameliorating potentiality and safety profile of Gynura procumbens leaves. Evid.-Based Complement. Altern. Med. 2022, 2022, 9095504. [Google Scholar] [CrossRef]
- Lam, T.-P.; Tran, N.-V.N.; Pham, L.-H.D.; Lai, N.V.-T.; Dang, B.-T.N.; Truong, N.-L.N.; Nguyen-Vo, S.-K.; Hoang, T.-L.; Mai, T.T.; Tran, T.-D. Flavonoids as dual-target inhibitors against α-glucosidase and α-amylase: A systematic review of in vitro studies. Nat. Prod. Bioprospecting 2024, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Limanto, A.; Simamora, A.; Santoso, A.W.; Timotius, K.H. Antioxidant, α-glucosidase inhibitory activity and molecular docking study of gallic acid, quercetin and rutin: A comparative study. Mol. Cell Biomed. Sci. 2019, 3, 67–74. [Google Scholar] [CrossRef]
- Foo, S.; Chan, S.; Sharryl, T.; Puah, J.; Hong, Y.; Thibiya, D.; Baskaram, G.; Shamala, S. Hypoglycemic effects od plant flavonoids: A Review. Evid.-Based Complement. Altern. Med. 2021, 2021, 2057333. [Google Scholar]
- Pham, A.; Malterud, K.; Paulsen, B.; Diallo, D.; Wangensteen, H. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Yang, C.Y.; Hsiu, S.L.; Wen, K.C.; Lin, S.P.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005, 13, 5. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jin, H.; Fan, H.R.; Wang, B.L. Simultaneous assessment of absorption and pharmacokinetic characteristics of four active flavonoids from Chimonanthus nitens Leaf Granules using LC-MS determination: In vivo and in vitro. J. Asian Nat. Prod. Res. 2024, 26, 930–944. [Google Scholar] [CrossRef]
- Pollastri, M.P. Overview on the Rule of Five. Curr. Prot. Pharmacol. 2010, 49, 9–12. [Google Scholar] [CrossRef]
- Park, M.J.; Kang, Y.H. Isolation of Isocoumarins and Flavonoids as α-Glucosidase Inhibitors from Agrimonia pilosa L. Molecules 2020, 25, 2572. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, X. Synthesis of activity evaluation of flavonoid derivatives as ɑ-glucosidase inhibitors. Front. Chem. 2022, 10, 1041328. [Google Scholar] [CrossRef]
- Yi, X.; Dong, M.; Guo, N.; Tian, J.; Lei, P.; Wang, S.; Yang, Y.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Dunya, A.; Duhaidahawi, S.; Haider, F. Flavonoids in the Treatment of Diabetes: Clinical Outcomes and Mechanism to Ameliorate Blood Glucose Levels. Curr. Diabetes Rev. 2021, 17, e120720188794. [Google Scholar] [CrossRef]
- Abdelhakim, B.; Abdelaali, B.; Asaad, K.; Hafiz, A.; Hassan, A.; Mohamed, A.; Hermansyah, A.; Long, C.; Khang, W.; Nasreddine, E. Clinical applications and mechanism insights of natural flavonoids against type 2 diabetes mellitus. Heliyon 2024, 10, e29718. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. NOM-062-ZOO-1999: Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999. Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 5 February 2024).
- OECD. Guideline for Testing of Chemicals 423. Acute Oral Toxicity-Acute Toxic Class Method. Organización Para la Cooperación y el Desarrollo Economicos, OECD/OCDE. 2001. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948378.pdf (accessed on 9 April 2024).
