9-Methylfascaplysin, a Marine-Derived Bioactive Compound, Promotes Neurite Outgrowth via the Inhibition of ROCK2
Abstract
1. Introduction
2. Results
2.1. Subsection
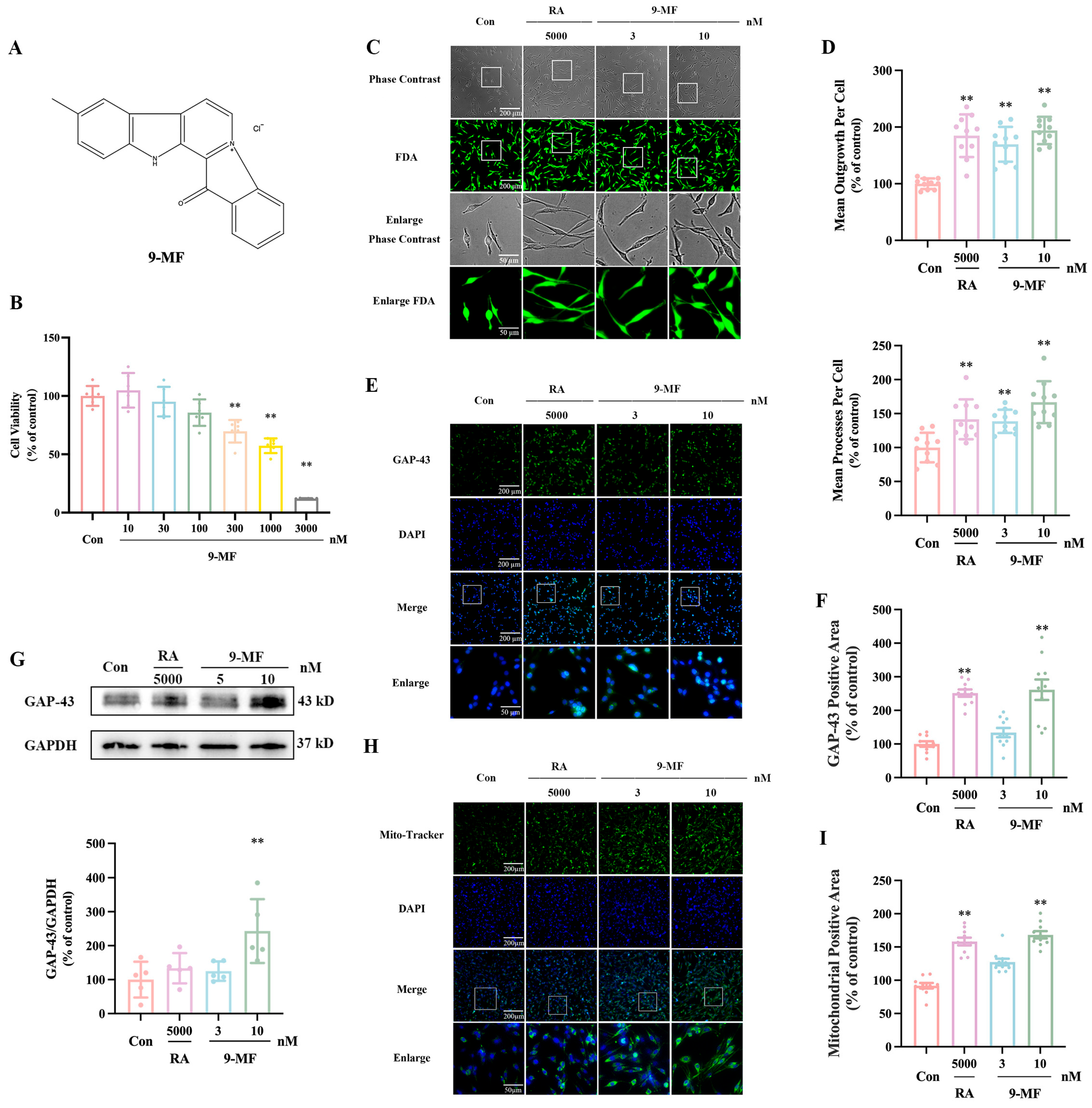
2.1.1. 9-MF Induces Neurite Outgrowth in PC12 Cells
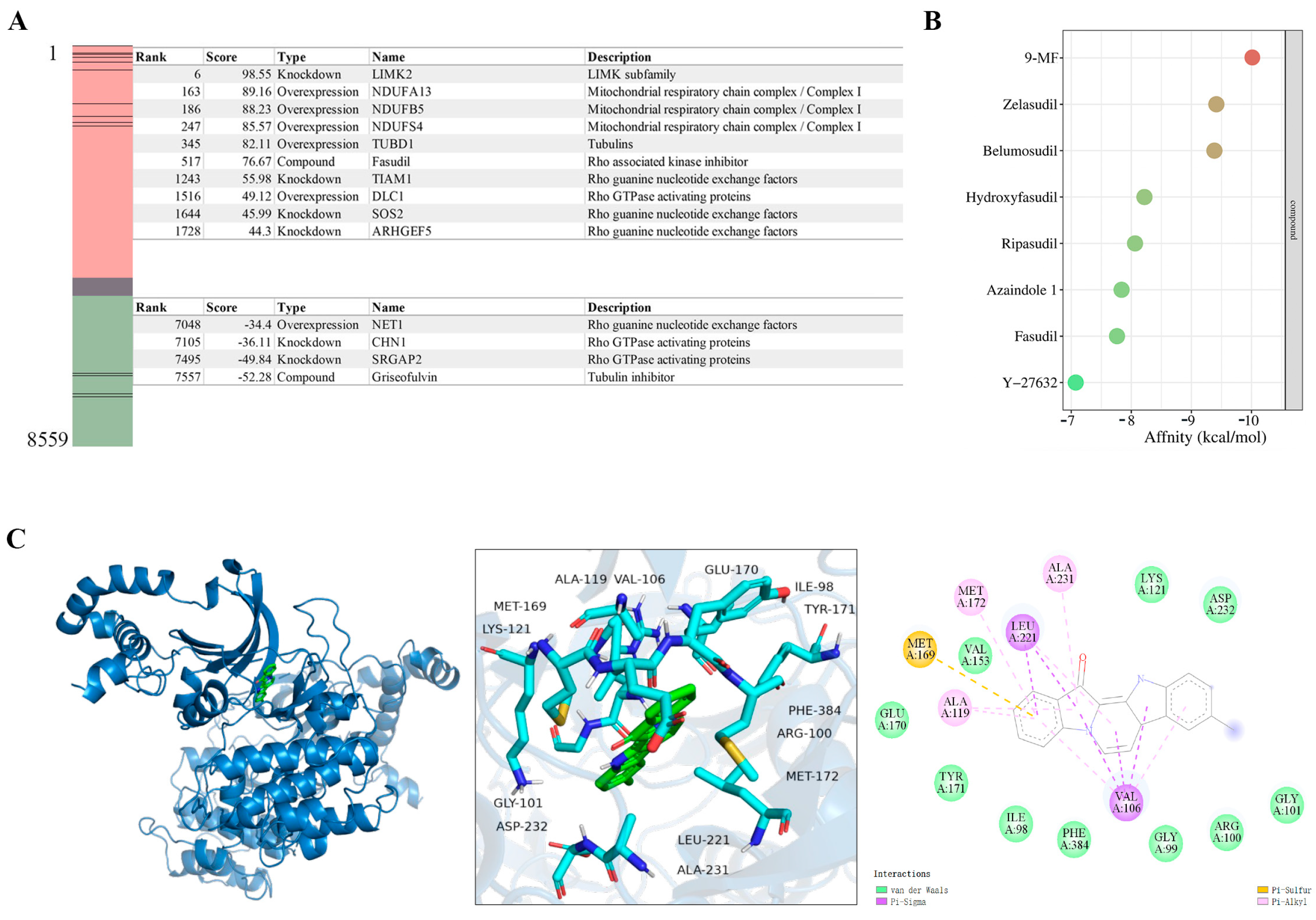
2.1.2. 9-MF Induces Neurite Outgrowth via the Action on a ROCK2-Centralized Network
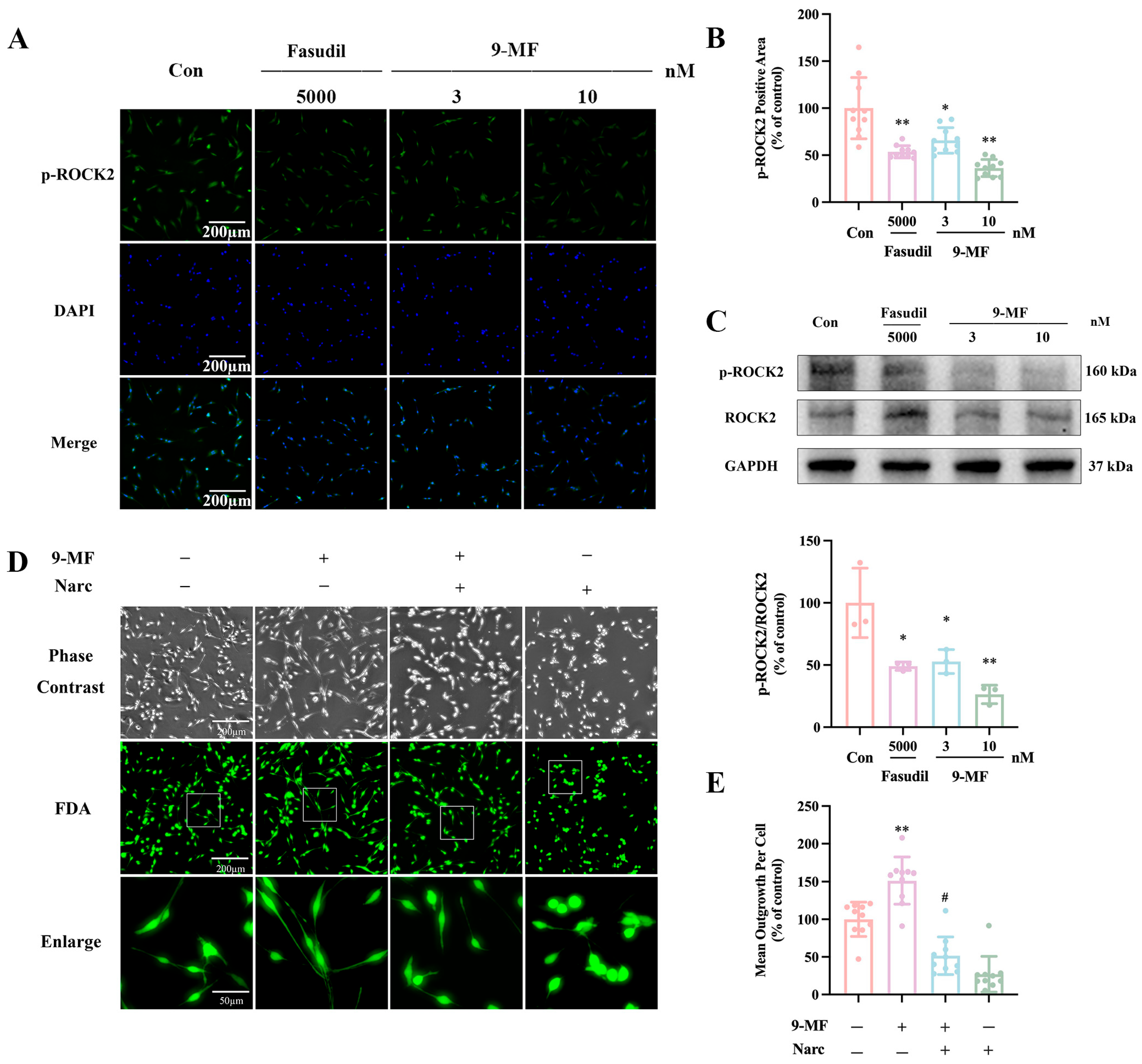
2.1.3. 9-MF Inhibits ROCK2
2.1.4. 9-MF Induces Neurite Outgrowth via the Inhibition of ROCK2
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. PC12 Cells
4.3. Cell Viability Assay
4.4. Fluorescein Diacetate (FDA) Staining
4.5. Mitochondrial Staining
4.6. RNA-Seq
4.7. Bioinformatical Analysis
4.8. CMap Analysis
4.9. Molecular Docking Analysis
4.10. Western Blotting Analysis
4.11. Immunocytochemical Staining
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhidkov, M.E.; Kaune, M.; Kantemirov, A.V.; Smirnova, P.A.; Spirin, P.V.; Sidorova, M.A.; Stadnik, S.A.; Shyrokova, E.Y.; Kaluzhny, D.N.; Tryapkin, O.A.; et al. Study of Structure-Activity Relationships of the Marine Alkaloid Fascaplysin and Its Derivatives as Potent Anticancer Agents. Mar. Drugs 2022, 20, 185. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, H.; Hou, Y.; Cao, H.; Hua, H.; Li, D. Marine-Derived Lead Fascaplysin: Pharmacological Activity, Total Synthesis, and Structural Modification. Mar. Drugs 2023, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, F.; Sang, J.; Lin, M.; Ma, J.; Xiao, X.; Yan, S.; Naman, C.B.; Wang, N.; He, S.; et al. 9-Methylfascaplysin Is a More Potent Aβ Aggregation Inhibitor than the Marine-Derived Alkaloid, Fascaplysin, and Produces Nanomolar Neuroprotective Effects in SH-SY5Y Cells. Mar. Drugs 2019, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Feng, Y.; Pan, H.; Xuan, Z.; Yan, S.; Mao, Y.; Xiao, X.; Huang, X.; Zhang, H.; Zhou, F.; et al. 9-Methylfascaplysin exerts anti-ischemic stroke neuroprotective effects via the inhibition of neuroinflammation and oxidative stress in rats. Int. Immunopharmacol. 2021, 97, 107656. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Xia, C.; Xu, J.; Cai, J.; Hu, C.; Bai, Y.; Chen, H.; Rong, W.; Jiang, Y.; Wu, X.; et al. 9-Methylfascaplysin Prevents Neuroinflammation and Synaptic Damage via Cell-Specific Inhibition of Kinases in APP/PS1 Transgenic Mice. CNS Neurosci. Ther. 2024, 30, e70100. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Sun, X.; Shi, X.; Liang, H.; Chen, X.; Cui, W.; Fan, Y.; Ma, J.; Wang, H. Pharmacokinetics, Tissue Distribution, and Excretion of 9-Methylfascaplysin, a Potential Anti-Alzheimer’s Disease Agent. Electrophoresis 2025, 46, 452–461. [Google Scholar] [CrossRef]
- Salvadores, N.; Sanhueza, M.; Manque, P.; Court, F.A. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front. Neurosci. 2017, 11, 451. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current Trends in Neurodegeneration: Cross Talks between Oxidative Stress, Cell Death, and Inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Chong, C.M.; Ai, N.; Lee, S.M. ROCK in CNS: Different Roles of Isoforms and Therapeutic Target for Neurodegenerative Disorders. Curr. Drug Targets 2017, 18, 455–462. [Google Scholar] [CrossRef]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef]
- Martín-Cámara, O.; Cores, Á.; López-Alvarado, P.; Menéndez, J.C. Emerging targets in drug discovery against neurodegenerative diseases: Control of synapsis disfunction by the RhoA/ROCK pathway. Eur. J. Med. Chem. 2021, 225, 113742. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.M.; Teusch, N.; Imhof, C.; Bakker, M.H.; Schurdak, M.; Burns, D.J.; Warrior, U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J. Neurosci. Res. 2008, 86, 2214–2226. [Google Scholar] [CrossRef] [PubMed]