- Hsu, J.; Wu, C.; Hung, C.; Wang, C.; Huang, H. Myrciaria cauliflora extract improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-nicotinamide mice. J. Food Drug Anal. 2016, 24, 730–737. [Google Scholar] [CrossRef]
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.; Lindstrom, W.; Sanner, M.; Belew, R.; Goodshell, D.; Olson, A. Autodock4 and AutodockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. 2017 Calculation of Molecular Properties and Bioactivity Score. Available online: http://www.molinspiration.com/services/logp.html. (accessed on 5 January 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Cao, D. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, gkae303. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Glycemia (mg/dL) | |||||
|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 3 h | 5 h | 7 h | |
| Healthy | 107 ± 2.4 | 124.6 ± 3.0 Δ | 129.8 ± 3.38 # | 124.8 ± 3.7 ## | 123 ± 7.4 θ | 122.8 ± 5.5 θθ |
| ST2D | 349.8 ± 7.2 | 398 ± 32.4 | 413.6 ± 20.4 * | 464 ± 21 * | 491.8 ± 37.5 * | 475.6 ± 38.9 * |
| AEAcL (100 mg/kg) | 330.3 ± 21.1 | 429.6 ± 33.1 * | 384.3 ± 37.1 | 362 ± 32.6 ## | 372 ± 40.5 θ | 330 ± 43.9 θθ |
| AEAcL (200 mg/kg) | 307 ± 16.4 | 288.3 ± 21.4 Δ | 286.6 ± 22.1 # | 273.3 ± 31.2 ## | 248 ± 17.1 *θ | 263 ± 14.9 θθ |
| AEAcL (300 mg/kg) | 324.4 ± 33.6 | 348.2 ± 25.4 | 350.4 ± 20.5 | 288 ± 14.5 ## | 294.4 ± 23.6 θ | 315.6 ± 20.8 θθ |
| AEAcS (100 mg/Kg) | 299.7 ± 5 | 400.7 ± 20.1 * | 367 ± 27.9 * | 333.3 ± 33.7 ## | 361.6 ± 35.3 *θ | 372.4 ± 32.6 *θθ |
| AEAcS (200 mg/Kg) | 329 ± 12.4 | 324.4.6 ± 34.9 | 297.7 ± 31.7 # | 282 ± 40.3 ## | 275.6 ± 33.5 θ | 270.8 ± 34.7 *θθ |
| AEAcS (300 mg/Kg) | 340.4 ± 12.2 | 404.04 ± 19.4 | 276.8 ± 23.1 # | 368 ± 6 ## | 355.5 ± 6.9 θ | 267.5 ± 12.6 *θθ |
| AEAcL + Met (100/850 mg/kg) | 313.3 ± 12 | 144 ± 26 *Δ | 110 ± 21.2 *# | 68 ± 5.2 *## | 73.3 ± 3.7 *θ | 102.3 ± 2.5 *θθ |
| AEAcL + Met (200/850 mg/kg) | 351 ± 22.6 | 366 ± 26.6 | 287.5 ± 21.1 *# | 179 ± 11.6 *## | 70.5 ± 4.8 *θ | 80 ± 5 *θθ |
| AEAcL + Met (100/500 mg/kg) | 346 ± 15.4 | 357.6 ± 22 | 240.8 ± 36.2 *# | 164.4 ± 35.5 *## | 143.2 ± 29.1 *θ | 161.2 ± 38.6 *θθ |
| AEAcL + Met (200/500 mg/kg) | 356.3 ± 7.3 | 146.6 ± 24.2 *Δ | 109.6 ± 11 *# | 67.6 ± 18.4 *## | 48.6 ± 9.7 *θ | 113.3 ± 35.6 *θθ |
| AEAcL + Aca(100/50 mg/kg) | 382.7 ± 4.7 | 333.5 ± 21.8 * | 300.2 ± 21.3 *# | 294.7 ± 22.3 *## | 338.75 ± 26 *θ | 396 ± 34.9 |
| AEAcL + Gli(100/5 mg/kg) | 334.3 ± 12.8 | 454.6 ± 23.3 * | 464.3 ± 16.8 * | 402.3 ± 8.6 *## | 364.6 ± 6.9 θ | 399.3 ± 19.4 * |