- Boland, S.; Bourin, A.; Alen, J.; Geraets, J.; Schroeders, P.; Castermans, K.; Kindt, N.; Boumans, N.; Panitti, L.; Vanormelingen, J.; et al. Design, synthesis and biological characterization of selective LIMK inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Croft, D.R.; Crighton, D.; Samuel, M.S.; Lourenco, F.C.; Munro, J.; Wood, J.; Bensaad, K.; Vousden, K.H.; Sansom, O.J.; Ryan, K.M.; et al. p53-mediated transcriptional regulation and activation of the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway promotes cell survival. Cell Res. 2011, 21, 666–682. [Google Scholar] [CrossRef]
- Glotfelty, E.J.; Tovar, Y.R.L.B.; Hsueh, S.C.; Tweedie, D.; Li, Y.; Harvey, B.K.; Hoffer, B.J.; Karlsson, T.E.; Olson, L.; Greig, N.H. The RhoA-ROCK1/ROCK2 Pathway Exacerbates Inflammatory Signaling in Immortalized and Primary Microglia. Cells 2023, 12, 1367. [Google Scholar] [CrossRef]
- Li, W.P.; Ma, K.; Jiang, X.Y.; Yang, R.; Lu, P.H.; Nie, B.M.; Lu, Y. Molecular mechanism of panaxydol on promoting axonal growth in PC12 cells. Neural Regen. Res. 2018, 13, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Nishina, A.; Kimura, H.; Tsukagoshi, H.; Kozawa, K.; Koketsu, M.; Ninomiya, M.; Furukawa, S. Neurite Outgrowth in PC12 Cells Stimulated by Components from Dendranthema × grandiflorum cv. “Mottenohoka” Is Enhanced by Suppressing Phosphorylation of p38MAPK. Evid. Based Complement. Alternat Med. 2013, 2013, 403503. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, e00045. [Google Scholar] [CrossRef]
- Duffy, P.; Schmandke, A.; Schmandke, A.; Sigworth, J.; Narumiya, S.; Cafferty, W.B.; Strittmatter, S.M. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J. Neurosci. 2009, 29, 15266–15276. [Google Scholar] [CrossRef]