| AEAcS + Met (100/500 mg/kg) | 331.75 ± 14 | 293.75 ± 19.7 Δ | 178.5 ± 17.2 *# | 65.75 ± 10.1 *## | 151.25 ± 31 *θ | 241.25 ± 34.5 *θθ |
| AEAcS + Aca(100/50 mg/kg) | 375.6 ± 2.73 | 298.3 ± 5.9 *Δ | 329.6 ± 3.7 *# | 311 ± 11 *## | 332.6 ± 4.3 *θ | 336.6 ± 4.2 θθ |
| AEAcS + Gli(100/5 mg/kg) | 350.3 ± 13 | 433.3 ± 10.3 * | 404.6 ± 2.6 * | 383.6 ± 12.8 | 386.3 ± 21.5 θ | 387.6 ± 17.5 |
| Lipid Profile | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cholesterol (mg/dL) | Triglycerides (mg/dL) | |||||||
| Treatment | Week | Week | ||||||
| 0 | 4 | 8 | 12 | 0 | 4 | 8 | 12 | |
| Healthy | 97.6 ± 0.8 | 99 ± 1 | 99.6 ± 1.7 | 100.3 ± 0.8 | 69.6 ± 4.7 | 73 ± 1 | 72 ± 2.3 | 71.3 ± 0.8 |
| ST2D | 101.3 ± 1.2 | 100.6 ± 1.2 | 101.3 ± 1.4 | 104 ± 2 | 68 ± 1.1 | 125 ± 1.1 # | 138 ± 10 # | 140 ± 4 # |
| AEAcL | 100.6 ± 1.2 | 96.6 ± 1.4 | 105 ± 1.1 | 101 ± 1.1 | 75.3 ± 2.3 | 89.6 ± 4.1 #* | 87.3 ± 2 #* | 92.6 ± 1.4 #* |
| Met | 101.3 ± 1.2 | 113.3 ± 5.4 #* | 101.3 ± 0.8 | 100.3 ± 0.8 | 68 ± 1.1 | 156 ± 6 #* | 146 ± 2 # | 156 ± 1.1 #* |
| AEAcL + Met | 100.6 ± 1.2 | 102.3 ± 1.7 | 103.3 ± 3.5 * | 102 ± 1.7 | 68 ± 1.1 | 83.3 ± 2.4 * | 85.3 ± 2.1 * | 76.6 ± 1.2 * |
| HDL-c (mg/dL) | LDL-c (mg/dL) | |||||||
| Treatment | Week | Week | ||||||
| 0 | 4 | 8 | 12 | 0 | 4 | 8 | 12 | |
| Healthy | 78.7 ± 3.5 | 65 ± 5.7 | 65.3 | 63.7 | 53 ± 1.7 | 46 ± 1.7 | 52 ± 4 | 64.3 ± 2 * |
| ST2D | 80 ± 1.1 | 27.3 ± 1.2 # | 33.6 ± 2 # | 23 ± 4 # | 56.1 ± 1.4 # | 100 ± 1.5 # | 86 ± 1.1 # | 100 ± 1.5 # |
| AEAcL | 77.6 ± 1.3 | 61 ± 3 * | 66 ± 6.4 * | 72.3 ± 1.4 * | 55 ± 2.6 * | 48 ± 1.1 * | 49 ± 3.4 * | 65 ± 5.6 * |
| Met | 78.6 ± 4.3 | 81.3 ± 1.2 #* | 80 ± 2.3 #* | 82.3 ± 1.6 #* | 58.1 ± 2.6 # | 108.6 ± 8.7 # | 105.6 ± 1.4 #* | 102 ± 1.1 # |
| AEAcL + Met | 79 ± 6.2 | 67 ± 1.1 * | 67 ± 1.1 * | 65 ± 1.1 * | 57 ± 2.5 * | 55.6 ± 1.7 * | 60 ± 8 * | 65 ± 2.6 * |
| Treatment | Glycemia (mg/dL) | ||
|---|---|---|---|
| 0 | 2 | 4 | |
| Healthy | 145 ± 0.6 Δ | 149 ± 9 | 150.2 ± 1.6 |
| ST2D | 340.4 ± 6.5 | 474 ± 33.6 * | 477.3 ± 29 * |
| AEAcL | 329 ± 9 | 373.1 ± 28.7 # | 367 ± 31.6 ## |
| Met | 361.9 ± 7.6 | 384.5 ± 1.6 *# | 371.4 ± 2.33 ## |
| Aca | 346.5 ± 3.9 | 329.5 ± 10 # | 275 ± 11 *## |
| Gli | 348.1 ± 11 | 267.1 ± 0.32 *# | 353.2 ± 0.4 ## |
| Rutin | 321.2 ± 3 | 185 ± 6.4 *# | 168.5 ± 2.9 *## |
| Nicotiflorin | 307.3 ± 0.28 | 180.4 ± 1.6 *# | 185 ± 2 *## |
| Narcissin | 311.6 ± 3 | 188 ± 2 *# | 178.2 ± 5 *## |
| Compound | α-Glucosidase | ||
|---|---|---|---|
| ΔG (kcal/mol) | H-Binding Residues | NPI | |
| Rutin | −4.44 | Aps 203, Thr 204, Thr 205, Tyr 299, Asp 327, Ile 364, Trp 441, Ser 448, Phe 450, Lys 480, Arg 526, Trp 539, Asp 542, Ala 576, Leu 577, His 600, Gly 602, Gln 603, Tyr 605 | Trp 406, Met 444, Phe 575 |
| Nicotiflorin | −5.23 | Asp 203, Thr 205, Pro 206, Tyr 299, Asp 327, Asp 366, Trp 441, Asp 443, Met 444, Arg 526, Asp 542, Asn 543, Thr 544, Thr 544, His 600, Gln 603, Tyr 605 | Ile 328, Ile 364, Trp 406, Phe 575, Ala 576 |
| Narcissin | −5.61 | Asp 203, Thr 204, Tyr 299, Asp 327, Asp 366, Trp 441, Asp 443, Met 444, Arg 526, Asp 542, His 600, Gly 602, Gln 603 | Ile 328, Ile 364, Trp 406, Phe 450, Phe 575, Ala 576, Tyr 605 |
| Acarbose | −4.36 | Asp 203, Thr 204, Thr 205, Asn 207, Asp 327, Ile 328, Ile 364, Trp 441, Asp 443, Met 444, Lys 480, Arg 526, Trp 539, Gly 541, Asp 542, Thr 544, Ala 576, Arg 598, His 600 | Tyr 299, Trp 406, Phe 575 |
| Compound | Sodium–Glucose Cotransporter (SGLT1) | ||
| Rutin | 24.12 | Phe 251, Asp 273, Cys 345, Val 346, Pro 348, Val 359, Gly 360, Thr 362, Asn 363, Gly 450, Gln 451, Asp 454, Gly 509, Ser 510, Met 512, His 525, Tyr 526 | Cys 255, Cys 351, Cys 511 |
| Nicotiflorin | −2.15 | Thr 90, Ala 93, Ser 94, Lys 254, Cys 255, Tyr 256, Asp 273, Ala 344, Cys 345, Val 346, Cys 351, Val 359, Gly 360, Cys 361, Thr 362, Gly 450, Gln 451, Asp 454, Leu 274, Tyr 526 | His 525 |
| Narcissin | −1.17 | Gly 82, His 83, Gly 86, Leu 87, Thr 90, Ser 94, Ala 97, Asp 273, Leu 274, Thr 362, Gln 451, Asp 454, Gln 457, Tyr 526 | Ala 93, Ile 98, Phe 101, Phe 453 |
| Canagliflozin | −6.77 | Gly 82, His 83, Leu 87, Glu 102, Thr 156, Met 283, Thr 287, Tyr 290, Trp 291, Lys 321, Phe 453, Gln 457, Thr 460 | Ala 105, Lys 157, Aala 160, Leu 286, Trp 289, Ile 456 |
| Rutin | Nicotiflorin | Narcissin | Rutin | Nicotiflorin | Narcissin | ||
|---|---|---|---|---|---|---|---|
| Physicochemical | Pharmacokinetics | ||||||
| TPSA | 269.43 | 249.2 | 258.4 | Human intestinal absorption | Medium | Excellent | Excellent |
| Lipophilicity (logP) | 0.98 | 1.16 | 0.72 | BBBp | No | No | No |
| Water solubility (logS) | −2.39 | −2.55 | −2.64 | Volume of distribution | 0.87 | 0.91 | 0.79 |
| Rotatable bonds | 6 | 6 | 7 | Plasma protein binding | 85.0% | 85.1% | 84.5% |
| Number of H donors | 10 | 9 | 9 | CYP1A2 inhibitor | No | No | No |
| Number of H-bond acceptors | 16 | 15 | 16 | CYP2C19 inhibitor | No | No | No |
| Druglikeness | CYP2C19 substrate | No | No | No | |||