- Chong, C.M.; Kou, M.T.; Pan, P.; Zhou, H.; Ai, N.; Li, C.; Zhong, H.J.; Leung, C.H.; Hou, T.; Lee, S.M. Discovery of a novel ROCK2 inhibitor with anti-migration effects via docking and high-content drug screening. Mol. Biosyst. 2016, 12, 2713–2721. [Google Scholar] [CrossRef]
- Zaim, M.; Isik, S. DNA topoisomerase IIβ stimulates neurite outgrowth in neural differentiated human mesenchymal stem cells through regulation of Rho-GTPases (RhoA/Rock2 pathway) and Nurr1 expression. Stem Cell Res. Ther. 2018, 9, 114. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef]
- Fischer, L.R.; Glass, J.D. Axonal degeneration in motor neuron disease. Neurodegener. Dis. 2007, 4, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Caroni, P. Selective neuronal vulnerability in neurodegenerative diseases: From stressor thresholds to degeneration. Neuron 2011, 71, 35–48. [Google Scholar] [CrossRef]
- Scheibe, R.J.; Ginty, D.D.; Wagner, J.A. Retinoic acid stimulates the differentiation of PC12 cells that are deficient in cAMP-dependent protein kinase. J. Cell Biol. 1991, 113, 1173–1182. [Google Scholar] [CrossRef]
- da Rocha, J.F.; da Cruz e Silva, O.A.; Vieira, S.I. Analysis of the amyloid precursor protein role in neuritogenesis reveals a biphasic SH-SY5Y neuronal cell differentiation model. J. Neurochem. 2015, 134, 288–301. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, Y.; Hu, C.; Zhai, Y.; Pan, N.; Ding, L.; Han, W.; Cui, W. Chryxanthone A, an extracted substance from endophytic fungal Aspergillus versicolor, produces anti-oxidant neuroprotection possibly via the action on mTOR/CREB axis. Gene 2025, 944, 149298. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Lantz, S.; Paule, M.G. In vivo imaging and quantitative analysis of changes in axon length using transgenic zebrafish embryos. Neurotoxicol. Teratol. 