| Lipinski | No, 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | No, 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | No 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | CYP2C9 inhibitor | No | No | No |
| CYP2D6 inhibitor | No | No | No | ||||
| CYP2D6 substrate | No | No | No | ||||
| Ghose | No 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | No 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | No 4 violations: MW > 480, WLogP < −0.4, MR > 130, #atoms > 70 | CYP3A4 inhibitor | No | No | No |
| Clearance | Low | Low | Low | ||||
| T1/2 | 4.6 | 4.27 | 4.34 | ||||
| Veber | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 | Toxicity | |||
| Egan | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | Mutagenic | No | No | No |
| Carcinogenic | No | No | No | ||||
| Neurotoxicity | No | No | No | ||||
| Rat oral acute toxicity | No | No | No | ||||
| H-HT | Low | Low | Low | ||||
| Predicted toxicity class b | 5 | 5 | 5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Santos, J.; Calzada, F.; Martínez-Rodríguez, J.; Valdes, M.; Barbosa, E.; Velázquez, C. Aqueous Extracts and Flavonoids Obtained from Annona cherimola Miller as Antidiabetic Treatments Alone and in Combination with Antidiabetic Drugs: In Vivo and In Silico Studies. Pharmaceuticals 2025, 18, 1754. https://doi.org/10.3390/ph18111754
Ramírez-Santos J, Calzada F, Martínez-Rodríguez J, Valdes M, Barbosa E, Velázquez C. Aqueous Extracts and Flavonoids Obtained from Annona cherimola Miller as Antidiabetic Treatments Alone and in Combination with Antidiabetic Drugs: In Vivo and In Silico Studies. Pharmaceuticals. 2025; 18(11):1754. https://doi.org/10.3390/ph18111754
Chicago/Turabian StyleRamírez-Santos, Jesica, Fernando Calzada, Julita Martínez-Rodríguez, Miguel Valdes, Elizabeth Barbosa, and Claudia Velázquez. 2025. "Aqueous Extracts and Flavonoids Obtained from Annona cherimola Miller as Antidiabetic Treatments Alone and in Combination with Antidiabetic Drugs: In Vivo and In Silico Studies" Pharmaceuticals 18, no. 11: 1754. https://doi.org/10.3390/ph18111754
APA StyleRamírez-Santos, J., Calzada, F., Martínez-Rodríguez, J., Valdes, M., Barbosa, E., & Velázquez, C. (2025). Aqueous Extracts and Flavonoids Obtained from Annona cherimola Miller as Antidiabetic Treatments Alone and in Combination with Antidiabetic Drugs: In Vivo and In Silico Studies. Pharmaceuticals, 18(11), 1754. https://doi.org/10.3390/ph18111754