2011, 33, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Huang, X. Transcriptome analysis of human dental pulp cells cultured on a novel cell-adhesive fragment by RNA sequencing. Gene 2024, 927, 148709. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, W.; Lu, X.; Ma, S.; Dong, L.; Zou, B. Weighted gene co-expression network analysis and connectivity map identifies lovastatin as a treatment option of gastric cancer by inhibiting HDAC2. Gene 2019, 681, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.; Cai, J.; Xu, J.; Hu, C.; Chen, H.; Hong, Y.; Pan, N.; Jiang, Y.; Zhou, C.; et al. The activation of RARα prevents surgery-induced cognitive impairments via the inhibition of neuroinflammation and the restoration of synaptic proteins in elderly mice. Int. Immunopharmacol. 2024, 130, 111772. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Gao, K.; Hong, Y.; Le, J.; Cai, J.; Liang, H.; Cui, W. 9-Methylfascaplysin, a Marine-Derived Bioactive Compound, Promotes Neurite Outgrowth via the Inhibition of ROCK2. Pharmaceuticals 2025, 18, 1751. https://doi.org/10.3390/ph18111751
Zheng M, Gao K, Hong Y, Le J, Cai J, Liang H, Cui W. 9-Methylfascaplysin, a Marine-Derived Bioactive Compound, Promotes Neurite Outgrowth via the Inhibition of ROCK2. Pharmaceuticals. 2025; 18(11):1751. https://doi.org/10.3390/ph18111751
Chicago/Turabian StyleZheng, Meilin, Kangyang Gao, Yirui Hong, Jingyang Le, Jingjing Cai, Hongze Liang, and Wei Cui. 2025. "9-Methylfascaplysin, a Marine-Derived Bioactive Compound, Promotes Neurite Outgrowth via the Inhibition of ROCK2" Pharmaceuticals 18, no. 11: 1751. https://doi.org/10.3390/ph18111751
APA StyleZheng, M., Gao, K., Hong, Y., Le, J., Cai, J., Liang, H., & Cui, W. (2025). 9-Methylfascaplysin, a Marine-Derived Bioactive Compound, Promotes Neurite Outgrowth via the Inhibition of ROCK2. Pharmaceuticals, 18(11), 1751. https://doi.org/10.3390/ph18111751







